Abstract
Herein, we have investigated the toxicity of SWCNTs and MWCNTs in vitro and in vivo, and assessed their therapeutic effects on a typical animal model of breast cancer in order to obtain: first, the cytotoxicity effects of CNTs on MC4L2 cell and mice, second the impact of CNTs on ablation of breast tumor. CNTs especially SWCNTs were toxic to organs and induced death at high dosages. In this case, some of the liver cells showed a relative shrinkage which was also confirmed by Annexin test in MC4L2 cells. Moreover, CNTs decreased the tumor volume. BCL2 gene was down-regulated, and BAX and Caspase-3 were also up-regulated in the treated groups with CNTs. As a result, CNTs especially MWCNT in lower dosages can be used as a promising drug delivery vehicle for targeted therapy of abnormal cells in breast cancer.
Subject terms: Breast cancer, Breast cancer
Introduction
Carbon nanotubes (CNTs) are one of the well-known and prominent materials that express the new ideas in the biomedicine by offering the potential approaches of diagnosis and treatment of the diseases1. They belongs to nano-materials with tiny sizes which can be divided into two main categories according to the structure; single-wall carbon nanotubes (SWCNTs) and multi-wall carbon nanotube (MWCNTs)2. SWCNTs are contained the tube-shaped carbon benzene rings and MWCNTs are made of multiple layers of CNTs. Both cylindrical-shaped CNTs have the unique properties including the high diameter ratio, remarkable surface density, low weight, electrical properties, and high thermal and chemical stability3. CNTs can play a critical role in the biotechnological and biomedical applications such as tissue engineering, biosensors, drug delivery, and cancer cell death4,5. In fact, they can act as mediators and nano-carriers for the targeted cancer therapy. In this condition, CNTs can deliver the chemotherapy drugs such as doxorubicin, cisplatin and etc., or genes including plasmid DNA, small-interfering RNA, oligonucleotides and RNA/DNA aptamers, proteins, and immunotherapy components to the target cell or tissue6. However, CNTs toxicity is one of the major concerns of their applying in the diagnostic or therapeutic areas. Cytotoxicity of CNTs is determined by various factors including purity, functionalization moieties, dimension, as well as concentration7. In vivo and in vitro studies have confirmed that CNTs can cause the cytotoxicity effects via oxidative stress induction8. There are so many studies on the pulmonary toxicity of CNTs by using animal models. Depending on the doses of CNTs, some studies showed the persistent pulmonary inflammation9–14, transient inflammation15–17, and no inflammation11,18,19. It has been confirmed that CNTs can cause the cardiovascular toxicity20 and increase susceptibility to cardiovascular diseases21 such as ischemia22 as well as an increase in the resting heart rate23. A very low neurotoxicity of CNTs was observed by Chen et al.24 and Ema et al.25, while Gholamine et al. reported that, depending on the type of CNTs, they can result in the neurotoxicity along with depression or anxiety26. It has been demonstrated that the high dosages of CNTs result in the liver and the spleen inflammation, but they are relatively safe in lower dosages27. The in vivo splenic toxicity of MWCNTs has been also investigated and no signs of the toxicity was observed in the spleen even by high doses of MWCNTs28. Results of another study confirm that SWCNTs can cause the oxidative damage to the kidney and the brain tissues29. The previous studies have also shown the contradictory results about the toxicity of CNTs on different cells and organs. Since, SWCNT and MWCNT have their own unique structures and features, they can show the different toxicity on the cells30. A few reports have been published concerning the difference between SWCNTs and MWCNTs impacts on the cells and major organs. For instance, Fujita et al. reported that SWCNTs result in the persistent pulmonary inflammation, while MWCNTs can cause the transient pulmonary inflammation31. Moreover, Jia et al. investigated the in vitro cytotoxicity of SWCNTs and MWCNTs in the alveolar macrophages, and observed an impaired phagocytosis at a low dose of SWCNT, while MWCNT resulted in the identical outcomes at a higher dose, revealing higher toxicity of SWCNT than MWCNT32.
Although in vitro observations clarify the potential of CNTs in the biological systems, the thorough examinations of the animals are needed to associate the observed in vitro impacts of CNTs with in vivo outcomes. In the present study, we investigated the toxicity of CNTs with the different doses to identify the toxic doses in vitro and in vivo. Furthermore, the effects of CNTs were assessed on cancerous cell lines through apoptosis pathways. The clinical observations, hematological/blood chemistry tests, and the histological examinations were conducted to evaluate their efficacy in a typical animal model of breast cancer.
Results
The effects of CNTs on the cell viability
CNTs significantly suppressed the cell viability of MC4L2 in a time and dose-dependent manner. Half-maximal inhibitory concentration (IC50) of SWCNT and MWCNT was 50 and 400 µg/ml after 48 h, respectively (Figs 1 and 2).
Figure 1.
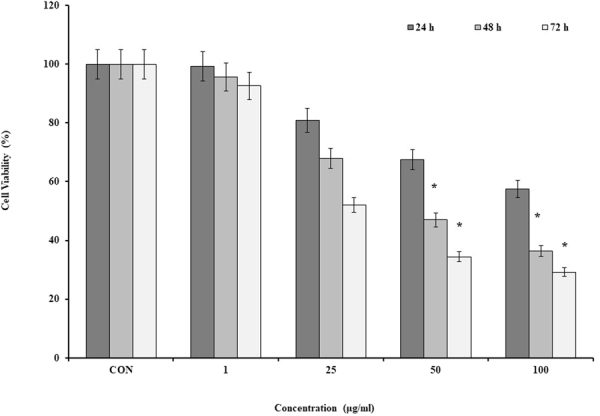
The cell viability of the MC4L2 by SWCNT. Data are the mean values for triplicate experiments. The cells were treated with the various concentrations of SWCNT for 24, 48 and 72 h. The results were expressed as mean ± SD. Significance levels are *P < 0.05 compared to the control.
Figure 2.
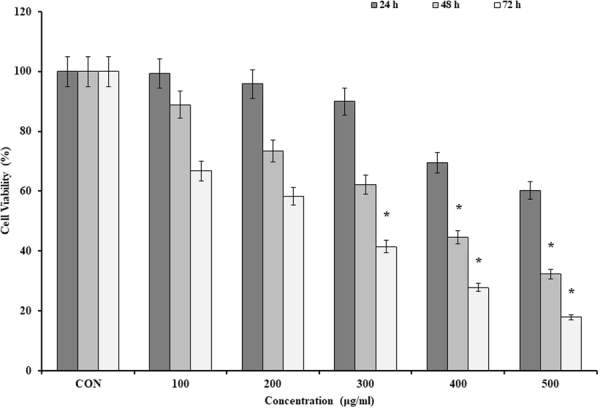
The cell viability of the MC4L2 by MWCNT. Data are the mean values for triplicate experiments. The cells were treated with the various concentrations of MWCNT for 24, 48 and 72 h. The results were expressed as mean ± SD. Significance levels are *P < 0.05 compared to control.
CNTs-induced apoptosis in MC4L2 cells
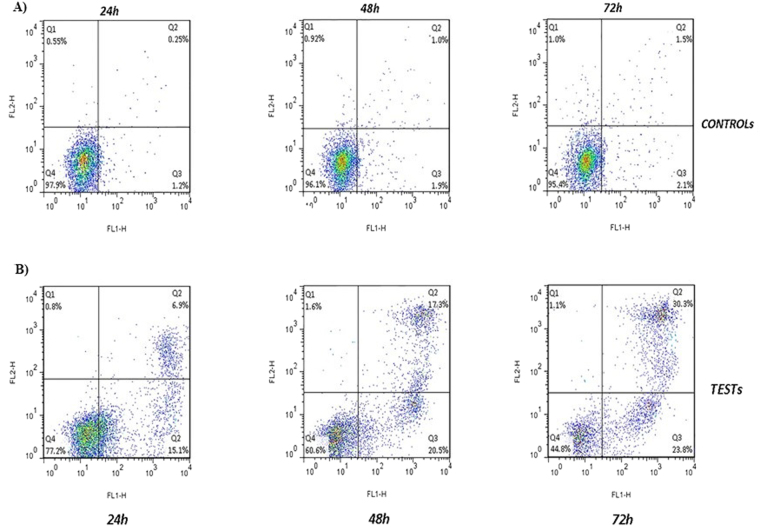
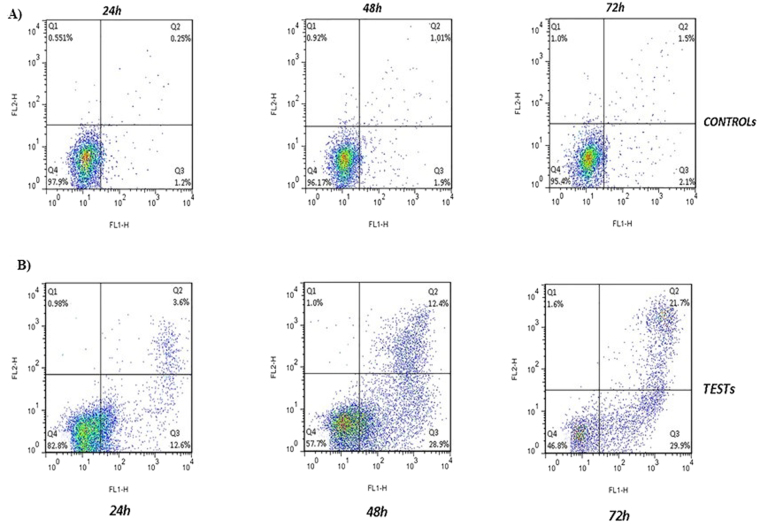
In order to perform the Annexin test, 50 and 400 µg/ml of the SWCNT and MWCNT were used and the percentage of the cell apoptosis after 24, 48, and 72 h was assessed by the flow cytometry (Figs 3 and 4). The treated cells with 50 µg/ml SWCNT showed the apoptotic cell induction in Q3 region compared to the control group. The apoptotic cells in this region were increased from %1.7 to %12.6 after 24 h, and %28.9 and %29.9 after 48 and 72 h, respectively. About the Q2 region, they were increased from %3.6 at 24 h to %12.4 and %21.7 at 48 and 72 h, respectively (p < 0.01) (Figs 5 and 6). Moreover, the treated cells with 400 µg/ml MWCNT showed an induction of the cell apoptosis in the Q3 region compared to the control group. The apoptotic cells in this region were increased from %1.7 to %15.1 after 24 h and %20.5 and %23.8 after 48 and 72 h, respectively. About the Q2 region, they were increased from %6.9 at 24 h to %17.3 and %30.3 at 48 and 72 h, respectively (p < 0.01). Generally, the Q2 and Q3 regions in the treated cells with CNTs have shown an increased percentage of the apoptotic cells over time which can confirm that the cell apoptosis induction was the time-dependent (Figs 3 and 4).
Figure 3.
Flow cytometry assay to evaluate the effects of the MWCNT on the cell apoptosis induction. (A) Control cells: the cells were harvested, stained with Annexin V-FITC (FL-I) after 24, 48 and 72 h and analyzed by the flow cytometry. (B) MWCNT-treated cells: after incubation with MWCNT for 24, 48, and 72 h, the cells were harvested, stained with Annexin V-FITC (FL-I) and analyzed by the flow cytometry.
Figure 4.
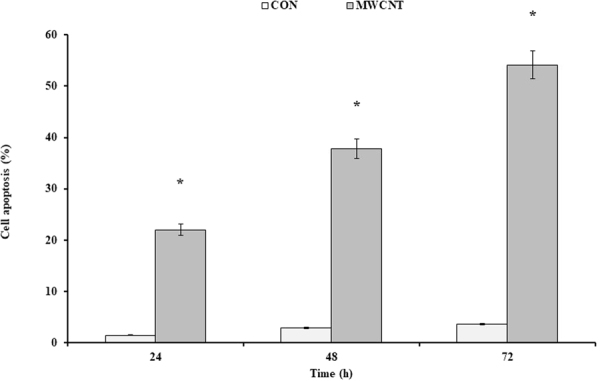
The effects of the MWCNT on the cell apoptosis induction. Annexin test was done at the IC50 concentration of the MWCNT (400 μg/ml). The results were expressed as mean ± SD. Significance levels are *P < 0.05 compared to control.
Figure 5.
Flow cytometry assay to evaluate the effects of the SWCNTs on the MC4L2 cell death. (A) Control cells: the cells were harvested, stained with Annexin V-FITC (FL-I) after 24, 48, and 72 h and analyzed by flow cytometry. Four populations are resolved. Viable cells or Annexin-FITC (−) are seen in the lower left quadrant (Q4 area). The cells with Annexin V-FITC (+) are apoptotic (upper right, Q2 area). The cells with Annexin V-FITC (+) have been described as necrotic or advanced apoptotic (upper left, Q1 area) and Annexin V-FITC (−) may be bare nuclei in first apoptotic (lower right, Q3 area). (B) SWCNT-treated cells: after incubation with the SWCNT for 24, 48, and 72 h, the cells were harvested, stained with Annexin V-FITC (FL-I) and analyzed by flow cytometry.
Figure 6.
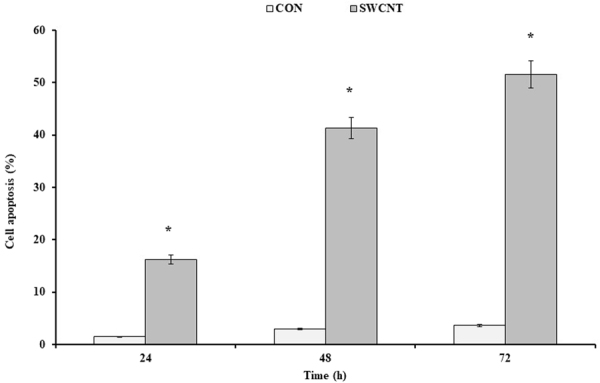
The effects of the SWCNT on the cell apoptosis induction. Annexin test was done at the IC50 concentration of the SWCNT (50 μg/ml). The results were expressed as mean ± SD. Significance levels are *P < 0.05 compared to control.
Dosing procedure
Some doses of CNTs including 200, 100, 40, 20 and 5 mg/kg were associated with the death of mice, while under 1 mg/kg CNTs no death was observed. The weight of the treated animals didn’t change significantly within 7 days post-injection in comparison with the control groups (P = 0.3554).
CBC analysis was used to measure the main hematological markers of the whole blood. The hemoglobin, RBC, WBC and HCT counts were within the expected range in the treated animals. The treated animals with 1 mg/kg SWCNT showed a significant increase in MCH and MCHC levels (P < 0.0001), but animals which were treated with the MWCNT didn’t show any significant changes. All the treated animals with SWCNT and MWCNT showed about the 0.9-fold decrease in MCV count (P = 0.0025) and 1.5-fold increase in PLT count (P < 0.0001) compared to the control group.
We measured some biochemical parameters including the total protein, glucose, ALP, albumin, creatinine, direct bilirubin, HDL, cholesterol, urea and triglyceride, which were significantly decreased in the treated groups compared to control, while SGPT showed about 0.8- and 1.5-fold increase in the treated animals with MWCNT and SWCNT, respectively, compared to the control group (p = 0.0421). SGOT was also showed a significant increase in the treated groups (about 2-fold in MWCNT and 3-fold in SWCNT treated animals) in comparison with the control group (P < 0.0001).
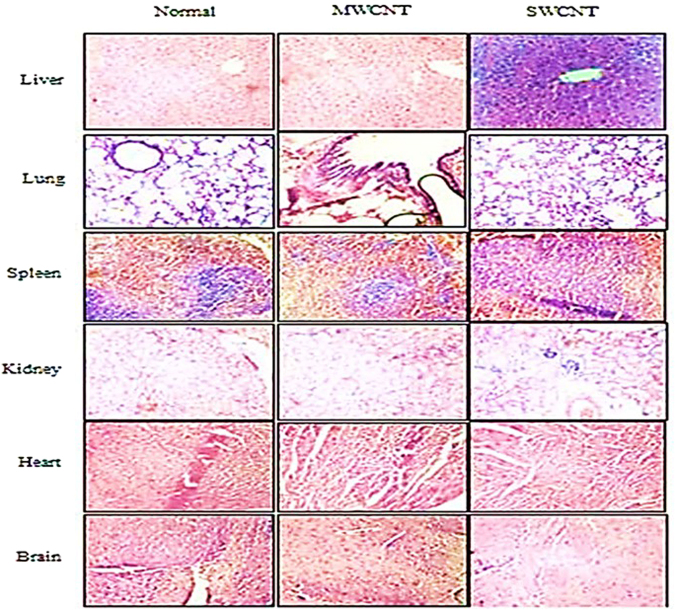
In order to evaluate the CNTs toxicity on the major organs such as the liver, kidney, heart, lung, spleen, and brain, these organs were studied at the histopathological level. No histological abnormalities were found in the tissues of the treated mice except the liver which showed a relative shrinkage in some of its cells compared to the control group (Fig. 7).
Figure 7.
Light microscopic analysis of 6 major tissues the incubated by CNTs. The tissue samples were fixed and preserved in 4% buffered formaldehyde for at least 24 h. Paraffin blocks were then sectioned by 3–5 μm thickness for hematoxylin and eosin staining. Slides were studied by OLYMPUS-BX51 microscope. A relative shrinkage was observed in some of the liver cells (×10).
The tumor weight and size
No significant difference in the animal weight was observed among the groups during the study (Fig. 8).
Figure 8.
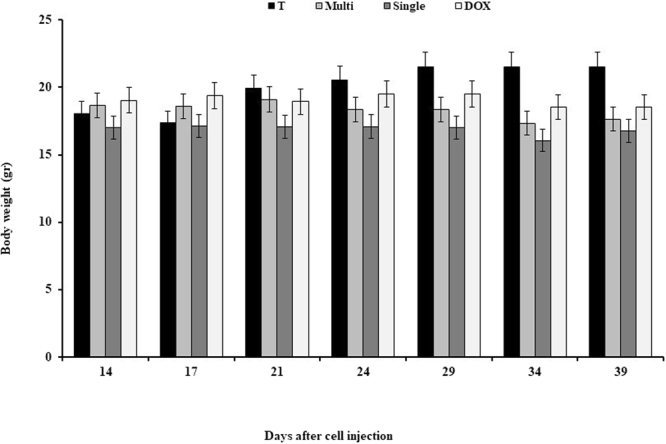
Effects of CNTs on the body weight on days 14, 21, 29, and 39 after cell injection in an animal model of breast cancer. Data were reported mean ± SD. *P < 0.05 in comparison to the tumor group. T: tumor, DOX: doxorubicin, M: MWCNT, S: SWCNT.
The treated mice had smaller tumor volume (mm3) at the end of the second week compared to the control group. Average tumor volume in the treated groups with SWCNT and MWCNT was significantly less than Doxorubicin group, while no significant difference has been observed between SWCNT and MWCNT groups (Fig. 9). In Fig. 10, we have demonstrated the macroscopic views of the tumor extension in the control and treated groups on days 14, 21, 29, and 39 after cell injection.
Figure 9.
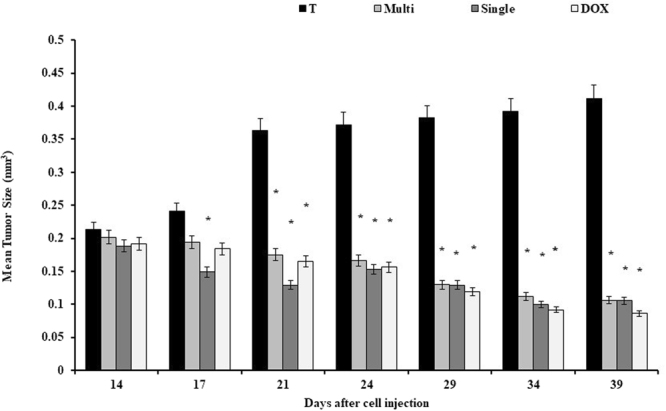
Effects of CNTs on the tumor volume on days 14, 21, 29, and 39 after cell injection in an animal model of breast cancer. Data were reported mean ± SD. *P < 0.05 in comparison to the tumor group. T: tumor, DOX: doxorubicin, M: MWCNT, S: SWCNT.
Figure 10.
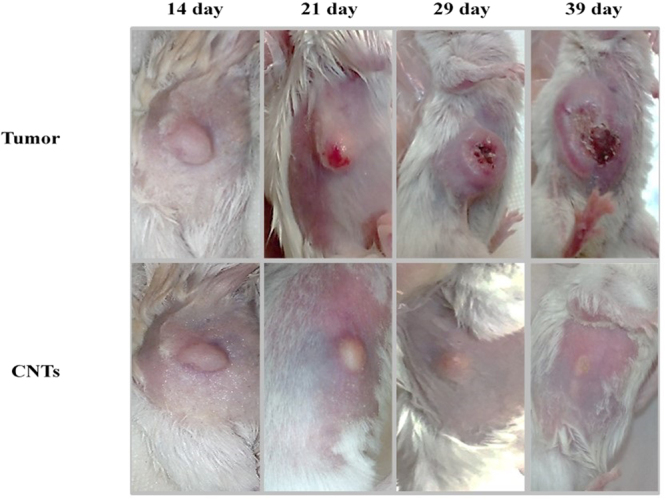
Effects of CNTs on the macroscopic views of the tumor extension on days 14, 21, 29, and 39 after cell injection in an animal model of breast cancer. Data were reported mean ± SD. *P < 0.05 in comparison to the tumor group. T: tumor, DOX: doxorubicin, M: MWCNT, S: SWCNT.
The expression of the anti/pro-apoptotic genes of the tumor tissues
The expression of BCL2, BAX, and Caspase-3 in the tumor samples (the treated mice with 0.25 mg/kg SWCNT, 0.5 mg/kg MWCNT, and 2.5 mg/kg Doxorubicin) compared to the control was as follow: the expression of BCL2 in the tumor treated with MWCNT was significantly decreased in comparison with the control group (p < 0.05). BAX showed a significant increase in the treated tumor with MWCNT compared to the control group, but no significant difference was observed between other groups. Caspase-3 expression showed a significant difference in the MWCNT group compared to the tumor (Fig. 11).
Figure 11.
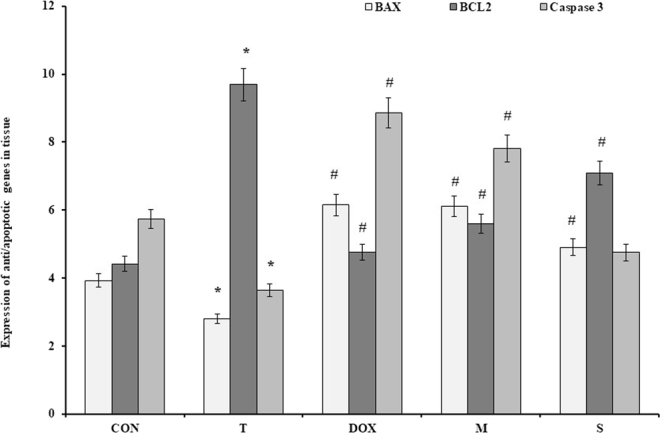
Effects of CNTs on the expression of BAX, BCL2 and Caspase-3 genes in mice breast cancer. Data were reported mean ± SD. *P < 0.05 compared to the control group, #P < 0.05 compared to the tumor group. CON: Control, T: tumor, DOX: doxorubicin, M: MWCNT, S: SWCNT.
Discussion
The main goal of this study was to develop a novel strategy for treatment of breast cancer by CNTs. The pristine CNTs are insoluble in aqueous solutions and chemically inert, so they are not appropriate for utilization in biological or medical applications. In order to use CNTs in these applications, they are usually oxidized in the strong acids to create carboxyl and hydroxyl groups on their surface33. In this condition, CNTs are dispersed in the aqueous solution and other nanomaterials or biomolecules such as oligonucleotides, proteins or peptides. This kind of CNTs can be applied in the biological or medical applications34,35. On the other hand, evaluation and characterization of the toxicity of CNTs are critical because they can cause the adverse health effects in human and other organisms36,37. So far, a few studies have been reported the toxic effects of CNTs either in vivo or in vitro, and the results are usually divergent38–42. Thus in this study, we investigated the toxicity effects of the SWCNTs and MWCNTs both in vitro and in vivo.
The different impacts of SWCNT and MWCNT on the normal and tumor tissues and the expression of anti/pro-apoptotic genes (BCL2, BAX, and Caspase-3) were assessed. We investigated the CNTs effects on cell viability of MC4L2 cells. The IC50 values for MC4L2 cells were 50 µg/ml (SWCNT) after 24 h and 400 µg/ml (MWCNT) after 48 h. The viability of the cells was the maximum after 24 h which reduced after 48 h and eventually all concentrations were led to the reduction of the cell viability after 72 h. In this case, 100 µg/ml SWCNT and 500 µg/ml MWCNT concentrations resulted in the minimum cell viability. It suggests a direct relation between time and concentration of CNTs to induce toxicity. Likewise, flow cytometry analysis determined that CNTs induced the apoptosis in MC4L2 cells over time and confirmed that the CNTs-mediated apoptosis is time-dependent. Our results are in accordance with the findings of Ursini et al. revealing that MWCNT is toxic for the human lung epithelial cells (A549), and with increasing the concentration of MWCNT the cell viability decreases43. Di Giorgio et al. have also investigated the cyto-and genotoxic effects of SWCNTs and MWCNTs on the mouse macrophage cell line RAW 264.7. It has been also demonstrated that the exposure of rat glioma cell line (C6 cells) to MWCNT (200–400 mg/ml) results in a concentration-dependent cell apoptosis, G1 cell cycle arrest, and subsequent cytotoxicity. So, it has been suggested that MWCNT in the smaller size is probably more toxic than that of the larger one44. In the current study, the high doses of CNTs (5 mg/kg and higher) could be the reason for the death of mice. The treated mice with doses of 0.05 and 0.5 mg/kg MWCNT, and 0.25 and 1 mg/kg SWCNT didn’t show the statistically significant weight changes during 7 days post-injection compared to the control group. The higher levels of SGOT and SGPT in the treated mice confirm that the CNTs are toxic to the liver. The treated mice with SWCNT showed higher levels of these two parameters compared to MWCNT group, confirming that the toxicity of SWCNT can be more pronounced than the MWCNT. Based on this data it may be deduced that MWCNTs are more appropriate for the medical applications. Furthermore, some of the liver cells in the treated mice showed a relative shrinkage at a microscopic level, indicating the initiation of the cell apoptosis. In this context, the shape, size, purity, and the ways of exposure to the CNTs affect the toxicity of this nanoparticle45,46. In some studies was reported that CNTs are toxic to the lungs and these tissues are the most susceptible organs to the CNTs-induced cytotoxicity among the other organs7,8,47.
In the other part of this study, we have investigated the Doxorubicin and CNTs effects on the breast cancer tumor. We observed that the treated mice with the CNTs and Doxorubicin had smaller tumor volume (mm3) at the end of the study compared to the control animals. Average tumor volume was significantly less in the treated groups with SWCNT and MWCNT compared to Doxorubicin group, while no significant difference has been observed between SWCNT and MWCNT groups. Breast cancer is a disease in which a number of the signaling pathways such as apoptosis, proliferation, angiogenesis, DNA repair, and metastasis can be affected by nanomaterials. In these pathways, a wide variety of the genes including tumor suppressors, oncogenes, transcription factors, and pro- and anti-apoptotic genes can be deregulated which in turn cause tumorigenesis48–50. In breast cancer, an imbalance in the pro- and anti-apoptotic member of the BCL2 family has been observed due to many genetic alterations. In fact, a decline in BCL2/BAX ratio indicates the overall propensity of cells to undergo apoptosis51. Furthermore, in the mitochondrial pathway of apoptosis Caspase-3 causes the cell apoptosis by cleaving cellular proteins52,53. It has been demonstrated that exposure to CNTs can inhibit cell proliferation, induce membrane destabilization, reduce cell adherence ability, and cause oxidative stress35,54–57. Oxidative stress, in turn, leads to induction of the mitochondrial pathway of apoptosis58. There are the controversial findings on the CNTs-initiated apoptosis, in which some researchers found that CNTs caused apoptosis and inflammation19,42,59, while others demonstrated that CNTs caused apoptosis without inflammation or lead to the cytotoxicity without causing apoptosis60–62. These differences are due to the CNT structure, fabrication route, size, surface chemistry, purity, and their applied concentration63. In order to gain insight into mitochondrial apoptosis induction mediated by CNTs in breast cancer, in the present study we studied the impact of CNTs on the expression of BCL2, BAX, and Caspase-3 under in vivo situation. Since Doxorubicin is a powerful anti-tumoral drug and one of the most active agents for the treatment of breast cancer64. We have also evaluated the effects of this drug on the expression of these three genes. The expression of BCL2 in the tumor treated with MWCNT (0.5 mg/kg) was significantly decreased in comparison to the control. BAX showed a significant increase in the treated tumors with MWCNT compared to the control group, while no significant difference observed among other groups (0.25 mg/kg SWCNT and 2.5 mg/kg Doxorubicin). Our findings are consistent with the results of Sohaebuddin et al. reporting that MWCNT can induce the cell apoptosis in vitro by activating Caspase-3/765. According to Pilco-Ferreto et al. study, Doxorubicin could induce the cell apoptosis by up-regulating BAX, Caspase-8 and Caspase-3 and down-regulation of BCL2 protein expression66. Gorji et al. also observed that Doxorubicin decreased BCL2 expression levels (0.1 ± 0.07) and increased BAX expression levels (2.1 ± 0.1) in the myocardium compared to control group67.
Conclusion
The CNTs (especially SWCNT) are toxic to organs at the high dosages and can cause mice death, while their toxicity decreased by reducing their concentration. In this case, just some of the liver cells showed a relative shrinkage, which is indicative of the initiation of cell apoptosis supported by Annexin test in MC4L2 cells. Moreover, CNTs cause more shrinkage in breast tumor than Doxorubicin. The tumor tissues treated with 0.5 mg/kg MWCNT showed down-regulation of BCL2 and up-regulation of BAX compared to controls which in turn induces apoptosis in cells, but Caspase-3 didn’t show a significant difference among groups. A treated group with Doxorubicin didn’t show significant the expression changes compared to control group. As a result, CNTs specially MWCNT in lower dosages can be used as a promising drug delivery carrier for targeted therapy of abnormal cells in breast cancer, and treating these patients would be more effective with fewer side effects than Doxorubicin.
Materials and Methods
Materials
CNTs with <99% purity (SWCNT: 1–2 nm length, MWCNT: 20–30 nm length) were purchased from Notrino Co (St. Sadeghiye, Tehran, Iran). The MC4L2 cell line was purchased from Iranian Biological Resource Center (Tehran, Iran). Dulbecco’s Modified Eagle Medium (DMEM) cell culture and FBS were purchased from Scotland (Gibco, Scotland). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) powder and Doxorubicin were purchased from Sigma Co. Annexin kit was purchased from Iq products, Poland. Ketamine and Xylazine were purchased from (Sigma Aldrich Co, USA). The BALB/C mouse was purchased from Iran Pasteur Institute (Tehran, Iran). TRIzol solution and 2× Real Rime SYBER GREEN Master Mix were purchased from Invitrogen, USA. The first standard cDNA synthesis kit and nuclease-free water were purchased from Representing of Fementas Co. (USA).
The study design
The present study has been done on four series of experiments in order to obtain: first, the cytotoxicity effect of CNTs on cancerous cell line and detection of apoptosis; second, in vivo toxicity effects of CNTs on the histopathology and the blood parameters in BALB/c mice; third, the impact of CNTs on breast tumor volume and eventually the effects of CNTs on the expression of BCL2, BAX and Caspase-3 in a typical animal model of breast cancer.
Animals
All inbred wild-type female BALB/c mice (6–8 weeks old, purchased from Pasteur Institute, Karaj, Iran) preserved in large group house under 12-h dark and light cycles and had access to food and water ad libitum.
Statement
All methods and experiments were performed in compliance with the guidelines of the Declaration of Helsinki (DOH), and its later amendments or Comparable ethical standards. The experimental procedures and the animal use and care protocols were approved by review board committee of IAUPs (Islamic Azad University, pharmaceutical science branches, Tehran, Iran).
The preparation of CNT stock
CNTs were dissolved in DMSO 1% and homogeneous in sonication machine for 5 min and kept at room temperature30. Also, SWCNT and MWCNT were fully characterized by transmission electron microscopy (TEM), scanning electron microscopy (SEM), dynamic light scattering (DLS), surface tension, UV-visible spectroscopy, and zeta potential investigations in our previous paper30.
Cell culture
MC4L2 cell line (mice estrogen receptor-positive breast carcinoma cells, obtained from Iranian Biological Resource Center, Tehran, Iran) was cultured in DMEM medium, supplemented with FBS (10%), penicillin (100 units/mL) and streptomycin (100 μg/mL). The cells were grown in a CO2 incubator (Memmert, Germany) at 37 °C.
MTT assay
MTT colorimetric assay was used to measure the anti-proliferative effects of SWCNT and MWCNT. Concisely, MC4L2 cells were seeded in 96-well microplates at a density of (1 × 106 cells/mL). After cell attachment, the plates were washed twice with phosphate buffered saline (PBS), and then cells were cultured in DMEM medium with varying concentrations of SWCNT (1, 25, 50 and 100 µg/ml) and MWCNT (100, 200, 300, 400 and 500 µg/ml) for 24, 48, and 72 h30. These concentrations of CNTs were selected based on literature survey and our experimental conditions. Indeed, blow 1 and 100 µg/ml of SWCNT and MWCNT, respectively no marked cytotoxicity was observed. Higher doses of CNTs also result in agglomeration of NPs and subsequent to their colloidal stability. Therefore, the selected concentration range was selected. MC4L2 cells in culture medium without SWCNT and MWCNT were designated as reference blanks. 20 μl of MTT (5 mg/mL) was then added to each well and the plates were incubated at 37 °C for 4 h in a 5% CO2 incubator. The medium was then carefully removed, and the purple products were lysed in 100 μl dimethyl sulfoxide. Relative cell viability was then determined using a microplate reader (Expert 96, Asys Hitch, Ec Austria) at 570 nm. Cytotoxicity was expressed as a percentage of the growth inhibition, relative to the control culture value, and the concentration of CNTs required to inhibit MC4L2 cell growth by 50% (IC50) was calculated. Each experiment was repeated in triplicate to calculate the standard error and finally, statistical analysis was performed using the one way ANOVA test68.
Annexin-PI assay
Annexin-PI assay was used to detect the plausible percentage of apoptosis induced by SWCNT and MWCNT in MC4L2 cell line. A suspension of the cells including 15000 cells per ml was prepared. One ml of the suspension and 2 ml of medium containing %10 FBS were added to 24 well plates, then incubated for 24 h (%5 CO2, %98 humidity, and 37 °C). After that, 50 µg/ml SWCNT and 400 µg/ml MWCNT (concentrations which led to %50 cell death) were added to the wells and incubated for 24, 48 and 72 h. After washing plates with PBS and centrifugation, the sediments were transferred into the 1.5 ml microtubes and 2 µl binding buffer was then added to samples except for the control tube. 10 µl Annexin was added and kept in the dark on ice for 30 min. Then, 10 µL PI was added to tubes. Finally, 1 ml PBS added to samples and rapidly assessed by flow cytometer (FACSCalibur, BD Bioscience, CA, USA)30.
The dosing procedure
150 mice were used to study in vivo toxic effects of CNTs. In order to obtain the desired concentration with the maximal toxicity of CNTs, we chose the starting dose administered to mice which were 200 mg/kg and continued increasing the dosage to the amount in which no dying or symptoms of poisoning occurred. If major adverse reactions in animals identified at a certain dose (toxic dose) within 24 h, a decreased dose (usually the mean value between toxic dose and last tolerated dose) were applied to a new group of mice. Animals (one or more) may have shown the onset of major adverse reactions even before all animals (six) in the same group injected; when this occurred, no more animal was injected. Histological damages, abnormal hematological/blood chemical indices, reduced organ weight ratios, and body weight changes were among the signs favoring major toxicity. Therefore, doses of 1, 0.5, 0.25 and 0.05 mg/kg of CNTs were injected, and the animals were euthanized one week after injection. The animals were under observation for abnormal sequels. Animals that survived post CNTs treatment weighed on a daily basis and euthanized one week later. Hematology, blood chemistry, and pathology tests were carried out. Vital organs including heart, liver, spleen, lung, brain, and kidney excised and weighed separately, and fixed with 10% formalin. We prepared the tissue blocks and then slides were evaluated for histopathological changes69.
Pathology, blood and biochemistry parameters tests
At the end of the treatment, we decapitated the animals under general anesthesia. Blood samples were taken and added to ethylene-diamine-tetra-acetic-acid (EDTA)-coated tubes for hematology and heparin-coated tubes for clinical chemistry. Blood samples were collected in anticoagulant tubes to determine the parameters of erythrocyte count (RBC), hematocrit (HCT), hemoglobin, mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), and total leukocyte count (WBC) using cis-Max device. In addition, albumin, cholesterol, creatinine, glucose, hematocrit (HCT), high-density lipoprotein (HDL), serum glutamic pyruvic transaminase (SGPT), serum glutamic-oxaloacetic transaminase (SGOT), triglyceride, total protein, urea, alkaline phosphatase (ALP), and total and direct bilirubin concentration of blood serum from the blood clot of animals using auto-analyzer were obtained for each parameters70.
Histological assay
Formaldehyde (10%) was used to fix the treated and non-treated tissues followed by passage and embedding in paraffin. In order to perform the hematoxylin and eosin (H & E) staining, paraffin blocks were sectioned by 3–5 μm thickness71. Slides were studied at the microscopic level (OLYMPUS-BX51 microscope), then an Olympus-DP12 camera was used to take digital photos and graded by the Scharff-Bloom-Richardson Scale72.
Tumorgenicity
MC4L2 was trypsinized and re-suspended in10-fold excess culture medium. After centrifugation, the cells were re-suspended in a serum-free medium. Prepared cells (1 × 106/0.1 ml) were subcutaneously injected into the right inguinal flank of the female mice under ketamine (50 mg/kg i.p) and xylazine (10 mg/kg i.p.) anesthesia. Two weeks post cell injection, 40 mice were taken and equally divided into the control and tumor groups (treated with Doxorubicin (2.5 mg/kg), SWCNT (0.25 mg/kg) and MWCNT (0.5 mg/kg)). The doses of 0.25 mg/kg SWCNT and 0.5 mg/kg MWCNT were considered as the safe doses. CNTs were injected once a week for two consecutive weeks using a 21-gauge needle under ketamine and xylazine anesthesia in the treated group, while in the control animals, saline solution was administered. Animals were euthanatized on day 28 post cell injection, and the tumors were extracted73.
Tumor size and body weight measurement
The animals were weekly weighed and regularly monitored for abnormal sequels. In order to measure the size of tumors, a digital vernier caliper (Mitutoyo, Japan) was used. Tumor volume was measured on a weekly basis and reported as mm3 using the following formula73:
RNA extraction and Real-Time PCR analysis
In order to define the expression level of BCL2, Caspase-3 and BAX genes, TRIzol solution (Invitrogen, USA) was used to extract total RNA from tissues according to the manufacturer’s instructions. Afterward, cDNA was synthesized by RevertAid first-strand cDNA synthesis kit (Thermo Scientific Fermentas, USA) according to the manufacturer’s instructions. Primer pairs which were used in this study were designed by Beacon Designer 7 software. The primer sequences were as follows: forward, 5′-GAGCCTGTGAGAGACGTGG-3′ and reverse, 5′-CGAGTCTGTGTATAGCAATCCCA-3′ for BCL2 gene; forward, 5′-GATGATTGCTGACGTGGAC-3′ and reverse, 5′-ACGGAGGAAGTCCAGTGTC-3′ for BAX gene; forward, 5′-ATGGGAGCAAGTCAGTGGACTC-3′ and reverse, 5′-GTCTCTCTGAGGTTGGCTGC-3′ for Caspase-3 gene; and forward, 5′-AATGGATTTGGACGCATTGGT-3′ and reverse, 5′-TTTGCACTGGTACGTGTTGAT-3′ for GAPDH gene.
Quantitative RT-PCR (qRT-PCR) was performed on cDNAs using Rotor-Gene Q 2plex HRM platform real-time PCR system (Corbett Life Science). The relative expression levels of BCL2, Caspase-3 and BAX were evaluated in comparison with GAPDH as an endogenous control gene. Each 15 μl reaction volume contained 7.5 μl of the 2× SYBR green master mix (Invitrogen, USA), 1 μl of cDNA, and 0.6 μl (10 ppm) of each pair of oligonucleotide primers. RT-PCR reactions were done in duplicate. The PCR cycling began with an initial step of 95 °C for 10 min followed by 35 cycles of 95 °C for 20 s, 57 °C for 20 s and 72 °C for 15 s; then a melting curve was analyzed. Rotor-gene Q sequence detection system determined the threshold cycle (CT) values. Comparative threshold cycle (2−ΔΔCT) method was used in order to analyze the data74.
Data analysis
Data (gene expression, hematological and biochemical data) were analyzed with GraphPad Prism 7 statistical software (La Jolla, USA) using Analysis of variance (ANOVA) test in order to compare the nominal variables between treated and non-treated mice. Results were expressed as mean ± SD. P < 0.05 was considered statistically significant.
Ethical approval
All procedures performed in the studies involving animal participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgements
The authors would like to thank the Cancer Research Center related to Tehran University of Medical Sciences, for cooperation in the study. The data was provided from the thesis with number “ 160 “ of Ms. A. Kavosi, MSc student of Department of Cellular and Molecular Biology, Faculty of Advanced Sciences and Technology, Islamic Azad University, Tehran. None of the funding sources had any role in the study design, the collection, the analysis and interpretation of data, the writing of the report, or in the decision to submit the article for publication.
Author Contributions
A.K., S.H.G.N. and S.M. study conception and design. S.K., S.K. and H.K. sample collection, sample processing, and article revision. M.M., M.R.K. and M.Y. data analysis and sample processing. A.M.A. and M.F. study conception and design, and manuscript preparation.
Competing Interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41598-023-46596-w
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/15/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-023-46596-w
Contributor Information
Ali Mohammad Alizadeh, Email: aalizadeh@sina.tums.ac.ir.
Mojtaba Falahati, Email: falahati@ibb.ut.ac.ir.
References
- 1.Whitesides GM. The’right’size in nanobiotechnology. Nature biotechnology. 2003;21:1161. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 2.Rashad A, Noaman R, Mohammed S, Yousif E. Journal of Nanoscience and Technology. Journal of Nanoscience and Technology. 2016;2:155–162. [Google Scholar]
- 3.Demczyk B, et al. Direct mechanical measurement of the tensile strength and elastic modulus of multiwalled carbon nanotubes. Materials Science and Engineering: A. 2002;334:173–178. doi: 10.1016/S0921-5093(01)01807-X. [DOI] [Google Scholar]
- 4.Liu Z, Tabakman S, Welsher K, Dai H. Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano research. 2009;2:85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim BY, Rutka JT, Chan WC. Nanomedicine. The New England journal of medicine. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 6.Son KH, Hong JH, Lee JW. Carbon nanotubes as cancer therapeutic carriers and mediators. International journal of nanomedicine. 2016;11:5163. doi: 10.2147/IJN.S112660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madani SY, Mandel A, Seifalian AM. A concise review of carbon nanotube’s toxicology. Nano reviews. 2013;4:21521. doi: 10.3402/nano.v4i0.21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayat J, Gajbhiye V, Tekade RK, Jain NK. Pulmonary toxicity of carbon nanotubes: a systematic report. Nanomedicine: Nanotechnology, Biology and Medicine. 2011;7:40–49. doi: 10.1016/j.nano.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 9.DeLorme MP, et al. Ninety-day inhalation toxicity study with a vapor grown carbon nanofiber in rats. Toxicological Sciences. 2012;128:449–460. doi: 10.1093/toxsci/kfs172. [DOI] [PubMed] [Google Scholar]
- 10.Aiso S, et al. Pulmonary toxicity of intratracheally instilled multiwall carbon nanotubes in male Fischer 344 rats. Industrial health. 2010;48:783–795. doi: 10.2486/indhealth.MS1129. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto, Y. et al. In Journal of Nano Research. 9–25 (Trans Tech Publ).
- 12.Murray AR, et al. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Particle and fibre toxicology. 2012;9:10. doi: 10.1186/1743-8977-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter DW, et al. Mouse pulmonary dose-and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Shvedova AA, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 15.Pauluhn J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicological Sciences. 2009;113:226–242. doi: 10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- 16.Warheit DB, et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicological sciences. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 17.Han SG, Andrews R, Gairola CG. Acute pulmonary response of mice to multi-wall carbon nanotubes. Inhalation toxicology. 2010;22:340–347. doi: 10.3109/08958370903359984. [DOI] [PubMed] [Google Scholar]
- 18.Morimoto Y, et al. Pulmonary toxicity of well-dispersed single-wall carbon nanotubes after inhalation. Nanotoxicology. 2012;6:766–775. doi: 10.3109/17435390.2011.620719. [DOI] [PubMed] [Google Scholar]
- 19.Elgrabli D, et al. Induction of apoptosis and absence of inflammation in rat lung after intratracheal instillation of multiwalled carbon nanotubes. Toxicology. 2008;253:131–136. doi: 10.1016/j.tox.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, et al. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environmental health perspectives. 2007;115:377. doi: 10.1289/ehp.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helfenstein M, et al. Effects of combustion-derived ultrafine particles and manufactured nanoparticles on heart cells in vitro. Toxicology. 2008;253:70–78. doi: 10.1016/j.tox.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Urankar RN, et al. Expansion of cardiac ischemia/reperfusion injury after instillation of three forms of multi-walled carbon nanotubes. Particle and fibre toxicology. 2012;9:38. doi: 10.1186/1743-8977-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosseinpour M, et al. The cardiac effects of carbon nanotubes in rat. BioImpacts: BI. 2016;6:79. doi: 10.15171/bi.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y-S, Hsiue G-H. Directing neural differentiation of mesenchymal stem cells by carboxylated multiwalled carbon nanotubes. Biomaterials. 2013;34:4936–4944. doi: 10.1016/j.biomaterials.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 25.Ema M, et al. Evaluation of genotoxicity of multi-walled carbon nanotubes in a battery of in vitro and in vivo assays. Regulatory Toxicology and Pharmacology. 2012;63:188–195. doi: 10.1016/j.yrtph.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Gholamine B, Karimi I, Salimi A, Mazdarani P, Becker LA. Neurobehavioral toxicity of carbon nanotubes in mice: Focus on brain-derived neurotrophic factor messenger RNA and protein. Toxicology and industrial health. 2017;33:340–350. doi: 10.1177/0748233716644381. [DOI] [PubMed] [Google Scholar]
- 27.Liang G, et al. Effects of subchronic exposure to multi-walled carbon nanotubes on mice. Journal of Toxicology and Environmental Health, Part A. 2010;73:463–470. doi: 10.1080/15287390903523378. [DOI] [PubMed] [Google Scholar]
- 28.Deng X, et al. The splenic toxicity of water soluble multi-walled carbon nanotubes in mice. Carbon. 2009;47:1421–1428. doi: 10.1016/j.carbon.2008.12.032. [DOI] [Google Scholar]
- 29.Shang S, Yang S-Y, Liu Z-M, Yang X. Oxidative damage in the kidney and brain of mice induced by different nano-materials. Frontiers in biology. 2015;10:91–96. doi: 10.1007/s11515-015-1345-3. [DOI] [Google Scholar]
- 30.Zeinabad HA, Zarrabian A, Saboury AA, Alizadeh AM, Falahati M. Interaction of single and multi wall carbon nanotubes with the biological systems: tau protein and PC12 cells as targets. Scientific reports. 2016;6:26508. doi: 10.1038/srep26508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita K, et al. Pulmonary and pleural inflammation after intratracheal instillation of short single-walled and multi-walled carbon nanotubes. Toxicology letters. 2016;257:23–37. doi: 10.1016/j.toxlet.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Jia G, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environmental science & technology. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, et al. Fullerene pipes. Science. 1998;280:1253–1256. doi: 10.1126/science.280.5367.1253. [DOI] [PubMed] [Google Scholar]
- 34.Bottini, M. et al. Covalent decoration of multi-walled carbon nanotubes with silica nanoparticles. Chemical Communications, 758–760 (2005). [DOI] [PubMed]
- 35.Bottini M, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121–126. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Oberdörster G, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle and fibre toxicology. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donaldson K, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicological sciences: an official journal of the Society of Toxicology. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 38.Lam C-W, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicological sciences. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 39.Warheit DB. What is currently known about the health risks related to carbon nanotube exposures? Carbon. 2006;44:1064–1069. doi: 10.1016/j.carbon.2005.10.013. [DOI] [Google Scholar]
- 40.Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicology letters. 2005;155:73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Muller J, et al. Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis. 2008;29:427–433. doi: 10.1093/carcin/bgm243. [DOI] [PubMed] [Google Scholar]
- 42.Patlolla A, Knighten B, Tchounwou P. Multi-walled carbon nanotubes induce cytotoxicity, genotoxicity and apoptosis in normal human dermal fibroblast cells. Ethnicity & disease. 2010;20:S1. [PMC free article] [PubMed] [Google Scholar]
- 43.Ursini CL, et al. Comparative cyto-genotoxicity assessment of functionalized and pristine multiwalled carbon nanotubes on human lung epithelial cells. Toxicology in Vitro. 2012;26:831–840. doi: 10.1016/j.tiv.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Han Y-G, Xu J, Li Z-G, Ren G-G, Yang Z. In vitro toxicity of multi-walled carbon nanotubes in C6 rat glioma cells. Neurotoxicology. 2012;33:1128–1134. doi: 10.1016/j.neuro.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Hussain S, Hess K, Gearhart J, Geiss K, Schlager J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicology in vitro. 2005;19:975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 46.Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. International journal of pharmaceutics. 2002;233:51–59. doi: 10.1016/S0378-5173(01)00923-1. [DOI] [PubMed] [Google Scholar]
- 47.Lanone S, et al. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Particle and fibre toxicology. 2009;6:14. doi: 10.1186/1743-8977-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suter R, Marcum JA. The molecular genetics of breast cancer and targeted therapy. Biologics: targets & therapy. 2007;1:241. [PMC free article] [PubMed] [Google Scholar]
- 49.Wiechec E. Implications of genomic instability in the diagnosis and treatment of breast cancer. Expert review of molecular diagnostics. 2011;11:445–453. doi: 10.1586/erm.11.21. [DOI] [PubMed] [Google Scholar]
- 50.Wiechec E, Overgaard J, Kjeldsen E, Hansen LL. Chromosome 1q25. 3 copy number alterations in primary breast cancers detected by multiplex ligation-dependent probe amplification and allelic imbalance assays and its comparison with fluorescent in situ hybridization assays. Cellular Oncology. 2013;36:113–120. doi: 10.1007/s13402-012-0117-1. [DOI] [PubMed] [Google Scholar]
- 51.Baell JB, Huang DC. Prospects for targeting the Bcl2 family of proteins to develop novel cytotoxic drugs. Biochemical pharmacology. 2002;64:851–863. doi: 10.1016/S0006-2952(02)01148-6. [DOI] [PubMed] [Google Scholar]
- 52.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annual review of biochemistry. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 53.Farsinejad S, Gheisary Z, Samani SE, Alizadeh AM. Mitochondrial targeted peptides for cancer therapy. Tumor Biology. 2015;36:5715–5725. doi: 10.1007/s13277-015-3719-1. [DOI] [PubMed] [Google Scholar]
- 54.Shvedova A, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. Journal of toxicology and environmental health Part A. 2003;66:1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 55.Kagan V, et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicology letters. 2006;165:88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicology letters. 2005;155:377–384. doi: 10.1016/j.toxlet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Manna SK, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-κB in human keratinocytes. Nano letters. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 59.Hu X, et al. In vitro evaluation of cytotoxicity of engineered carbon nanotubes in selected human cell lines. Science of the Total Environment. 2010;408:1812–1817. doi: 10.1016/j.scitotenv.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 60.Tabet L, et al. Adverse effects of industrial multiwalled carbon nanotubes on human pulmonary cells. Journal of Toxicology and Environmental Health, Part A. 2008;72:60–73. doi: 10.1080/15287390802476991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Giorgio ML, et al. Effects of single and multi walled carbon nanotubes on macrophages: cyto and genotoxicity and electron microscopy. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2011;722:20–31. doi: 10.1016/j.mrgentox.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Yehia HN, et al. Single-walled carbon nanotube interactions with HeLa cells. Journal of Nanobiotechnology. 2007;5:8. doi: 10.1186/1477-3155-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, et al. Multi-walled carbon nanotubes induce apoptosis via mitochondrial pathway and scavenger receptor. Toxicology in Vitro. 2012;26:799–806. doi: 10.1016/j.tiv.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological reviews. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 65.Sohaebuddin SK, Thevenot PT, Baker D, Eaton JW, Tang L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Particle and fibre toxicology. 2010;7:22. doi: 10.1186/1743-8977-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilco-Ferreto N, Calaf GM. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. International journal of oncology. 2016;49:753–762. doi: 10.3892/ijo.2016.3558. [DOI] [PubMed] [Google Scholar]
- 67.Gorji, S. M. & Malekshah, A. Effect of doxorubicin on Bcl2 and Bax expression in Rat heart. Journal of Gorgan University of Medical Sciences15 (2013).
- 68.Johari-Ahar M, et al. Methotrexate-conjugated quantum dots: synthesis, characterisation and cytotoxicity in drug resistant cancer cells. Journal of drug targeting. 2016;24:120–133. doi: 10.3109/1061186X.2015.1058801. [DOI] [PubMed] [Google Scholar]
- 69.Alizadeh, A. M. et al. Encapsulation of curcumin in diblock copolymer micelles for cancer therapy. BioMed research international2015 (2015). [DOI] [PMC free article] [PubMed]
- 70.Mohsenikia M, et al. Therapeutic effects of dendrosomal solanine on a metastatic breast tumor. Life sciences. 2016;148:260–267. doi: 10.1016/j.lfs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 71.Alizadeh AM, et al. Apoptotic and proliferative activity of mouse gastric mucosa following oral administration of fumonisin B1. Iranian journal of basic medical sciences. 2015;18:8. [PMC free article] [PubMed] [Google Scholar]
- 72.Amat S, et al. Scarff-Bloom-Richardson (SBR) grading: a pleiotropic marker of chemosensitivity in invasive ductal breast carcinomas treated by neoadjuvant chemotherapy. International journal of oncology. 2002;20:791–796. [PubMed] [Google Scholar]
- 73.Khori V, et al. Effects of exercise training together with tamoxifen in reducing mammary tumor burden in mice: Possible underlying pathway of miR-21. European journal of pharmacology. 2015;765:179–187. doi: 10.1016/j.ejphar.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 74.Isanejad A, et al. MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life sciences. 2016;151:30–40. doi: 10.1016/j.lfs.2016.02.090. [DOI] [PubMed] [Google Scholar]