Abstract
Ustilaginoidea virens is the causal agent of rice false smut, one of the major fungal diseases of rice. However, there are only limited molecular studies with this important pathogen due to the lack of efficient approaches for generating targeted gene disruption mutants. In this study, we used the CRISPR-Cas9 system to efficiently generate mutants deleted of the USTA ustiloxin and UvSLT2 MAP kinase genes. Three gRNA spacers of USTA, UA01, UA13, and UA21, were expressed with the RNAP III promoter of Gln-tRNA. For all of them, the homologous gene replacement frequency was higher when the Cas9 and gRNA constructs were transformed into U. virens on the same vector than sequentially. UA01, the spacer with the highest on-target score, had the highest knockout frequency of 90%, which was over 200 times higher than that of Agrobacterium tumefaciens-mediated transformation (ATMT) for generating ustA mutants. None of these USTA spacers had predicted off-targets with 1 or 2-nt variations. For predicted off-targets with 3 or 4-nt variations, mutations were not detected in 10 ustA mutants generated with spacer UA13 or UA21, indicating a relatively low frequency of off-target mutations in U. virens. For UvSLT2, the homologous gene replacement frequency was 50% with CRISPR-Cas9, which also was significantly higher than that of ATMT. Whereas ustA mutants had no detectable phenotypes, Uvslt2 mutants were slightly reduced in growth rate and reduced over 70% in conidiation. Deletion of UvSLT2 also increased sensitivity to cell wall stresses but tolerance to hyperosmotic or oxidative stresses. Taken together, our results showed that the CRISPR-Cas9 system can be used as an efficient gene replacement or editing approach in U. virens and the UvSlt2 MAP kinase pathway has a conserved role in cell wall integrity.
Keywords: rice false smut, MAP kinase, pathogenesis, gene knockout, ustiloxins
Introduction
Rice false smut is a destructive disease caused by the ascomycete Ustilaginoidea virens (Teleomorph Villosiclava virens). In the past decade, this disease has becoming one of the major fungal diseases that threaten rice production (Zhou et al., 2008). In infected kernels, smut balls containing darkly-pigmented chlamydospores are developed instead of rice seeds. U. virens is also a producer of toxic secondary metabolites, including ustiloxins that are toxic to plants and animals by interfering with the microtubule functions (Tsukui et al., 2015).
Unlike many other plant pathogenic fungi, there are only limited molecular genetic studies in U. virens although its genome was sequenced and published in Zhang et al. (2014). To date, only the UvHOG1, UvSUN2, Uvt3277, and UvPRO1 genes have been functionally characterized by deletion or disruption in this important plant pathogenic fungus (Yu et al., 2015; Lv et al., 2016; Zheng et al., 2016, 2017). One major bottleneck for molecular genetic studies with U. virens is its low homologous recombination frequency using the conventional gene replacement approaches. Mutants disrupted of the UvSUN2, Uvt3277, and UvPRO1 genes were generated by random insertional mutagenesis instead of targeted gene deletion or disruption (Yu et al., 2015; Lv et al., 2016; Zheng et al., 2017). For UvHOG1, the only U. virens gene with mutants generated by targeted gene deletion, less than 0.5% of hygromycin-resistant transformants generated by Agrobacterium tumefaciens-mediated transformation (ATMT) were confirmed to true deletion mutants (Zheng et al., 2016). The homologous gene replacement frequency is about 10 times higher in many other plant pathogenic fungi such as the rice blast fungus Magnaporthe oryzae and wheat scab fungus Fusarium graminearum, in which 100s of pathogenicity factors having been characterized (Wang et al., 2011; Choi et al., 2013).
The clustered regularly interspaced short palindromic repeats (CRISPR)-associated RNA-guided DNA endonuclease Cas9 has been extensively used for gene editing in plants and animals by taking advantage of the simple design of a single crRNA:tracrRNA chimeric guide RNA (gRNA). The gRNA contains a 20-bp target sequence that can guide Cas9 to the target locus and cause double-strand breaks (DSB) by Cas9, which triggers targeted gene editing or replacement by homologous recombination (HR) in organisms that have no or very low HR frequency (Cong et al., 2013). Although most filamentous fungi have a higher homologous recombination frequency than animals and plants, the CRISPR-Cas9 system has been reported to improve the HR frequency for targeted gene deletion in several ascomycetes, including Trichoderma reesei, M. oryzae, Neurospora crassa, Alternaria alternata, Penicillium chrysogenum, and Aspergillus niger (Arazoe et al., 2015; Liu et al., 2015; Matsu-ura et al., 2015; Kuivanen et al., 2016; Pohl et al., 2016; Wenderoth et al., 2017).
In this study, we used the CRISPR-Cas9 system to functionally characterize the USTA ustiloxin and UvSLT2 MAP kinase genes in U. virens. Because the U6 promoter is not conserved in U. virens, the promoter of its Gln-tRNA gene was used as the RNAP III promoter to express the sgRNA constructs. For both USTA and UvSLT2, the homologous gene replacement frequency was significantly higher with the CRISPR-Cas9 system than the conventional ATMT approach. For the three USTA gRNA spacers tested, the gene replacement frequency was higher when the Cas9 and gRNA constructs were transformed into U. virens on the same vector than sequentially. The gRNA spacer with the high on-target score had the highest HR frequency and none of the ustA mutants assayed had mutations at predicted off-targets, suggesting a low frequency of off-target mutations. Whereas ustA mutants had no detectable phenotypes in cultures, Uvslt2 mutants were reduced in growth rate and conidiation and had increased sensitivity to cell wall stresses. Taken together, our results showed that the CRISPR-Cas9 system can be used as an efficient gene replacement or editing approach in U. virens and the UvSlt2 MAP kinase pathway has a conserved role in cell wall integrity.
Results
Construction of the tRNA-gRNA Vectors for U. virens Transformation
To develop gRNA vectors suitable for the rice false smut fungus, we first identified the glutaminyl-tRNA (Gln-tRNA) gene in the U. virens genome (Zheng et al., 2014; Mefferd et al., 2015; Xie et al., 2015) with the tRNAscan-SE program (Lowe and Eddy, 1997). The 72-bp Gln-tRNA region was synthesized and fused with the 108-bp gRNA by fusion PCR with primers listed in Supplementary Table S1. The resulting PCR product with two BsaI sites between Gln-tRNA and gRNA was then cloned between the HindIII and EcoRI sites of plasmid pUC-H1-gRNA that carries the geneticin-resistance marker (GenR) (Zheng et al., 2014) to generate the pUC19-tRp-gRNA vector (Figure 1). A short cassette with two BsmBI sites was generated by annealing the sense and antisense oligonucleotides LINK-F and LINK-R (Supplementary Table S1) and inserted between the two BsaI sites of pUC19-tRp-gRNA by Golden Gate cloning (Ran et al., 2013; Arazoe et al., 2015). In the resulting construct, the two BsaI sites in the Gln-tRNA-gRNA cassette were replaced with two BsmBI sites. The modified Gln-tRNA-gRNA cassette was then inserted between the KpnI and EcoRI sites of pCRISPR/Cas-U6-1 (Arazoe et al., 2015) to generate the pCas9-tRp-gRNA vector (Figure 1).
FIGURE 1.
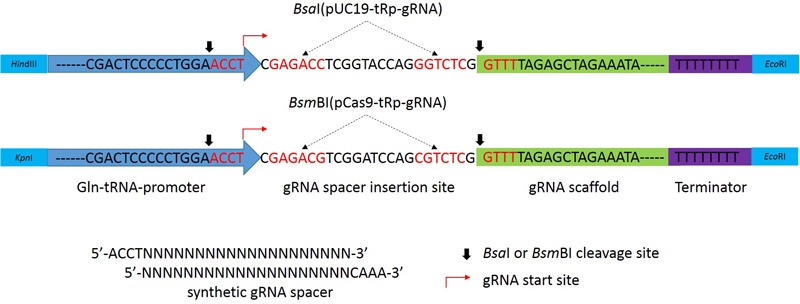
Diagram of the modified Gln-tRNA-gRNA cassettes in pUC19-tRp-gRNA and pCas9-tRp-gRNA vectors. The blue arrow on the left is the Gln-tRNA promoter that is followed by the gRNA spacer insert site, gRNA scaffold (green), and RNA polyIII terminator (purple) sequences. The gRNA spacers are synthesized by annealing the sense and antisense oligonucleotides with 5′-ACCT and 3′-CAAA overhangs and inserted into the gRNA spacer insertion site of BsaI-digested pUC19-tRp-gRNA (upper) or BsmBI-digested pCas9-tRp-gRNA (lower). The gRNA start site is marked with a red arrow. The cleavage sites of type II restriction enzyme BsaI and BsmBI are marked with black arrows.
Generation of U. virens Transformants Expressing the Cas9 Enzyme
To generate U. virens transformants expressing the Cas9 enzyme alone, the pDHt/sk-PC Cas9 vector (Liu et al., 2015) carrying the hygromycin phosphotransferase (hph) cassette was transformed into the wild-type strain P1 (Zheng et al., 2017) by A. tumefaciens-mediated transformation (ATMT). Transformants resistant to hygromycin were isolated and analyzed by PCR. Strain CS-2 (Table 1) was one of the transformants that were confirmed to contain the transforming Cas9 vector.
Table 1.
Strains and vectors used in this study.
| Strain | Brief description | Reference |
|---|---|---|
| P1 | Wild-type | Zheng et al., 2017 |
| CS-2 | Transformant of P1 expressing the pDHt/sk-PC | This study |
| MS-1 | Uvslt2 deletion mutant of P1 | This study |
| MS-2 | Uvslt2 deletion mutant of P1 | This study |
| MS-4 | Uvslt2 deletion mutant of P1 | This study |
| MS-5 | Uvslt2 deletion mutant of P1 | This study |
| MS-8 | Uvslt2 deletion mutant of P1 | This study |
| MS-9 | Uvslt2 deletion mutant of P1 | This study |
| MS-11 | Uvslt2 deletion mutant of P1 | This study |
| MS-12 | Uvslt2 deletion mutant of P1 | This study |
| MS-15 | Uvslt2 deletion mutant of P1 | This study |
| MU-45 | ustA deletion mutant of P1 | This study |
| MU-47 | ustA deletion mutant of P1 | This study |
| MU-49 | ustA deletion mutant of P1 | This study |
| MU-52 | ustA deletion mutant of P1 | This study |
| MU-54 | ustA deletion mutant of P1 | This study |
| MU-60 | ustA deletion mutant of P1 | This study |
| Vectors | ||
| pUC-H1-gRNA | Vector with the H1 promoter for gRNA expression | Zheng et al., 2014 |
| pDHt/sk-PC | pDH1/tk-Ppdc-toCas9-Tpdc | Liu et al., 2015 |
| pCRISPR/Cas-U6-1 | Cas9-gRNA vector with the U6 promoter | Arazoe et al., 2015 |
| pCBDW | Agrobacterium binary vector | Zheng et al., 2016 |
| pUC19-tRp-gRNA | Gln-tRNA promoter of gRNA | This study |
| pCAS9-tRp-gRNA | Cas9-gRNA vector with the tRNA promoter | This study |
| pCas9-tRp-UA01 | Cas9-gRNA vector with the UA01 spacer | This study |
| pCas9-tRp-UA13 | Cas9-gRNA vector with the UA13 spacer | This study |
| pCas9-tRp-UA21 | Cas9-gRNA vector with the UA21 spacer | This study |
| pCas9-tRp-SLT01 | Cas9-gRNA vector with the SLT01 spacer | This study |
| pHY2016A | pCAS9: tRp-SLT01 gRNA | This study |
The Gene Replacement Frequency for USTA Is Over 60% With the CRISPR-Cas9 System
To test the efficiency of CRISPR-Cas9 for generating knockout mutants in U. ravens, we first identified the predicted gene UV8b_7487 as the ortholog of ustA of Aspergillus flavus (Tsukui et al., 2015) and named it USTA in this study. In A. flavus, ustA encodes an oligopeptide that is processed post-translationally to ustiloxins (Tsukui et al., 2015). Three gRNA spacers, UA01, UA13, and UA21 (Supplementary Table S1), were designed with the sgRNA designer program for best on-target scores (Doench et al., 2014, 2016). Among them, UA01 had the highest predicted on-target score (Figure 2A). All of these three spacers were cloned into the BsaI-digested pUC19-tRp-gRNA vector by Golden Gate Cloning (Arazoe et al., 2015). The resulting constructs were verified by sequencing analysis and transformed into protoplasts of the Cas9-expressing transformant CS-2 (Table 1). Transformants resistant to geneticin G418 were isolated and screened by PCR with primers USTAF5 and USTA6R (Supplementary Table S1) for deletion of USTA (Figure 2B), and further verified by PCR with primer pairs USTA7F/G855R and USTA8R/G856F for homologous recombination in both upstream and downstream flanking sequences (Figure 2C).
FIGURE 2.
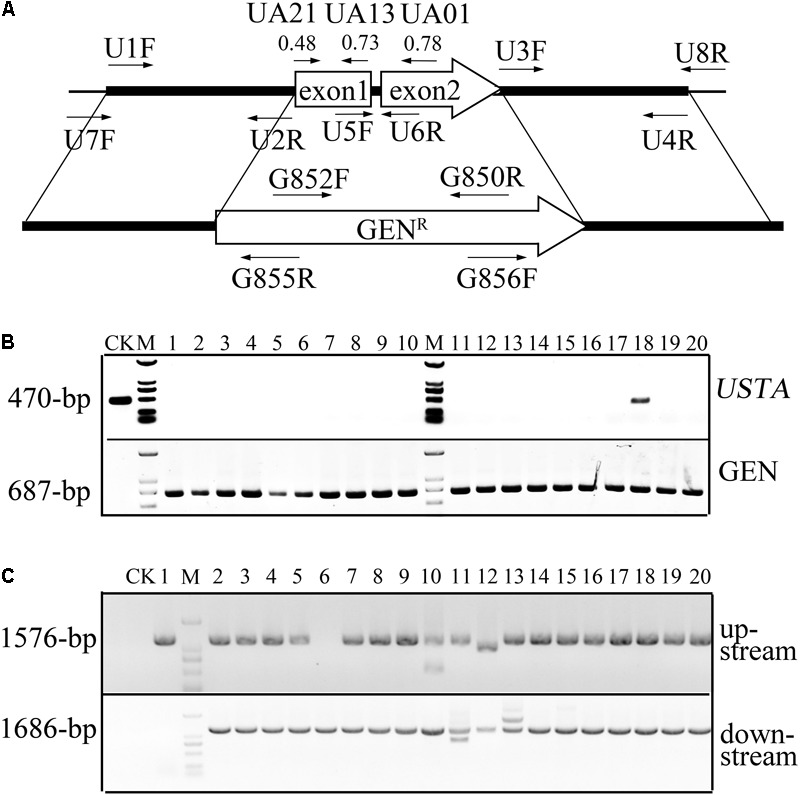
The USTA gene and deletion mutants. (A) The USTA gene and gRNA spacers. The position and direction of gRNA spacers and primers used to generate and screen ustA deletion mutants are marked with arrows. The on-target scores of spacers UA01, UA13, and UA21 are labeled in the bracket. (B) PCR assays for the deletion of USTA (upper panel) and presence of the geneticin-resistance gene (lower panel) in 20 transformants generated with pCas9-tRp-gRNA-UA01 (spacer UA01). The 470-bp USTA fragment was only amplified in the wild-type (CK) and transformant 18. M: 1-kb DNA ladder marker. (C) PCR assays to verify gene replacement events in 20 putative ustA deletion mutants. The 1576-bp upstream and 1686-bp downstream recombination products were amplified with primer pairs USTA7F(U7F)/G855R and USTA8R(U8R)/G856F, respectively. Amplification with P1 was used as the negative control. M: 1-kb DNA ladder marker.
For spacer UA01, 27 of the 36 G418-resistant transformants were confirmed to be ustA deletion mutants, suggesting that the homologous gene replacement frequency was as high as 75%. For spacers UA13 and UA21, 21 ustA deletion mutants each were identified by PCR after screening 34 and 36 G418-resistant transformants, respectively. Five of these ustA deletion mutants identified by PCR were selected for further verification by Southern blot analysis. All of them were confirmed to be deleted of the USTA gene (Supplementary Figure S1). The ustA mutants were normal in growth, colony morphology, and conidiation and had no defects in response to different stresses. These results indicated that the homologous gene replacement frequency varied among different gRNA spacers from 60 to 75% in U. virens. Space UA01 with the highest on-target score had the highest gene replacement frequency.
For comparison, we also generated ustA deletion mutants by the conventional ATMT approach (Zheng et al., 2016). The USTA gene replacement construct was generated by overlapping PCR and cloned into the Agrobacterium vector pCBDW-HPH (Zheng et al., 2016), which was then transformed into the wild-type strain P1. After screening over 600 ATMT transformants, only one ustA deletion mutant was identified, indicating a homologous replacement frequency of <0.2%. Therefore, the CRISPR-Cas9 system significantly increases the homologous gene replacement frequency in U. virens in comparison with ATMT.
Transformation of the Cas9 and gRNA Constructs on the Same Vector Further Increases the Gene Replacement Frequency in U. virens
Three gRNA spacers of USTA, UA01, UA13, and UA21, also were cloned into the BsmBI-digested pCas9: tRp-gRNA vector by Golden Gate Cloning (Arazoe et al., 2015). All the resulting constructs were verified by sequencing analysis and transformed into protoplasts of the wild-type strain P1. For spacer UA01, 27 of the 30 G418-resistant transformants were ustA deletion mutants. For spacers UA13 and UA21, 25, and 26 ustA deletion mutants were identified after screening 29 and 30 G418-resistant transformants, respectively. Therefore, the homologous gene replacement frequency was 90, 86, and 87%, respectively, for spacers UA01, UA13, and UA21, which was higher than transforming these gRNA spacers into the Cas9-expressing transformant CS-2. These results indicate that transformation of the Cas9 and gRNA constructs into U. virens on the same vector further increased the homologous gene replacement frequency.
No Off-Target Mutations Are Detected in ustA Mutants Generated With CRISPR-Cas9
For the three gRNA spacers of USTA, potential off-targets of the RNA-guided nuclease (RNG) Cas9 were analyzed with the Cas9off program (Guo et al., 2014). None of them had off-targets with less than two nucleotide variations, indicating that they are highly specific in the U. virens genome. Spacers UA01, UA13, and UA21 have 1, 4, and 2 off-targets with four or less nucleotide variations, respectively (Figure 3A).
FIGURE 3.
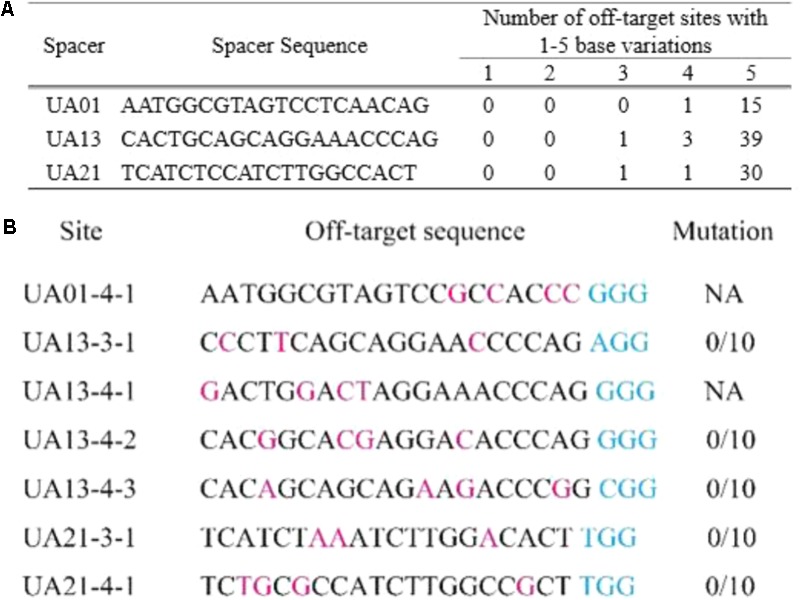
The predicted off-targets of three USTA gRNA spacers. (A) The sequences of three USTA gRNA spacers and numbers of their off-targets with 1–5 nucleotide differences. The off-target sites of gRNA spacers UA01, UA13, and UA21 were predicted with the Cas9off program. All of them have no off-targets with only one or two nucleotide variations. Spacer UA01 has only one off-target with four nucleotide variations. (B) Sequences of the off-targets of with fewer than five nucleotide variations from USTA gRNA spacers and experimental verification results. Nucleotides in the off-targets differing from the gRNA sequences are in pink. The PAM sequences (NGG) are in blue. For each gRNA spacer, the off-target sites were amplified and sequenced from 10 corresponding ustA deletion mutants. No mutations were detected in any of the 10 ustA mutants (0/10) at UA13-3-1, UA13-4-2, UA13-4-3, UA21-3-1, and UA21-4-1. NA, no data available due to PCR failures.
We then selected the ustA mutants generated with the pCas9: tRp-gRNA constructs (Cas9 + gRNA spacers) for experimental verification. For each spacer, 10 ustA mutants were selected for PCR and sequencing analysis for possible off-targets with 3 or 4 mismatches (Figure 3A). For spacer UA13, no mutations were detected at the predicted off-targets UA13-3-1, UA13-4-2, and UA13-4-3 in any of the 10 ustA mutants sequenced (Figure 3B). Similar results were obtained with transformants generated with spacer UA21. None of the 10 ustA mutants had mutations at the off-targets UA21-3-1 and UA21-4-1 (Figure 3B). These results suggested that mutations at the off-targets of these gRNA spacers likely occur at a relatively low frequency in U. viren, which may be related to the low complexity of U. virens genome and high specificity of gRNA spacers.
The CRISPR-Cas9 System Also Increases the Knockout Efficiency for the UvSLT2 MAPK Gene
To further test the gene knockout efficiency of the CRISPR-Cas9 system in U. virens, we then selected the UvSLT2 MAP kinase (MAPK) gene that is orthologous to yeast SLT2 (Gustin and Albertyn, 1998; Xu et al., 1998). Three UvSLT2 gRNA spacers were designed (Supplementary Table S1) with the gRNA designer program (Doench et al., 2014, 2016). SLT02 had the highest predicted on-target score of 0.75 (Figure 4A) and was the only gRNA spacer used to for UvSLT2 deletion. Spacer SLT02 was cloned into the BsmBI-digested pCas9-tRp-gRNA vector by Golden Gate Cloning. The resulting construct pHY2016A was verified by sequencing analysis and transformed into protoplasts of the wild-type strain P-1. Among the 50 G418-resistant transformants analyzed, 25 of them were confirmed to be Uvslt2 deletion mutants by PCR screening (Figure 4B), and verified for homologous recombination in both flanking sequences (Figure 4C), indicating a homologous gene replacement frequency of 50%. Therefore, the homologous gene replacement frequency of the CRISPR-Cas9 system varied among different spacers and different genes in U. virens.
FIGURE 4.
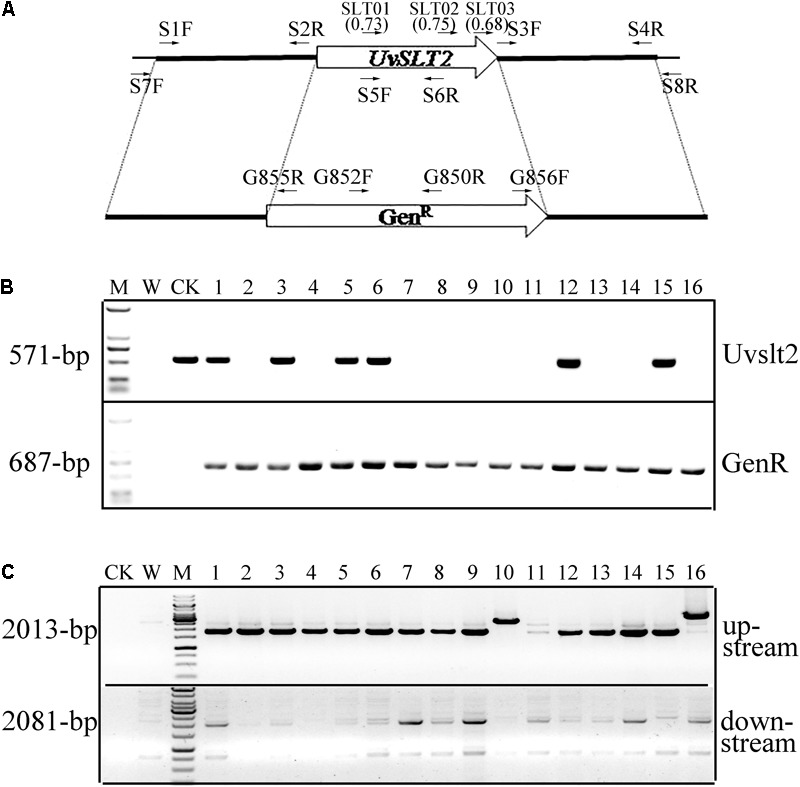
The UvSTL2 gene and deletion mutants. (A) The UvSLT2 gene and gRNA spacers. The position and direction of gRNA spacers and primers used to generate and screen Uvslt2 deletion mutants are marked with arrows. The on-target scores of spacers SLT01, SLT02, and SLT03 are labeled in the bracket. (B) PCR assays for the deletion of UvSTL2 (upper panel) and presence of the geneticin-resistance gene (lower panel) in 16 transformants generated with pCas9-tRp-gRNA-SLT01 (spacer SLT01). The 571-bp UvSTL2 fragment was only amplified in the wild-type (CK) and transformant 1, 3, 5, 6, 12, and 15. M: 1-kb DNA ladder marker. (C) PCR assays to verify gene replacement events in 16 putative Uvslt2 deletion mutants. The 2013-bp upstream and 2081-bp downstream recombination products were amplified with primer pairs SLT27F(S7F)/G855R and SLT8R(S8R)/G856F, respectively. Amplification with P1 was used as the negative control. M: 1-kb DNA ladder marker.
For comparison, we also generated the UvSLT2 gene replacement construct and transformed it into the wild-type strain UV-8b by ATMT. After screening 238 hygromycin-resistant transformants, two Uvslt2 deletion mutants were identified by PCR analysis. Therefore, the homologous gene replacement frequency was approximately 0.8% for UvSLT2 with the ATMT approach, which was significantly lower than that of the CRISPR-Cas9 system.
The Uvslt2 Mutant Has Increased Sensitivity to Cell Wall and Cytoplasm Membrane Stresses
The MAP kinases orthologous to Slt2 are known to regulate cell wall integrity and other differentiation processes in fungal pathogens (Hamel et al., 2012). The Uvslt2 mutant was slightly reduced in growth rate (Table 2) and produced colonies with shorter aerial hyphae on PSA and 5xYEG plates in comparison with the wild-type (Figure 5). Conidiation was reduced over 70% in the Uvslt2 mutant (Table 2). In the presence of 300 mg/L Congo red or 0.2% SDS, the Uvslt2 mutant was significantly more reduced in growth rate than the wild-type on PSA plates (Figure 5 and Table 3), indicating that deletion of UvSLT2 increased sensitivities to cell wall and cytoplasm membrane stresses. However, the Uvslt2 mutant grew faster than the wild-type on PSA plates with 0.6 M NaCl, 0.6 M Sorbitol, or 0.05% H2O2 (Figure 5 and Table 3). It appeared that deletion of UvSLT2 increased tolerance to hyperosmotic and oxidative stresses.
Table 2.
Defects of the Uvslt2 mutant in growth and conidiation.
| Strain | Growth rate (mm/d)a |
Conidiationb (106 conidia/ml) | |
|---|---|---|---|
| PDA | 5xYEG | ||
| P1 (wild-type) | 2.88 ± 0.04A | 2.86 ± 0.02A | 3.0 ± 0.5A |
| MS-2 (Uvslt2) | 2.31 ± 0.13B | 2.23 ± 0.28B | 0.9 ± 0.1B |
aThe average growth rate and standard deviation (mean ± SD) were calculated from at least three independent measurements. bConidiation in 6-day-old PSB cultures. Data from three replicates were analyzed with the protected Fisher’s least significant difference (LSD) test. Different letters mark statistically significant differences (P ≤ 0.05).
FIGURE 5.
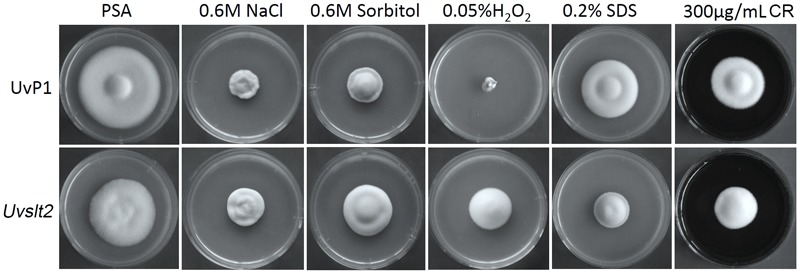
Defects of the Uvslt2 mutant in response to different stresses. The wild-type strain P1 and Uvslt2 mutant were cultured on regular PSA plates or PSA with 0.6 NaCl, 0.6 M sorbitol, 0.05% H2O2, 0.2% SDS, or 300 μg/ml Congo red (CR). Typical cultures were photographed after incubation for 14 days.
Table 3.
Defects of the Uvslt2 mutant in response to different stresses.
| Strain | Percentage of growth rate reduction |
||||
|---|---|---|---|---|---|
| NaCl | Sorbitol | H2O2 | SDS | CR | |
| P1 (wild-type) | 65.3 ± 3.5%A | 54.1 ± 1.0%A | 80.2 ± 3.5%A | 26.9 ± 2.7%B | 40.5 ± 1.8%A |
| MS-2 (Uvslt2) | 44.3 ± 6.7%B | 16.5 ± 4.6%B | 48.5 ± 10.7%B | 43.3 ± 1.5%A | 38.7 ± 0.7%A |
Growth rate was assayed with the wild-type strain P1 and Uvslt2 mutant cultured on regular PSA or PSA with 0.6 NaCl, 0.6 M sorbitol, 0.05% H2O2, 0.2% SDS, or 300 μg/ml Congo red (CR). Mean and standard deviation were calculated with data from three replicates. For each strain, the growth rate on PSA with individual stresses was compared with that on regular PSA (arbitrarily set to 100%) to estimate the percentage of growth rate reduction. Differences in growth rate reduction between the wild-type and Uvslt2 mutant were analyzed with the protected Fisher’s least significant difference (LSD) test. Different letters mark statistically significant differences (P ≤ 0.05).
Like in other filamentous fungi, UvSlt2 is one of the two MAP kinases with the TEY dual-phosphorylation motif in U. virens. Western blot analysis with the anti-TpEY antibody showed that Uvslt2 deletion mutants had no detectable phosphorylation of UvSlt2 MAP kinase (Figure 6). However, phosphorylation of the other MAP kinase with the TEY motif that is orthologous to Pmk1 in the rice blast fungus M. oryzae (Li et al., 2017) was not affected (Figure 6). Similar amount of proteins from each sample was detected by anti-H3 antibody (Figure 6). These results further indicated the conserved nature of UvSlt2 as a MAP kinase in U. virens.
FIGURE 6.
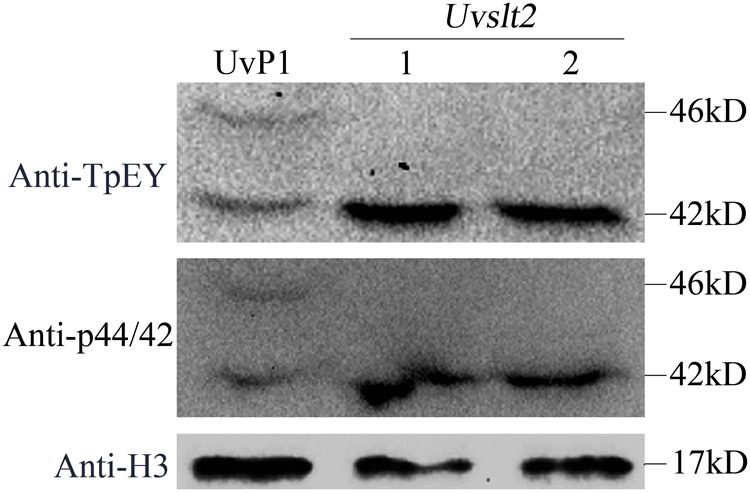
Assays for the phosphorylation of UvPmk1 and UvSlt2 MAP kinases. Western blots of total proteins isolated from the wild-type strain P1 and Uvslt2 mutant were detected with an anti-TpEY phosphorylation specific antibody and an anti- p44/42 antibody. Phosphorylation and expression of UvSlt2 was detected in the wild-type but not in the Uvslt2 mutant. Detection with an anti-H3 antibody (Cell Signaling Technology) was to show that similar amount of proteins were loaded for each sample.
Discussion
Targeted gene deletion and modification are important molecular tools to study gene functions in fungi and other organisms. In the budding yeast Saccharomyces cerevisiae, targeted gene deletion can be achieved by transforming a selectable marker flanked by homologous sequences as short as 20 bp (Ahn et al., 1988). In general, filamentous fungi have much lower homologous recombination frequency than S. cerevisiae. In M. oryzae, a model plant pathogenic fungus, the homologous gene replacement frequency is approximately 5% with 1-kb flanking sequences (Arazoe et al., 2015). For the rice false smut fungus U. virens, gene replacement was not effective by PEG-mediated transformation and occurred at <0.5% frequency by ATMT with 1-kb flanking sequences (Zheng et al., 2016). In this study, we showed that the efficiency of gene replacement by homologous recombination was significantly increased with the CRISPR-Cas9 system in U. virens. For the USTA gene, the gene replacement frequency was as high as 90% with gRNA spacer UA01. The significant increase in the gene replacement frequency by the CRISPR-Cas9 system will likely enable functional characterization of genes related to pathogenesis and mycotoxin production efficiently in U. virens, which is one of the major fungal pathogens of rice. In other filamentous fungi, the CRISPR-Cas9 system also increased the homologous gene replacement frequency (Arazoe et al., 2015; Liu et al., 2015). For example, the gene knockout frequency was increased from <5 to 25–53% in Aspergillus fumigatus with theCRISPR/Cas9 system (Fuller et al., 2015). Another major advantage of CRISPR is its capacity to introduce mutations to multiple genes with different gRNA (Liu et al., 2015). Because we used geneticin resistance to select for gene replacement mutants, the availability of useful selectable markers will be a limitation for applying this approach in U. virens. However, it remains possible that targeted deletion of two or more genes may be achieved by CRISPR with the same selectable marker in the rice false smut fungus.
In comparison with transforming the Cas9 and gRNA constructs sequentially into U. virens, the homologous gene replacement frequency was higher when they were transformed together on the same vector for all three USTA gRNA spacers. When spacer constructs were introduced into U. virens transformants expressing the Cas9 cassette, the homologous gene replacement frequency was 75, 60, and 60% for USTA gRNA spacers UA01, US13, and UA21, respectively. It was increased to 90, 86, and 87% when the Cas9 and gRNA spacers were transformed into U. virens on the same plasmid. In mammalian cells, similar observations have been reported because the Cas9 protein needs gRNA for the stabilization20. Among the three USTA spacers, UA01 had the highest predicted on-target score. It also had the highest gene replacement efficiency for deletion of USTA in U. virens. Therefore, it will be desirable to use gRNA spacers with the highest on-target score in other filamentous fungi for generating targeted gene knockout mutants by CRISPR-Cas9.
The U6 promoter is commonly used as the RNAP III promoter to control the expression of gRNA spacers in eukaryotes organisms (Gaj et al., 2013; Mefferd et al., 2015). However, the U6 promoter is not well-conserved in different fungi and it is approximately 500-bp in length. Furthermore, the gRNA spacers expressed from the U6 promoter have a G base at the 5′ end (Mefferd et al., 2015). Therefore, tRNA promoters also have been used as the RNAP III promoter to express gRNA spacers (Mefferd et al., 2015; Xie et al., 2015). In this study, we used the 72-bp Gln-tRNA promoter that has a typical cloverleaf structure11,14 to control the expression of gRNA spacers. Our results showed that the Gln-tRNA RNAP III promoter worked well to express gRNA spacers in U. virens. As a well-conserved promoter, it should also work in other plant pathogenic fungi for CRISPR-Cas9 studies.
One common concern is the off-target mutations of RGNs (Cho et al., 2014; Doench et al., 2016). However, none of the 20 ustA mutants assayed by sequencing analysis had mutations at seven predicted off-targets sites with 3–4 nucleotide variations for gRNA spacers UA13 and UA21 (UA13-3-1, UA13-4-2, UA13-4-3, UA21-3-1, and UA21-4-1). These results suggested that the mutation rate at off-targets with the CRISPR-Cas9 system is relatively low in U. virens. One possible explanation is that the U. virens genome is small (33.6 Mb) and not as complex as higher eukaryotic organisms (Zhang et al., 2014). In fact, all three USTA gRNA spacers have no predicted off-targets with only 1 or 2 nucleotide variations. The specificity of gRNA spacers is likely helpful to reduce mutations at off-targets. For the predicted off-targets of gRNA spacer UA01-1-1 and UA13-4-1, we failed to amplify the target sequences from the wild-type and ustA mutants with at least three different primer pairs tested for each. It is likely that the predicted off-target sequences of UA01-1-1 and UA13-4-1 in UV-8b, the strain used for genome sequencing4, may be different or absent in the wild-type strain P1 used in this study.
Unlike most small peptide mycotoxins synthesized by non-ribosomal peptide synthase (NRPS), ustiloxins are produced by post-translational modifications of UstA proteins synthesized on the ribosome. Although ustiloxins are present in the smut balls formed on infected rice kernels, to our knowledge, ustiloxin production have not been reported in in vitro cultures of U. virens. Under different culture conditions used to assay mycotoxin productions in Fusarium species, such as rice grain and cracked corn cultures (He et al., 2007; Jiang et al., 2016), we failed to detect ustiloxin production by the wild-type strain P1. Nevertheless, USTA is a single copy gene and encodes the only protein containing the ustiloxin peptide sequence in U. virens. Therefore, we expected that ustA deletion mutants will no longer produce ustiloxins. Unfortunately, because infection assays with U. virens is difficult and un-reliable, we failed to infect developing rice heads with the wild-type strain P1 and ustA mutants in repeated attempts. Thus, it remains to be determined whether ustiloxin production is important for virulence on rice in U. virens.
The Slt2 MAP kinase pathway is known to regulate cell wall integrity in S. cerevisiae, M. oryzae, and other fungi (Li et al., 2012; Jiang et al., 2018). The Uvslt2 deletion mutant had increased sensitivities to cell wall and cytoplasmic membrane stresses in U. virens, which is consistent with its function in cell wall integrity. Interestingly, the Uvslt2 mutant was more tolerant to hyperosmotic and oxidative stressors. In U. virens, UvHog1 is known to regulate responses to hyperosmotic and oxidative stresses (Zheng et al., 2016). It is possible that deletion of UvSLT2 resulted in the hyper-activation of the UvHog1 pathway, which in turn increased the tolerance to hyperosmotic and oxidative stresses. Therefore, it will be important to characterize the crosstalk between these two MAP kinase pathways in U. virens in the future.
Materials and Methods
Strains, Plasmids, and Culture Conditions
All the U. virens strains were routinely cultured on YT (0.1% yeast extract, 0.1% tryptone, and 1% glucose) at 25°C. Conidiation was assayed with 6-day-old PSB (Potato Sucrose Broth) cultures. For ATMT (Zheng et al., 2016), A. tumefaciens strain AGL1 was used for the transformation of the wild-type strain P1 (Zheng et al., 2017), a field isolate provided by Dr. Yuanfeng Liu at Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences. The binary T-DNA vector pDHt/sk-Ppdc-toCas9-Tpdc (pDHt/sk-PC) (Liu et al., 2015) was provided by Dr. Zhi-Hua Zhou at Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Protoplast preparation and PEG-mediated transformation of U. virens strains were performed as described (Zheng et al., 2016). For transformation selection, hygromycin B (Calbiochem, La Jolla, CA, United States) and G418 (MP Biomedicals, Santa Ana, CA, United States) were added to the final concentration of 180 and 700 μg/ml, respectively, in the medium. The pCRISPR/Cas-U6-1 vector (Arazoe et al., 2015) was provided by Dr. Kuwata at Meiji University.
Construction of the Cas9-gRNA Vectors With the U. virens Gln-tRNA Promoter
The Gln-tRNA-gRNA cassette containing two BsaI sites was generated by fusion PCR with primers GRNA-F1, GRNA-R1, GRNA-F2, and GRNA-R2 (Supplementary Table S1). It was then cloned between the HindIII and EcoRI sites of vector pUC-H1-gRNA (Zheng et al., 2014) to generate the pUC19-tRp-gRNA plasmid. A short cassette with two BsmBI sites was generated by annealing the sense (LINK-SEQ-F) and antisense (LINK-SEQ-R) oligonucleotides (Supplementary Table S1) and inserted between the two BsaI sites of pUC19-tRp-gRNA by Golden Gate cloning (New England Biolabs, Ipswich, MA, United States) as described (Ran et al., 2013; Arazoe et al., 2015) to generate the pUC19- LINK vector. The PGln-tRNA-gRNA cassette flanked by two BsmBI sites was then released from pUC19-LINK and cloned between the KpnI and EcoRI sites of pCRISPR/Cas-U6-1 (Arazoe et al., 2015) to generate the pCas9-tRp-gRNA vector. All the resulting plasmid vectors were verified by sequencing analysis.
Construction of the Cas9-gRNA Vectors for Deletion of USTA
The gRNA spacers were designed with the gRNA designer program1 for best on-target scores (Doench et al., 2014, 2016) and then analyzed with the Cas9off program2 to identify potential off-targets (Guo et al., 2014). Three USTA gRNA spacers, UA01, UA13, and UA21, were selected by weighing both on-target scores and potential off-targets. The sense and antisense oligonucleotides of each gRNA spacer (Supplementary Table S1) were synthesized and annealed to generate corresponding gRNA spacers as described (Arazoe et al., 2015). The resulting gRNA spacers were cloned between the two BsaI sites of pUC19-tRp-gRNA or the two BsmBI sites of pCas9-tRp-gRNA by Golden Gate cloning (New England Biolabs).
Generation of the USTA Gene Replacement Constructs and Mutants
The 1.01-kb upstream and 1.09-kb downstream flanking sequences of USTA were amplified with primer pairs of USTA1F/USTA2R and USTA3F/USTA4R (Supplementary Table S1), respectively, and fused with the geneticin-resistance (GenR) cassette from pFL2 (Zhou et al., 2011) by double-joint PCR. For sequential transformation, the resulting PCR products were cloned into pUC19-tRp-gRNA and transformed into protoplasts of transformant CS-2 expressing the pDHt/sk-PC Cas9 construct as described (Zheng et al., 2016). The same USTA knockout PCR products also were cloned into the pCas9-tRp-UA01, pCas9-tRp-UA13, and pCas9-tRp-UA21 vectors and then transformed into protoplasts of the wild-type strain P1. G418-resistant transformants were screened for deletion of USTA by PCR with primers USTA/5F and USTA/6R, and further verified by PCR with primer pairs USTA7F/G855R and USTA8R/G856F (Supplementary Table S1).
To delete USTA by the conventional gene replacement approach, its upstream and downstream flanking sequences were amplified as described above and fused with the hygromycin phosphotransferase gene (hph) from pCB1003 (Carroll et al., 1994) by overlapping PCR as described (Zheng et al., 2016). The resulting PCR products were cloned into the binary vector pCBDW and transformed into stain P1 by ATMT as described (Zheng et al., 2016). Hygromycin-resistant transformants were screened for ustA deletion mutants by PCR.
Analysis of Off-Target Mutations by CRISPR-Cas9
The potential off-target sites of USTA spacers UA01, UA13, and UA21 with 1 to 5 nucleotide variations were predicted with the Cas9off program (Guo et al., 2014). For each gRNA spacer, the predicted off-targets with less than four nucleotide differences were amplified from 10 corresponding ustA deletion mutants and sequenced for possible mutations.
Generation of the UvSLT2 Gene Replacement Construct and Mutants
For UvSLT2, the gRNA spacer SLT01 was selected for its highest on-target score and generated by annealing the sense and antisense oligonucleotides (Supplementary Table S1). The resulting products were cloned between the two BsmBI sites of pCas9-tRp-gRNA by Golden Gate cloning to generate pHY2016A (Table 1). The 1.25-kb upstream and 1.04-kb downstream flanking sequences of UvSLT2 were amplified and fused to the GenR cassette by double-joint PCR (Hou et al., 2002). The resulting PCR products were cloned into pHY2016A and transformed into protoplasts of strain P1. To generate UvSTL2 deletion mutants by ATMT, its upstream and downstream flanking sequences were fused with the hph cassette from pCB1003 (Carroll et al., 1994) by overlapping PCR and cloned into pCBDW for transformation of strain P1 as described (Zheng et al., 2016). The Uvslt2 deletion mutants were screened and verified by PCR with primers listed in Supplementary Table S1.
Western Blot Analysis for Assaying the Phosphorylation of UvSlt2
Total proteins were isolated from vegetative hyphae as described (Hou et al., 2015). For western blot analysis, total proteins (20 μg) were separated on a 10% SDS-PAGE gel and transferred to the nitrocellulose membrane. Phosphorylation of the UvPmk1 and UvMps1 MAP kinases was detected with the PhophoPlus p44/42 MAP kinase antibody (Cell Signaling Technology, Danvers, MA, United States) as described (Liu et al., 2011). Detection with an anti-p44/42 antibody (Cell Signaling Technology, Danvers, MA, United States) was to show the expression levels of Slt2 in these samples.
Author Contributions
YL and J-RX conceived the experiments. YL, YH, and CJ conducted the experiments and analyzed data. YL, CJ, CW, and J-RX prepared and revised this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Sheng-Lin Huang at Fudan University for the pUC-H1-gRNA vector, Dr. Zhi-Hua Zhou at Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences for the pDHt/sk-PC vector, and Dr. Shigeru Kuwata at Meiji University for the pCRISPR/Cas-U6-1 vector. We also thank Dr. Yong-Feng Liu at Jiangsu Academy of Agricultural Sciences for providing the U. virens field isolate P1.
Funding. This work was supported by Sino-German Center for Research (Grant No. GZ928).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00699/full#supplementary-material
References
- Ahn B., Dornfeld K. J., Fagrelius T. J., Livingston D. M. (1988). Effect of limited homology on gene conversion in a Saccharomyces cerevisiae plasmid recombination system. 8 2442–2448. 10.1128/MCB.8.6.2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arazoe T., Miyoshi K., Yamato T., Ogawa T., Ohsato S., Arie T., et al. (2015). Tailor-made CRISPR/Cas system for highly efficient targeted gene replacement in the rice blast fungus. 112 2543–2549. 10.1002/bit.25662 [DOI] [PubMed] [Google Scholar]
- Carroll A. M., Sweigard J. A., Valent B. (1994). Improved vectors for selecting resistance to hygromycin. 41:22 10.4148/1941-4765.1367 [DOI] [Google Scholar]
- Cho S. W., Kim S., Kim Y., Kweon J., Kim H. S., Bae S., et al. (2014). Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. 41 132–141. 10.1101/gr.162339.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Park S. Y., Kim B. R., Roh J. H., Oh I. S., Han S. S., et al. (2013). Comparative analysis of pathogenicity and phylogenetic relationship in Magnaporthe griseaspecies complex. 8:e57196. 10.1371/journal.pone.0057196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L., Ran F. A., Cox D., Lin S., Barretto R., Habib N., et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. 339 819–823. 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench J., Hartenian E., Graham D., Tothova Z., Hegde M., Smith I., et al. (2014). Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. 32 1262–1267. 10.1038/nbt.3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench J. G., Fusi N., Sullender M., Hegde M., Vaimberg E. W., Donovan K. F., et al. (2016). Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. 34 184–191. 10.1038/nbt.3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller K. K., Chen S., Loros J. J., Dunlap J. C. (2015). Development of the CRISPR/Cas9 system for targeted gene disruption in Aspergillus fumigatus. 14 1073–1080. 10.1128/EC.00107-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T., Gersbach C. A., Barbas C. F. (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. 31 397–405. 10.1016/j.tibtech.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. G., Zhang T., Hu Z., Zhang Y., Shi Z., Wang Q., et al. (2014). Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. 141 707–714. 10.1242/dev.099853 [DOI] [PubMed] [Google Scholar]
- Gustin M. C., Albertyn J. (1998). MAP kinase pathways in the yeast Saccharomyces cerevisiae. 62 1264–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel L., Nicole M., Duplessis S., Ellis B. E. (2012). Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. 24 1327–1351. 10.1105/tpc.112.096156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. W., Yang R., Zhou T., Tsao R., Young J. C., Zhu H. H., et al. (2007). Purification of deoxynivalenol from Fusarium graminearum rice culture and mouldy corn by high-speed counter-current chromatography. 1151 187–192. 10.1016/j.chroma.2007.01.112 [DOI] [PubMed] [Google Scholar]
- Hou R., Jiang C., Zheng Q., Wang C., Xu J. R. (2015). The AreA transcription factor mediates the regulation of deoxynivalenol (DON) synthesis by ammonium and cyclic adenosine monophosphate (cAMP) signalling in Fusarium graminearum. 16 987–999. 10.1111/mpp.12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Xue C., Peng Y., Katan T., Kistler H. C., Xu J.-R. (2002). A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. 15 1119–1127. 10.1094/MPMI.2002.15.11.1119 [DOI] [PubMed] [Google Scholar]
- Jiang C., Zhang C. K., Wu C. L., Sun P. P., Hou R., Liu H. Q., et al. (2016). TRI6 and TRI10 play different roles in the regulation of deoxynivalenol (DON) production by cAMP signalling in Fusarium graminearum. 18 3689–3701. 10.1111/1462-2920.13279 [DOI] [PubMed] [Google Scholar]
- Jiang C., Zhang X., Liu H. Q., Xu J. R. (2018). Mitogen-activated protein kinase signaling in plant pathogenic fungi. 14:e1006875. 10.1371/journal.ppat.1006875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivanen J., Wang Y. M. J., Richard P. (2016). Engineering Aspergillus niger for galactaric acid production: elimination of galactaric acid catabolism by using RNA sequencing and CRISPR/Cas9. 15:210. 10.1186/s12934-016-0613-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Zhou X., Xu J. R. (2012). Genetic control of infection-related development in Magnaporthe oryzae. 15 678–684. 10.1016/j.mib.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang X., Hu S., Liu H., Xu J. R. (2017). PKA activity is essential for relieving the suppression of hyphal growth and appressorium formation by MoSfl1 in Magnaporthe oryzae. 13:e1006954. 10.1371/journal.pgen.1006954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Chen L., Jiang Y., Zhou Z., Zou G. (2015). Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. 1 15007. 10.1038/celldisc.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. D., Zhou X. Y., Li G. T., Li L., Kong L. G., Wang C. F., et al. (2011). Multiple plant surface signals are sensed by different mechanisms in the rice blast fungus for appressorium formation. 7:e1001261. 10.1371/journal.ppat.1001261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe T. M., Eddy S. R. (1997). tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. 25 955–964. 10.1093/nar/25.5.0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv B., Zheng L., Liu H., Tang J., Hsiang T., Huang J. (2016). Use of random T-DNA mutagenesis in identification of gene UvPRO1, aregulator of conidiation, stress response, and virulence in Ustilaginoidea virens. 7:2086. 10.3389/fmicb.2016.02086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsu-ura T., Baek M., Kwon J., Hong C. (2015). Efficient gene editing in Neurospora crassa with CRISPR technology. 2:4. 10.1186/s40694-015-0015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefferd A. L., Kornepati A. V., Bogerd H. P., Kennedy E. M., Cullen B. R. (2015). Expression of CRISPR/Cas single guide RNAs using small tRNA promoters. 21 1683–1689. 10.1261/rna.051631.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl C., Kiel J. A. K. W., Driessen A. J. M., Bovenberg R. A. L., Nygard Y. (2016). CRISPR/Cas9 based genome editing of Penicillium chrysogenum. 5 754–764. 10.1021/acssynbio.6b00082 [DOI] [PubMed] [Google Scholar]
- Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., Zhang F. (2013). Genome engineering using the CRISPR-Cas9 system. 8 2281–2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukui T., Nagano N., Umemura M., Kumagai T., Terai G., Machida M., et al. (2015). Ustiloxins, fungal cyclic peptides, are ribosomally synthesized in Ustilaginoidea virens. 31 981–985. 10.1093/bioinformatics/btu753 [DOI] [PubMed] [Google Scholar]
- Wang C., Zhang S., Hou R., Zhao Z., Zheng Q., Xu Q., et al. (2011). Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. 7:e1002460. 10.1371/journal.ppat.1002460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth M., Pinecker C., Voss B., Fischer R. (2017). Establishment of CRISPR/Cas9 in Alternaria alternata. 101 55–60. 10.1016/j.fgb.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Xie K., Minkenberg B., Yang Y. (2015). Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. 17 3570–3575. 10.1073/pnas.1420294112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Staiger C. J., Hamer J. E. (1998). Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. 95 12713–12718. 10.1073/pnas.95.21.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Yu J., Hu J., Huang L., Wang Y., Yin X., et al. (2015). Identification of pathogenicity-related genes in the rice pathogen Ustilaginoidea virens through random insertional mutagenesis. 76 10–19. 10.1016/j.fgb.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang K., Fang A., Han Y., Yang J., Xue M., et al. (2014). Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. 5:3849. 10.1038/ncomms4849 [DOI] [PubMed] [Google Scholar]
- Zheng D., Wang Y., Han Y., Xu J. R., Wang C. (2016). UvHOG1 is important for hyphal growth and stress responses in the rice false smut fungus Ustilaginoidea virens. 6:24824. 10.1038/srep24824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M. T., Ding H., Huang L., Wang Y. H., Yu M. N., Zheng R., et al. (2017). Low-affinity iron transport protein Uvt3277 is important for pathogenesis in the rice false smut fungus Ustilaginoidea virens. 63 131–144. 10.1007/s00294-016-0620-4 [DOI] [PubMed] [Google Scholar]
- Zheng Q., Cai X., Tan M. H., Schaffert S., Arnold C. P., Gong X., et al. (2014). Precise gene deletion and replacement using the CRISPR/Cas9 system in human cells. 57 115–124. 10.2144/000114196 [DOI] [PubMed] [Google Scholar]
- Zhou X., Li G., Xu J. R. (2011). Efficient approaches for generating GFP fusion and epitope-tagging constructs in filamentous fungi. 722 199–212. 10.1007/978-1-61779-040-9_15 [DOI] [PubMed] [Google Scholar]
- Zhou Y.-L., Pan Y.-J., Xie X.-W., Zhu L.-H., Xu J.-L., Wang S., et al. (2008). Genetic diversity of rice false smut fungus, Ustilaginoidea virens and its pronounced differentiation of populations in North China. 156 559–564. 10.1111/j.1439-0434.2008.01387.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


