Abstract
Although kin selection is central to the modern study of social evolution, recent studies of social species have revealed that no simple relationship exists between levels of kinship and sociality. The soldier-producing aphids are unique among highly social animals because, barring movement by aphids between colonies, they occur in clonal groups of genetically identical individuals. Potentially, clonality simplifies efforts to understand social evolution in aphids by obviating issues of intragroup conflict. However, we report here high levels of clonal mixing and conflict in an aphid society. The gall-dwelling colonies of a social aphid species (Pemphigus obesinymphae) are not pure clones, but are invaded by large numbers of aphids from other clones. Intruders behave and develop selfishly once they have invaded a colony of nonkin. They refrain from risky defensive behaviors and accelerate their own development into reproductive rather than defensive stages. This conditionality in the social life of P. obesinymphae reveals complex dynamics and a degree of behavioral plasticity not previously known in aphid societies.
There are various scenarios for the evolution of soldiers in aphids (1), but two elements thought to be decisive are the high relatedness afforded by the isolation of clonally produced colonies within galls (2, 3) and the need for defending long-lived galls from attack by natural enemies (4, 5). In purely clonal groups, the social traits exhibited by soldiers, like risky defense against enemies and reproductive altruism, reflect clone-level allocations to defense over reproduction, without conflict between group members (1). But if clones mix, the advantages of defense are weakened because the benefits of sacrificial behaviors accrue in part to unrelated aphids. Thus, the genetic structure of colonies is crucial to understanding the selective advantages of social traits in aphids (e.g., refs. 6 and 7).
Pemphigus obesinymphae is the only North American aphid known to have soldiers (1, 4). Its complex life cycle involves phases of sexual and asexual reproduction on alternative host plants. In the spring, a “foundress” initiates a globular gall at the base of a leaf of a cottonwood (Populus spp.). Permanently entombed in the hollow cavity of the gall, she asexually produces a caste of up to 300 first-instar soldiers, which can exit and reenter through a small ostiole. Aphid soldiers effectively deter attack by much larger enemies, often sacrificing themselves in the process (8, 9). Colonies of P. obesinymphae, for example, suffer less than half the predation experienced by colonies of nondefending Pemphigus species on the same trees (P.A., unpublished data).
Near the end of the growing season, all colonies make a conspicuous transition from defense to reproduction as nymphs mature beyond the first-instar soldier stage. The maturing nymphs, which as adults will fly from the gall to reproduce on another host plant, no longer participate in group defense (4). Before they mature, the development of P. obesinymphae soldiers into more advanced instars is inhibited in the presence of the foundress (10). Thus, soldiers are altruistic, because delayed development and aggressive behaviors toward other arthropods result in an extended period of risk and lowered likelihood of successful reproduction (4).
Although altruism and defense in aphids probably reflect selection acting on entire clones rather than on individuals (1), the benefits of self-sacrifice by soldiers depend critically on whether soldiers are actually defending clone mates or unrelated aphids from other clones. Several studies have reported aphids invading galls of conspecifics, mostly in species lacking soldiers (11–13), and limited field observations suggested the occurrence of first-instar “intruders” in P. obesinymphae (4). But there have been no quantitative measures of intruder frequencies in social aphid colonies and no assays of cheating or conflict in aphid societies, making it difficult to evaluate the roles of clonal mixing and kinship in shaping soldier evolution in aphids.
We used a combination of field studies and colony censuses based on genetic markers to characterize between-gall movement by P. obesinymphae and to explore the balance between cooperation and conflict in this species by determining the fate of intruders once they join other colonies. One option for intruders is to assist cooperatively in defense of the gall, either because of the mutualistic benefits of group defense (14) or because of the lack of the behavioral plasticity necessary to adjust their behavior among unrelated aphids. However, in doing so, they would sacrifice their own reproduction for that of nonrelatives. Thus, within-colony selection should favor intruders that behave selfishly by not defending once they leave their natal galls. Moreover, because arrested development in the soldier stage is presumably a means for the clone to maintain a large army (10), intruders would gain little by lingering at immature stages. As autumn approaches, galling aphids become increasingly vulnerable to the escalating risks of predation and leaf abscission (P.A., unpublished data), and selection should favor nymphal intruders that accelerate development and depart the gall for their alternative host plant as soon as possible.
Materials and Methods
Collections.
The galls surveyed by genetic markers were collected from two sites near Tucson, Arizona in late August 1998: from one tree at Empire Cienega Resource Conservation Area (EC) on August 28, 1998 (31:45N, 110:37W), and from two adjacent trees at Presidio Santa Cruz de Terranate (TR) on October 10, 1998 (31:43N, 110:11W). Galls were placed individually in screw-cap vials and stored at −80°C until needed for analysis.
Field Studies.
We conducted both field studies at EC. First, we placed a sticky paste (Tanglefoot) on stems 10 cm proximal to 40 galls; we counted and removed trapped nymphs every 2 weeks for 10 weeks between July and September 1996. Then, in late August 1997, we labeled 72 galls in 15 groups with up to six colors of powdered dyes (3 to 6 galls per set; all labeled galls were within 2 m of one another, but not all galls on leaves were labeled). We measured the shortest walking distance and the straight-line aerial distance between each pair of galls. We collected all galls after 10 days, and scored the number of detectable intruders in each gall.
Laboratory Methods.
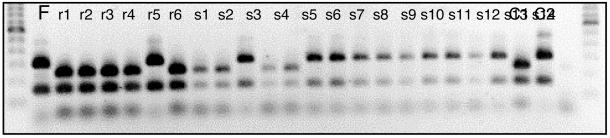
We used a single nucleotide polymorphism (SNP) and multilocus intersimple sequence repeat markers to survey galls. The SNP was discovered by sequencing genes of the bacterial endosymbiont Buchnera aphidicola from multiple foundresses. Buchnera are strictly maternally transmitted (15, 16) and, thus, can provide clonal markers during the asexual phase of aphid life cycles (17). Only one polymorphic locus was found; that was in a fragment of the Buchnera ATPase operon. We scored the SNP by PCR-restriction fragment length polymorphism. We amplified a PCR fragment containing the SNP from whole-genomic DNA-extracted individual aphids. We incubated the fragment at 37°C with a diagnostic restriction enzyme (VspI; 5′-ATTAAT-3′). Depending on the genotype of the aphid, the fragment contained one or two restriction sites and, when visualized on 2.5% agarose gels stained with GelStar, provided unambiguous typing of each aphid successfully amplified (Fig. 1; the small, third fragment from the genotypes with two sites is not visible in the figure).
Figure 1.
Clonal variation in a P. obesinymphae gall, subdivided by developmental stage of resident aphids, revealed by PCR-restriction fragment length polymorphism (see Materials and Methods for details). All gels contained a 100-bp ladder as a size standard, followed by the gall foundress (F) and the larvae being tested against the foundress: those in the nondefensive, reproductive stages (lanes r1–r6) and those in the first-instar soldier stage (lanes s1–s14). Lanes C1 and C2 are control genotypes previously typed by direct sequencing. In the absence of intruders, all individuals would share the same genotype, which is identical to the clone foundress. Thus, nymphs of P. obesinymphae clearly move between galls, and intruders tend to accelerate development once they become residents in nonnatal galls.
Intersimple sequence repeats are a recently developed technique for generating PCR-based markers (18) that provide anonymous, typically dominant Mendelian markers (19). We screened whole-genomic DNA from single individuals for polymorphic banding patterns (20). Only one primer, 5′-(AC) (8)G-3′, consistently distinguished individual genotypes, providing three polymorphic loci and six distinct banding profiles. Gels were scored twice for errors, and ambiguous loci were not scored.
Genetic Experiments and Analyses.
In surveying the clonal composition of galls, we sampled 10 to 15 aphids from 15 galls (12 from EC, 3 from TR). To test for loss of defensive behaviors by intruders, Drosophila larvae were inserted into galls containing large numbers of soldiers (>100) at EC in August by lightly pinching the gall to increase the size of the aperture. After 5 min, the galls were halved and the Drosophila and attacking soldiers were removed and placed into a labeled vial. The remaining contents were emptied into a separate labeled vial. Soldiers readily attacked the Drosophila larvae, which are equivalent in size to larvae of predaceous flies that commonly attack galls in southern Arizona (4). By using the SNP, we then compared the genotype of each defender and of nonattacking nymphs to the natal genotype, as represented by the resident foundress.
To measure intruder development, we collected 102 galls from the TR site in early October as clones in southern Arizona were beginning to make the transition from defense to reproduction and surveyed them for aphids at advanced developmental stages. We compared the proportions of SNP heterotypes in the soldier stage (first-instar) and the more advanced developmental stages (second, third, and fourth instars and alates; collectively called “reproductives”) in galls containing mixtures of the two. Galls with very few mature nymphs or with very low intruder frequencies (four or fewer intruders) were not used in this experiment.
Results
We documented extensive movement out of natal galls and intrusion into nearby clones by first-instar nymphs (Table 1). With the Tanglefoot, we trapped an average of 182.1 ± 30.9 SE first-instar nymphs leaving each gall over a 10-week period, a number that approaches the typical number of individuals in P. obesinymphae galls in late summer (10). In the surveys using colored powders, half of the galls received at least one marked migrant over a 5-day sample period, and 35% received migrants from two or more clones. Both aerial and walking distances between galls decreased the likelihood of exchanging migrants, although some aphids moved nearly 2 m. Galls with more aphids produced more migrants, but there was no effect of gall size on the number of aphids leaving galls, indicating that migration is not strictly a function of crowding in the gall.
Table 1.
Measures of movement of P. obesinymphae nymphs among colonies, based on direct field observations and on censuses of gall populations using genetic markers for recognition of intruders
| Measure of intergall movement | Method | Galls (aphids) surveyed | Outcome |
|---|---|---|---|
| Census aphids leaving natal gall | traps | 24 | 182.1 ± 30.9 emigrants per gall (cumulative) |
| Detect intruders in recipient gall | mark/recapture | 72 | 44% of galls received at least one intruder |
| Quantify intruders in recipient gall | genetic (SNP) | 15 (201) | 47–49% of aphids in galls are intruders* |
| Quantify intruders in recipient gall | genetic (ISSRs) | 7 (93) | 38% of aphids in galls are intruders |
The pooled, unadjusted variation was 0.24 (range between galls = 0–0.50). Because migration into a gall with the same allele would not be detected directly, we estimated the actual migration levels based on the assumption that the alleles are present in the migrant pool in proportions equal to those in the population overall. Using a ratio of genotype frequencies of 0.75 C:1.33 T gives an estimate of variation in C galls of 0.27 T migrants + 0.27(0.75) C migrants = 0.47 total migrants, and in T galls of 0.21 C migrants + 0.21(1.33) T migrants = 0.49 total migrants.
The results from the field surveys were confirmed by the genetic markers. Both markers revealed extremely high levels of within-gall variation (Table 1; Fig. 1). In the 15 galls surveyed with the SNP, the foundresses were typically unrelated to almost one-half of the individuals in their galls, and only two galls lacked detectable heterotypes. When we genotyped the same individuals from seven galls selected arbitrarily from this group with the more sensitive intersimple sequence repeat (ISSR) markers, we found that all had at least one ISSR heterotype, with the frequency of heterotypes ranging from 0.09 to 0.66 per gall. Furthermore, the intruders arrived from multiple source colonies: two galls contained intruders from at least three different clones, and five of seven galls held intruders from two or more different clones. The two markers were in close agreement, and combining the results from the two gave a joint unadjusted estimate of intruder frequency across the seven galls of 0.41 (range = 0.21–0.71). Thus, a large fraction of individuals in galls are intruders from other galls and represent distinct and diverse genotypes.
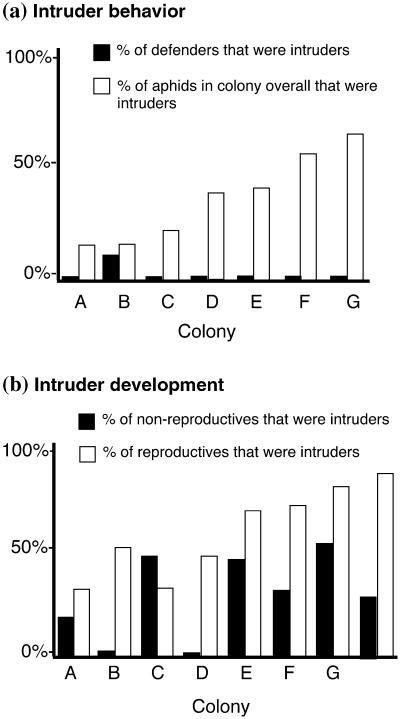
Based on the results of the experiment on the expression of attack behavior in response to Drosophila larvae, these intruders fail to contribute proportionately to gall defense. Intruders were significantly underrepresented in the attacking class in all seven galls, relative to their background frequency in galls (Fig. 2a). Overall, 36% of aphids in these galls were intruders, but only 2% of the attackers were heterotypic.
Figure 2.
(a) Percentages of intruders defending colonies (black bars) compared with the overall percentage of intruders in the same colonies (white bars). (b) Percentages of nonreproductive soldiers in colonies that were intruders (black bars) compared with the percentages of reproductives that were intruders in the same colonies (white bars), based on assays of the SNP marker. Note that categories are independent samples and, thus, should not sum to 100%, and that assays of behavior and development were not made on the same colonies. Both comparisons support the interpretation that intruders behave selfishly by parasitizing the altruistic tendencies expressed by unrelated, natal clones (a, P = 0.002; b, P = 0.015; paired t tests on arcsine–square root transformed proportions).
Intruders also spend less time in the first-instar defending stage and, instead, accelerate development into reproductive adults. In seven of eight galls, heterotypes were significantly overrepresented in the reproductive class (Fig. 2b). In all, intruders were more than three times as likely to be reproductive than were nymphs that remained in their natal gall.
Discussion
Relative to other social insects, soldier-producing aphids are understood only poorly. An issue outstanding has been the degree to which clonal mixing and relatedness can explain why closely related species differ in soldier production. Although parallel studies of the genetic composition of other social aphid colonies are needed, these results—on the only North American aphid with soldiers yet described—suggest that degrees of clonal mixing and kinship in themselves are unlikely to be particularly informative axes on which to discriminate soldier-producing species from other gall-forming aphids lacking cooperative defense. Rather, these results point to nongenetic factors thought to underlie social evolution in aphids (1, 21). The prolonged galling stage in P. obesinymphae, as compared with other Pemphigus species, probably has favored cooperative defense by increasing the vulnerability to predation (4, 5)—a pattern that is mirrored in other defense-based social taxa with expandable and long-lived domiciles (21, 22).
Although we found developmental and behavioral shifts that were distinctive to intruders, the proximal mechanism triggering these changes is not yet clear. In particular, we have not determined directly the ability of intruders to assess whether colony mates have the same or different genotypes. Kin recognition is unknown in any aphid (23–25). In P. obesinymphae, intruders are likely to be a random assortment of genotypes from the larger population: neighboring foundresses are sexually produced and are derived from winged grandmothers that arrive from widely dispersed localities and produce few descendant galls [six or fewer (4) on trees that can have hundreds of galls]. A previous study on P. obesinymphae showed that the death of the foundress and, to a lesser extent, gall damage induced developmental acceleration of soldiers (10). The experience of traveling between galls may have similar developmental effects by removing the influence of the foundress or by triggering a generalized developmental response to disturbance. The failure to attack, also characteristic of intruders, might be part of a coordinated shift toward feeding and growth and away from defense of the gall.
The cost of intruders for natal clones is not yet known. Such costs are likely, however, because intruders must compete with natal clones for space and resources in the gall (e.g., ref. 25), thereby reducing the productivity or survivorship of clones (9). What then is the basis for the evolution and maintenance of soldiers in P. obesinymphae, when colonies contain large numbers of unrelated aphids? One possible explanation lies in an evolutionary association between soldiers and intruders (1). Social aphids like P. obesinymphae readily can afford to invest some offspring toward defense because the reproductive capacity of the foundress outstrips the limited size of the gall. This same excess reproductive capacity favors intruders as an additional response to the high risk of clonal extinction by destruction of the natal gall. Intruders may be a cryptic caste of “self-propelled cuckoo eggs” (26) that extends the reproduction of the clone by parasitizing the galls of neighbors. Interestingly, both the soldier and the intruder strategies are favored under the same selective conditions—long-lived galls and high predation rates—and both require movement outside the gall. Thus, both are enabled by the same phenotypic traits: the gall ostiole, allowing movement into and out of galls, and adaptations for resisting desiccation and increasing mobility of first-instar stages, thereby conferring increased survival outside of the gall (1). The end result of combining these two survival strategies is that P. obesinymphae, and possibly some other social aphid species, may show lower within-gall relatedness yet higher levels of sociality relative to species without cooperative defense.
Considered together, our findings indicate that the intracolony dynamics of aphid social groups can be more complex than previously recognized, and that these little-studied taxa can furnish unique insights into the dynamics of social cooperation and conflict (27–29). Although kinship within colonies may not be decisive in determining which aphid species evolve sociality, the behavioral and developmental shifts toward selfishness exhibited by intruders indicate a central role for kin selection in shaping colony dynamics within P. obesinymphae. For example, one possible outcome of this study was that P. obesinymphae clones would coexist in socially cohesive, cooperative groups (e.g., refs. 6 and 30). However, relatedness asymmetries between intruders and resident aphids create opportunities for defection and conflict—scenarios characteristic of P. obesinymphae societies that are predicted outcomes of kin selection operating within social groups.
Acknowledgments
We thank M. Kaplan for advice on laboratory work and B. Crespi, Y. Carriere, A. Mira, and two anonymous reviewers for comments on the manuscript. This work was supported in part by the National Science Foundation (IBN-9808703) and the Center for Insect Science at the University of Arizona.
Abbreviation
- SNP
single nucleotide polymorphism
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See Commentary on page 11839.
References
- 1.Stern D L, Foster W A. Biol Rev. 1996;71:27–79. doi: 10.1111/j.1469-185x.1996.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 2.Aoki S. Kontyu. 1977;45:333–334. [Google Scholar]
- 3.Hamilton W D. In: The Biology of Social Insects. Breed M D, Michener C D, Evans H E, editors. Boulder, CO: Westview; 1997. pp. 81–102. [Google Scholar]
- 4.Moran N A. Insect Soc. 1993;40:391–402. [Google Scholar]
- 5.Foster W A, Northcott P A. In: Plant Galls: Organisms, Interactions, Populations. Williams M A J, editor. Oxford: Clarendon; 1994. pp. 161–182. [Google Scholar]
- 6.Queller D C, Zacchi F, Cervo R, Turillazzi S, Henshaw M T, Santorelli L A, Strassmann J E. Nature (London) 2000;405:784–787. doi: 10.1038/35015552. [DOI] [PubMed] [Google Scholar]
- 7.Strassman J E, Zhu Y, Queller D C. Nature (London) 2000;408:965–967. doi: 10.1038/35050087. [DOI] [PubMed] [Google Scholar]
- 8.Foster W A. Behav Ecol Sociobiol. 1990;27:421–430. [Google Scholar]
- 9.Foster W A, Rhoden P K. Anim Behav. 1998;55:761–765. doi: 10.1006/anbe.1997.0664. [DOI] [PubMed] [Google Scholar]
- 10.Withgott J H, Abbot D K, Moran N A. Proc R Soc London Ser B. 1997;264:1197–1203. [Google Scholar]
- 11.Aoki S. In: The Biology of Social Insects. Breed M D, Michener C D, Evans H E, editors. Boulder, CO: Westview; 1982. pp. 154–158. [Google Scholar]
- 12.Ozaki K. Evol Ecol. 1995;9:542–549. [Google Scholar]
- 13.Setzer R W. Ann Entomol Soc Am. 1980;73:327–331. [Google Scholar]
- 14.Bernasconi G, Strassmann J E. Trends Ecol Evol. 1999;14:477–482. doi: 10.1016/s0169-5347(99)01722-x. [DOI] [PubMed] [Google Scholar]
- 15.Funk D J, Helbling L, Wernegreen J J, Moran N A. Proc R Soc London Ser B. 2000;267:2517–2521. doi: 10.1098/rspb.2000.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moran N A, Baumann P. Curr Opin Microbiol. 2000;3:270–275. doi: 10.1016/s1369-5274(00)00088-6. [DOI] [PubMed] [Google Scholar]
- 17.Simon J C, Martinez Torres D, Latorre A, Moya A, Hebert P D N. Proc R Soc London Ser B. 1996;263:481–486. doi: 10.1098/rspb.1996.0072. [DOI] [PubMed] [Google Scholar]
- 18.Zietkiewicz E, Fafalski A, Labuda D. Genomics. 1994;20:176–183. doi: 10.1006/geno.1994.1151. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe A D, Liston A. In: Molecular Systematics of Plants II: DNA Sequencing. Soltis D E, Soltis P S, Doyle J J, editors. Boston: Kluwer; 1998. pp. 43–86. [Google Scholar]
- 20.Abbot P. J. Insect Sci. 2001. http://insectscience.org/1.8 http://insectscience.org/1.8. . [PMC free article] [PubMed] [Google Scholar]
- 21.Crespi B J, Choe J C. In: The Evolution of Social Behavior in Insects and Arachnids. Choe J C, Crespi B J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1996. pp. 499–524. [Google Scholar]
- 22.Alexander R D, Noonan K M, Crespi B J. In: The Biology of the Naked Mole-Rat. Sherman P W, Jarvis J U M, Alexander R D, editors. Princeton: Princeton Univ. Press; 1991. pp. 3–44. [Google Scholar]
- 23.Carlin N F, Gladstein D S, Berry A J, Pierce N E. J N Y Entomol Soc. 1994;102:287–298. [Google Scholar]
- 24.Shibao H. J Ethol. 1999;17:17–24. [Google Scholar]
- 25.Miller D G. Behav Ecol Sociobiol. 1998;43:95–103. [Google Scholar]
- 26.Hamilton W D. In: Animal Societies: Theories and Facts. Ito Y, Brown J L, Kikkawa J, editors. Tokyo: Japan Sci. Soc. Press; 1987. pp. 81–102. [Google Scholar]
- 27.Keller L, Chapuisat M. Bioscience. 1999;49:899–1000. [Google Scholar]
- 28.Queller D C. Phil Trans R Soc London B. 2000;355:1647–1655. doi: 10.1098/rstb.2000.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Queller D C, Strassman J E. Bioscience. 1998;48:165–174. [Google Scholar]
- 30.Clutton-Brock T H, Brotherton P N M, O'Riain M J, Griffin A S, Gaynor D, Kansky R, Sharpe L, McIlrath G M. Anim Behav. 2001;61:705–710. [Google Scholar]