Abstract
Introduction
It is inaccurate to assess blood glucose with glycated hemoglobin (HbA1c) in patients with diabetes and chronic kidney disease (CKD), and whether glycated albumin (GA) is better than HbA1c in these patients remains unclear.
Methods
We searched PubMed, Embase, Web of Science, Scopus, the Cochrane Library, and MEDLINE to July 2017 for studies that investigated the correlation between GA or HbA1c and the average glucose levels (AG) relevant to this theme. Statistical analysis was performed using RevMan5.3 and Stata12.0. The outcome was the correlation coefficient between GA or HbA1c and AG. For the first time, we made a comparison of GA and HbA1c in different CKD stages.
Results
A total of 24 studies with 3928 patients were included. Early stages of CKD refer to CKD stage 1 to 3. Advanced CKD refer to CKD stage 4 and 5 including patients receiving dialysis. The meta-analysis suggested that in early stages of CKD, the pooled R between GA and AG was 0.61 (95% CI = 0.49−0.73) and 0.71 (95% CI = 0.55−0.87) for HbA1c (P > 0.05). In advanced CKD patients, the pooled R between GA and AG was 0.57 (95% CI = 0.52−0.62), and 0.49 (95% CI = 0.45−0.52) for HbA1c (P = 0.0001).
Conclusion
GA is superior to HbA1c in assessing blood glucose control in diabetes patients with advanced CKD.
Keywords: chronic kidney disease, diabetes mellitus, glycated albumin, HbA1c
Chronic kidney disease (CKD) is a worldwide public health problem that affects millions of people of all racial and ethnic groups. Diabetes mellitus is a leading cause of CKD, and it is also an important comorbidity in established CKD.1 The rapidly increasing prevalence of diabetes worldwide virtually ensures that the proportion of CKD attributable to diabetes will continue to rise.2, 3 Glycemic control may decrease the incidence of new-onset microalbuminuria,4 delay the progression of diabetic nephropathy,5 limit end-organ damage, and reduce cardiovascular morbidity and mortality in uremic patients on hemodialysis.6 Compared to the general population, glycemic control in patients with CKD is complicated by alterations in glucose and insulin homeostasis.7 Therefore, choosing reliable clinical biomarkers to monitor glycemic control is critical in patients with both diabetes and CKD.
Glycated hemoglobin (HbA1c), glycated albumin (GA), fructosamine, and 1,5-anhydroglucitol (1,5-AG) are biomarkers used for evaluating glycemic control. At present, HbA1c, which reflects average glucose levels (AG) over the 120 days preceding the test, is widely used as a gold standard index for glycemic control in clinical practice. However, the HbA1c levels may be erroneous in patients with CKD8 because of factors such as anemia (due to reduced erythrocyte life span or iron deficiency), and the administration of erythropoietin.9, 10 1,5-AG reflects the degree of excretion of urinary glucose and is influenced by food ingestion and by threshold of glucose in the kidney. Fructosamine is a generic term that refers to all glycated serum proteins including GA in blood serum. It has a shorter half-life than HbA1c, as it reflects 1 to 3 weeks of glycemic status. However, fructosamine depends not only on glucose concentrations but also on the concentration of individual plasma proteins, and these may vary greatly in CKD.11
GA measures specifically the glycation product of albumin; it has been developed as an index for glycemic control,12 but it is not affected by serum albumin levels because its ratio to total serum albumin is calculated.13 To date, serum GA has been suggested as a more reliable and sensitive glycemic index to replace HbA1c in diabetic patients with CKD,14, 15, 16, 17 because it is not influenced by anemia and associated treatments. In addition, GA may also reflect the status of blood glucose more rapidly than HbA1c, and it is beneficial to patients with wide variations in blood glucose or those at higher risk for hypoglycemia.7 However, these benefits have not been verified by large-scale clinical trials and systemic meta-analyses. Therefore, it is necessary to perform a meta-analysis to address these issues.
Methods
Search Strategy
On 31 July 2017, we searched PubMed, Embase, Web of Science, Scopus, the Cochrane Library, and MEDLINE databases for articles about GA or HbA1c as an index of glycemic control in diabetic patients with CKD. The predefined searching key words were [“Glycated albumin” OR “Glycated hemoglobin”] AND “Kidney”, [“Glycated albumin” OR “Glycated hemoglobin”] AND “Renal”, [“Glycated albumin” OR “Glycated hemoglobin”] AND “Dialysis” through keywords searching systems. The search was limited to publications written in English to match our translation capacity.
Selection Criteria
Inclusion criteria were as follows: original and observational research; investigation of the relationship between the GA or HbA1c and AG levels; participation of diabetic patients with CKD; inclusion of the correlation coefficient and the number of patients; and full manuscript publication.
Exclusion Criteria
Exclusion criteria were as follows: animals used as research subjects; systematic review or meta-analysis; studies without the correlation coefficient and the number of patients; and articles not written in English.
Quality Assessment and Data Extraction
Data were extracted independently according to the above-mentioned selection and exclusion criteria; selection process details are shown in the Figure 1. The information extracted from each publication, in the form of a table, included the following: authors, year of publication, nation of origin, number and mean age of patients, patients’ CKD status, Pearson or Spearman correlation coefficient, and methods used to measure the average glucose levels.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of identification process for eligible articles. CKD, chronic kidney disease.
The correlation coefficients were obtained from each publication, including Pearson correlation (R), Spearman correlation coefficient (Rs), and R2. We converted the published Spearman correlation coefficient into Pearson correlation coefficient [Rs = 6π−1 sin−1(R/2)], and calculated the R value based on R2. The sampling distribution of Pearson correlation coefficients is problematic because the SE depends on the value of the correlation coefficients. Thus, a Fisher r-to-z transformation was conducted to obtain variance-stabilized correlation coefficients. The transformed Pearson coefficient was used in the meta-analysis, and finally the pooled correlation coefficient was transformed back to the raw scale for presentation.
Also, the methodological quality of the included studies was independently assessed by 2 observers (T.G. and X.L.) using the quality assessment tool recommended by the Agency for Healthcare Research and Quality (AHRQ), a tool specifically developed for systematic reviews of cross-sectional studies. Disagreements between the 2 reviewers were resolved by a majority opinion after a third reviewer (G.X.) assessed all of the involved items.
Statistical Analysis
After appropriate conversion, data from each publication were selected to perform meta-analysis by using RevMan 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) and Stata 12.0 (StataCorp, College Station, TX). The comparison between 2 correlation coefficients was analyzed by using the SPSS version 19.0 statistical package (SPSS Inc, Chicago, IL), and a P value of <0.05 was considered to indicate statistical significance. For each study, the correlation coefficients expressed as the effect size (ES) and 95% confidence interval (CI) were used and summarized by forest plots. The heterogeneity of the R values between studies was determined by calculating the Q statistic, derived from the χ2 test, and the inconsistency index (I2). A P value of ≤0.1 or an I2 value of ≥50% suggested heterogeneity. The standard fixed effects model was selected in the absence of heterogeneity. On the contrary, the random effects model was used. If notable heterogeneity was detected, sensitivity analysis and meta-regression were performed to further investigate the study heterogeneity. Subgroup analysis was performed to explore the source of heterogeneity based on different CKD stages of patients. Early stages of CKD refer to CKD stage 1 to 3. Advanced CKD refers to CKD stage 4 and 5, including patients receiving dialysis. Egger and Begg funnel plots were generated to assess the existence of publication bias and examine the differences in the studies.
Results
In all, 24 articles9, 10, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 with a total of 3928 patients with CKD were eventually identified according to the search strategy and the inclusion and exclusion criteria. Characteristics and other information extracted from each publication are summarized in Table 1. The quality was assessed as moderate in the 24 studies according to the AHRQ items, and the results are shown in Table 2. For 2 studies,23, 35 the R values were calculated based on the R2 provided in the papers. For 1 study,25 the R (Pearson correlation coefficient) value was calculated indirectly from the Spearman correlation coefficient provided in the paper. Of note, of the selected 24 studies, 15 were Asian.
Table 1.
Characteristics of studies included in the meta-analysis
| First author, reference | Year | Nation | n (F/M) | Age (yr) | CKD status | Ra (HbA1c) | Ra (GA) | Method |
|---|---|---|---|---|---|---|---|---|
| Yajima18 | 2017(a) | Japan | 15 (13/2) | 70.3 | Long HD group | 0.522 | 0.506 (P = NS) | CGMS |
| 2017(b) | Japan | 16 (11/5) | 71.3 | Short HD group | 0.098 (P = NS) | 0.337 (P = NS) | CGMS | |
| Chujo19 | 2010(a) | Japan | 49 (36/13) | 63.9 | ESRD (predialysis) | 0.47 | 0.56 | Not CGMS |
| 2010(b) | Japan | 37 (25/12) | 64.4 | ESRD (dialysis) | 0.42 | 0.5 | Not CGMS | |
| Hayashi20 | 2007 | Japan | 41 (27/14) | 60.2 | HD | 0.59 | 0.42 | CGMS |
| Vos21 | 2011 | New Zealand | 25 (18/7) | 60.2 | CKD 4−5 | 0.38 (P = NS) | 0.54 | CGMS |
| Kobayashi22 | 2013(a) | Japan | 20 (16/4) | 58.6 | HD | 0.121 (P = NS) | 0.67 | Not CGMS |
| 2013(b) | Japan | 20 (17/3) | 59.6 | PD | 0.166 (P = NS) | 0.62 | Not CGMS | |
| Inaba9 | 2015 | Japan | 538 (NA) | NA | HD | 0.52 | 0.539 | Not CGMS |
| Meyer23 | 2013 | France | 23 (13/10) | 65.7 | HD | 0.36c | 0.44c | CGMS |
| Sany24 | 2014(a) | Egypt | 25 (9/16) | 43.8 | CKD 1−3 | 0.56 | 0.58 | Not CGMS |
| 2014(b) | Egypt | 25 (15/10) | 49.6 | HD | 0.51 | 0.54 | Not CGMS | |
| Harada25 | 2009(a) | Japan | 28 (22/6) | 57 | CKD 1−2 | 0.739b | 0.67b | Not CGMS |
| 2009(b) | Japan | 69 (51/18) | 67.3 | CKD3 | 0.561b | 0.556b | Not CGMS | |
| 2009(c) | Japan | 42 (28/14) | 68.3 | CKD 4−5 | 0.289b (P = NS) | 0.361b | Not CGMS | |
| Kim26 | 2016 | Korea | 185 (60/125) | 60.3 | ESRD | 0.54 | 0.7 | Not CGMS |
| Fukami27 | 2002 | Japan | 30 (22/8) | 63 | CKD 4−5 | 0.24 (P = NS) | 0.41 | Not CGMS |
| Riveline28 | 2006 | France | 19 (8/11) | 64 | HD | 0.47 | NA | CGMS |
| Lee29 | 2013 | China | 25 (13/12) | 59 | PD | 0.51 | NA | CGMS |
| Kim30 | 2016 | USA | 347 (177/170) | 59 | HD | 0.48 | NA | Not CGMS |
| Mittman31 | 2015(a) | USA | 100 (46/54) | 63 | HD | 0.31 (P = NS) | NA | Not CGMS |
| 2015(b) | USA | 100 (46/54) | 66 | HD | 0.45 | NA | Not CGMS | |
| Uzu32 | 2012 | Japan | 87 (56/31) | NA | HD | 0.539 | 0.52 | Not CGMS |
| Qayyum33 | 2009 | Singapore | 60 (46/14) | 60.2 | PD | 0.48 | NA | CGMS |
| Tsuruta34 | 2012 | Japan | 46 (34/12) | 66.3 | HD | 0.363 | 0.385 | Not CGMS |
| Lo35 | 1996(a) | Australia | 14 (NA) | NA | CKD 3 | 0.89c | NA | CGMS |
| 1996(b) | Australia | 29 (NA) | NA | CKD 4−5 | 0.58c | NA | CGMS | |
| Joy10 | 2013 | USA | 23 (13/10) | 56 | HD | 0.57883 | NA | Not CGMS |
| Ichikawa36 | 2010 | Japan | 31 (20/11) | 66.9 | HD | 0.6705 | 0.5883 | Not CGMS |
| Wang37 | 2014 | China | 71 (41/30) | 66 | CKD 2−5 | 0.537 | 0.628 | Not CGMS |
| Williams38 | 2017 | USA | 1758 (932/826) | 62.1 | Dialysis | 0.69 | 0.63 | Not CGMS |
| Chen39 | 2016 | China | 30 (19/11) | 75 | CKD 3−4 | 0.796 | NA | Not CGMS |
CGMS, continuous glucose monitoring systems; CKD, chronic kidney disease; F, female; GA, glycated albumin; HbA1c, glycated hemoglobin; HD, hemodialysis; M, male; NA, not available; NS, not significant (P > 0.05); PD, peritoneal dialysis.
Letters in parentheses [(a), (b), and (c)] following the year represent different groups in the same study.
Pearson correlation coefficient.
Spearman correlation coefficient.
Values calculated based on R2 values.
Table 2.
Methodological quality of the included studies
| First author, reference | (1) Define the source of information | (2) List inclusion and exclusion criteria for subjects | (3) Indicate time period used for identifying patients | (4) Subjects were consecutive | (5) Evaluators of subjective components of study were masked to other aspects of the status of the participants | (6) Any assessments undertaken for quality assurance purposes | (7) Explain any patient exclusions from analysis | (8) Describe how confounding was assessed and/or controlled | (9) Explain how missing data were handled in the analysis | (10) Summarize patient response rates and completeness of data collection | (11) The percentage of patients for which incomplete data or follow-up was obtained | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yajima18 | Y | N | N | Y | N | Y | N | Y | N | Y | Y | 6 |
| Chujo19 | Y | N | N | Y | N | Y | N | Y | N | Y | Y | 6 |
| Hayashi20 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Vos21 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Kobayashi22 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Inaba9 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Meyer23 | Y | N | N | Y | N | Y | N | N | N | Y | Y | 5 |
| Sany24 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Harada25 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Kim26 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Fukami27 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Riveline28 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Lee29 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Kim30 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Mittman31 | Y | N | N | Y | N | Y | N | Y | N | Y | Y | 6 |
| Uzu32 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Qayyum33 | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | 9 |
| Tsuruta34 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Lo35 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Joy10 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Ichikawa36 | Y | N | N | Y | N | Y | N | Y | N | Y | Y | 6 |
| Wang37 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
| Williams38 | Y | N | N | Y | N | Y | N | Y | N | Y | Y | 6 |
| Chen39 | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | 8 |
N, no; Y, yes.
An item would be scored “0” if it was answered “No” or “Unclear”; an item would be scored “1” if it was answered “Yes.” Article quality was assessed as follows: low quality = 0−3; moderate quality = 4−7; high quality = 8−11.
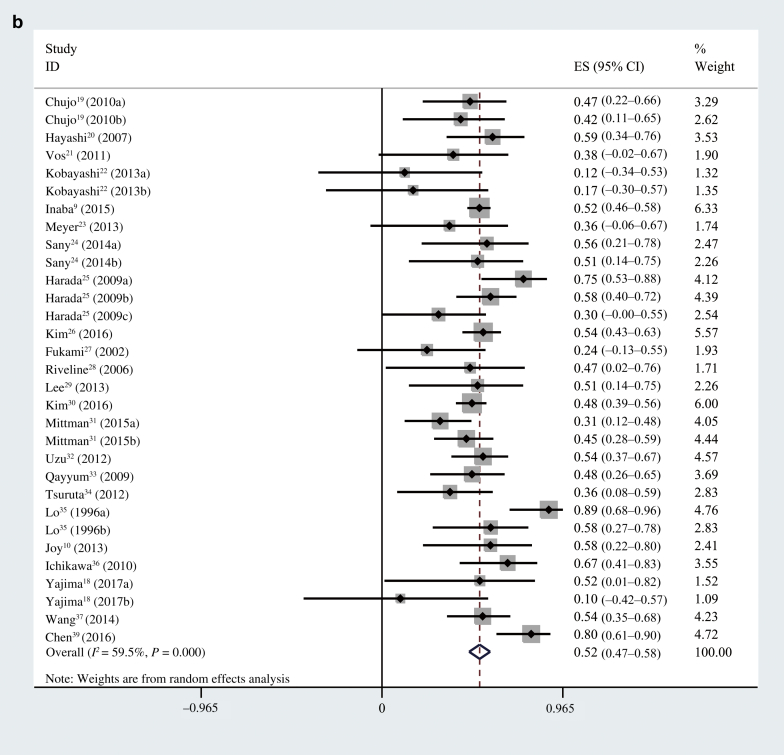
First, we performed an overall analysis of the correlation coefficient between GA or HbA1c and AG in all of the patients. The pooled R between GA and AG was 0.61 (95% CI = 0.59−0.63; I2 = 27.8%, P = 0.111) (Figure 2a) and 0.53 (95% CI = 0.47−0.59; I2 = 76.8%, P < 0.001) for HbA1c (Figure 2b). Because the pooled R between HbA1c and AG exhibited notable heterogeneity, we conducted a sensitivity analysis, meta-regression, and subgroup analysis to investigate the sources of heterogeneity. The results showed that 1 study38 and different CKD status were the main reasons. After removing the homogeneous study,38 in the overall CKD patients, the pooled R between GA and AG was 0.58 (95% CI = 0.54−0.61; I2 = 16.8%, P = 0.241) (Figure 3a) and 0.52 (95% CI = 0.47−0.58; I2 = 59.5%, P < 0.001) for HbA1c (Figure 3b).
Figure 2.
(a) Pooled R between glycated albumin and average glucose levels in all of the patients. (b) Pooled R between HbA1c and average glucose levels in all of the patients. CI, confidence interval; ES, effect size.
Figure 3.
(a) Pooled R between glycated albumin and average glucose levels after 1 study38 was excluded. (b) Pooled R between HbA1c and average glucose levels after 1 study38 was excluded. CI, confidence interval; ES, effect size.
Heterogeneity was still present in the included subjects due to the different CKD status, so we performed a subgroup analysis and made a comparison of GA and HbA1c in different CKD stages. Two studies37, 39 were subsequently excluded from subgroup analysis, because these studies provided only an overall R value to represent the correlation coefficient in different CKD stages. There were a total of 96 patients in early stages of CKD and 3731 patients in advanced CKD. In the early stages of CKD patients, the pooled R between GA and AG was 0.61 (95% CI = 0.49−0.73; I2 = 0%, P = 0.687) (Figure 4a) and 0.71 (95% CI = 0.55−0.87; I2 = 69.1%, P = 0.021) for HbA1c (Figure 4b). The results of a statistical analysis showed there was no difference between the 2 correlation coefficients (P > 0.05).
Figure 4.
(a) Pooled R between glycated albumin and average glucose levels in patients with different chronic kidney disease (CKD) status. (b) Pooled R between HbA1c and average glucose levels in patients with different CKD status. CI, confidence interval; ES, effect size.
In advanced CKD patients, the pooled R between GA and AG was 0.57 (95% CI = 0.52−0.62; I2 = 39.9%, P = 0.042) (Figure 4a) and 0.49 (95% CI = 0.45−0.52; I2 = 1.7%, P = 0.437) for HbA1c after 1 study38 was excluded (Figure 4b). A higher explanatory power was observed between AG and GA compared to HbA1c (P < 0.05). Egger and Begg funnel plots were used to detect publication bias. The Egger linear regression test (P = 0.001) and Begg rank correlation test (Pr >|z| = 0.496) showed publication bias (Figure 5). Sensitivity analysis showed that the pooled R of HbA1c had low sensitivity (Supplementary Figure S1A) and that the pooled R of GA had less satisfied stability (Supplementary Figure S1B).
Figure 5.
Publication bias of Egger and Begg test funnel plots.
Discussion
The aims of the present meta-analysis were to explore the correlation between AG and GA compared with HbA1c in diabetic patients with CKD. It is also the first study to compare GA and HbA1c in different CKD stages. Anemia not secondary to renal disease, active bleeding, recent blood transfusion within 30 days, hemoglobinopathy, chronic liver disease, and various other conditions with abnormal metabolism of GA or HbA1c were excluded from the analysis. In addition, subjects were required to have stable glycemic control and treatment regimens, because unstable glycemic levels and changes in treatment regimens may influence the GA or HbA1c levels and the accuracy of measurement methods,40, 41 which made the results of the present study more reliable.
Our meta-analysis showed that in the early stages of CKD, there was no statistically significant difference between GA and HbA1c, but GA is superior to HbA1c in advanced CKD. To test the difference between the 2 correlations more directly, we compared them in a joint analysis. We calculated the difference between the correlation coefficients (the correlation coefficient of GA subtracted from the correlation coefficient of HbA1c) and used it to perform a meta-analysis. According to the results, as shown in Supplementary Figure S2A, the joint analysis could be a supplementary analysis to verify the previous conclusion. In addition, the sensitivity analysis showed low sensitivity and satisfactory stability (Supplementary Figure S2B).
In advanced CKD, GA is superior to HbA1c because HbA1c underestimates and inaccurately reflects the glycemic conditions of patients. Several features may contribute to the inaccuracy of HbA1c, including the lifespan of red blood cells, use of iron and/or erythropoietin therapy, uremia, and need for frequent blood transfusions.7 Iron and/or erythropoietin treatment may cause an immediate fall in HbA1c levels without significant changes in glycemic status,42, 43 which is likely due to the stimulation of erythropoiesis with an increased ratio of young to old erythrocytes, and which leads to a reduction in the proportion of glycated hemoglobin.40 Moreover, carbamylated hemoglobin, which is formed under uremic conditions, may interfere with some HbA1c assays and result in an overestimation of HbA1c values.44 Compared with HbA1c, GA has a strong correlation with AG and provides a more reliable index of glycemic control because it is not affected by red blood cell lifespan or erythropoietin administration. Because advanced CKD is an extreme microvascular complication of diabetic nephropathy, CKD patients with diabetes should be carefully managed to prevent disease progression. GA allows rapid changes in overall glucose to be detected at an earlier stage, so that countermeasures can be taken promptly. In addition, it has been shown that increased levels of GA are associated with both the presence and severity of cardiovascular disease and impaired kidney function.45 Thus, GA may be a more reliable measure of glycemic control as well as a predictor of developing vascular complications, in people with diabetes and nephropathy.46
When we investigated the relationship between HbA1c and AG, heterogeneity was noticed in both the early and advanced stages of CKD, so we investigated the sources of the heterogeneity. In the early stages of CKD, we think that the limited number of studies, small numbers of patients, and studies from different nations (3 studies were from 3 different nations) were the main reasons. In advanced CKD, the sensitivity analysis and meta-regression identified the study38 that caused heterogeneity (I2 = 77.5%, data not show in figures). We noticed that the number of patients in the study38 was much larger than those in other studies, and this may be why the result of the sensitivity analysis was not very stable. In addition, in this study, the majority of the American patients were white or African American, and Asians were a small part (43.6%, 52.2%, and 1.7% respectively); however, 15 of the 24 selected studies were Asian. Some previous studies have pointed out that the value of HbA1c may be affected in individuals living in countries where the prevalence of sickle hemoglobin (HbS) is high.8 African Americans have an increased risk of carrying the hemoglobin S and thalassemia genes, which are associated with decreased erythrocyte survival. Also, therapeutic regimens were different in the United States and other nations. For example, hemodialysis patients in the United States received a high dose of erythropoietin, which was more than 3 times the dose in Japan. Previous studies have reported that both iron and erythropoietin can cause a significant fall in HbA1c values.40
Other sources of heterogeneity may be present, including the different patient characteristics and measuring methods for GA, HbA1c, and AG. The different patient characteristics included age, ratio of male to female, nationality, CKD status, and therapeutic regimen. Indeed, these factors may influence the GA or HbA1c levels. As is well known, age and sex affect the number and survival of erythrocytes. In addition, our study involved 9 countries, and the value of HbA1c and therapeutic regimen may be affected in countries where the prevalence of sickle hemoglobin (HbS) is high. Except for 1 study36 that used high-performance liquid chromatography (HPLC) for the measurement of GA, the remaining 23 studies used bromocresol purple (BCP). To date, there is no assay standardization for the measurement of GA. Furthermore, both assays have high accuracy, and we found that this difference in measurement is not linked with the heterogeneity by performing a meta-regression (Supplementary Figure S3).
Several inherent limitations existed in our study design and should be considered when interpreting the results. First, the number of patients in several of the included studies was relatively small, and data are limited on the relationship of GA or HbA1c and AG in earlier stages of CKD, which may reduce the strength of the conclusions of the present study. In earlier stages of CKD, the small number of patients and the variation in ethnicity are the main reasons for the wide confidence intervals. Second, our meta-analysis was based on cross-sectional studies. Moreover, this review was restricted to publications written in English because other languages,47, 48 such as Japanese, could not be translated by the study authors, which may introduce bias. Finally, continuous glucose monitoring systems (CGMS) are a reliable indicator of real-time blood glucose concentrations in the general population and are not affected by kidney disease.46 However, among selected studies, only 8 studies have incorporated this method for accurate determination of glycemia when exploring the correlation between markers for glycemic control. In the future, large-sample controlled trials using CGMS to investigate the relationship between GA and AG are needed to verify our findings.
In conclusion, despite the study limitations, our findings showed that GA is superior to HbA1C in assessing blood glucose control in diabetes patients with advanced CKD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. H0517/81560132), the Supporting Project for the Foregoers of Main Disciplines of Jiangxi Province (No. 20162BCB22023), and the “5511” Innovative Drivers for Talent Teams of Jiangxi Province (No. 20165BCB18018).
Acknowledgments
Author Contributions
All authors have contributed significantly. Ting Gan performed the meta-analysis, Xin Liu was responsible for the statistical analysis, and Gaosi Xu prepared the manuscript. All authors are in agreement with the content of the manuscript.
Footnotes
Figure S1. (A) Sensitivity analysis of the pooled R between HbA1c and average glucose levels in early and advanced stages of CKD. (B) Sensitivity analysis of the pooled R between glycated albumin and average glucose levels in early and advanced stages of CKD.
Figure S2. (A) The joint analysis comparing the correlations of glycated albumin and HbA1c. (B) Sensitivity analysis of the joint analysis comparing the correlations of glycated albumin and HbA1c.
Figure S3. Meta-regression based on the different assays for the measurement of glycated albumin.
Figure S4. The result of the trim and fill method.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Figure S1.
(A) Sensitivity analysis of the pooled R between HbA1c and average glucose levels in early and advanced stages of CKD. (B) Sensitivity analysis of the pooled R between glycated albumin and average glucose levels in early and advanced stages of CKD.
Figure S2.
(A) The joint analysis comparing the correlations of glycated albumin and HbA1c. (B) Sensitivity analysis of the joint analysis comparing the correlations of glycated albumin and HbA1c.
Figure S3.
Meta-regression based on the different assays for the measurement of glycated albumin.
Figure S4.
The result of the trim and fill method.
References
- 1.Kovesdy C.P., Sharma K., Kalantar-Zadeh K. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis. 2008;52:766–777. doi: 10.1053/j.ajkd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Kovesdy C.P., Kalantar-Zadeh K. Enter the dragon: a Chinese epidemic of chronic kidney disease? Lancet. 2012;379:783–785. doi: 10.1016/S0140-6736(12)60115-9. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Kawazu S., Tomono S., Shimizu M. The relationship between early diabetic nephropathy and control of plasma glucose in non-insulin-dependent diabetes mellitus. The effect of glycemic control on the development and progression of diabetic nephropathy in an 8-year follow-up study. J Diabetes Complications. 1994;8:13–17. doi: 10.1016/1056-8727(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 5.Hovind P., Rossing P., Tarnow L. Progression of diabetic nephropathy. Kidney Int. 2001;59:702–709. doi: 10.1046/j.1523-1755.2001.059002702.x. [DOI] [PubMed] [Google Scholar]
- 6.McMurray S.D., Johnson G., Davis S., McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis. 2002;40:566–575. doi: 10.1053/ajkd.2002.34915. [DOI] [PubMed] [Google Scholar]
- 7.Zheng C.-M., Ma W.-Y., Wu C.-C., Lu K.-C. Glycated albumin in diabetic patients with chronic kidney disease. Clin Chim Acta. 2012;413:1555–1561. doi: 10.1016/j.cca.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Abe M., Matsumoto K. Glycated hemoglobin or glycated albumin for assessment of glycemic control in hemodialysis patients with diabetes? Nat Clin Pract Nephrol. 2008;4:482. doi: 10.1038/ncpneph0881. [DOI] [PubMed] [Google Scholar]
- 9.Inaba M., Okuno S., Kumeda Y. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 10.Joy M.S., Cefalu W.T., Hogan S.L., Nachman P.H. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis. 2002;39:297–307. doi: 10.1053/ajkd.2002.30549. [DOI] [PubMed] [Google Scholar]
- 11.Montagna M.P., Laghi F., Cremona G. Influence of serum proteins on fructosamine concentration in multiple myeloma. Clin Chim Acta. 1991;204:123–130. doi: 10.1016/0009-8981(91)90223-y. [DOI] [PubMed] [Google Scholar]
- 12.Guthrow C.E., Morris M.A., Day J.F. Enhanced nonenzymatic glucosylation of human serum albumin in diabetes mellitus. Proc Natl Acad Sci U S A. 1979;76:4258–4261. doi: 10.1073/pnas.76.9.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga M. 1,5-Anhydroglucitol and glycated albumin in glycemia. In: Makowski G.S., editor. Advances in Clinical Chemistry. Elsevier Science & Technology; Amsterdam: 2014. pp. 269–301. [DOI] [PubMed] [Google Scholar]
- 14.Kim I.Y., Kim M.J., Lee D.W. Glycated albumin is a more accurate glycemic indicator than hemoglobin A1c in diabetic patients with pre-dialysis chronic kidney disease. Nephrology. 2015;20:715–720. doi: 10.1111/nep.12508. [DOI] [PubMed] [Google Scholar]
- 15.Inoue K., Goto A., Kishimoto M. Possible discrepancy of HbA1c values and its assessment among patients with chronic renal failure, hemodialysis and other diseases. Clin Exp Nephrol. 2015;19:1179–1183. doi: 10.1007/s10157-015-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peacock T.P., Shihabi Z.K., Bleyer A.J. Comparison of glycated albumin and hemoglobin A1c levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73:1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 17.Williams M.E., Lacson E., Jr., Teng M. Hemodialyzed type I and type II diabetic patients in the US: characteristics, glycemic control, and survival. Kidney Int. 2006;70:1503–1509. doi: 10.1038/sj.ki.5001789. [DOI] [PubMed] [Google Scholar]
- 18.Yajima T., Yajima K., Hayashi M. Serum albumin-adjusted glycated albumin as a better indicator of glycemic control in type 2 diabetes mellitus patients with short duration of hemodialysis. Diabetes Res Clin Pract. 2017;130:148. doi: 10.1016/j.diabres.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Chujo K., Shima K., Tada H. Indicators for blood glucose control in diabetics with end-stage chronic renal disease: GHb vs. glycated albumin (GA) J Med Investig. 2006;53:223–228. doi: 10.2152/jmi.53.223. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi A., Takano K., Masaki T. Distinct biomarker roles for HbA1c and glycated albumin in patients with type 2 diabetes on hemodialysis. J Diabetes Complications. 2016;30:1494. doi: 10.1016/j.jdiacomp.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Vos F.E., Schollum J.B., Coulter C.V. Assessment of markers of glycemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology. 2012;17:182–188. doi: 10.1111/j.1440-1797.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H., Abe M., Yoshida Y. Glycated albumin versus glycated hemoglobin as a glycemic indicator in diabetic patients on peritoneal dialysis. Int J Mol Sci. 2016;17:619. doi: 10.3390/ijms17050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer L., Chantrel F., Imhoff O. Glycated albumin and continuous glucose monitoring to replace glycated hemoglobin in patients with diabetes treated with hemodialysis. Diabet Med. 2013;30:1388–1389. doi: 10.1111/dme.12294. [DOI] [PubMed] [Google Scholar]
- 24.Sany D., Elshahawy Y., Anwar W. Glycated albumin versus glycated hemoglobin as glycemic indicator in hemodialysis patients with diabetes mellitus: variables that influence. Saudi J Kidney Dis Transplant. 2013;24:260–273. [PubMed] [Google Scholar]
- 25.Harada K., Sumida K., Yamaguchi Y., Akai Y. Relationship between the accuracy of glycemic markers and the chronic kidney disease stage in patients with type 2 diabetes mellitus. Clin Nephrol. 2014;82:107–114. doi: 10.5414/CN108027. [DOI] [PubMed] [Google Scholar]
- 26.Kim J.K., Park J.T., Oh H.J. Estimating average glucose levels from glycated albumin in patients with end-stage renal disease. Yonsei Med J. 2012;53:578. doi: 10.3349/ymj.2012.53.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukami K., Shibata R., Nakayama H. Serum albumin-adjusted glycated albumin reflects glycemic excursion in diabetic patients with severe chronic kidney disease not treated with dialysis. J Diabetes Complications. 2015;29:913. doi: 10.1016/j.jdiacomp.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Riveline J.P., Teynie J., Belmouaz S. Glycemic control in type 2 diabetic patients on chronic hemodialysis: use of a continuous glucose monitoring system. Nephrol Dial Transplant. 2009;24:2866. doi: 10.1093/ndt/gfp181. [DOI] [PubMed] [Google Scholar]
- 29.Lee S.Y., Chen Y.C., Tsai I.C. Glycosylated hemoglobin and albumin-corrected fructosamine are good indicators for glycemic control in peritoneal dialysis patients. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y., Park J.C., Molnar M.Z. Correlates of low hemoglobin A1c in maintenance hemodialysis patients. Int Urol Nephrol. 2013;45:1079. doi: 10.1007/s11255-012-0208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittman N., Desiraju B., Fazil I. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int. 2010;78:S41. doi: 10.1038/ki.2010.193. [DOI] [PubMed] [Google Scholar]
- 32.Uzu T., Hatta T., Deji N. Target for glycemic control in type 2 diabetic patients on hemodialysis: effects of anemia and erythropoietin injection on hemoglobin A 1c. Ther Apher Dial. 2009;13:89–94. doi: 10.1111/j.1744-9987.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- 33.Qayyum A., Chowdhury T.A., Oei E.L., Fan S.L. Use of continuous glucose monitoring in patients with diabetes mellitus on peritoneal dialysis: correlation with glycated hemoglobin and detection of high incidence of unaware hypoglycemia. Blood Purif. 2015;41:18. doi: 10.1159/000439242. [DOI] [PubMed] [Google Scholar]
- 34.Tsuruta Y., Ichikawa A., Kan K. Glycated albumin is a better indicator of the glucose excursion than predialysis glucose and hemoglobin A1c in hemodialysis patients. Renal Replace Ther. 2016;2:1–5. [Google Scholar]
- 35.Lo C., Lui M., Ranasinha S. Defining the relationship between average glucose and HbA1c in patients with type 2 diabetes and chronic kidney disease. Diabetes Res Clin Pract. 2014;104:84–91. doi: 10.1016/j.diabres.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Ichikawa H., Nagake Y., Takahashi M. What is the best index of glycemic control in patients with diabetes mellitus on hemodialysis? Nihon Jinzo Gakkai Shi. 1996;38:305–308. [PubMed] [Google Scholar]
- 37.Wang N., Xu Z., Han P., Li T. Glycated albumin and ratio of glycated albumin to hemoglobin are good indicators of diabetic nephropathy in type 2 diabetes mellitus. Diabetes Metab Res Rev. 2017;33(2) doi: 10.1002/dmrr.2843. [DOI] [PubMed] [Google Scholar]
- 38.Williams M.E., Mittman N., Ma L. The Glycemic Indices in Dialysis Evaluation (GIDE) study: comparative measures of glycemic control in diabetic dialysis patients. Hemodial Int. 2015;19:562–571. doi: 10.1111/hdi.12312. [DOI] [PubMed] [Google Scholar]
- 39.Chen H.S., Wu T.E., Lin H.D. Hemoglobin A 1c, and fructosamine for assessing glycemic control in diabetic patients with CKD stages 3 and 4. Am J Kidney Dis. 2010;55:867–874. doi: 10.1053/j.ajkd.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 40.Ng J.M., Cooke M., Bhandari S. The effect of iron and erythropoietin treatment on the A1c of patients with diabetes and chronic kidney disease. Diabetes Care. 2010;33:2310. doi: 10.2337/dc10-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakao T., Matsumoto H., Okada T. Influence of erythropoietin treatment on hemoglobin A1c levels in patients with chronic renal failure on hemodialysis. Intern Med. 1998;37:826–830. doi: 10.2169/internalmedicine.37.826. [DOI] [PubMed] [Google Scholar]
- 42.Koga M., Murai J., Saito H., Kasayama S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care. 2010;33:270–272. doi: 10.2337/dc09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng J.M., Jennings P.E., Laboi P., Jayagopal V. Erythropoetin treatment significantly alters measured glycated hemoglobin (HbA1c) Diabet Med. 2008;25:239–240. doi: 10.1111/j.1464-5491.2007.02336.x. [DOI] [PubMed] [Google Scholar]
- 44.Weykamp C.W., Penders T.J., Siebelder C.W. Interference of carbamylated and acetylated hemoglobins in assays of glycohemoglobin by HPLC, electrophoresis, affinity chromatography, and enzyme immunoassay. Clin Chem. 1993;39:138–142. [PubMed] [Google Scholar]
- 45.Pu L.J., Lu L., Shen W.F. Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J. 2007;71:1067–1073. doi: 10.1253/circj.71.1067. [DOI] [PubMed] [Google Scholar]
- 46.Vos F.E., Schollum J.B., Walker R.J. Glycated albumin is the preferred marker for assessing glycemic control in advanced chronic kidney disease. NDT Plus. 2011;4:368. doi: 10.1093/ndtplus/sfr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada T., Yamanaka T., Tomaru R. Relationship among glycated albumin, hemoglobin A1c, and daily blood glucose profile in diabetic patients on chronic hemodialysis. Nihon Toseki Igakkai Zasshi. 2010;43:433–441. [Google Scholar]
- 48.Motor S., Dokuyucu R., Sefil F. Relationship between HbA1c and blood glucose level in hemodialysis patients with diabetes mellitus. Dicle Med J. 2013;40:616–620. [Google Scholar]