Abstract
Introduction
Abrupt declines in kidney function often occur in patients with advanced chronic kidney disease and may exacerbate the need to initiate dialysis treatment. It is unclear how frequently such events occur in patients transitioning to chronic dialysis therapy, and what outcomes they are associated with.
Methods
We examined a national cohort of 23,349 US veterans with incident end-stage renal disease (ESRD) and with available pre-ESRD estimated glomerular filtration rate (eGFR) to identify abrupt declines in kidney function, defined as an unexpected >50% decrease in eGFR at the time of chronic dialysis transition. Associations with all-cause mortality and with renal recovery were examined in Cox proportional hazard and competing risk regression models.
Results
A total of 4804 (21%) patients experienced an abrupt decline in kidney function at dialysis transition. Renal recovery occurred in 586 (12.2%) and 297 (1.6%) patients with and without an abrupt decline, respectively (adjusted subhazard ratio: 4.42; 95% confidence interval [CI]: 3.72–5.27; P < 0.001). In the first 6 months after dialysis transition 1178 patients (24.5%) with abrupt decline died (annualized mortality rate 574/1000 patient-years), compared with 2354 deaths (12.7%) in patients without abrupt decline (274 deaths/1000 patient-years). An abrupt decline was associated with 45% higher mortality after multivariable adjustments (hazard ratio: 1.45; 95% CI: 1.33–1.57).
Conclusion
Abrupt declines in kidney function are common in patients transitioning to chronic dialysis, and are associated with higher mortality. Patients with abrupt declines also experience a higher rate of renal recovery; hence, careful attention to residual kidney function is warranted in these patients.
Keywords: acute kidney injury, chronic kidney disease, dialysis, end-stage renal disease, kidney function recovery, mortality
Chronic kidney disease (CKD) affects >10% of the general population, and >100,000 patients transition to maintenance hemodialysis annually in the United States.1 The transition to maintenance dialysis therapy is a watershed event for patients with CKD, which ideally should be preceded by education about preferred renal replacement modalities and other aspects of end-stage renal disease (ESRD), and preparations, such as the creation of a permanent dialysis access.2 Despite widespread agreement about the ideal transition to dialysis, >80% of patients initiating renal replacement therapy in the United States do so using a tunneled dialysis catheter, and dialysis patients’ mortality rates following transition are extremely high.1, 3, 4, 5
Part of the reason for this may be due to the uncertainty about when a patient with deteriorating kidney function may require initiation of renal replacement therapy.6, 7, 8 The interindividual variation in CKD progression and in the tolerance of the various consequences of advanced CKD (e.g., volume overload, electrolyte abnormalities, protein-energy wasting, or other uremic complications) makes it difficult to accurately predict an individual’s ideal time to transition.9 As a result, a substantial proportion of patients with advanced CKD develop acute medical complications related to worsening kidney function combined with underlying comorbid conditions, and transition to dialysis in an acute hospitalized setting.10, 11, 12
Adverse clinical events occurring in patients with advanced CKD can induce or worsen an acute decline of kidney function, which, when superimposed on advanced CKD,13 may result in sufficient deterioration in kidney function and/or in the development of complications that warrant initiation of renal replacement therapy. In patients with sufficiently advanced underlying CKD or a more severe acute event, the deterioration in kidney function may be perceived as irreversible, and the initiation of dialysis may be considered as transition to chronic renal replacement therapy, with reporting to dialysis registries as ESRD. Furthermore, many of these patients may have their ESRD reported as caused by the underlying CKD, and hence the identification of exacerbating abrupt deteriorations in kidney function from traditional data sources may be difficult.
Notwithstanding the desire to only capture patients with irreversible kidney failure, it is known that some patients in ESRD registries do recover kidney function and discontinue dialysis.14 Although some of these cases may be due to acute events, such as acute kidney injury (AKI) precipitating the initiation of dialysis, it is currently unclear how often such events are present among patients who transition to dialysis, what the characteristics of patients with abrupt pre-ESRD deteriorations in kidney function are, and what the outcomes associated with such events during transition are. To investigate this, we examined a large cohort of US veterans to determine the incidence of abrupt deterioration in kidney function occurring at the time of dialysis transition, its clinical characteristics, and the posttransition outcomes (renal functional recovery and mortality) of patients affected by such events.
Methods
Cohort Definition
We analyzed data from the Transition of Care in Chronic Kidney Disease (TC-CKD) study, a retrospective cohort study of US veterans with incident ESRD, who transitioned to renal replacement therapy from October 1, 2007, through March 31, 2014.15, 16, 17 A total of 85,505 US veterans with incident ESRD were identified from the US Renal Data System (USRDS)1 as our initial cohort. We subsequently excluded patients who had insufficient information about serum creatinine measurements before transition to dialysis (n = 62,037) and patients whose follow-up ended on the day of dialysis transition (n = 119), resulting in a final analytical sample of 23,349 patients. Compared with included patients, those who were excluded were older (71.1 vs. 67.5 years old), more likely to be female (8% vs. 2%) and white (75% vs. 66%), and less likely to have diabetes mellitus (56% vs 72%). Other comorbidities and the Charlson comorbidity index were similar between excluded and included patients (data not shown).
Data Collection
Data from the USRDS Patient and Medical Evidence Form 2728 were used to determine baseline demographic characteristics at the time of dialysis transition, primary cause of ESRD (AKI vs. others), renal replacement modality and vascular access type. Information about hospitalizations and comorbidities was extracted from the Department of Veterans Affairs (VA) Inpatient and Outpatient Medical SAS Datasets, using International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic and procedure codes and current procedural terminology codes, as well as from Centers for Medicare and Medicaid Services Data files, as previously described.15, 16, 17 We calculated the Charlson comorbidity index score using the Deyo modification for administrative data sets, without including kidney disease.18 Information about serum creatinine was obtained both from USRDS Form 2728 (for last serum creatinine value before transition) and from the VA LabChem files19 (for all serum creatinine levels measured up to 1 year before [also referred to as “prelude”] and 1 year after dialysis transition). Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation.20 Medication dispensation during the year before dialysis transition was recorded from both VA pharmacy dispensation records and from Medicare files, and information about all-cause mortality was obtained from the VA Vital Status files.21 Information about renal recovery was obtained from USRDS Form 2728 (which is used in the United States to report [among others] discontinuation of renal replacement therapy due to recovery of kidney function), using only recovery events that were recorded within 180 days of dialysis transition, and lasted at least 90 days.
Definition of Abrupt Deterioration in Kidney Function
The presence of an abrupt deterioration in kidney function at the time of transition to dialysis was defined by comparing the recorded eGFR at the time of transition with the eGFR value that was expected at transition based on the eGFR trajectory of each individual during the last year before transition. We used the eGFR at the time of transition that was recorded on the USRDS Form 2728, or the eGFR obtained from the VA LabChem files,22 retaining only values that were recorded no more than 7 days before the transition date, and retaining the value that was closest to the transition date. In cases in which an eGFR from the USRDS Form 2728 and from the VA LabChem files were recorded on the same date, we retained the value from the VA LabChem files. The pretransition eGFR trajectory was defined as the slope of all eGFR values recorded during days −30 to −365 (i.e., between 1 and 12 months before transition), which were calculated from mixed effect models in patients with at least 2 eGFR records during this time interval (median of 7 eGFR measures, 25th and 75th percentile: 2 and 13). The predicted eGFR at the time of transition was calculated by extending each patient’s eGFR slope from the last recorded pretransition eGFR to the transition date. We calculated the percent difference between the predicted and the recorded eGFR at transition ([predicted eGFR − recorded eGFR]/predicted eGFR × 100), and defined an abrupt deterioration in kidney function as a ≥50% difference in our primary analyses.
Statistical Analysis
Data are presented as number (percent) for categorical variables and mean ± SD or median (interquartile range [IQR]), as appropriate. Comparison between characteristics in patients with and without abrupt deterioration was done using t-tests for continuous variables and χ2 tests for categorical variables. The association of select characteristics with abrupt deterioration in kidney function was examined using multivariable logistic regression, using the same set of selected characteristics as used for multivariable models for all outcomes of interest. Our exposure variable was abrupt deterioration in kidney function, and the co-primary outcomes were post-ESRD mortality and renal recovery. We examined the association of abrupt deterioration with all-cause mortality in the first 6 months following dialysis transition using the Kaplan-Meier method and the log-rank test, and we calculated hazard ratios in unadjusted and multivariable adjusted Cox models. The association of abrupt deterioration at transition with posttransition recovery of kidney function was examined in multivariable adjusted competing risk regression models using the Fine and Gray method,23 with mortality as the competing event. Hierarchical multivariable models were constructed by making sequential adjustments (based on a priori considerations) for age, gender, race, and ethnicity (Model 2); comorbid conditions (history of diabetes mellitus, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic lung disease, liver disease and malignancies, and the Charlson comorbidity index as an omnibus measure of illness; Model 3); marital status; vascular access type; dialysis modality; hospitalization status at dialysis transition; eGFR level at transition; eGFR slope in the pretransition period; the number of serum creatinine measurements used to calculate slopes of eGFR; use of various medication classes linked to AKI risk during the year preceding dialysis transition; and for recovery of renal function within 6 months of dialysis transition (for mortality analyses only) (Model 4). Of the variables included in the main multivariable model, data points were missing for race (3.7%), marital status (0.1%), dialysis modality (0.1%), and vascular access type (5.1%). A total of 21,317 patients (91%) had complete data for multivariable analysis; because of the relatively low proportion of missingness, missing data were not imputed.
In sensitivity analyses, we examined the same associations in patients who had abrupt deterioration in kidney function defined as ≥25% lower detected eGFR compared with predicted eGFR at transition, and in subgroups of patients categorized based on presence or absence of pretransition nephrology care and the number of serum creatinine measurements used to calculate slopes of eGFR. Analyses were conducted using STATA MP Version 14 (STATA Corporation, College Station, TX). The study was approved by the institutional review boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
Results
Overall, patients were 67.5 ± 11.0 years old, 98% were men, 33% were African American, and 72% had diabetes. A total of 4804 (21%) patients experienced an abrupt deterioration in kidney function at the time of dialysis transition with a stable annual proportion from 2007 to 2014 (Supplementary Figure S1). Patients’ baseline characteristics at the time of dialysis transition overall and by abrupt deterioration status are presented in Table 1. Compared with patients without abrupt deterioration, patients with abrupt deterioration were more likely to be white and less likely to have diabetes mellitus, and were more likely to use a tunneled dialysis catheter as vascular access and to transition to dialysis in a hospitalized setting. AKI was listed as the primary cause of ESRD in 12.4% of patients with an abrupt deterioration and 1.8% of patients without an abrupt deterioration. In a multivariable logistic regression model, younger age, white race, non-Hispanic ethnicity, use of an arteriovenous graft or a tunneled catheter, nondiabetic status, chronic lung disease, liver disease, malignancies, a lower eGFR at transition, and the use of renin-angiotensin-aldosterone system inhibitors, thiazide, and K-sparing diuretics and i.v. contrast were significantly associated with the presence of an abrupt deterioration in kidney function (Supplementary Table S1).
Table 1.
Baseline characteristics at transition to dialysis overall and according to the absence or presence of abrupt deterioration in kidney function in 23,349 veterans who transitioned to maintenance dialysis therapy between 2007 and 2014
| Characteristics | All (n = 23,349) | Abrupt deterioration (n = 4,804) | No abrupt deterioration (n = 18,545) | P |
|---|---|---|---|---|
| Age (yr) | 67.5 ± 10.9 | 68.3 ± 10.9 | 67.3 ± 10.9 | <0.001 |
| Sex (male) | 22,850 (97.9) | 4700 (97.8) | 18,150 (97.9) | 0.881 |
| Race | <0.001 | |||
| White | 14,786 (65.8) | 3346 (71.7) | 11,440 (64.2) | |
| African American | 7424 (33.0) | 1292 (27.7) | 6132 (34.4) | |
| Other | 268 (1.2) | 30 (0.6) | 238 (1.3) | |
| Hispanic ethnicity | 1889 (8.1) | 306 (6.4) | 1583 (8.5) | <0.001 |
| Married | 11,901 (51.0) | 2432 (50.6) | 9469 (51.1) | 0.24 |
| Vascular Access Type | <0.001 | |||
| AV fistula | 4663 (21.0) | 215 (4.6) | 4448 (25.4) | |
| AV graft | 526 (2.4) | 47 (1.0) | 479 (2.7) | |
| Catheter | 16,977 (76.6) | 4413 (94.4) | 12,564 (71.8) | |
| Pre-ESRD nephrology care | 5247 (26.1) | 2027 (50.6) | 3220 (20.0) | <0.001 |
| AKI as primary cause of ESRD | 925 (4.0) | 594 (12.4) | 331 (1.8) | <0.001 |
| Myocardial infarction | 5390 (23.1) | 1169 (24.3) | 4221 (22.8) | 0.02 |
| Congestive heart failure | 12,300 (52.7) | 2506 (52.2) | 9794 (52.8) | 0.4 |
| Peripheral vascular disease | 8131 (34.8) | 1720 (35.8) | 6411 (34.6) | 0.11 |
| Cerebrovascular disease | 6663 (28.5) | 1376 (28.6) | 5287 (28.5) | 0.8 |
| Diabetes mellitus | 16,901 (72.4) | 3195 (66.5) | 13,706 (73.9) | <0.001 |
| Dementia | 497 (2.1) | 103 (2.1) | 394 (2.1) | 0.93 |
| Chronic pulmonary disease | 9225 (39.5) | 2150 (44.8) | 7075 (38.1) | <0.001 |
| Liver disease | 2706 (11.6) | 658 (13.7) | 2048 (11.0) | <0.001 |
| Malignancy | 5148 (22.1) | 1334 (27.8) | 3814 (20.6) | <0.001 |
| Charlson comorbidity index | 4.0 ± 2.4 | 4.2 ± 2.5 | 3.9 ± 2.3 | <0.001 |
| Renal replacement modality | <0.001 | |||
| Hemodialysis | 20,704 (88.8) | 3948 (82.3) | 16,756 (90.4) | |
| Peritoneal dialysis | 754 (3.2) | 43 (0.9) | 711 (3.8) | |
| Not recorded | 1870 (8.0) | 807 (16.8) | 1063 (5.7) | |
| Hospitalization at first dialysis | 14,348 (61) | 3531 (74) | 10,817 (58) | <0.001 |
| Medication use (1 yr prelude) | ||||
| ACE inhibitor/ARB | 14,166 (60.7) | 3078 (64.1) | 11,088 (59.8) | <0.001 |
| Loop diuretic | 18,524 (79.3) | 3191 (66.4) | 15,333 (82.7) | <0.001 |
| Thiazide diuretic | 7039 (30.1) | 1481 (30.8) | 5558 (30.0) | 0.25 |
| Potassium-sparing diuretic | 2442 (10.5) | 636 (13.2) | 1806 (9.7) | <0.001 |
| NSAIDs | 19 (0.1) | 9 (0.2) | 10 (0.1) | 0.004 |
| Contrast | 460 (2.0) | 128 (2.7) | 332 (1.8) | <0.001 |
Abrupt deterioration defined as a 50% drop in detected eGFR compared with predicted eGFR at dialysis transition.
Data are presented as number (percentage), mean ± SD, or median (interquartile range).
ACE, angiotensin-converting enzyme; AKI, acute kidney injury; ARB, angiotensin receptor blocker; AV, arteriovenous; BP, blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; NSAID, nonsteroidal anti-inflammatory drug.
Measures of Kidney Function and Renal Recovery
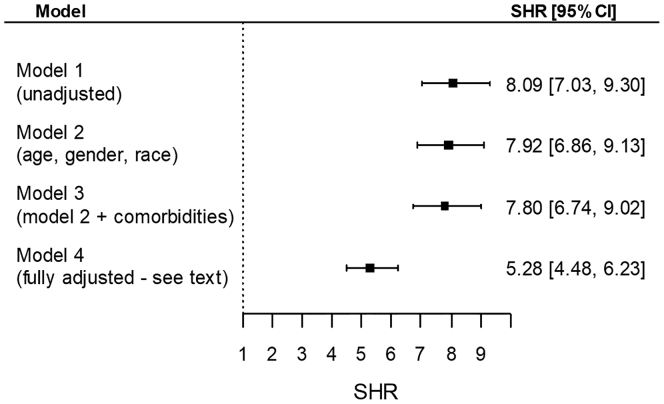
Table 2 shows the various measures of kidney function before, at the time, and after dialysis transition in the overall cohort and in patients with and without abrupt deterioration. Compared with patients without abrupt deterioration, patients with abrupt deterioration displayed a significantly steeper slope of eGFR during the prelude period, their predicted eGFR at transition was significantly higher, and their detected eGFR was significantly lower. Furthermore, the mean eGFR during the year following dialysis transition and the proportion of patients with mean eGFR levels >15 and >30 ml/min per 1.73 m2 during the year after transition were all significantly higher in patients with abrupt deterioration. Finally, renal recovery in the first 6 months was present in 12.2% of patients with abrupt deterioration and 1.6% of patients without abrupt deterioration (P < 0.001). In competing risk regression models, abrupt deterioration in kidney function at transition (vs. no abrupt deterioration) was associated with significantly higher subhazard ratios of renal recovery in the first 6 months after dialysis transition (multivariable adjusted subhazard ratio: 5.28; 95% confidence interval [CI]: 4.48–6.23; P < 0.001) (Figure 1).
Table 2.
Kidney function parameters before and after dialysis transition
| Characteristics | All (n = 23,349) | Abrupt deterioration (n = 4804) | No abrupt deterioration (n = 18,545) | P |
|---|---|---|---|---|
| Prelude eGFR slope (ml/min per 1.73 m2/yr) | −11.8 ± 13.7 | −14.0 ± 18.5 | −11.2 ± 12.1 | <0.001 |
| Predicted eGFR at transition (ml/min per 1.73 m2) | 16.4 ± 15.2 | 34.2 ± 23.5 | 11.8 ± 6.9 | <0.001 |
| Detected eGFR at transition (ml/min per 1.73 m2) | 10.6 ± 6.3 | 8.4 ± 4.7 | 11.1 ± 6.5 | <0.001 |
| Vintage eGFR (ml/min per 1.73 m2) | 13.6 ± 10.2 | 19.8 ± 17.3 | 12.1 ± 6.8 | <0.001 |
| Vintage eGFR >15 ml/min per 1.73 m2 | 4646 (25) | 1557 (45) | 3089 (21) | <0.001 |
| Vintage eGFR >30 ml/min per 1.73 m2 | 904 (4.9) | 586 (17) | 318 (2) | <0.001 |
| Renal recovery | 883 (3.8) | 586 (12) | 297 (2) | <0.001 |
Abrupt deterioration defined as a 50% drop in detected eGFR compared with predicted eGFR at dialysis transition. Predialysis eGFR slope defined from eGFR values between 365 and 30 days before transition. Predicted eGFR at transition defined as the eGFR value calculated by the extension of prelude eGFR slope to the transition day. Vintage eGFR represents the mean of all eGFR measurements obtained between days 30 and 365 after transition, irrespective of renal recovery status and without knowledge of temporal relationship to dialysis treatments. Renal recovery recorded from USRDS.
Values expressed as means ± SD and number (percent). eGFR, estimated glomerular filtration rate.
Figure 1.
Subhazard ratios (SHR) (95% confidence intervals [CI]) of renal functional recovery during the first 6 months after dialysis transition associated with the abrupt deterioration in kidney function at dialysis transition (vs. no abrupt deterioration). Results are from competing risk regression analyses, with all-cause mortality being the competing event. Model 1: unadjusted; model 2: age, gender, race; model 3: model 2 variables + comorbidities; model 4: model 3 variables + marital status, vascular access type, dialysis modality, hospitalization status at dialysis transition, eGFR level at transition, eGFR slope in the pretransition period, the number of serum creatinine measurements used to calculate slopes of eGFR, and the use of various medication classes linked to AKI risk during the year preceding dialysis transition. AKI, acute kidney injury; eGFR, estimated glomerular filtration rate.
Mortality
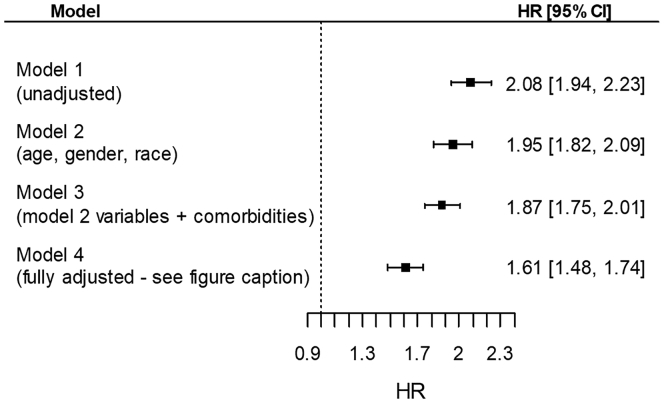
A total of 3532 patients died overall (annualized mortality rate 332/1000 patient-years, 95% CI: 321–342) during the first 6 months after dialysis transition; patients with abrupt deterioration experienced 1178 deaths (574/1000 patient-years, 95% CI 542–608) and patients without abrupt deterioration experienced 2354 deaths (274/1000 patient-years, 95% CI: 263–285). Figure 2 shows unadjusted and multivariable adjusted hazard ratios of 6-month mortality in patients with abrupt deterioration, compared with patients with no abrupt deterioration. Patients with abrupt deterioration experienced significantly higher mortality even after multivariable adjustments (hazard ratio: 1.61; 95% CI: 1.48–1.74).
Figure 2.
Hazard ratios (HR) (95% confidence intervals [CI]) of all-cause mortality during the first 6 months after dialysis transition associated with the abrupt deterioration in kidney function at dialysis transition (vs. no abrupt deterioration). Model 1: unadjusted; model 2: age, gender, race; model 3: model 2 variables + comorbidities; model 4: model 3 variables + marital status, vascular access type, dialysis modality, hospitalization status at dialysis transition, eGFR level at transition, eGFR slope in the pretransition period, the number of serum creatinine measurements used to calculate slopes of eGFR, the use of various medication classes linked to AKI risk during the year preceding dialysis transition, and for recovery of renal function within 6 months of dialysis transition. AKI, acute kidney injury; eGFR, estimated glomerular filtration rate.
Results were similar in sensitivity analyses when abrupt deterioration in kidney function was defined as a 25% difference between detected and predicted eGFR at dialysis transition (Supplementary Tables S2–S4; Supplementary Figures S2 and S3), and in subgroups divided by presence/absence of pretransition nephrology care and the number of serum creatinine measurements used to calculate slopes of eGFR (Supplementary Table S5).
Discussion
In this large national cohort of US veterans transitioning to hemodialysis during 2007–2014, 20% experienced an abrupt decrease in eGFR of ≥50% at the time of transition to chronic dialysis, and 40% experienced an abrupt decrease in eGFR of ≥25%. The frequent occurrence of abrupt deteriorations among patients transitioning to chronic dialysis suggests that such events may play a role in the decision to initiate chronic renal replacement therapy, and may be one of the reasons why many patients start dialysis under less than optimal circumstances (e.g., with a tunneled dialysis catheter as vascular access). In spite of the common nature of abrupt deteriorations in kidney function leading up to ESRD, AKI was listed as a primary cause of ESRD on the USRDS Form 2728 in only 12.4% of patients with abrupt deteriorations, suggesting that the detection of such events from databases that do not collect detailed information on kidney function trajectories is difficult.
Our results align with other previous publications that examined predialysis trajectories of kidney function. Rapid or very rapid (sometimes termed “catastrophic”24) declines in kidney function in patients with CKD have been reported in 3% to 12% of patients,24, 25, 26, 27, 28, 29 and were associated with higher mortality. To the best of our knowledge, none of the aforementioned studies examined the association between predialysis deterioration in kidney function and recovery of renal function after starting chronic dialysis.
Patients who experience abrupt deterioration in kidney function may recover function once the cause of the acute event is eliminated. In our study, abrupt deterioration at transition was strongly associated with renal recovery in the first 6 months following dialysis transition, but in terms of absolute numbers, relatively few patients who experienced such events were able to discontinue dialysis (12.2%). Previous studies examining large dialysis registries reported that overall approximately 6.7% of contemporary incident dialysis patients recover kidney function.14 These results suggest that most patients who experience abrupt deteriorations in kidney function at the time of dialysis transition may have irreversible renal injury, or their underlying CKD may be too advanced to allow meaningful functional recovery. However, the routine management of patients receiving chronic dialysis is not optimized for renal recovery after abrupt deteriorations in kidney function, in that personal supervision of chronic dialysis treatments by nephrologists is sparse, monitoring of kidney function can be infrequent, and protection of kidney function (e.g., by avoiding intradialytic hypotensive episodes, by avoiding exposure to nephrotoxic agents such as contrast material) is not always a priority. It is conceivable that close follow-up of patients who experienced abrupt deteriorations during transition might improve their chances of renal recovery postdialysis. A recent single-center study of 119 patients in the United States who started dialysis due to AKI described renal recovery and dialysis independence in 42% of them.30 The examined patients were not referred to regular chronic dialysis units following hospital discharge, but were instead dialyzed in a specialized unit under the close supervision of nephrologists, where their management was optimized toward renoprotection and renal recovery.30 These results suggest that attention to kidney function and a focus on strategies to promote renal recovery should be priorities even after transitioning to a chronic renal replacement environment in patients experiencing an abrupt deterioration in their kidney function at dialysis transition. Further studies are needed to determine the characteristics of patients who might benefit most from such strategies (e.g., differentiating patients with an abrupt deterioration who are more likely to die from those who are more likely to recover kidney function), the best interventions that could be applied toward renal recovery, and the duration of their implementation following transition to a chronic setting.
An abrupt deterioration in kidney function was also significantly associated with higher all-cause mortality in the first 6 months of dialysis, indicating that the propensity for abrupt deterioration may signal the presence of more severe underlying disease states and/or a higher likelihood for acute complications even after transitioning to chronic renal replacement therapy. Another possible contributor to the observed higher mortality is the less-than-optimal dialysis transition in patients with an abrupt deterioration, such as the lack of predialysis nephrology care or the high proportion of patients using a tunneled catheter as vascular access, which are known to portend a poorer prognosis in incident dialysis patients.31, 32, 33, 34, 35 It is currently unclear if prevention of AKI events in patients with advanced CKD might allow for better predialysis preparations (e.g., by extending the time available for nephrology referral and vascular access creation), or for improved outcomes by virtue of preventing acute medical complications precipitated by AKI. Although there are currently no specific therapies aimed at preventing or treating AKI, this is a field of intense investigation,36 and the development of future AKI therapies might allow their application in patients with advanced CKD.
Our study is notable for its large sample size, for being representative of veterans in the entire United States, and for the ability to define abrupt deteriorations in kidney function at dialysis transition based on changes in patients’ eGFR level compared with a predicted level calculated based on their past CKD trajectory. The laboratory-based diagnosis of AKI is superior to using diagnostic codes,37, 38 and the use of past CKD trajectories to predict expected eGFR levels prevents the misdiagnosis of a low eGFR resulting from the rapid progression of CKD as an abrupt deterioration. Our study also has limitations that need to be acknowledged. Even though we adjusted for numerous available confounders, the possibility of residual confounding remains. Our cohort consisted of mostly male US veterans and patients with available serum creatinine measurements in the predialysis period; therefore, the results may not be generalizable to the general population and to patients from other countries. We used predialysis laboratory records to diagnose abrupt deteriorations in kidney function, which necessitated the exclusion of many patients who did not have such measurements available for assessment. Although abrupt deteriorations in kidney function are in general called AKI, the conventional AKI definition requires both a pre-event steady-state baseline serum creatinine, and an acute rise in serum creatinine following a set time point (such as a hospitalization date or a procedure date). This conventional framework of AKI definition could not be applied in our cohort, whose pretransition baseline creatinine is a moving target due to the potentially rapid decline in kidney function caused by the underlying CKD process, and in whom there may not be a well-defined time point to anchor subsequent rises in serum creatinine before dialysis initiation. We overcame these limitations by predicting baseline kidney function using slopes of eGFR in the predialysis period, and by using the date of dialysis transition as a uniform time point when predialysis kidney function is reported in all patients, but this approach prevented us from using the AKI terminology to describe the detected abrupt deterioration events. We assumed a linear trajectory of patients’ underlying CKD during the year before ESRD transition, which is not always present, as described in previous studies assessing the renal function trajectory of patients with advanced CKD.24, 39 Finally, because of the observational nature of our study, we can only detect associations, which may or may not represent causal links.
In conclusion, abrupt deteriorations in kidney function occur frequently at the time of chronic dialysis initiation, and could jeopardize an ideal transition to renal replacement therapy. An abrupt deterioration at the time of transition is associated with both higher early postdialysis mortality, and also with a higher probability of renal functional recovery. Prevention of such events and enhanced focus on renal recovery in those who developed an abrupt deterioration could be used to improve outcomes in incident ESRD patients.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study is supported by grant 5U01DK102163 from the National Institutes of Health (NIH) to KKZ and CPK, and by resources from the US Department of Veterans Affairs (VA). The data reported here have been supplied by the US Renal Data System. Support for VA/Centers for Medicare and Medicaid Services data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
CPK and KKZ are employees of the Department of Veterans Affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs or the US government. The results of this paper have not been published previously in whole or part.
Footnotes
Table S1. Variables associated with abrupt deterioration in kidney function on transition to dialysis in 23,349 veterans who transitioned to maintenance dialysis therapy between 2007 and 2014.
Table S2. Baseline characteristics at transition to dialysis overall and according to the absence or presence of abrupt deterioration in kidney function, defined as a 25% drop in detected eGFR compared with predicted eGFR at dialysis transition.
Table S3. Variables associated with abrupt deterioration in kidney function, defined as a 25% drop in detected eGFR compared with predicted eGFR at dialysis transition.
Table S4. Kidney function parameters before and after dialysis transition.
Table S5. Subhazard ratios (SHR) (95% confidence intervals [CI]) of renal recovery and hazard ratios (95% confidence intervals) of all-cause mortality during the first 6 months after dialysis transition associated with abrupt deterioration in kidney function, defined as a 50% decrease in kidney function at dialysis transition, in subgroups of patients with and without pretransition nephrology care, and in patients divided by the number of serum creatinine measurements available for calculation of pretransition eGFR slopes.
Figure S1. Yearly incidence of abrupt deterioration in kidney function defined as a 25% decrease in kidney function (AD1) and a 50% decrese in kidney function (AD2) at dialysis transition.
Figure S2. Subhazard ratios (SHR) (95% confidence intervals [CI]) of renal recovery during the first 6 months after dialysis transition associated with abrupt deterioration in kidney function defined as a 25% decrease in kidney function at dialysis transition.
Figure S3. Hazard ratios (HR) (95% confidence intervals [CI]) of all-cause mortality during the first 6 months after dialysis transition associated with abrupt deterioration in kidney function defined as a 25% decrease in kidney function at dialysis transition.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Variables associated with abrupt deterioration in kidney function on transition to dialysis in 23,349 veterans who transitioned to maintenance dialysis therapy between 2007 and 2014.
Baseline characteristics at transition to dialysis overall and according to the absence or presence of abrupt deterioration in kidney function, defined as a 25% drop in detected eGFR compared with predicted eGFR at dialysis transition.
Variables associated with abrupt deterioration in kidney function, defined as a 25% drop in detected eGFR compared with predicted eGFR at dialysis transition.
Kidney function parameters before and after dialysis transition.
Subhazard ratios (SHR) (95% confidence intervals [CI]) of renal recovery and hazard ratios (95% confidence intervals) of all-cause mortality during the first 6 months after dialysis transition associated with abrupt deterioration in kidney function, defined as a 50% decrease in kidney function at dialysis transition, in subgroups of patients with and without pretransition nephrology care, and in patients divided by the number of serum creatinine measurements available for calculation of pretransition eGFR slopes.
Figure S1.
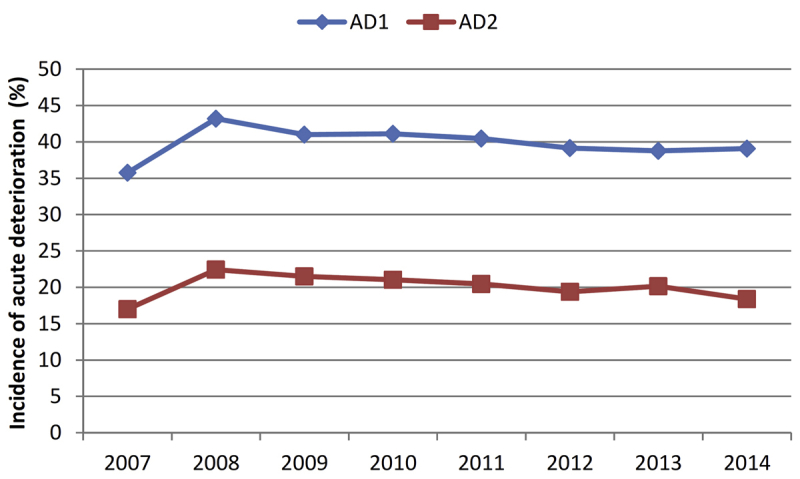
Yearly incidence of abrupt deterioration in kidney function defined as a 25% decrease in kidney function (AD1) and a 50% decrese in kidney function (AD2) at dialysis transition.
Figure S2.
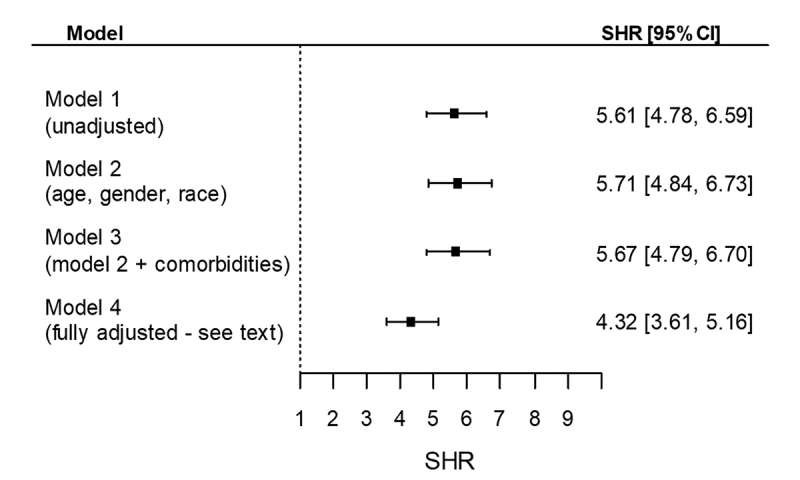
Subhazard ratios (SHR) (95% confidence intervals [CI]) of renal recovery during the first 6 months after dialysis transition associated with abrupt deterioration in kidney function defined as a 25% decrease in kidney function at dialysis transition.
Figure S3.
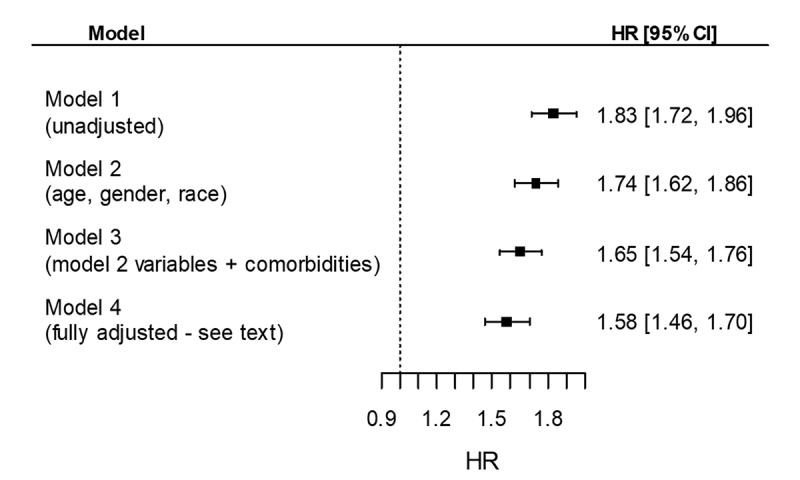
Hazard ratios (HR) (95% confidence intervals [CI]) of all-cause mortality during the first 6 months after dialysis transition associated with abrupt deterioration in kidney function defined as a 25% decrease in kidney function at dialysis transition.
References
- 1.Saran R., Li Y., Robinson B. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67 doi: 10.1053/j.ajkd.2015.12.014. Svii, S1-Svii, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 3.Foley R.N., Chen S.C., Solid C.A. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86:392–398. doi: 10.1038/ki.2014.15. [DOI] [PubMed] [Google Scholar]
- 4.Lukowsky L.R., Kheifets L., Arah O.A. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35:548–558. doi: 10.1159/000338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson B.M., Zhang J., Morgenstern H. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85:158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollock C.A., Cooper B.A., Harris D.C. When should we commence dialysis? The story of a lingering problem and today's scene after the IDEAL study. Nephrol Dial Transplant. 2012;27:2162–2166. doi: 10.1093/ndt/gfs125. [DOI] [PubMed] [Google Scholar]
- 7.Rosansky S.J., Cancarini G., Clark W.F. Dialysis initiation: what's the rush? Semin Dial. 2013;26:650–657. doi: 10.1111/sdi.12134. [DOI] [PubMed] [Google Scholar]
- 8.Wilson B., Harwood L., Locking-Cusolito H. Optimal timing of initiation of chronic hemodialysis? Hemodial Int. 2007;11:263–269. doi: 10.1111/j.1542-4758.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooper B.A., Branley P., Bulfone L. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 10.Arif F.M., Sumida K., Molnar M.Z. Early mortality associated with inpatient versus outpatient hemodialysis initiation in a large cohort of US veterans with incident end-stage renal disease. Nephron. 2017;137:15–22. doi: 10.1159/000473704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y.M., Wang Y.C., Hwang S.J. Patterns of dialysis initiation affect outcomes of incident hemodialysis patients. Nephron. 2016;132:33–42. doi: 10.1159/000442168. [DOI] [PubMed] [Google Scholar]
- 12.Wong S.P., Vig E.K., Taylor J.S. Timing of initiation of maintenance dialysis: a qualitative analysis of the electronic medical records of a national cohort of patients from the Department of Veterans Affairs. JAMA Intern Med. 2016;176:228–235. doi: 10.1001/jamainternmed.2015.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chawla L.S., Eggers P.W., Star R.A. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohan S., Huff E., Wish J. Recovery of renal function among ESRD patients in the US Medicare program. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molnar M.Z., Gosmanova E.O., Sumida K. Predialysis cardiovascular disease medication adherence and mortality after transition to dialysis. Am J Kidney Dis. 2016;68:609–618. doi: 10.1053/j.ajkd.2016.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumida K., Molnar M.Z., Potukuchi P.K. Association between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal disease. Nephrol Dial Transplant. 2017;32:1330–1337. doi: 10.1093/ndt/gfw220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumida K., Molnar M.Z., Potukuchi P.K. Association of slopes of estimated glomerular filtration rate with post-end-stage renal disease mortality in patients with advanced chronic kidney disease transitioning to dialysis. Mayo Clin Proc. 2016;91:196–207. doi: 10.1016/j.mayocp.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Kovesdy C.P., Alrifai A., Gosmanova E.O. Age and outcomes associated with BP in patients with incident CKD. Clin J Am Soc Nephrol. 2016;11:821–831. doi: 10.2215/CJN.08660815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 206;4:2. [DOI] [PMC free article] [PubMed]
- 22.Kovesdy C.P., Norris K.C., Boulware L.E. Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation. 2015;132:1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24.O'Hare A.M., Batten A., Burrows N.R. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis. 2012;59:513–522. doi: 10.1053/j.ajkd.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Aly Z., Zeringue A., Fu J. Rate of kidney function decline associates with mortality. J Am Soc Nephrol. 2010;21:1961–1969. doi: 10.1681/ASN.2009121210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovesdy C.P., Coresh J., Ballew S.H. Past decline versus current eGFR and subsequent ESRD risk. J Am Soc Nephrol. 2016;27:2447–2455. doi: 10.1681/ASN.2015060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu R.K., Chai B., Roy J.A. Abrupt decline in kidney function before initiating hemodialysis and all-cause mortality: The Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2016;68:193–202. doi: 10.1053/j.ajkd.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naimark D.M., Grams M.E., Matsushita K. Past decline versus current eGFR and subsequent mortality risk. J Am Soc Nephrol. 2016;27:2456–2466. doi: 10.1681/ASN.2015060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young B.A., Katz R., Boulware L.E. Risk factors for rapid kidney function decline among African Americans: The Jackson Heart Study (JHS) Am J Kidney Dis. 2016;68:229–239. doi: 10.1053/j.ajkd.2016.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautam S.C., Brooks C.H., Balogun R.A. Predictors and outcomes of post-hospitalization dialysis dependent acute kidney injury. Nephron. 2015;131:185–190. doi: 10.1159/000441607. [DOI] [PubMed] [Google Scholar]
- 31.Khan S.S., Xue J.L., Kazmi W.H. Does predialysis nephrology care influence patient survival after initiation of dialysis? Kidney Int. 2005;67:1038–1046. doi: 10.1111/j.1523-1755.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- 32.Winkelmayer W.C., Liu J., Chertow G.M. Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern Med. 2011;171:1371–1378. doi: 10.1001/archinternmed.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacson E., Jr., Wang W., Hakim R.M. Associates of mortality and hospitalization in hemodialysis: potentially actionable laboratory variables and vascular access. Am J Kidney Dis. 2009;53:79–90. doi: 10.1053/j.ajkd.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Pisoni R.L., Arrington C.J., Albert J.M. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis. 2009;53:475–491. doi: 10.1053/j.ajkd.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 35.Xue J.L., Dahl D., Ebben J.P. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–1019. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Basile D.P., Bonventre J.V., Mehta R. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. 2016;27:687–697. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grams M.E., Waikar S.S., MacMahon B. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9:682–689. doi: 10.2215/CJN.07650713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waikar S.S., Wald R., Chertow G.M. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 39.Sumida K., Kovesdy C.P. Disease trajectories before ESRD: implications for clinical management. Semin Nephrol. 2017;37:132–143. doi: 10.1016/j.semnephrol.2016.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variables associated with abrupt deterioration in kidney function on transition to dialysis in 23,349 veterans who transitioned to maintenance dialysis therapy between 2007 and 2014.
Baseline characteristics at transition to dialysis overall and according to the absence or presence of abrupt deterioration in kidney function, defined as a 25% drop in detected eGFR compared with predicted eGFR at dialysis transition.
Variables associated with abrupt deterioration in kidney function, defined as a 25% drop in detected eGFR compared with predicted eGFR at dialysis transition.
Kidney function parameters before and after dialysis transition.
Subhazard ratios (SHR) (95% confidence intervals [CI]) of renal recovery and hazard ratios (95% confidence intervals) of all-cause mortality during the first 6 months after dialysis transition associated with abrupt deterioration in kidney function, defined as a 50% decrease in kidney function at dialysis transition, in subgroups of patients with and without pretransition nephrology care, and in patients divided by the number of serum creatinine measurements available for calculation of pretransition eGFR slopes.