Abstract
Introduction
The renal angina index (RAI) is determined based on changes in the creatinine and condition scores of patients. The aim of this study is to evaluate the efficacy of the RAI in predicting persistent acute kidney injury (AKI) in Asian intensive care unit (ICU) patients.
Methods
This is a subanalysis of 3 prospective studies conducted in Japan and Thailand. The RAI was calculated for all enrolled patients using the method of Goldstein and colleagues, with a minor modification for adults on day 2. To determine the accuracy of RAI further, we evaluated a subgroup of patients for whom baseline serum creatinine values were available at ICU admission (i.e., those with hospital-acquired AKI). AKI biomarkers were evaluated for their efficacy in improving the performance of RAI. The outcome was defined as AKI stage 2 or 3 over 48 hours.
Results
Of the 263 patients analyzed, a total of 22 progressed to stage 2 or 3 AKI over 48 hours. The RAI was associated with an area under the curve (AUC) of 0.63 in receiver-operating characteristics analysis, with a cutoff of 10. In those admitted from general wards, the RAI had good performance, with an AUC of 0.73 and a cutoff of 6. A combination of L-type fatty acid–binding protein with the RAI improved the predictive performance for assessing persistent AKI with an AUC of 0.79.
Conclusion
The RAI may be effective in predicting persistent AKI in adult patients admitted from general wards. Incorporation of AKI biomarkers into the RAI may potentially improve prediction.
Keywords: acute kidney injury, AKI biomarkers, prediction, renal angina
Acute kidney injury (AKI) is a common complication with miserable consequences in critically ill patients worldwide.1, 2 To prevent and reduce the incidence of severe AKI, risk stratification is deemed important for providing care to patients at high risk of AKI.
Although the Kidney Disease Improving Global Outcomes (KDIGO) guideline defines AKI according to serum creatinine and urine output, serum creatinine is an imperfect marker for detecting severe AKI, and novel AKI biomarkers are emerging. Thus, prediction of AKI or risk stratification of patients in danger of kidney damage is crucial for initiating preventive measures for AKI. AKI biomarkers, such as cell cycle arrest markers (tissue inhibitor of metalloproteinases 2 and insulin-like growth factor binding protein 7),3, 4, 5 neutrophil gelatinase-associated lipocalin, and L-type fatty acid–binding protein (L-FABP)6, 7, 8 were reported to predict AKI. However, it is necessary that these biomarkers be used in an appropriate setting, because they may be affected by comorbidities, and their performance may decrease in a different setting.9, 10 Thus, an appropriate risk assessment for AKI is required in every patient admitted to the intensive care unit (ICU).
Recently, the renal angina index (RAI), which is determined based on changes in renal function, was proposed to risk stratify critically ill children at high risk of AKI.11, 12 The concept of renal angina has come into use to highlight the characteristics of renal injury as an analogy to the concept of angina pectoris, which is used to increase the suspicion of acute coronary syndrome in cardiology. The RAI is assumed to serve as a potential biomarker for detecting early signs of persistent AKI. The RAI in adults was proposed, which involved a consistent, albeit more complicated, definition compared with that used in pediatric ICU patients.13 Although the RAI appears to be an attractive concept, it remains yet to be tested for its applicability and validated in non-Western populations, such as Asian populations.
Of all the multiple chronic comorbidities that represent risk factors for AKI, diabetes mellitus has been reported to be a high risk14 and has been included in AKI prediction models.15 Although multiple risk factors for AKI progression have been included in an earlier report,13 we included factors that were thought likely to influence creatinine production, such as diabetes mellitus and severe illness that required vasopressor therapy or ventilation, to make the RAI simpler in this analysis.
The aim of this study was to evaluate the performance of RAI for predicting patients who were at higher risk of persistent severe AKI in the Asian population. Additional clinical parameters evaluated at ICU admission were also examined for their ability to predict severe AKI in this study.
Methods
Study Population
This study was a subanalysis of 3 prospective observational studies conducted in a mixed medical and/or surgical ICU settings (i.e., 2 prospective observational studies in Japan16, 17, 18 and the SEA-AKI study in Thailand, South East Asia-Acute Kidney Injury, https://www.theisn.org/programs/isn-programs/item/2645-clinical-research-the-epidemiology-and-prognostic-factors-for-mortality-in-intensive-care-unit-patients-with-acute-kidney-injury-in-south-east-asia19). As previously reported, serum creatinine was prospectively measured in all ICU patients in these studies every day for 1 week after ICU admission. In the first analysis, all patients were included, except for those who had already progressed to AKI stage 2 or 3 at ICU admission, those who stayed in ICU for <48 hours, and those whose serum creatinine values at ICU admission or on day 2 were not available. In the next analysis, patients were included if they met the following criteria: (i) those admitted from general wards with no AKI or in AKI stage 1; (ii) those who had their serum creatinine measured at 2 points (within 3 days before ICU admission and at ICU admission); and (iii) those who stayed in ICU for >48 hours. This study was approved by the institutional review committees of The University of Tokyo Hospital (Japan) and King Chulalongkorn Memorial Hospital (Thailand), and was conducted in accordance with the Declaration of Helsinki.
Renal Angina Index
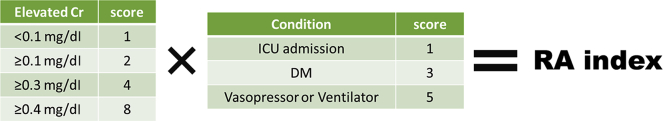
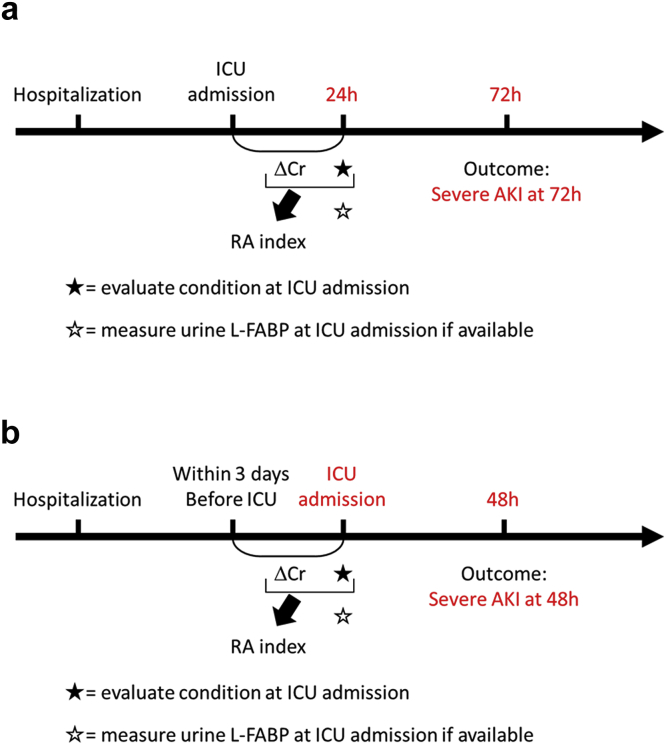
The RAI in previous reports13, 20 was used with a minor modification (Figure 1 and Supplementary Figure S1). The condition of each patient was scored as follows: those with ventilation and/or vasopressor therapy, 5 points; those with ≥1 comorbidities (diabetes mellitus, advanced age [70 years or older], chronic kidney disease [CKD], or hypertension), 3 points; and those admitted to ICU, 1 point. The data on the history of cardiovascular diseases, including chronic heart failure, were not collected in these studies, and therefore, were not integrated into the RAI calculation. The creatinine score was determined by the difference in serum creatinine between that at ICU admission and that within 3 days before ICU admission at the latest, as follows: those with creatinine ≥0.4 mg/dl, 8 points; those with creatinine ≥0.3 mg/dl, 4 points; those with creatinine ≥0.1 mg/dl, 2 points; and those with creatinine <0.1 mg/dl, 1 point. The RAI score was defined as the worst condition score multiplied by the creatinine score and consisted of 1, 2, 3, 4, 6, 8, 10, 12, 24, and 40. All patients were evaluated for the RAI on the next day after ICU admission in the first analysis and at ICU admission in the second analysis (Figure 2).
Figure 1.
Renal angina (RA) index. The scheme was derived from Basu et al.20 for use with a minor modification for diabetes mellitus (DM) as an adult-specific disease. The RA index-based approach was originally reported by Claure-Del Granado et al.21Supplementary Figure S1 shows the influence of additional comorbidities. ICU, intensive care unit.
Figure 2.
Study protocol for (a) all patients and (b) patients admitted from general wards. (a) The renal angina (RA) index (RAI) was calculated at 24 hours after intensive care unit (ICU) admission based on each patient’s condition determined at ICU admission and the slight changes observed in serum creatinine between ICU admission and 24 hours later. (b) The RAI was calculated at ICU admission based on each patient’s condition determined at ICU admission and the slight changes observed in serum creatinine from before and at ICU admission. In both protocols, the outcome measure was persistent moderate-to-severe acute kidney injury (AKI) over 48 hours based on RAI scoring. Cr, creatinine; L-FABP, L-type fatty acid–binding protein.
Urinary L-FABP Measurement
Because urine output was measured hourly by an indwelling catheter, a fresh urine sample was obtained from all patients at ICU admission. The urinary L-FABP level was measured using in vitro diagnostic medical tests that formed part of enzyme-linked immunosorbent assay kits (Human L-FABP Assay Kit; CMIC Co. Ltd., Tokyo, Japan) in which the ability to detect AKI was proven in clinical and experimental settings in previous studies.8, 22, 23, 24
Assessment of Kidney Function
The baseline serum creatinine value was defined as the last value measured in an outpatient setting within 6 months before ICU admission. For patients with an unavailable creatinine value before ICU admission, the baseline value was defined as the minimum among inpatient values obtained before ICU admission, the last value before hospital discharge, or the estimated value using the Modification of Diet in Renal Disease equation, which assumes a baseline estimated glomerular filtration rate (eGFR) of 75 ml/min per 1.73 m2. Serum creatinine was measured every day. AKI was defined and classified according to the KDIGO Clinical Practice Guideline for Acute Kidney Injury.25
Outcome
The primary outcome measure was defined as onset of persistent severe AKI (stage 2 or 3 AKI) over 48 hours.
Statistical Analyses
Continuous variables were analyzed by the Wilcoxon ranked test, and categorical variables were analyzed by the χ2 test. The breakdown of RAI derivation was determined using a decision-tree analysis, and the RAI performance was ascertained using a receiver-operating characteristic (ROC) curve analysis. Calculations were conducted using statistical analysis software (JMP version 13.0; SAS Institute Inc., Cary, NC). A conventional α level of 0.05 was used to assess statistical significance.
Results
Patient Characteristics
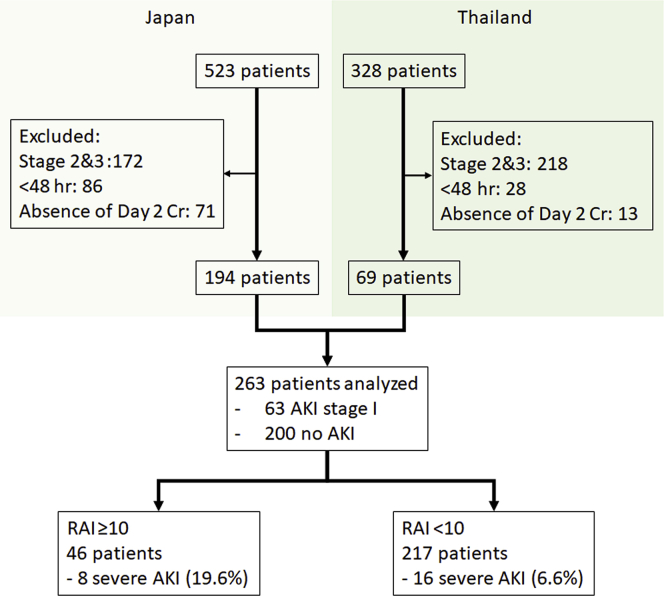
Of the 851 patients (523 in Japan and 328 in Thailand), 263 patients met the inclusion criteria for initial analysis, and 24 achieved the primary outcome after 48 hours (Figure 3). The baseline characteristics of these patients are shown in Table 1. Sepsis was the most common cause of ICU admission, which varied in incidence between the 2 countries and was more predominant in Thailand than in Japan, where various other causes, as well as sepsis, accounted for ICU admission.
Figure 3.
The flowchart of the enrolled patients. Decision-tree analysis stratified the population with a cutoff of 10. AKI, acute kidney injury; Cr, creatinine; RAI, renal angina index.
Table 1.
Baseline characteristics
| Characteristics | All (n = 263) | Japan (n = 194) | Thailand (n = 69) |
|---|---|---|---|
| Age, mean ± SD | 61.6 ± 17.2 | 60.1 ± 17.3 | 65.7 ± 16.5 |
| Male, % | 59.3 | 62.4 | 50.7 |
| Main causes of ICU admission, n (%) | |||
| Sepsis | 68 (25.9) | 36 (18.6) | 32 (46.3) |
| Abdominal | 37 (14.1) | 32 (16.5) | 5 (7.2) |
| Cardiovascular | 20 (7.6) | 12 (6.2) | 8 (11.5) |
| Neurologic | 59 (22.4) | 56 (28.9) | 3 (4.3) |
| Pulmonary | 30 (11.4) | 15 (7.7) | 15 (21.8) |
| Trauma | 14 (5.3) | 14 (7.2) | 0 (0.0) |
| Malignancy | 15 (5.7) | 12 (6.2) | 3 (4.3) |
| Others | 20 (7.6) | 17 (8.7) | 3 (4.3) |
| APACHE II | 17.5 ± 6.2 | 16.3 ± 6.3 | 20.9 ± 4.4 |
| Baseline Cr, mg/dl | 0.85 ± 0.51 | 0.72 ± 0.35 | 1.22 ± 0.70 |
| ICU mortality, n (%) | 36 (13.7) | 12 (6.2) | 24 (34.8) |
| Hospital mortality, n (%) | 62 (23.5) | 22 (11.3) | 40 (57.9) |
APACHE, acute physiology and chronic health evaluation; Cr, creatinine; ICU, intensive care unit.
Results Among All ICU Patients
Figure 3 shows the results of the decision-tree analysis performed to identify at-risk individuals in the AKI cohort using the best cutoff values. The RAI exhibited a fair, but not good, AUC of 0.63 (95% confidential interval [CI]: 0.53–0.73) in ROC analysis, with a cutoff of 10; two-thirds of all severe AKI patients were associated with RAI of <10. The RAI exhibited an AUC of 0.68 (95% CI: 0.61–0.75) with a cutoff of 8 when those with AKI stage 2 at ICU admission were also included in the analysis, thus showing no remarkable improvement.
Results Among the ICU Patients Admitted From General Wards
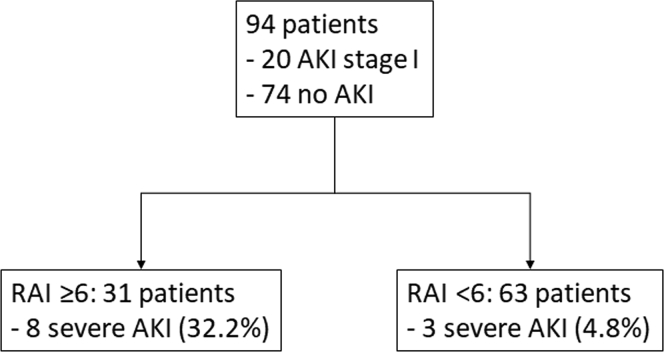
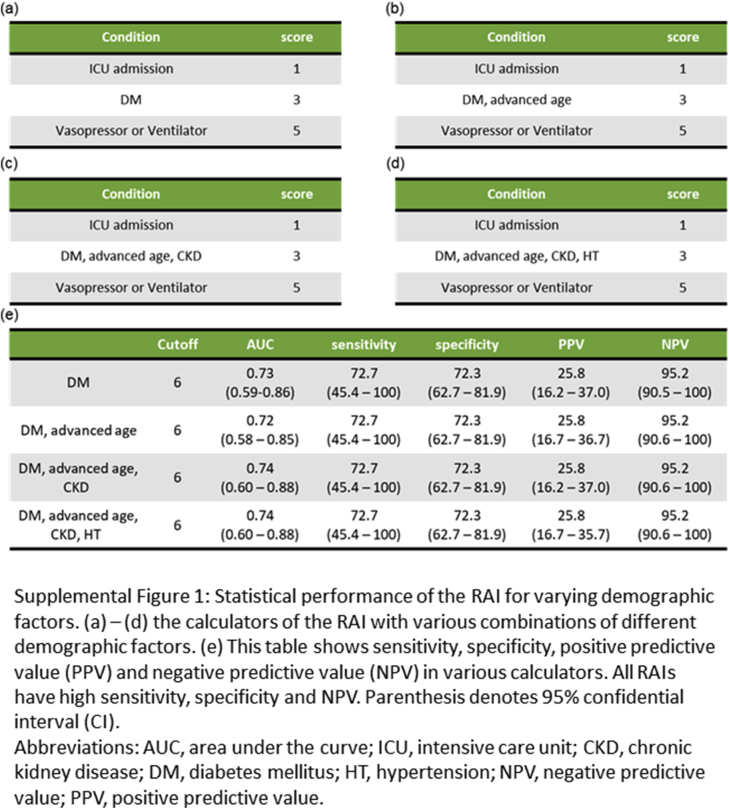
In the initial analysis, the RAI did not perform better than in previous studies involving critically ill children and adults at high risk of AKI. This might reflect differences in clinical practice between our ICUs and those described in earlier reports, as well as in the proportion of patients admitted to the ICU from emergency rooms and those from general wards after onset of AKI. Thus, 94 of the 263 patients admitted from general wards who had had their serum creatinine measured before ICU admission were included in the next analysis. They were expected to demonstrate hospital-acquired persistent AKI based on their measured baseline serum creatinine values. Decision-tree analysis indicated the use of the RAI was 6 (Figure 4). Although the acute physiology and chronic health evaluation (APACHE) II score had a fair predictive performance with an AUC of 0.65 (95% CI: 0.48–0.81), the RAI showed reasonably good predictive performance for persistent AKI, with an AUC of 0.73 (95% CI: 0.58–0.87) (Figure 4) and had a higher negative predictive value (NPV) with good sensitivity and specificity (Supplementary Figure S1). The RAI showed a similar performance even when other demographic factors, such as advanced age, CKD, and hypertension, were included as its components, which suggested that diabetes mellitus alone was a sufficient demographic component in the RAI.
Figure 4.
The decision-tree analysis stratified patients admitted from general wards with a cutoff of 6. AKI, acute kidney injury; RAI, renal angina index.
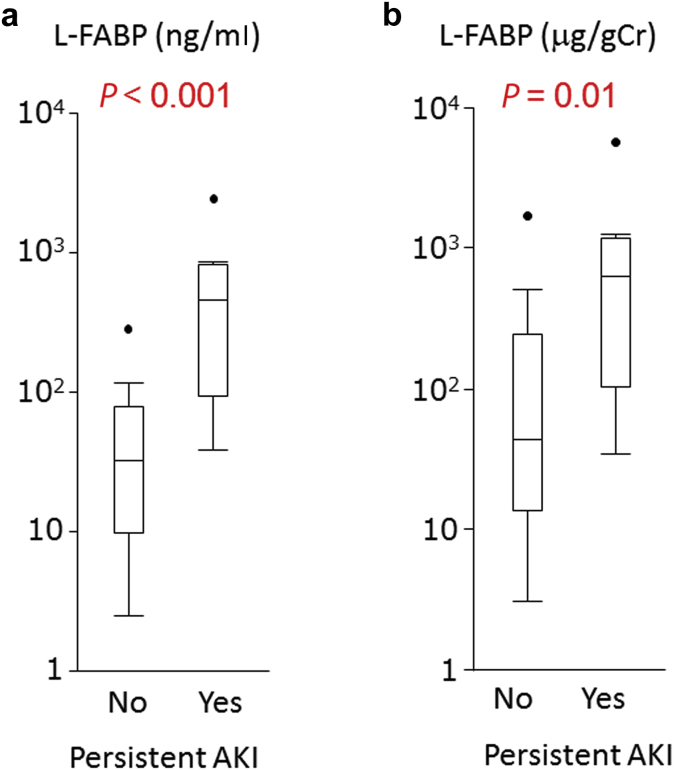
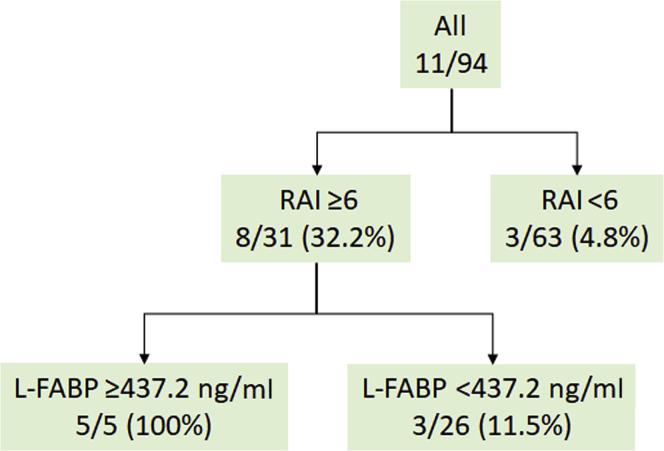
In addition, the urinary L-FABP level was shown to be higher among patients with persistent severe AKI with or without creatinine adjustment in the high RAI group (≥6) (Figure 5). Decision-tree sensitivity analysis revealed that a combination of the RAI and L-FABP could contribute much to stratifying higher risk patients with severe persistent AKI, with a cutoff of 437.2 ng/ml (Figure 6) and an AUC of 0.79 (95% CI: 0.58–0.91). When the most superior performance was optimized with the combination of Youden’s index and the highest NPV for screening purposes, the cutoff value was chosen to be 37.8 ng/ml. The AUC was found to be 0.82 (95% CI: 0.63–0.92).
Figure 5.
(a) Urine L-type fatty acid–binding protein (L-FABP) and (b) L-FABP/creatinine (Cr) in patients with or without progression to severe acute kidney injury (AKI). Patients who achieved the outcome had a higher level of L-FABP and L-FABP/Cr.
Figure 6.
The proposed algorithm for prediction for persistent moderate-to-severe acute kidney injury with the renal angina index (RAI) and L-type fatty acid–binding protein (L-FABP) as determined by decision-tree analysis.
RAI as a Potential Predictor of Severe AKI Within 1 Week
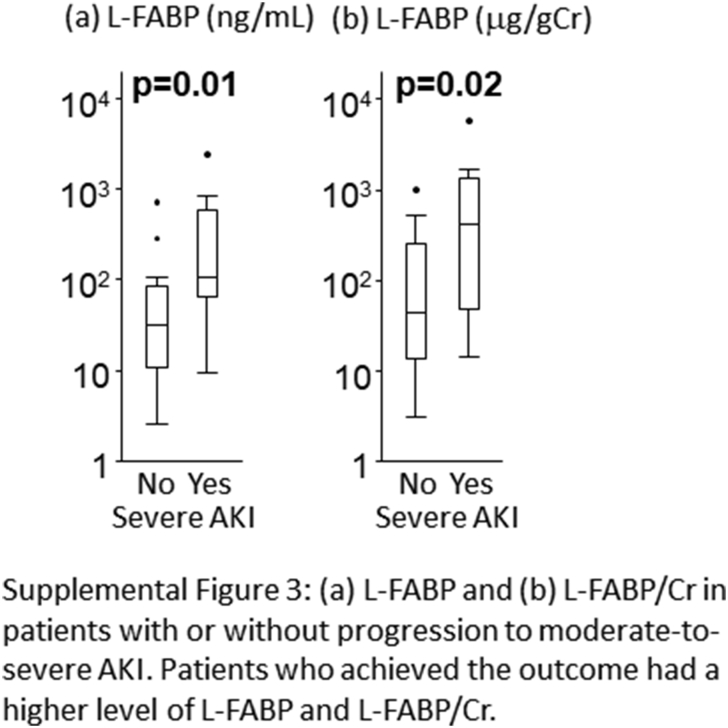
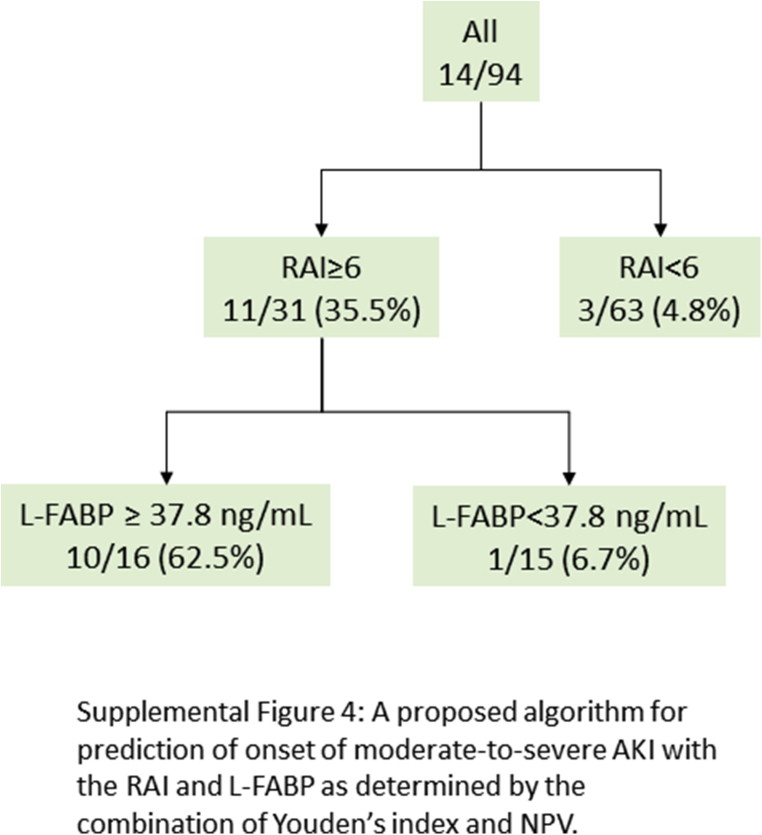
Although the RAI is an instrument designed for predicting persistent AKI, we assessed the applicability of the RAI for detection of severe AKI development within 1 week of admission in patients with hospital-acquired AKI. With the outcome defined as the development of stage 2 to 3 AKI within 1 week of admission, decision-tree analysis indicated the potential applicability of the RAI in detection of new-onset severe AKI with a cutoff of 6. The RAI showed reasonably good performance for assessment of new-onset severe AKI with an AUC of 0.76 (95% CI: 0.63–0.87) (Supplementary Figure S2). In the high-RAI group (≥6), the urinary L-FABP level was shown to be higher among those with severe AKI with or without creatinine adjustment (Supplementary Figure S3). A combination of the RAI and L-FABP also contributed greatly to stratifying higher risk patients with severe AKI, with a cutoff of 437.2 ng/ml and an AUC of 0.81 (95% CI: 0.65–0.91). When the most superior performance was optimized with the combination of Youden’s index and the highest NPV for screening purposes, the cutoff value was chosen as 37.8 ng/ml. The AUC was found to be 0.82 (95% CI: 0.66–0.91) (Supplementary Figure S4).
Discussion
There is a pressing need for early diagnosis of AKI among clinicians so they ascertain timely intervention in AKI. It has been shown that AKI biomarkers can assist in early diagnosis of AKI, but that their predictive performance may be lacking when used in a wrong clinical context. Any interpretation of AKI biomarkers without consideration of its clinical implications often leads to decision errors, and the interpretation of biomarkers giving full consideration to disease severity is in the natural order of real clinical practice. The RAI, as determined by condition of the patients and subtle changes in renal function, is a specific concept for approaching patients at high risk of persistent AKI.20 To improve the performance of AKI biomarkers, the use of the RAI in all AKI patients may be warranted to select those suspected of having persistent AKI.
The RAI has already been validated in children26, 27 and adults13 in the Western populations, but not in the Asian populations. In this analysis, we assessed the performance of the simplified RAI in a multicenter study of Asian ICUs, but the RAI scores on the day after ICU admission did not sufficiently predict persistent severe AKI. There are several reasons for this. First, the RAI used in this study was simpler than that used in the study of Cruz et al. In this previous report,13 multiple risk factors for AKI progression were included to stratify the ICU patients more accurately. In contrast, the RAI used in this study was similar to that used for critically ill children and simplified the ease of use, which might have led to the RAI performing less well in critically ill adults, who often have a variety of comorbidities, such as diabetes, hypertension, and cardiovascular diseases, which are rarely seen in children. However, as shown in Supplementary Figure S1, adding other comorbidities to diabetes mellitus in the RAI components contributed little to persistent AKI being predicted better. Second, there might be differences in clinical practice between Asian and Western countries. In our study, almost all cases of severe AKI (94.4%) occurred at ICU admission, whereas most cases of severe AKI (54.7%) occurred within 7 days of ICU admission in their studies,13 which suggested that the timing of ICU admission might vary depending on the center, country, or other specific geographic factors involved. Again, the RAI scoring on the day of ICU admission, not on the next day, was found to be useful for predicting persistent severe AKI in Asia, and urine L-FABP, a representative AKI biomarker, was shown to improve the predictive performance of the RAI. Third, renal replacement therapy (RRT), which is initiated at the discretion of individual clinicians, leads to all patients undergoing RRT being automatically categorized into stage 3 AKI, regardless of their serum creatinine level, which suggests that a certain number of patients might be grouped into stage 3 AKI without the elevation of creatinine required for the stage. For instance, fluid overload is among the grounds for initiating RRT early. Thus, early initiation of RRT might lead to the RAI performing less well in those undergoing RRT.
Despite these limitations, the RAI showed good performance for patients admitted from general wards in our study. In earlier reports,13, 20, 27 the RAI was successfully used to detect hospital-acquired AKI in in-patients whose postadmission baseline creatinine and creatinine trajectories were known. Again, our study demonstrated the ability of the RAI to detect persistent severe AKI in a similar clinical context. Thus, study findings suggest a rationale for the use of the simplified RAI at ICU admission, as well as on the next day, along with precise creatinine assessment.
AKI biomarkers are generally used for early detection of patients at high risk of AKI. Although they have been shown to have good performance for predicting severe AKI in some settings, such as postcardiac surgery, they have also been shown to predict severe AKI, with an AUC of 0.6 to 0.8 in other settings.28 Moreover, cost considerations argue against the use of AKI biomarkers in all ICU patients in clinical practice. In earlier reports on pediatric ICUs,26, 27 the RAI was validated in a context that allowed novel urinary biomarkers to be directly measured and severe persistent AKI to be better predicted in a heterogeneous population. In our analysis as well, incorporation of the AKI biomarker L-FABP into the RAI improved prediction of severe AKI in a small heterogeneous population, thus ensuring better prediction of severe AKI in the higher RAI cohort. Notably, the decision-tree analysis is prone to increase the sensitivity of the most discriminatory features, and the combination of Youden’s index and NPV is the specificity suitable to screening. The cutoff value depends on the precedence of 2 factors based on the clinical scenario.
This study had several limitations. First, this was a subanalysis of prospective observational studies and might have experienced the inherent bias common to all observational studies. For instance, selected patients might not have been representative of the general ICU population. Moreover, the numbers of those who reached the primary outcome in hospital-acquired AKI was not large enough for analysis compared with that in other cohorts, such as the Acute Kidney Injury-Epidemiologic Prospective Investigation (AKI-EPI) study.29 Second, the RAI calls for further study in other ICU settings and remains to be validated in larger cohorts, as does our concise version of the RAI for adults, which might help manage adult patients at high risk of persistent AKI. Third, we had no access to urine output in our patients. The ideal RAI would therefore include criteria for urine output to better stratify the risk for AKI. Fourth, we did not include patients in coronary care units who were also at high risk of AKI. Despite these limitations, preliminary findings in this study suggested that the RAI might be able to predict high-level AKI progression at least in patients admitted from general wards, and that the cutoff value for the RAI needs to be determined individually upon careful review of critically ill patients in each facility.
Conclusion
The RAI was shown to be effective in predicting persistent AKI in adult patients admitted to ICU from general wards. RAI scoring at ICU admission might be effective for predicting onset of moderate-to-severe AKI. Incorporation of an AKI biomarker into the RAI might improve prediction of severe AKI. Further study is required in a larger cohort to validate the potential of the RAI.
Disclosure
All the authors declared no competing interests.
Acknowledgment
This study was approved by the Institutional Review Board of The University of Tokyo Hospital, Japan and King Chulalongkorn Memorial Hospital, Thailand. The requirement to obtain informed consent for this study was waived because of its retrospective nature.
RM and EN conceived the study, participated in its design and coordination, conducted sample collection, measured biomarkers, analyzed the data, and drafted the manuscript. RCDG advised RAI use. SN conducted data collection. KD and MN participated in its design and coordination, and drafted the manuscript. All authors have read and approved the final manuscript.
Footnotes
Figure S1. Statistical performance of the renal angina index (RAI) for varying demographic factors. (A–D) The calculators of the RAI with various combinations of different demographic factors. (E) This table shows sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) in various calculators. All RAIs have high sensitivity, specificity, and NPV. Parenthesis denotes 95% confidential intervals (CIs). AUC, area under the curve; CKD, chronic kidney disease; DM, diabetes mellitus; HT, hypertension; ICU, intensive care unit; NPV, negative predictive value; PPV, positive predictive value.
Figure S2. The decision-tree analysis for the prediction of moderate-to-severe AKI development within 1 week stratified patients admitted from general wards, with a cutoff of 6.
Figure S3. (A) L-type fatty acid–binding protein (L-FABP) and (B) L-FABP/creatinine (Cr) in patients with or without progression to moderate-to-severe acute kidney injury (AKI). Patients who achieved the outcome had a higher level of L-FABP and L-FABP/Cr.
Figure S4. A proposed algorithm for prediction of onset of moderate-to-severe acute kidney injury (AKI), with the renal angina index (RAI) and L-type fatty acid–binding protein (L-FABP) as determined by the combination of Youden’s index and NPV.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Figure S1.
Statistical performance of the renal angina index (RAI) for varying demographic factors. (A–D) The calculators of the RAI with various combinations of different demographic factors. (E) This table shows sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) in various calculators. All RAIs have high sensitivity, specificity, and NPV. Parenthesis denotes 95% confidential intervals (CIs). AUC, area under the curve; CKD, chronic kidney disease; DM, diabetes mellitus; HT, hypertension; ICU, intensive care unit; NPV, negative predictive value; PPV, positive predictive value.
Figure S2.
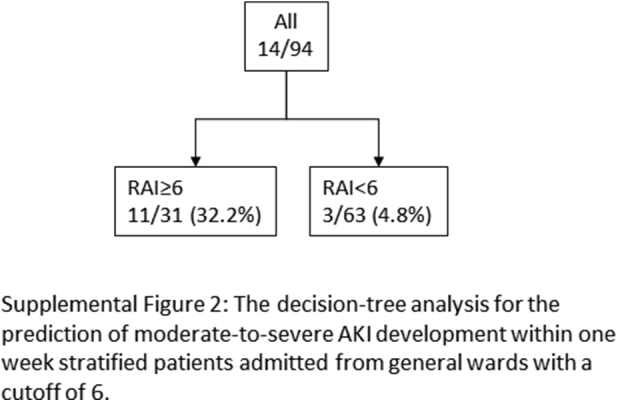
The decision-tree analysis for the prediction of moderate-to-severe AKI development within 1 week stratified patients admitted from general wards, with a cutoff of 6.
Figure S3.
(A) L-type fatty acid–binding protein (L-FABP) and (B) L-FABP/creatinine (Cr) in patients with or without progression to moderate-to-severe acute kidney injury (AKI). Patients who achieved the outcome had a higher level of L-FABP and L-FABP/Cr.
Figure S4.
A proposed algorithm for prediction of onset of moderate-to-severe acute kidney injury (AKI), with the renal angina index (RAI) and L-type fatty acid–binding protein (L-FABP) as determined by the combination of Youden’s index and NPV.
References
- 1.Mehta R.L., Burdmann E.A., Cerda J. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet (London, England) 2016;387:2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 2.Susantitaphong P., Cruz D.N., Cerda J. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koyner J.L., Shaw A.D., Chawla L.S. Tissue inhibitor metalloproteinase-2 (TIMP-2) IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015;26:1747–1754. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashani K., Al-Khafaji A., Ardiles T. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoste E.A.J., McCullough P.A., Kashani K. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014;29:2054–2061. doi: 10.1093/ndt/gfu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer E., Elger A., Elitok S. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405–414. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nejat M., Pickering J.W., Devarajan P. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012;81:1254–1262. doi: 10.1038/ki.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi K., Katagiri D., Negishi K. Mild elevation of urinary biomarkers in prerenal acute kidney injury. Kidney Int. 2012;82:1114–1120. doi: 10.1038/ki.2012.266. [DOI] [PubMed] [Google Scholar]
- 9.Van Biesen W. Con: cautionary tales and reservations about the adoption of new technologies and biomarkers for the management of acute kidney injury. Nephrol Dial Transplant. 2017;32:414–417. doi: 10.1093/ndt/gfx017. [DOI] [PubMed] [Google Scholar]
- 10.Vanmassenhove J., Kielstein J., Jorres A. Management of patients at risk of acute kidney injury. Lancet (London, England) 2017;389:2139–2151. doi: 10.1016/S0140-6736(17)31329-6. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein S.L., Chawla L.S. Renal angina. Clin J Am Soc Nephrol. 2010;5:943–949. doi: 10.2215/CJN.07201009. [DOI] [PubMed] [Google Scholar]
- 12.Chawla L.S., Goldstein S.L., Kellum J.A. Renal angina: concept and development of pretest probability assessment in acute kidney injury. Crit Care. 2015;19:93. doi: 10.1186/s13054-015-0779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz D.N., Ferrer-Nadal A., Piccinni P. Utilization of small changes in serum creatinine with clinical risk factors to assess the risk of AKI in critically lll adults. Clin J Am Soc Nephrol. 2014;9:663–672. doi: 10.2215/CJN.05190513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyner J.L., Adhikari R., Edelson D.P. Development of a multicenter ward-based AKI prediction model. Clin J Am Soc Nephrol. 2016;11:1935–1943. doi: 10.2215/CJN.00280116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flechet M., Guiza F., Schetz M. AKI predictor, an online prognostic calculator for acute kidney injury in adult critically ill patients: development, validation and comparison to serum neutrophil gelatinase-associated lipocalin. Intensive Care Med. 2017;43:764–773. doi: 10.1007/s00134-017-4678-3. [DOI] [PubMed] [Google Scholar]
- 16.Asada T., Aoki Y., Sugiyama T. Organ system network disruption in nonsurvivors of critically ill patients. Crit Care Med. 2016;44:83–90. doi: 10.1097/CCM.0000000000001354. [DOI] [PubMed] [Google Scholar]
- 17.Hayase N., Yamamoto M., Asada T. Association of heart rate with N-terminal pro-B-type natriuretic peptide in septic patients: a prospective observational cohort study. Shock. 2016;46:642–648. doi: 10.1097/SHK.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 18.Isshiki R., Asada T., Sato D. Association of urinary neutrophil gelatinase-associated lipocalin with long-term renal outcomes in ICU survivors: a retrospective observational cohort study. Shock. 2016;46:44–51. doi: 10.1097/SHK.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 19.Peerapornratana S., Mahamitra N., Srisawat N. 112 outcomes of renal replacement therapy in intensive care units in Thailand. Kidney Int Rep. 2017;2:S16. [Google Scholar]
- 20.Basu R.K., Zappitelli M., Brunner L. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85:659–667. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claure-Del Granado R., Pero A., Bouchard J., Mehta R.L. Renal angina index: a practical tool to identify patients at increased risk of acute kidney injury [abstract] J Am Soc Nephrol. 2015;26:669A. [Google Scholar]
- 22.Noiri E., Doi K., Negishi K. Urinary fatty acid-binding protein 1: an early predictive biomarker of kidney injury. Am J Physiol Renal Physiol. 2009;296:F669–F679. doi: 10.1152/ajprenal.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negishi K., Noiri E., Doi K. Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury. Am J Pathol. 2009;174:1154–1159. doi: 10.2353/ajpath.2009.080644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto T., Noiri E., Ono Y. Renal L-type fatty acid–binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 25.Section 2: AKI Definition. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu R.K., Wang Y., Wong H.R. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol. 2014;9:654–662. doi: 10.2215/CJN.09720913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon S., Goldstein S.L., Mottes T. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant. 2016;31:586–594. doi: 10.1093/ndt/gfv457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanmassenhove J., Vanholder R., Nagler E. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. 2013;28:254–273. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]
- 29.Hoste E.A.J., Bagshaw S.M., Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]