Abstract
Introduction
Alport syndrome (AS) is caused by mutations in α3/α4/α5 (IV) collagen genes, the severity of which determine the progression of AS. Posttransplantation outcome is good, although anti−glomerular basement membrane (anti-GBM) glomerulonephritis occurs in 3% to 5% of recipients, clustering in patients with a severe mutation. We assessed whether the severity of the underlying AS mutation affects graft and patients outcome after transplantation, including the occurrence of anti-GBM nephritis.
Methods
We included 73 AS patients with an identified mutation (COL4A5, 57 patients; COL4A3, 9 patients; COL4A4, 6 patients; heterozygous composite COL4A3 and A4, 1 patient) who underwent transplantation between 1971 and 2014 and who had received a total of 93 kidney grafts.
Results
In all, 41 patients had a severe mutation (COL4A5, 30 patients; COL4A3, 6 patients; COL4A4, 5 patients), and 32 had a nonsevere mutation (COL4A5, 27 patients; COL4A3, 4 patients; COL4A4, 1 patient). Patient survival was similar in patients with severe and nonsevere mutations (89% vs. 84% at 5 years, 83% vs. 75% at 10, 15, and 20 years; P = 0.46). Graft survival was not affected by the severity of mutation (77% vs. 63% at 5 years, 60% vs. 55% at 10 years, 55% vs. 55% at 15 years, and 55% vs. 50% at 20 years; P = 0.65). Clinically significant anti-GBM glomerulonephritis occurred in 1 male patient with severe COL4A5 mutation 6 years after transplantation recurred in a subsequent graft, leading twice to graft loss.
Conclusion
Although severe mutations affect the severity of AS, they do not have an impact on patient and graft survival after transplantation. De novo anti-GBM nephritis after transplantation was less frequent than previously reported, occurring in only 1.4% of AS patients, and in 2% of males with COL4A5 mutation.
Keywords: Alport syndrome, anti−glomerular basement membrane nephritis, mutations, recurrence, renal transplantation, survival
Alport syndrome (AS) is a hereditary kidney disease caused by mutations in type IV collagen genes.1 The most common form has X-linked (XLAS) dominant inheritance and involves mutations in the COL4A5 gene coding for the α5 chain of type IV collagen.2 A less common autosomal recessive form (ARAS) results from mutations in the α4 (COL4A4) and/or α3 (COL4A3) chains located on chromosome 2.3, 4 The autosomal dominant form arises from heterozygous mutations in COL4A4 or COL4A3 genes, and its frequency is more important than previously thought.5, 6, 7, 8 Nearly 800 pathogenic variants in AS genes have been reported so far,9, 10, 11 with strong genotype−phenotype correlations.12, 13 Mutation type has been associated with age at onset of end-stage renal disease (ESRD) in either XLAS or ARAS patients. Nonsense mutations or mutations resulting in downstream stop codons confer a higher risk for developing ESRD before the age of 30 years, compared with missense mutations. Extrarenal disease such as hearing loss and ocular lesions is also more frequent in patients with severe mutations.12, 13, 14 In females with COL4A5 mutations, AS is less severe than in males, and no clear genotype−phenotype correlation has been established.15
For patients with AS reaching ESRD, kidney transplantation is the best treatment option. Overall, patient and graft survival rates after transplantation are excellent.16, 17, 18, 19 However, transplanting a kidney graft with a normal glomerular basement membrane (GBM) to a patient with AS exposes the recipient’s immune system to “new” GBM collagen antigens and can lead rarely to posttransplantation de novo anti-GBM disease, as illustrated by a number of case reports or small series.16, 17, 18, 19, 20 However, no large series has yet assessed the occurrence of this complication as well as the posttransplantation outcomes of AS patients according to the type of underlying COL4A5/A4/A3 mutation.
Taking advantage of the identification of the causal mutation in our own cohort of AS patients who have undergone transplantation, we examined here whether the severity of the mutation in the AS gene affects graft and patient survival after kidney transplantation, including the occurrence of anti-GBM nephritis.
Methods
Inclusion Criteria and Outcomes
All patients who underwent transplantation by our team between January 1972 and December 2014 for AS with an identified mutation in the COL4A5/A4/A3 gene were included. Patients with clinical features of AS but lacking genetic proof of mutation were excluded (n = 32). Demographics, extrarenal features (ocular lesions or hearing impairment), age at ESRD, modalities of renal replacement therapy, time from ESRD to transplantation, age at transplantation, donor source, induction treatment, immunosuppressive regimen, and creatinine serum level at 1 year, 5 years, and at last follow-up were recorded. Cardiovascular events defined as cardiac, cerebral, or peripheral vascular disease, neoplastic disease, or infectious complications after transplantation were also recorded. All kidney graft biopsy findings were reviewed. Biopsies were performed for increased serum creatinine or new onset of significant proteinuria. Acute rejection was defined as a biopsy-proven rejection requiring treatment. De novo anti-GBM disease was defined as crescentic or necrotizing glomerulonephritis with linear glomerular IgG deposits in the graft biopsy. The study was approved by the Biomedical Ethics Committee of the Université Catholique de Louvain (Brussels, Belgium).
Genetic Testing and Definition of Severe and Nonsevere Mutations
Mutations in COL4A5/A4/A3 genes were identified by DNA analysis on blood samples obtained from patients followed up at the outpatient clinic at the time of the study. For patients who had died or were lost to follow-up, DNA analysis was performed on stored samples from kidney biopsies or nephrectomies.
We performed a combination of 4 multiplex polymerase chain reactions (ALPORT MASTR Multiplicom, MRC-Holland, Amsterdam, the Netherlands) and next-generation sequencing analysis of the COL4A5/A4/A3 genes as described.7 Large rearrangements were screened using 3 specific Multiplex Ligation-Dependent Probe Amplification, or MLPA, kits (MRC-Holland, Amsterdam, Netherlands), the P191/192 COL4A5 probemix, the P439 probemix containing probes for 33 of the 52 exons of the COL4A3 gene and the P444 probemix that contains probes for 35 of the 48 exons of the COL4A4 gene. Nucleotide numbering of variants reflects cDNA numbering, with +1 corresponding to the A of the ATG translation initiation codon in the reference sequences (COL4A3: NM_000091.3, COL4A4: NM_000042.4 and COL4A5: NM_000495.3). Large rearrangements, truncating mutations, splice-site defect, and nonsense mutations leading to a premature stop codon were classified as severe. Missenses mutations and in-frame deletion were considered to be nonsevere.13, 14
Statistical Analysis
Results are presented as median (minimum−maximum), mean ± SD, or as number and percentage as appropriate. Univariate analyses were performed using the Pearson correlation coefficient for continuous variables and the Student t test or Fisher exact test for binary variables as appropriate. Patient and graft survival rates were calculated according to Kaplan−Meier curves. For survival analysis, grafts were censored at the time of death or loss to follow-up. For multivariate analysis, exponential survival fit was used. Statistical analyses were performed using JMP Pro 12 software (SAS Institute Inc., Marlow, Buckinghamshire, England). All tests were 2-tailed, and a P value < 0.05 was considered as significant.
Results
Patient Characteristics
Among 3908 patients who underwent kidney transplantation between January 1972 and December 2014, a total of 105 patients had transplantation for AS diagnosed on clinical or pathological criteria, fulfilling expert guidelines recommendations.21, 22 Genetic testing was performed on blood samples in 49 patients and on stored tissues in 22 patients who had died or were lost to follow-up. A causal mutation was found in 42 and 20 patients, respectively, belonging to 58 families. Members of the same family were assumed to share the same mutation. We excluded 32 patients with no genetic proof of AS, comprising 7 patients in whom DNA analysis did not confirm AS and 25 patients lost to follow-up.
The 73 patients with AS and an identified mutation we included received 93 kidney grafts (Table 1). Patients were predominantly male and Caucasian/white. The median age at ESRD was 26 years (range, 11−71 years). Only 3 patients were on peritoneal dialysis. Seven patients had preemptive first kidney transplantation. The median age at first transplantation was 28 years (range, 12−73 years). All patients received induction treatment. Maintenance therapy was cyclosporine in 69%, tacrolimus in 31%, azathioprine in 73%, mycophenolate mofetil (MMF) in 25%, and corticosteroids in 98%. A total of 60 transplantations were performed before 1995, and 33 after 1995. The median follow-up was 16 years (range, 1−42 years). Characteristics of women with AS are detailed in Table 2.
Table 1.
Patient characteristics
| Sex, male/female, n | 59/14 |
| Race, Caucasian, % | 99 |
| Age, yr, at ESRD, median (min−max) | 26 (11−71) |
| Time on dialysis, mo median (min−max) | 33 (1−190) |
| Deafness (n) | 48 |
| Age, yr, at hearing aid, median (min−max) | 24 (6−55) |
| Age, years, at first TP, median (min−max) | 28 (12−73) |
| Duration of post-TP follow-up, yr, median (min−max) | 16 (1−42) |
| Living/deceased donor (n) | 13/80 |
| Number of TPs, n | 93 |
| Second/third TP | 16/2 |
| Immunosuppressive regimen, % | |
| Induction | 100 |
| Cyclosporine | 69 |
| Tacrolimus | 31 |
| Mycophenolate mofetil | 25 |
| Azathioprine | 73 |
| Sirolimus | 1 |
| Corticosteroids | 98 |
ESRD, end-stage renal disease; min−max, minimum−maximum; TP, transplantation.
Table 2.
Mutations and ESRD according to gender
| Gene | Mutation | Male (n = 59) | Femalea (n = 14) |
|---|---|---|---|
| COL4A5 | 50 | 7 | |
| Severe | 27 | 3 | |
| Nonsevere | 23 | 4 | |
| COL4A3 | 5 | 4 | |
| Severe | 5 | 1 | |
| Nonsevere | 0 | 3 | |
| COL4A4 | 3 | 3 | |
| Severe | 3 | 2 | |
| Nonsevere | 0 | 1 | |
| Deafness | 43 | 5 | |
| Age at ESRD, yr, median (min−max)) | 24 (12−61) | 33 (11−71) | |
| Severe | 22 (12−50) | 27 (11−61) | |
| Nonsevere | 26 (13−61) | 34 (28−71) |
ESRD, end-stage renal disease.
Women are identified in Table 3 (Genetics) as p6, p12s, p12s, p14, p15, p29, p33, p45, p49, p51, p52, p54, p54s, and p56.
Genotypes of AS patients
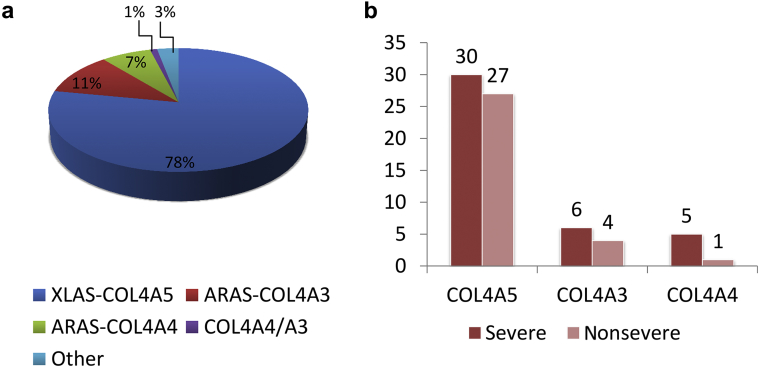
Most patients had a causative mutation in COL4A5 gene (57 of 73, 78%), whereas autosomal recessive inheritance was confirmed in 8 patients with COL4A3 mutations (11%) and in 5 patients with COL4A4 mutations (7%) (Figure 1a). Two heterozygous mutations were detected in 2 patients (53 and 59) compatible with an autosomal-dominant form of AS (3%). One patient was compound heterozygous for 2 causative mutations in COL4A3 and COL4A4 genes.
Figure 1.
(a) Distribution of mutations in the cohort. XLAS COL4A5, X-linked COL4A5 Alport syndrome (AS); ARAS COL4A3, autosomal recessive AS with mutations in COL4A3; ARAS COL4A4, autosomal recessive AS with mutations in COL4A4; COL4A4/A3, compound heterozygote with both mutation in COL4A3 and COL4A4. (b) COL4A5, COL4A3, and COL4A4 mutations classified as severe or nonsevere.
In COL4A5 gene, severe mutations included 7 splicing mutations in 8 patients, 5 nonsense variants in 6 patients, 7 small deletions, and 3 genomic rearrangements in 16 patients. Twelve of these severe mutations are novel. Nonsevere mutations included 22 missense variants, 16 of which were novel, in 27 patients.
In COL4A3 and COL4A4 genes, homozygous or compound heterozygous mutations resulting in a protein truncation were detected in 7 patients (5 in COL4A3 and 2 in COL4A4). Compound heterozygosity consisting of 1 null mutation and 1 missense variant was observed in 4 other patients. These 2 groups were classified as carrying a severe mutation (Figure 1b). Mutations are included in the Leiden Open Variation Database (LOVD) as described by Savige et al.,11 and are detailed for each patient in Table 3.
Table 3.
Detailed genetic results
| Identifier | Gene | Inheritance | Mutation(s)/cDNA | Mutation(s)/protein | Tissue |
|---|---|---|---|---|---|
| 1 | COL4A5 | hz | c.3958A>T | p.(Lys1230*) | K |
| 2 | COL4A5 | hz | c.1217G>T | p.(Gly406Val) | K |
| 3 | COL4A5 | hz | c.937del | p.(Gly313Aspfs*72) | B |
| 4 | COL4A5 | hz | c.4976+2T>C | p.(?) | B |
| 4s | COL4A5 | hz | c.4976+2T>C | p.(?) | NT |
| 5 | COL4A5 | hz | c.2078G>T | p.(Gly693Val) | B |
| 6 | COL4A5 | h | c.4757G>A | p.(Cys1586Tyr) | NT |
| 6s | COL4A5 | hz | c.4757G>A | p.(Cys1586Tyr) | B |
| 6s | COL4A5 | hz | c.4757G>A | p.(Cys1586Tyr) | B |
| 6s | COL4A5 | hz | c.4757G>A | p.(Cys1586Tyr) | B |
| 7 | COL4A5 | hz | c.1525G>A | p.(Gly509Ser) | B |
| 7s | COL4A5 | hz | c.1525G>A | p.(Gly509Ser) | B |
| 8 | COL4A5 | hz | c.944del | p.(Pro315Leufs*31) | K |
| 9 | COL4A5 | hz | c.412G>A | p.(Gly138Ser) | K |
| 10 | COL4A5 | hz | c.610-2A>G | p.(?) | K |
| 11 | COL4A5 | hz | c.1871G>A | p.(Gly624Asp) | B |
| 12 | COL4A5 | hz | c.3293G>A | p.(Gly1098Asp) | NT |
| 12s | COL4A5 | h | c.3293G>A | p.(Gly1098Asp) | B |
| 12s | COL4A5 | h | c.3293G>A | p.(Gly1098Asp) | NT |
| 13 | COL4A5 | hz | c.1018G>C | p.(gly340Arg) | K |
| 14 | COL4A5 | h | c.1A>C | p.(Met1?) | B |
| 15 | COL4A5 | h | c.3270C>A | p.(Tyr1090*) | B |
| 16 | COL4A5 | hz | c.1507G>C | p.(Gly503Arg) | B |
| 17 | COL4A5 | hz | c.4976+2T>C | p.(?) | B |
| 18 | COL4A5 | hz | c.(4065+1_4066-1)_(*5058_?)del | p.0? | B |
| 19 | COL4A5 | hz | c.(4065+1_4066-1)_(*5058_?)del | p.0? | B |
| 20 | COL4A5 | hz | c.2416C>T | p.(Gly1277Ser) | B |
| 21 | COL4A5 | hz | c.1525G>A | p.(Gly509Ser) | B |
| 22 | COL4A5 | hz | c.3035G>A | p.(Gly1012Asp) | NT |
| 22s | COL4A5 | hz | c.3035G>A | p.(Gly1012Asp) | NT |
| 23 | COL4A5 | hz | c.4391-1G>A | p.(?) | B |
| 24 | COL4A5 | hz | c.547-1G>C | p.(?) | K |
| 25 | COL4A5 | hz | c.2981del | p.(Gly994Aspfs*1) | B |
| 26 | COL4A5 | hz | c.2509G>A | p.(Gly837Ser) | K |
| 27 | COL4A5 | hz | c.2086G>C | p.(Gly696Arg) | K |
| 28 | COL4A5 | hz | c.1931G>A | p.(Gly644Asp) | K |
| 29 | COL4A5 | h | c.1226G>A | p.(Gly409Asp) | B |
| 30 | COL4A5 | hz | c.4975A>T | p.(Ser1659Cys) | B |
| 31 | COL4A5 | hz | c.2057del | p.(Pro686GInfs*50) | K |
| 32 | COL4A5 | hz | c.1483_1516del | p.(Gln495Aspfs*51) | NT |
| 32s | COL4A5 | hz | c.1483_1516del | p.(Gln495Aspfs*51) | K |
| 32s | COL4A5 | hz | c.1483_1516del | p.(Gln495Aspfs*51) | NT |
| 32s | COL4A5 | hz | c.1483_1516del | p.(Gln495Aspfs*51) | NT |
| 33 | COL4A5 | h | c.2057del | p.(Pro686GInfs*50) | K |
| 34 | COL4A5 | hz | c.(1777+1_1778-1)_(1951+1_1952-1)del | p.0? | B |
| 35 | COL4A5 | hz | c.1624G>T | p.(Gly542*) | K |
| 35s | COL4A5 | hz | c.1624G>T | p.(Gly542*) | NT |
| 36 | COL4A5 | hz | c.796C>T | p.(Arg266*) | B |
| 37 | COL4A5 | hz | c.1102G>A | p.(Gly368Arg) | K |
| 38 | COL4A5 | hz | c.3329del | p.(Gly1110Glufs*42) | K |
| 38s | COL4A5 | hz | c.3329del | p.(Gly1110Glufs*42) | NT |
| 39 | COL4A5 | hz | c.1018G>C | p.(gly340Arg) | B |
| 40 | COL4A5 | hz | c.4687C>T | p.(Arg1563*) | B |
| 41 | COL4A5 | hz | c.(3015+1_3016-1)_(3108+1_3109-1)del | p.0? | B |
| 42 | COL4A5 | hz | c.548G>T | p.(Gly183Val) | B |
| 43 | COL4A5 | hz | c.4688+1G>T | p.(?) | K |
| 44 | COL4A5 | hz | c.546+2T>C | p.(?) | B |
| 45 | COL4A3 | ch | c.934G>C/c.4564T>C | (p.(Gly312Arg)/p.(Trp1522Arg) | B |
| 46 | COL4A3 | ch | c.713del/c.1937del | p.(Pro238Argfs*8)/p.(Gly646Glufs*100) | B |
| 47 | COL4A3 | H | c.713del | p.(Pro238Argfs*8) | B |
| 48 | COL4A3 | ch | c.3244_3247del/c.-13G>C | p.(Lys1082Glufs*71)/p.0? | B |
| 49 | COL4A3 | ch | c.2083G>A/c.4772 | p.(Gly695Arg)/p.(Ser1591Phe) | B |
| 50 | COL4A3 | ch | c.1918G>A/ c.3211-1G>T | p.(Gly640Arg)/p.(?) | B |
| 51 | COL4A3 | h | c.1219G>C | p.(Gly407Arg) | B |
| 52 | COL4A3 | H | c.4441C>T | p.(Arg1481*) | B |
| 53 | COL4A3 | H | c.522dup | p.(Leu175Cysfs*47) | B |
| 54 | COL4A4 | ch | c.2908C>T/c.2756A>G + c.3725G>T | p.(Gln970*)/p.(Gly1242Val&p.Glu919Gly) | B |
| 54s | COL4A4 | ch | c.2908C>T/c.2756A>G + c.3725G>T | p.(Gln970*)/p.(Gly1242Val&p.Glu919Gly) | NT |
| 55 | COL4A4 | H | c.4129C>T | p.(Arg1377*) | K |
| 55s | COL4A4 | H | c.4129C>T | p.(Arg1377*) | K |
| 56 | COL4A4 | ch | c.1109G>A/c.43_54del | p.(Gly370Glu)/p.(Pro15_Leu18del) | B |
| 57 | COL4A4 | h | c.4787G>A | p.Trp1596X | B |
| 58 | COL4A3/COL4A4 | ch | c.599C>T/c.481G>C | p.(Pro200Leu)/p.(Gly161Arg) | B |
B, blood; ch, compound heterozygote; h, heterozygote; H, homozygote; hz, hemizygote; K, kidney; NT, not tested; S, sibling.
Female identifiers are shown in bold.
Outcomes According to Severity of Mutation
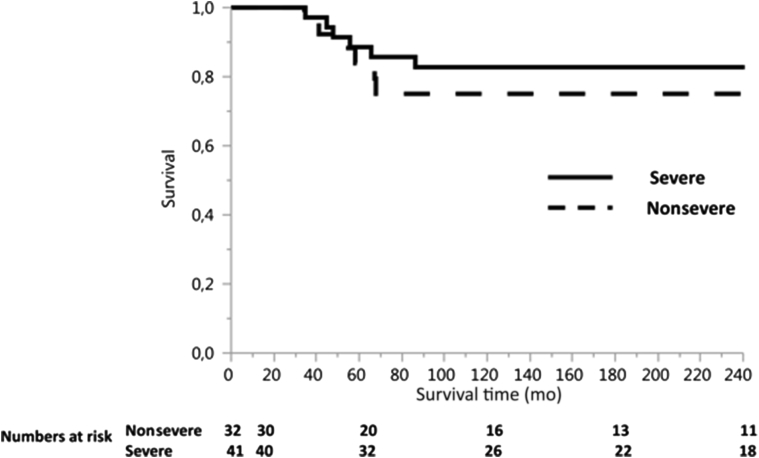
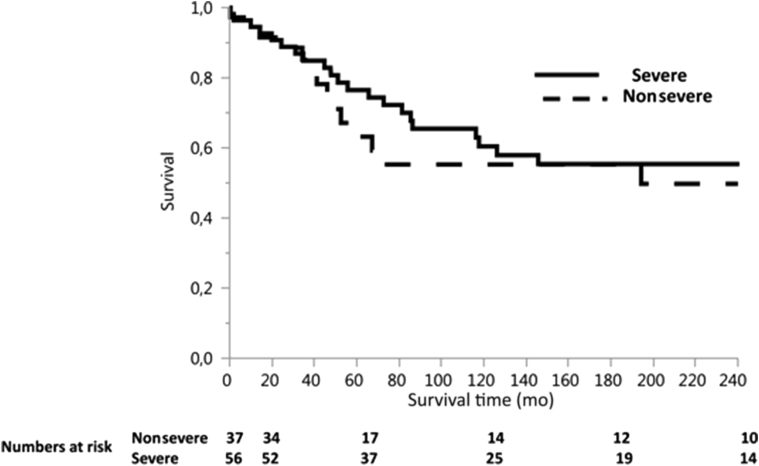
The median age at ESRD was significantly lower in patients with severe mutations compared to nonsevere mutations (median 23 years, range 11−61 years, vs. median 30 years, range 13−71 years; P = 0.0086). Age at first transplantation was also significantly lower in patients with severe mutations (median 27 years, range 11−67 years, vs. median 32 years, range 15−73 years; P = 0.011). Posttransplantation cardiovascular, infectious, and neoplastic complications were similar in patients with severe and nonsevere mutations; acute rejections were more frequent in patients with severe mutations (P = 0.03) (Table 4). Patient survival was similar in patients with severe and nonsevere mutations (100% at 1 year [89%] vs. 84% at 5 years; 83% vs. 75% at 10, 15, and 20 years; P = 0.46) (Figure 2). Graft survival was also not affected by the severity of mutation (77% for severe vs. 63% for nonsevere mutation at 5 years, 60% vs. 55% at 10 years, 55% vs. 55% at 15 years, and 55% vs. 50% at 20 years; P = 0.65) (Figure 3). Members of 4 families (3 with severe and 1 with nonsevere mutations) had excellent graft survival of more than 15 years, in the absence of rejection. Graft survival in the remaining 5 families (3 with severe and 2 with nonsevere mutations) was excellent for members free of rejection, whereas those who developed acute or chronic rejection experienced graft failure. By multivariable analysis, patient survival was not affected by age at ESRD. Twelve patients died during follow-up (6 with severe mutations and 6 with nonsevere): 3 of unknown cause, 3 of pneumonia with hypoxemic respiratory failure, 3 of neoplastic disease (T-cell lymphoma, bladder carcinoma, esophageal carcinoma), 2 of sepsis with multiple organ failure, and 1 of constrictive pericarditis.
Table 4.
Mutations severity and outcomes
| Patient characteristics | Mutations |
|
|---|---|---|
| Severe | Nonsevere | |
| Number of patients (N = 73) | 41 | 32 |
| Age at ESRD, yr, median (min−max) | 23 (11−61) | 30 (13−71) |
| Age at first TP, yr, median (min−max)) | 27 (11−67) | 32 (15−73) |
| Deafness, n | 29 | 19 |
| Death, n | 6 | 6 |
| Number of grafts (n = 93) | 55 | 38 |
| Immunosuppressive treatment | ||
| Cyclosporine/tacrolimus, n | 33/9 | 24/12 |
| Azathioprine/mycophenolate mofetil, n | 45/9 | 22/16 |
| Complications, n | ||
| Hypertension | 16 | 18 |
| Cardiovascular | 5 | 3 |
| Infections | 11 | 10 |
| Neoplastic | 5 | 8 |
| Acute rejection | 21 | 10 |
| Graft loss, n | 17 | 10 |
| Chronic allograft nephropathy | 14 | 6 |
| Acute rejection | 1 | 2 |
| TMA/arterial thrombosis | 1 | 2 |
| De novo anti-GBM nephritis | 1 | 0 |
ESRD, end-stage renal disease; GBM, glomerular basal membrane; TMA, thrombotic microangiopathy; TP, transplantation.
Figure 2.
Patient survival according to mutation severity.
Figure 3.
Graft survival according to mutation severity.
Anti-GBM Nephritis and Linear Glomerular IgG Deposits
A total of 50 biopsy samples (26 in patients with severe mutations) were available. Clinically significant anti-GBM glomerulonephritis occurred in only 1 patient, who had a truncating COL4A5 mutation (identifier 1, Table 2). This patient underwent transplantation in 1977 at age 18 years with a kidney from a deceased donor with 1 human leukocyte antigen (HLA) mismatch, and immunosuppression with azathioprine and steroids. He presented 6 years later with an increase in serum creatinine (2.5 mg/dl) accompanied by microscopic hematuria and proteinuria (4.6 g/l). A graft biopsy sample revealed crescentic glomerulonephritis and linear IgG deposits along the GBM. Circulating anti-GBM antibodies were negative by enzyme-linked immunosorbent assay and by immunoblotting as described by Savage.23 He lost his graft within a few months, and underwent transplantation again 6 months later, in 1984, with a kidney from a deceased donor. His immunosuppression consisted of cyclosporine and corticosteroids. An acute rejection episode on day 7 was treated by steroids and local radiotherapy. Anti-GBM disease recurred 3 years later, with the same clinical and histological presentation, leading to graft loss after 6 months.
Linear IgG glomerular deposits without glomerular lesions on light microscopy were observed in 4 grafts (identifiers 13, 34, 38, and 55 in Table 2) out of 48 graft biopsy samples. Two patients (identifiers 34 and 38) had small indel or genomic rearrangement in COL4A5. Patient 13 had a novel missense variant affecting a highly conserved glycine at residue 340 of α (IV) chain, and patient 55 was homozygous for a nonsense mutation in COL4A4. All patients were given immunosuppressive treatment with azathioprine. Three of these patients showed lesions of mild acute rejection, treated with corticosteroids (combined in 1 patient with antilymphocytic serum). One patient showed lesions of advanced chronic rejection and was lost to follow-up within 1 year. Two patients were lost to follow-up 6 and 16 years later with functioning grafts. One patient presented with acute rejection 2 years later: linear IgG deposits were no longer present on graft biopsy. He soon lost his graft.
Discussion
In this study, we report that patients with AS related to a severe mutation in COL4A5, COL4A3, and COL4A4 gene have very good outcomes after kidney transplantation, similar to AS patients with a nonsevere mutation. Patient and graft survival were excellent; however, clinically significant anti-GBM nephritis occurred in 1 male patient with a severe COL4A5 mutation, manifesting later than previously reported, recurring in a subsequent graft, and leading twice to graft loss.
Our cohort of patients is consistent with reported series of AS, with regard to the mode of inheritance and type of mutation as well as the spectrum of clinical manifestations. AS was X-linked in 78% and autosomal recessive in 18%. The distribution of the mutations found in the COL4A5 gene (47% missense with 88% as glycine substitution, 38% truncating, 14% splice-site defects) does not differ from series of AS cases previously reported.12, 13 The median age at ESRD in our patients with COL4A5 mutations was 25 years (range 12−66 years), and the patients reached ESRD earlier if the mutation was severe versus nonsevere (median 23 years, range 12−61 years, vs. median 28 years, range 13−66 years; P = 0.026). This is also in agreement with previous studies that have reported that patients with X-linked AS with severe mutations arrived at ESRD earlier than patients with nonsevere mutations. In their European cohort, Jais et al. reported that large deletions, nonsense mutations, or small mutations changing the reading frame conferred to affected male patients a 90% probability of developing ESRD before the age of 30 years, whereas this risk was 50% and 70% in patients with missense and splice site mutation, respectively.12 Likewise, a U.S. report showed that age at ESRD was 25 years for patients with truncating mutations versus 28 years for those with splice-site mutations and 37 years for those with missense mutations.13 The severity of the mutation also affects the extrarenal involvement of the disease in males with X-linked AS.12, 13
Outcomes after kidney transplantation are reportedly good, but no published data exist yet on the possible effect of mutation severity on long-term outcomes of AS patients. Our study shows that the severity of the mutation is not associated with increased complications after transplantation, and does not have an impact on patient and graft survival. As identifying the mode of inheritance of AS is important for providing genetic counseling in affected families, this observation is of interest. Indeed, informing on the severity of the mutation and its impact on age at ESRD can be stressful, but will then be balanced by awareness that the severity of the mutation does not have an impact on the long-term outcomes after kidney transplantation.
De novo anti-GBM nephritis after transplantation is a potential complication of AS that is unique to this disease. It has been reported to occur in 3% to 5% of males with AS who have undergone transplantation,16, 17, 24, 25 although more recent studies have pointed to a lower incidence of the disease: 2.4% of patients, and 3.1% in the subgroup of male AS patients in the Byrne et al. report19 and 0.4% in the Mallett et al. cohort.26 In our study, anti-GBM occurred in only 1.4% of AS patients, 1.7% in the subgroup of male AS patients, and 2% in male patients with COL4A5 mutation, which is lower than previously reported. It is interesting to note that our patient with de novo anti-GBM disease underwent transplantion in the early era of transplantation under azathioprine and corticosteroids. The advent of more potent immunosuppressive regimens in the past 3 decades (calcineurin inhibitors and MMF) might explain the lower incidence of the disease. Indeed, a review of published cases of anti-GBM disease showed that 70% had at least 1 episode of acute rejection, suggesting inadequate immunosuppression.19 Moreover, MMF interferes with purine synthesis in lymphocytes. It inhibits the proliferation of both T and B cells, which reduce the synthesis of antibodies. In 2002, a Spanish group showed that MMF had a preventive effect on mercury-induced anti-GBM nephritis in rats, as it blocked anti-GBM antibody synthesis, thereby avoiding glomerular IgG deposits, proteinuria, and the development of nephritis.27 Also, Takeda et al. reported, 2 years later, in a different rat model of anti-GBM nephritis, a significant reduction in proteinuria and crescent formation with MMF treatment.28 The onset of anti-GBM disease usually occurs within the first year after transplantation, leading to graft loss in 90% of patients within a few weeks to months after diagnosis.20 Our case of de novo anti-GBM shows that anti-GBM nephritis may manifest much later after transplantation (here 6 years) and may recur in a subsequent graft, leading to graft loss. The recurrence risk after retransplantation is very high. Recurrence can occur despite an interval of many years between transplantations and without any detectable circulating antibodies.29
The majority of reported patients who have developed posttransplantation anti-GBM nephritis are males with X-linked AS and severe COL4A5 mutations.12, 13, 30, 31, 32 In these patients, the transplanted GBM is recognized as foreign as a consequence of the absence of intact α3α4α5 (IV) trimers in their GBM. Anti-GBM antibodies recognize primarily the α5 (IV) chain, although anti-α3 (IV) antibodies were reported in 1 AS patient with a COL4A5 deletion.31, 32 X-linked AS males with missense mutations have preserved α-chain trimers and are at low risk for developing anti-GBM nephritis.33 In line with this observation, the 3 X-linked AS males (of 118 patients) reported by Jais et al. who developed anti-GBM disease had large deletions of the COL4A5 gene, as in our patient, confirming that the risk in these patients of developing anti-GBM nephritis is much higher compared to that in the the total AS population.12 Interestingly, however, 16 other patients in that report with a large rearrangement of COL4A5 and 32 with a small mutation expected to produce a truncated α5 (IV) protein did not develop anti-GBM glomerulonephritis in the graft. We add further information, showing, in our study, that 29 of 30 X-linked AS patients with a severe mutation did not develop anti-GBM disease. Other factors are thus involved in the development of anti-GBM nephritis.
Four of our patients presented with linear IgG on immunofluorescence without histological signs of glomerulonephritis. In these patients, this finding did not translate into poor graft outcome. This glomerular linear IgG deposition without deterioration of graft function was reported in 1986 by Quérin et al. and considered as a marker of mild alloimmunization.34 We show, in our patients, that IgG linear deposition occurs not only in COL4A5 but also in COL4A4, and in both severe and nonsevere mutations.
Our study is the first to report AS genotype in a kidney transplant recipient with an extended follow-up. Also, a large number of kidney graft biopsy samples were available, allowing the appreciation of linear IgG deposits. We acknowledge, however, the limitations of this study. Its retrospective nature makes it subject to collection bias. Also, we could not gather the genetic mutations for all of our cohort of AS patients, although these data were available in 76%. Nevertheless, we have included a substantial number of patients with genetically proven mutation, and our follow-up is very extensive, allowing an analysis of long-term outcomes.
In conclusion, we report that although severe mutations affect the severity of AS with younger age at ESRD, it does not have an impact on patient and graft survival after transplantation. In the present era, in which genetic testing is widely available, this information has its importance. Also, in our cohort, de novo anti-GBM nephritis after transplantation is less frequent than previously reported, occurring in only 1.4% of AS patients, and in 2% of males with COL4A5 mutation. Improved immunosuppression with potent agents including MMF may have contributed to the decrease in the disease.
Disclosure
All the authors declared no competing interests.
References
- 1.Pirson Y. Making the diagnosis of Alport’s syndrome. Kidney Int. 1999;56:760–777. doi: 10.1046/j.1523-1755.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- 2.Lemmink H.H., Schröder C.H., Monnens L.A.H., Smeets H.J.M. The clinical spectrum of type IV collagen mutations. Hum Mutat. 1997;9:477–499. doi: 10.1002/(SICI)1098-1004(1997)9:6<477::AID-HUMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki T., Lemmink H.H., Mariyama M. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet. 1994;8:77–81. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- 4.Lemmink H.H., Mochizuki T., van den Heuvel L.P. Mutations in the type IV collagen alpha 3 (COL4A3) gene in autosomal recessive Alport syndrome. Hum Mol Genet. 1994;3:1269–1273. doi: 10.1093/hmg/3.8.1269. [DOI] [PubMed] [Google Scholar]
- 5.van der Loop F.T., Heidet L., Timmer E.D. Autosomal dominant Alport syndrome caused by a COL4A3 splice site mutation. Kidney Int. 2000;58:1870–1875. doi: 10.1111/j.1523-1755.2000.00358.x. [DOI] [PubMed] [Google Scholar]
- 6.Heidet L., Arrondel C., Forestier L. Structure of the human type IV collagen gene COL4A3 and mutations in autosomal Alport syndrome. J Am Soc Nephrol. 2001;12:97–106. doi: 10.1681/ASN.V12197. [DOI] [PubMed] [Google Scholar]
- 7.Morinière V., Dahan K., Hilbert P. Improving mutation screening in familial hematuric nephropathies through next generation sequencing. J Am Soc Nephrol. 2014;25:2740–2751. doi: 10.1681/ASN.2013080912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallerini C., Baldassarri M., Trevisson E. Alport syndrome: impact of digenic inheritance in patients management. Clin Genet. 2017;92:34–44. doi: 10.1111/cge.12919. [DOI] [PubMed] [Google Scholar]
- 9.Crockett D.K., Pont-Kingdon G., Gedge F. The Alport syndrome COL4A5 variant database. Hum Mutat. 2010;31:E1652–E1657. doi: 10.1002/humu.21312. [DOI] [PubMed] [Google Scholar]
- 10.Fokkema I.F., Taschner P.E., Schaafsma G.C. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32:557–563. doi: 10.1002/humu.21438. [DOI] [PubMed] [Google Scholar]
- 11.Savige J., Storey H., Cheong H. X-linked and autosomal recessive Alport syndrome: pathogenic variant features and further genotype-phenotype correlations. PLoS One. 2016;9:e0161802. doi: 10.1371/journal.pone.0161802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jais J.P., Knebelmann B., Giatras I. X-linked Alport syndrome: natural history in 195 families and genotype-phenotype correlations in males. J Am Soc Nephrol. 2000;11:649–657. doi: 10.1681/ASN.V114649. [DOI] [PubMed] [Google Scholar]
- 13.Bekheirnia M.R., Reed B., Gregory M.C. Genotype-phenotype correlation in X-linked Alport syndrome. J Am Soc Nephrol. 2010;21:876–883. doi: 10.1681/ASN.2009070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storey H., Savige J., Sivakumar V. COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J Am Soc Nephrol. 2013;24:1945–1954. doi: 10.1681/ASN.2012100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jais J.P., Knebelmann B., Giatras I. X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a ‘European Community Alport Syndrome Concerted Action’ study. J Am Soc Nephrol. 2003;14:2603–2610. doi: 10.1097/01.asn.0000090034.71205.74. [DOI] [PubMed] [Google Scholar]
- 16.Göbel J., Olbricht C.J., Offner G. Kidney transplantation in Alport's syndrome: long-term outcome and allograft anti-GBM nephritis. Clin Nephrol. 1992;38:299–304. [PubMed] [Google Scholar]
- 17.Peten E., Pirson Y., Cosyns J.-P. Outcome of thirty patients with Alport’s syndrome after renal transplantation. Transplantation. 1991;52:823–826. doi: 10.1097/00007890-199111000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Temme J., Kramer A., Jager K.J. Outcomes of male patients with Alport syndrome undergoing renal replacement therapy. Clin J Am Soc Nephrol. 2012;7:1969–1976. doi: 10.2215/CJN.02190312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne M.C., Budisavljevic M.N., Fan Z. Renal transplant in patients with Alport's syndrome. Am J Kidney Dis. 2002;39:769–775. doi: 10.1053/ajkd.2002.31997. [DOI] [PubMed] [Google Scholar]
- 20.Kashtan C.E. Renal transplantation in patients with Alport syndrome. Pediatr Transplant. 2006;10:651–657. doi: 10.1111/j.1399-3046.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 21.Flinter F.A., Cameron J.S., Chantler C. Genetics of classic Alport's syndrome. Lancet. 1988;2:1005–1007. doi: 10.1016/s0140-6736(88)90753-2. [DOI] [PubMed] [Google Scholar]
- 22.Savige J., Gregory M., Gross O. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol. 2013;3:364–375. doi: 10.1681/ASN.2012020148. [DOI] [PubMed] [Google Scholar]
- 23.Savage C.O., Noel L.H., Crutcher E. Hereditary nephritis: immunoblotting studies of the glomerular basement membrane. Lab Invest. 1989;60:613–618. [PubMed] [Google Scholar]
- 24.Berardinelli L., Pozzoli E., Raiteri M. Renal transplantation in Alport’s syndrome: personal experience in twelve patients. Contrib Nephrol. 1990;80:131–134. [PubMed] [Google Scholar]
- 25.Hayes D.K., Majeski J.A., Alexander J.W. Renal transplantation in Alport’s syndrome. Am Surg. 1985;51:414–417. [PubMed] [Google Scholar]
- 26.Mallett A., Tang W., Clayton P.A. End-stage kidney disease due to Alport syndrome: outcomes in 296 consecutive Australia and New Zealand Dialysis and Transplant Registry cases. Nephrol Dial Transplant. 2014;29:2277–2286. doi: 10.1093/ndt/gfu254. [DOI] [PubMed] [Google Scholar]
- 27.Nieto E., Escudero E., Navarro E. Effects of mycophenolate mofetil in mercury-induced autoimmune nephritis. J Am Soc Nephrol. 2002;13:937–945. doi: 10.1681/ASN.V134937. [DOI] [PubMed] [Google Scholar]
- 28.Takeda S., Takahashi M., Sado Y. Prevention of glomerular crescent formation in glomerulonephritis by mycophenolate mofetil in rats. Nephrol Dial Transplant. 2004;19:2228–2236. doi: 10.1093/ndt/gfh302. [DOI] [PubMed] [Google Scholar]
- 29.Browne G., Brown P.A., Tomson C.R. Retransplantation in Alport post-transplant anti-GBM disease. Kidney Int. 2004;65:675–681. doi: 10.1111/j.1523-1755.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 30.Kashtan C.E., Butkowski R.J., Kleppel M.M. Posttransplant anti-glomerular basement membrane nephritis in related males with Alport syndrome. J Lab Clin Med. 1990;116:508–515. [PubMed] [Google Scholar]
- 31.Kalluri R., Weber M., Netzer K.-O. COL4A5 gene deletion and production of posttransplant-anti-a3(IV) collagen alloantibodies in Alport syndrome. Kidney Int. 1994;45:721–726. doi: 10.1038/ki.1994.96. [DOI] [PubMed] [Google Scholar]
- 32.Ding J., Zhou J., Tryggvason K., Kashtan C.E. COL4A5 deletions in three patients with Alport syndrome and posttransplant antiglomerular basement membrane nephritis. J Am Soc Nephrol. 1994;5:161–168. doi: 10.1681/ASN.V52161. [DOI] [PubMed] [Google Scholar]
- 33.Naito I., Kawai S., Nomura S. Relationship between COL4A5 gene mutation and distribution of type IV collagen in male X-linked Alport syndrome. Kidney Int. 1996;50:304–311. doi: 10.1038/ki.1996.316. [DOI] [PubMed] [Google Scholar]
- 34.Querin S., Noel L.H., Grünfeld J.P. Linear glomerular IgG fixation in renal allografts: incidence and significance in Alport syndrome. Clin Nephrol. 1986;25:134–140. [PubMed] [Google Scholar]