Abstract
Introduction
Diabetic nephropathy (DN) is a form of progressive kidney disease that often leads to end-stage renal disease (ESRD). It is initiated by microvascular complications due to diabetes. Although microalbuminuria (MA) is the earliest clinical indication of DN among patients with type 1 diabetes (T1D), it lacks the sensitivity and specificity to detect the early onset of DN. Recently, microRNAs (miRNAs) have emerged as critical regulators in diabetes as well as various forms of kidney disease, including renal fibrosis, acute kidney injury, and progressive kidney disease. Additionally, circulating extracellular miRNAs, especially miRNAs packaged in extracellular vesicles (EVs), have garnered significant attention as potential noninvasive biomarkers for various diseases and health conditions.
Methods
As part of the University of Pittsburgh Epidemiology of Diabetes Complications (EDC) study, urine was collected from individuals with T1D with various grades of DN or MA (normal, overt, intermittent, and persistent) over a decade at prespecified intervals. We isolated EVs from urine and analyzed the small-RNA using NextGen sequencing.
Results
We identified a set of miRNAs that are enriched in urinary EVs compared with EV-depleted samples, and identified a number of miRNAs showing concentration changes associated with DN occurrence, MA status, and other variables, such as hemoglobin A1c levels.
Conclusion
Many of the miRNAs associated with DN occurrence or MA status directly target pathways associated with renal fibrosis (including transforming growth factor-β and phosphatase and tensin homolog), which is one of the major contributors to the pathology of DN. These miRNAs are potential biomarkers for DN and MA.
Keywords: diabetic nephropathy, extracellular vesicles, microalbuminuria, microRNAs, RNA-seq
More than 2 million people worldwide currently suffer from end-stage renal disease (ESRD). The United States has the highest prevalence rate, and accounts for more than 30% of individuals with ESRD worldwide.1 One of the largest contributors to ESRD is diabetic nephropathy (DN), particularly in the western world where incidences of diabetes are higher than in developing countries, and diabetic patients live long enough to progress from DN to ESRD. However, as rates of diabetes in third-world countries continue to increase, so may the risk of developing DN, and consequently ESRD, worldwide.
DN is a microvascular complication associated with poor glycemic control. Early in the disease process, hyperglycemia induces glomerular hyperfiltration, which is followed by increased glomerular permeability to macromolecules and thickening of the glomerular basement membrane and eventually glomerular sclerosis and interstitial fibrosis. This disruption of glomerulus function leads to a decrease in the glomerular filtration rate and an increase in protein in the urine (proteinuria). Microalbuminuria (MA) has been used as an early biomarker of DN, but it lacks the specificity and sensitivity to detect early onset of DN: clinical factors unrelated to DN can affect MA status,2 and recently it has been observed that up to 30% of DN cases may occur in the absence of obvious MA.3, 4 Nonetheless, the exact relationship between MA, DN, and progression to ESRD remains an area of active research.
Over the past 2 decades, much work has demonstrated that a class of short (∼20 bp) noncoding RNAs, microRNAs (miRNAs), play an important role in shaping the cellular transcriptome,5, 6 are involved in a diverse set of cellular processes,7 and are often perturbed in many disease states, including type 1 diabetes (T1D) and type 2 diabetes.8, 9 Recently, the discovery of miRNAs in the extracellular environment10, 11, 12 and circulating in various biological fluids13 has suggested that miRNAs may be functioning as paracrine or endocrine signals between cells. Some of these circulating miRNAs are encapsulated in various extracellular vesicles (EVs), including microvesicles, exosomes, and apoptotic bodies.14 A number of studies have profiled these EV molecular contents and demonstrated their functional activities.12, 15, 16, 17, 18 It has been proposed that specific circulating miRNAs can act as noninvasive diagnostic biomarkers for a wide variety of diseases and conditions.19, 20
Several groups have looked at circulating miRNAs in patients with pre-T1D or patients with newly diagnosed T1D,21 in juveniles with T1D,22 and in urinary EVs of patients with incipient DN.23 We previously reported a quantitative polymerase chain reaction (qPCR)-based profiling study of the urinary miRNA spectra of patients with T1D with MA who would eventually develop DN, identifying miRNAs associated with biological pathways, such as transforming growth factor-β/bone morphogenetic protein signaling, which are perturbed in DN and other renal and kidney diseases.24
Recently, advances have been made in methods to isolate and characterize EVs, and in next generation sequencing approaches to profile miRNAs.20 To complement our previous qPCR-based miRNA study, we isolated and characterized EVs from the urine of patients with T1D with overt DN or MA, and used small-RNA sequencing (small-RNAseq) to comprehensively profile miRNAs in urine and urinary EVs. We detect miRNAs showing significant concentration changes in urine and urinary EVs that differ not only between MA and DN status, but also by factors such as hemoglobin A1c (HbA1c) levels. Follow-up validation using qPCR confirmed many of these findings.
Methods
Sample and Study Collection
Urine samples were collected from participants of the Pittsburgh Epidemiology of Diabetes Complications (EDC) study, as described previously.24 We analyzed urine from matched samples of 2 cohorts: (i) normoalbuminuric (N) diabetic patients who never developed microalbuminuria or nephropathy after prolonged (25-year) follow-up versus those who developed overt nephropathy (O), and (ii) patients who developed intermittent microalbuminuria (IMA) matched against EDC participants who developed persistent microalbuminuria (PMA), with 2 samples corresponding to a baseline urine sample from the last visit that tested negative for albumin and the subsequent follow-up, albuminuric sample that was collected 2 years after the first visit. A urine sample from healthy individuals (used as controls for the qPCR assays) was obtained from Innovative Research (Novi, MI). Samples were stored at −80°C until used for analysis. Before analysis, samples were thawed on ice and spun at 2000g at 4°C for 30 minutes to remove cellular debris. The resulting supernatant was then used for protein analysis, EV isolation, or RNA isolation as described later in this article. See supplementary data for detailed sample and study information.
Sodium Dodecyl Sulfate Protein Gel Electrophoresis
Individual urine samples were diluted in NuPAGE LDS sample buffer and NuPAGE Reducing reagent, and heated at 70°C for 10 minutes. The samples were then run on precast NuPAGE Novex Bis-Tris Mini gels with Novex Sharp prestained protein ladder and stained with SimplyBlue SafeStain, according to the manufacture’s protocol (Thermo Fisher, Waltham, MA).
EV Isolation
EVs were isolated from 250 μl of urine using qEV size-exclusion column (Izon Science, Cambridge, MA) with de-gassed 1X phosphate-buffered saline. Eluate fractions (∼500 μl) containing microvesicles (fractions 7–10) were collected individually. The subsequent fractions depleted of microvesicles (11–35) were collected into a single 15-ml tube. The vesicle fractions were pooled and concentrated to ∼100 μl using Amicon 10K centrifugation filters (EMD Millipore, Billerica, MA) spun in a swing-bucket rotor at 4000g at 4°C for 20 minutes. To confirm the purification of EVs from samples, the qEV-purified EVs were examined with transmission electron microscopy at the Fred Hutchinson Cancer Center, as previously described.25
miRNA Isolation
miRNA was isolated from 250 μl of urine, or concentrated EV/EV-depleted fractions from the same amount of urine of each patient using the miRNeasy Micro kit (QAIGEN, Germantown, MD). The RNA was eluted with 14 μl of nuclease-free H20 and quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) with a RNA (Pico) chip; 5 μl RNA was used as input for library construction.
Small-RNA Library Construction and Sequencing
We used an in-house small-RNAseq library construction method that uses adapters with 4 degenerated bases to reduce adapter-RNA ligation bias (see Supplementary Methods for the detailed protocol). Individual library concentrations were measured using the NEBNext Library Quant Kit for Illumina (New England Biolabs, Ipswich, MA) and adjusted to a final pooled concentration of 2 nM and run on a NextSeq sequencer (Illumina, San Diego, CA).
Data Analysis of Small-RNAseq Results
We used an in-house small-RNAseq data analysis pipeline (sRNAnalyzer26) to identify and compare miRNA levels for each sample (including urine, EV, and EV-depleted fractions). The quantity of miRNA is indicated by read counts that were normalized by counts per mapped million. To be considered as expressed miRNA, the raw read count of the miRNA has to be more than 10 reads and more than 10% of the mean of individual sample in 70% of the samples. Samples were compared using t-test, with a P < 0.05, and a log2 fold change cutoff of ±0.60. For determination of linear correlation between miRNAs and Hb1Ac levels, the coefficient of determination (R2), was calculated between these variables. All statistical analysis was done in Microsoft Excel (Redmond, WA).
qPCR
qPCR validation of miRNAs that showed concentration changes in the small-RNAseq datasets was performed using TaqMan Advanced miRNA assays (Thermo Fisher). Based on our small-RNAseq data, hsa-miR-16-5p was identified as invariant (did not show significant concentration changes across samples) and therefore was used as a normalization control for each assay. qPCR data were analyzed using Microsoft Excel. Relative mRNA or miRNA values are represented as the inverse of ΔCt values (maximum number of cycles – ΔCt) to give the linear range, which is directly proportional to the concentration of each mRNA in each sample, as previously described.24, 27 For qPCR assessment of specific kidney mRNA concentration, the QuantiTect SYBR Green PCR kit (QAIGEN) was used. The following PCR primers (forward and reverse, respectively) were used to detect mRNA present in urine, EV, or EV-depleted fractions: Aquaporin 2 (AQP2): GCTCCGCTCCATAGCCTTC, GGGTGCCAATACCCAAGCC; Nephrin (NPHS1): CTGCCTGAAAACCTGACGGT, GACCTGGCACTCATACTCCG; Podocin (NPHS2): ACCAAATCCTCCGGCTTAGG, CAACCTTTACGCAGAACCAGA; and β-actin: CGTCCACCGCAAATGCTT, TCTGCGCAAGTTAGGTTTTGTC. The level of β-actin was used to normalize the results because it has been previously used as a control for mRNA concentration measurements in urine and urinary EVs.28
Results
From the urine that was collected from participants of the Pittsburgh EDC study (see Supplementary Table S1), EVs were prepared using a size-exclusion chromatography (SEC)-based method that allowed for the separation of EVs, and EV-depleted fractions from each sample. We confirmed elevated protein levels (including a 60-to 80-kDa protein that likely represents albumin) in urine samples from patients with MA compared with patients without MA (Supplementary Figure S1A), and found their respective EV fractions isolated by SEC had reduced protein levels based on sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis (Supplementary Figure S1B).
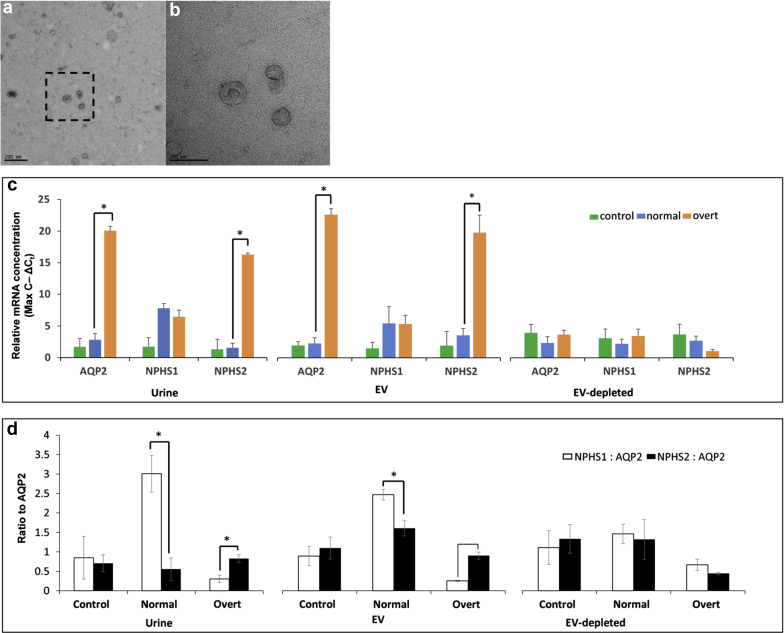
Isolated EVs were confirmed using electron microscopy, which revealed the presence of vesicles 30 to 100 nm in size that showed the typical morphological features of exosomes as described (Figure 1a and b).25 Previous studies have shown the urine-derived EVs contain mRNA transcripts of kidney origin, including AQP2, NPHS1, and NPHS2.28 We were able to detect these mRNAs in urine and EV, and EV-depleted samples, and interestingly found elevated levels of AQP2 and NPHS2 in patients with MA with overt nephropathy (overt) when compared with matched normoalbuminuric (non-MA) patients (normal) (Figure 1c) in urine and EV fractions (urine P = 0.005693 and 0.006899, respectively; EVs P = 0.002592 and 0.005345, respectively). Conversely, we did not see significant concentration differences of these transcripts between normal and overt patients in their respective EV-depleted fractions (Figure 1c), suggesting most of these mRNAs are present in EVs. The urine and EV ratio of NPHS1 and NPHS2 to AQP2 mRNA has been previously shown to be representative of MA status and can mark the progression of DN and ESRD.29, 30 Both urine and EV fractions of normal patients had higher NPHS1/AQP2 ratios compared with NPHS2/AQP2 ratios, whereas overt patients had higher NPHS2/AQP2 ratios compared with NPHS1/AQP2 ratios (urine P = 0.025409 and P = 0.042664, respectively; and EV P = 0.045542 and 0.009604, respectively; Figure 1d). We did not see significant normal versus overt differences in the NPHS1/AQP2 or NPHS2/AQP2 ratios in EV-depleted fractions (Figure 1d).
Figure 1.
Isolation of extracellular vesicles (EVs) from urine of patients with type 1 diabetes (T1D). (a) Scanning electron micrograph of EVs isolated using size-exclusion chromatography (SEC) from urine. Bar in lower left indicates 200 nm for reference. (b) Higher-resolution image from boxed region in (a). Bar in lower left indicates 100 nm for reference. (c) Quantitative polymerase chain reaction results for mRNA kidney markers Aquaporin2 (AQP2), Nephrin (NPSH1), and Podocin (NPHS2) in urine, EV, and EV-depleted (EV-dep) samples for control (green bars, non-T1D), normal (blue bars, T1D nonmicroalbuminuria), and overt (orange bars, T1D overt diabetic nephropathy status) patient samples. Values are represented as the inverse of ΔCt values (maximum number of cycles – ΔCt) to give the linear range, which is directly proportional to the concentration of each mRNA in each sample. (d) The ratio of NPHS1 (white bars) and NPHS2 (solid bars) to AQP2 (NPHS1: AQP2, NPHS2: AQP2) from linear range values in (c) is shown for control, normal, and overt patient samples in urine, EV, and EV-dep samples. Statistically significant (P ≤ 0.05) comparisons are indicated by an asterisk.
To identify miRNAs that exhibit specific concentration changes in the EVs, we made small-RNA libraries from each patient listed in Supplementary Table S1 from (i) total urine, (ii) EVs, and (iii) EV-depleted fractions. After sequencing, we obtained 7,250,689, 6,934,825, and 2,625,170 processed input reads from urine, EV, and EV-depleted fractions on average, respectively (Supplementary Table S2). Of those, 182,127, 270,688, and 187,920 reads mapped to miRNAs from these respective fractions (Supplementary Table S2).
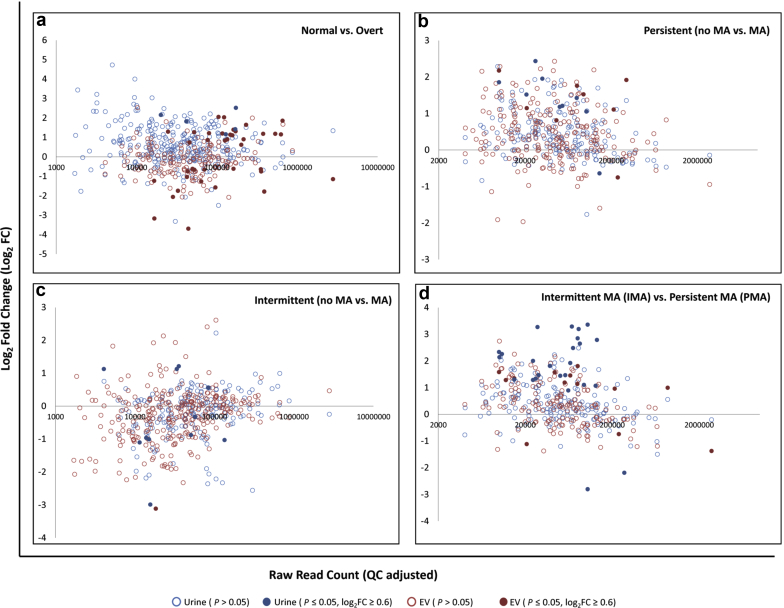
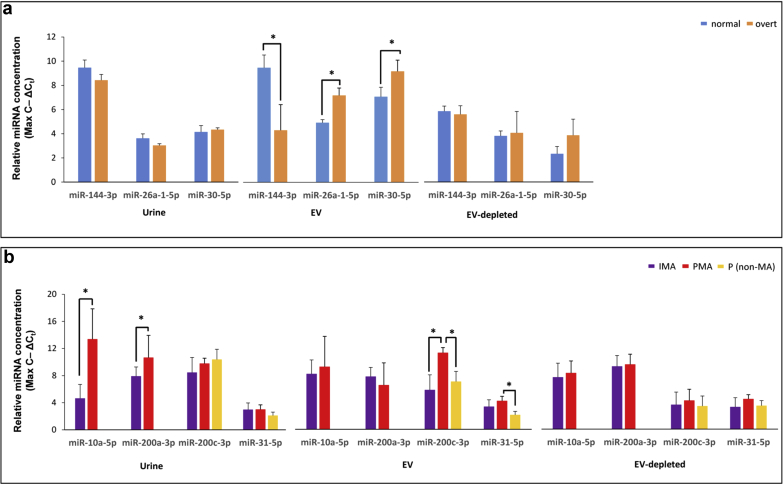
We analyzed the data using log2-transformed, counts per mapped million normalized read counts (Supplementary Table S3). When comparing normal with overt patients, we observed a number of miRNAs that showed statistically significant concentration changes in urine and corresponding EV fraction (Figure 2a; Table 1). In urine, the concentrations of the 5 affected miRNAs (miR-130a-3p, miR-142-3p, miR-223-3p, miR-22-3p, and miR-320a-3p) were all elevated in overt samples. The EV fraction showed more affected miRNAs (Figure 2a; Table 1), and we validated the changes of miR-144-3p, miR-26a-5p, and miR-30c-5p in the EV fraction using qPCR (Figure 3a).
Figure 2.
miRNA concentration changes from small-RNA sequencing across different type 1 diabetes (T1D) patient cohorts. Microalbuminuria (MA) plot of log2FC (fold change) of counts per million (CPM) versus raw read count for (a) normal versus overt, (b) persistent (no MA vs. MA), (c) intermittent (no MA vs. MA), and (d) intermittent MA (IMA) versus persistent MA (PMA) T1D patient cohorts. Light blue dots represent individual miRNAs from urine that do not have statistically significant (P ≤ 0.05) log2FC values, whereas dark blue dots indicate miRNAs from urine showing statistically significant log2FC values ≥ 0.6. Light red dots represent individual miRNAs from EVs that do not have statistically significant log2FC values, whereas dark red dots indicate miRNAs from EVs showing statistically significant log2FC values ≥ 0.6. EV, extracellular vesicle.
Table 1.
Selected miRNAs showing differential concentration changes between Normal and Overt patients
| Starting fraction | miRNA | NormAVE (log2CPM) | OvertAVE (log2CPM) | Log2FC | P |
|---|---|---|---|---|---|
| Urine | hsa-miR-130a-3p ♂ | 8.80 | 10.62 | 1.82 | 0.0238 |
| hsa-miR-142-3p | 8.20 | 10.35 | 2.16 | 0.0223 | |
| hsa-miR-223-3p | 11.33 | 13.85 | 2.52 | 0.0498 | |
| hsa-miR-22-3p | 11.87 | 13.29 | 1.42 | 0.0289 | |
| hsa-miR-320a-3p | 11.81 | 12.97 | 1.16 | 0.0332 | |
| EV (selected) | hsa-miR-941-1-3p | 9.71 | 6.01 | −3.70 | 0.0083 |
| hsa-miR-9-1-3p | 7.53 | 9.59 | 2.05 | 0.0479 | |
| hsa-let-7c-5p | 9.31 | 11.33 | 2.03 | 0.0050 | |
| hsa-miR-125b-1-5p | 11.48 | 13.34 | 1.86 | 0.0067 | |
| hsa-miR-486-1-5p | 15.05 | 13.26 | −1.79 | 0.0066 | |
| hsa-miR-144-3p | 11.98 | 10.24 | −1.75 | 0.0108 | |
| hsa-miR-30a-5p | 12.82 | 14.46 | 1.65 | 0.0400 | |
| hsa-miR-125a-5p | 10.97 | 12.36 | 1.39 | 0.0146 | |
| hsa-miR-30c-1-5p | 12.25 | 13.56 | 1.31 | 0.0450 | |
| hsa-miR-29b-1-3p ♂ | 9.99 | 11.28 | 1.29 | 0.0048 | |
| hsa-miR-99b-5p | 10.01 | 11.22 | 1.21 | 0.0246 | |
| hsa-miR-23b-3p | 11.10 | 12.30 | 1.20 | 0.0047 | |
| hsa-let-7a-1-5p | 13.37 | 14.55 | 1.18 | 0.0179 | |
| hsa-miR-26a-1-5p | 13.95 | 15.10 | 1.15 | 0.0023 | |
| hsa-miR-451a-5p | 17.85 | 16.70 | −1.15 | 0.0036 | |
| hsa-miR-27b-3p | 12.42 | 13.55 | 1.14 | 0.0164 | |
| hsa-miR-99a-5p | 10.60 | 11.70 | 1.10 | 0.0431 | |
| hsa-miR-26b-5p | 12.36 | 13.25 | 0.90 | 0.0002 | |
| hsa-miR-29c-3p | 12.20 | 13.07 | 0.86 | 0.0066 | |
| hsa-miR-363-3p | 10.30 | 9.54 | −0.76 | 0.0233 | |
| hsa-miR-185-5p | 7.93 | 5.40 | −0.67 | 0.0299 |
CPM, counts per million; EV, extracellular vesicle; miRNA, microRNA.
♂ (male) or ♀ (female) designates gender-specific enrichment for a given miRNAs. See Supplementary Table S3 for additional details.
Figure 3.
Quantitative polymerase chain reaction (qPCR) validation of microRNAs (miRNAs) from small-RNA sequencing of patients with type 1 diabetes (T1D). (a) qPCR results for miR-144-3p, miR-26a-5p, and miR-30c-5p in, urine, EV, and EV-depleted samples for normal (blue bars, non-MA), and overt (orange bars, overt MA status) patients. (b) qPCR results for miR-10a-5p, miR-200a-3p, miR-200c-3p, and miR-31c-5p in urine, EV, and EV-depleted samples for intermittent MA (purple bars), persistent MA (red bars), and persistent non-MA (yellow bars) patients. Values are represented as the inverse of ΔCt values (maximum number of cycles – ΔCt) to give the linear range, which is directly proportional to the concentration of each miRNA in each sample. Statistically significant (P ≤ 0.05) comparisons are indicated by an asterisk. EV, extracellular vesicle fraction; EV-dep, EV-depleted fraction; IMA, intermittent (microalbuminuria); MA, microalbuminuria; PMA, persistent (microalbuminuria); P (nonmicroalbuminuria), persistent (nonmicroalbuminuria).
When analyzing the miRNA profiles of intermittent and persistent patients, we compared patients who progressed to MA with their previous non-MA cycle within each cohort. Within the persistent cohort, where progression to MA status persisted for several disease cycles, we identified several urinary and EV miRNAs that were elevated in persistent patients’ MA (PMA) cycle compared with the previous non-MA cycle (Figure 2b; Table 2). qPCR validation of miR-31-5p and miR-200c-3p confirmed concentration changes between non-MA and PMA in EVs, but not in urine (Figure 3b).
Table 2.
Selected miRNAs showing differential concentration changes between non-MA and MA persistent patients
| Starting fraction | miRNA | PMA:NO-AVE (log2CPM) | PMA:MA-AVE (log2CPM) | Log2FC | P |
|---|---|---|---|---|---|
| Urine | hsa-miR-133a-1-3p | 5.97 | 8.40 | 2.43 | 0.0254 |
| hsa-miR-31-5p | 7.95 | 9.81 | 1.85 | 0.0498 | |
| hsa-miR-122-5p | 8.96 | 10.48 | 1.52 | 0.0097 | |
| hsa-miR-99b-5p | 10.57 | 11.99 | 1.43 | 0.0324 | |
| hsa-miR-92b-3p | 8.29 | 9.49 | 1.21 | 0.0282 | |
| hsa-miR-181b-1-5p | 9.44 | 10.49 | 1.05 | 0.0077 | |
| EV | hsa-miR-182-5p | 9.80 | 11.32 | 1.52 | 0.0446 |
| hsa-miR-200c-3p | 11.83 | 13.58 | 1.76 | 0.0483 | |
| hsa-miR-30d-5p | 13.48 | 14.59 | 1.11 | 0.0414 | |
| hsa-miR-31-5p | 8.74 | 10.92 | 2.17 | 0.0457 | |
| hsa-miR-335-5p | 10.40 | 11.21 | 0.81 | 0.0142 | |
| hsa-miR-96-5p | 7.75 | 8.90 | 1.15 | 0.0298 |
AVE, average; CPM, counts per million; EV, extracellular vesicle; FC, fold change; MA, microalbuminuria; miRNA, microRNA; PMA:NO, Persistent MA: non-MA cycle; PMA:MA, Persistent MA:MA cycle.
See Supplementary Table S3 for additional details.
Within the intermittent patient cohort, where progression to MA status was intermittent for several disease cycles, we identified some urinary and EV miRNAs that showed concentration changes in patient MA (IMA) cycles compared with the previous non-MA cycles (Figure 2c), but only 1 miRNA, miR-671-5p, was identified that showed a statically significant concentration change in EVs (Table 3). The smaller number of affected miRNAs in this cohort may reflect the more irregular and mild nature of MA seen in these intermittent patients. An overall reduction of protein in intermittent MA patient urine compared with persistent and overt patients was observed (Supplementary Figure S1A).
Table 3.
Selected miRNAs showing differential concentration between non-MA and MA intermittent patients
| Starting fraction | miRNA | IM:NO-AVE (log2CPM) | IM:MA-AVE (log2CPM) | Log2FC | P |
|---|---|---|---|---|---|
| Urine | hsa-miR-3168-5p | 10.23 | 7.24 | -2.99 | 0.0153 |
| hsa-miR-342-3p | 9.06 | 10.27 | 1.21 | 0.0487 | |
| hsa-miR-152-3p | 8.28 | 9.41 | 1.13 | 0.0164 | |
| hsa-miR-339-3p | 6.87 | 8.00 | 1.13 | 0.0392 | |
| hsa-miR-4286-5p | 9.58 | 8.47 | -1.11 | 0.0270 | |
| hsa-miR-192-5p | 10.17 | 9.13 | -1.03 | 0.0320 | |
| hsa-miR-362-5p | 8.43 | 7.42 | -1.01 | 0.0458 | |
| hsa-miR-197-3p | 9.53 | 8.56 | -0.97 | 0.0296 | |
| hsa-miR-1307-3p | 9.67 | 8.78 | -0.88 | 0.0159 | |
| hsa-miR-188-5p | 6.82 | 5.23 | -1.58 | 0.0128 | |
| hsa-miR-424-3p | 7.16 | 5.25 | -1.91 | 0.0192 | |
| EV | hsa-miR-671-5p | 6.43 | 3.32 | -3.12 | 0.0451 |
AVE, average; CPM, counts per million; EV, extracellular vesicle; FC, fold change; MA, microalbuminuria; miRNA, microRNA; IMA:NO, Intermittent MA: non-MA cycle; IMA:MA, Intermittent MA:MA cycle.
See Supplementary Table S3 for additional details.
When IMA transitions to PMA, it marks a critical clinical transition for patients with T1D, as comparison of albumin excretion rates and other clinical features between these 2 phases can be used to calculate risk of developing DN.31 We compared miRNA profiles between patients with PMA and those with IMA (patients at their MA disease cycle) and identified several miRNAs that showed concentration changes (Figure 2d; Table 4). In urine, miR-10a-5p was elevated in patients with IMA, and miR-200a-3p was elevated in patients with PMA; both were validated by qPCR. We also observed miR-200c-3p to be elevated in the EV fraction from patients with PMA compared to their previous non-MA cycle, and subsequently between patients with PMA and those with IMA in EV and urine. We were able to validate this result by qPCR; however, in only the EV fraction of patients with PMA and not in urine (Figure 3b).
Table 4.
Selected miRNAs showing differential concentration changes between IMA and PMA patients
| Starting fraction | miRNA | I(MA)-AVE (log2CPM) | P(MA)-AVE (log2CPM) | Log2FC | P |
|---|---|---|---|---|---|
| Urine | hsa-miR-10a-5p | 10.16 | 13.52 | 3.36 | 0.0048 |
| hsa-miR-10b-5p | 10.33 | 12.97 | 2.65 | 0.0408 | |
| hsa-miR-124-1-3p | 11.16 | 8.97 | -2.19 | 0.0448 | |
| hsa-miR-141-3p | 7.61 | 10.88 | 3.27 | 0.0129 | |
| hsa-miR-148a-3p | 11.72 | 12.79 | 1.07 | 0.0428 | |
| hsa-miR-183-5p | 7.10 | 9.58 | 2.48 | 0.0428 | |
| hsa-miR-192-5p | 9.13 | 11.92 | 2.79 | 0.0222 | |
| hsa-miR-200a-3p | 9.16 | 12.44 | 3.29 | 0.0069 | |
| hsa-miR-200c-3p | 10.19 | 13.04 | 2.85 | 0.0202 | |
| hsa-miR-29b-1-3p ♂ | 9.29 | 10.57 | 1.28 | 0.0017 | |
| hsa-miR-30b-5p | 10.80 | 12.27 | 1.46 | 0.0169 | |
| hsa-miR-31-5p | 7.47 | 9.81 | 2.33 | 0.0005 | |
| EV | hsa-miR-200c-3p | 11.78 | 13.58 | 1.80 | 0.0351 |
| hsa-miR-31-5p | 9.34 | 10.92 | 1.58 | 0.0343 | |
| hsa-miR-373-3p | 10.35 | 11.92 | 1.57 | 0.0495 | |
| hsa-miR-451a-5p | 17.32 | 15.95 | -1.38 | 0.0166 | |
| hsa-miR-362-5p | 8.63 | 9.98 | 1.34 | 0.0195 | |
| hsa-miR-28-3p | 8.20 | 9.48 | 1.28 | 0.0116 | |
| hsa-miR-660-5p | 9.95 | 11.14 | 1.19 | 0.0173 | |
| hsa-miR-99b-5p | 10.38 | 11.52 | 1.14 | 0.0250 | |
| hsa-miR-122-5p | 10.60 | 9.48 | -1.12 | 0.0284 | |
| hsa-miR-21-5p | 15.29 | 16.29 | 0.99 | 0.0291 | |
| hsa-miR-30d-5p | 13.63 | 14.59 | 0.96 | 0.0468 | |
| hsa-miR-101-1-3p | 13.57 | 12.82 | -0.75 | 0.0008 |
AVE, average; CPM, counts per million; EV, extracellular vesicle; FC, fold change; I(MA) = intermittent MA: MA cycle; MA, microalbuminuria; miRNA, microRNA; P(MA), persistent MA:MA cycle.
♂ (male) or ♀ (female) designates gender-specific enrichment for a given miRNAs. See Supplementary Table S3 for additional details.
Exosomal miRNAs Showing Correlation With Plasma Glucose Concentrations/HbA1c Levels in Patients With T1D
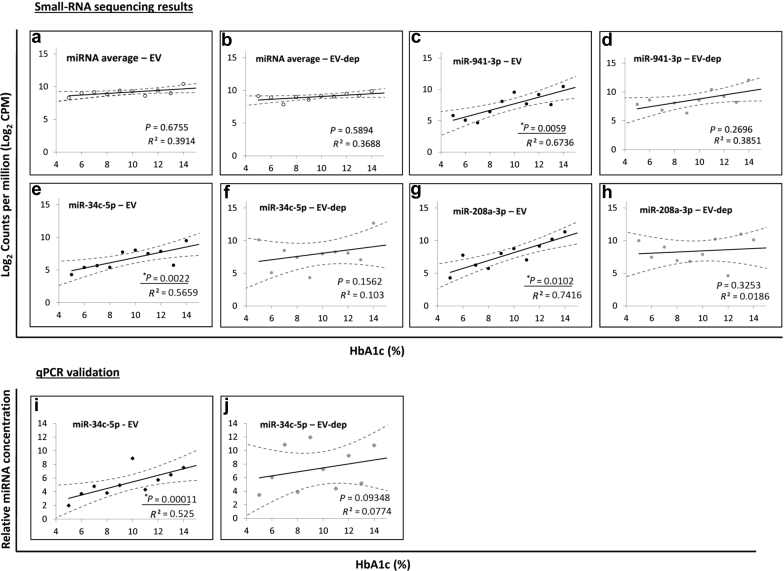
To determine if any miRNAs in EVs correlated with patient HbA1c level, we examined the linear correlation between total number of mapped reads and HbA1c levels (ranging from 5.2% to 14.3%; Supplementary Table S1). We did not observe any correlation between overall miRNA read counts and HbA1c levels (Figure 4a and b). Interestingly, several individual EV-associated miRNAs (miR-941-5p, miR-34c-5p, and miR-208a-3p) showed a significant correlation with the HbA1c level (Figure 4c, e, and g), whereas their EV-depleted equivalents did not (Figure 4d, f, and h). Because of the role for miR-34c-3p in glucose regulation in the kidney,32 we confirmed its association with HbA1c levels in EV but not in EV-depleted samples using qPCR (Figure 4i and j).
Figure 4.
Extracellular vesicle (EV) microRNAs (miRNAs) that show linear correlation with type 1 diabetes patient plasma glucose (hemoglobin A1c [HbA1c]). Plots of log2 counts per million (CPM) versus patient HbA1c (%) for (a) average of all miRNAs in patient EV samples, (b) average of all miRNAs in patient EV-depleted samples, (c) miR-941-3p in patient EV samples, (d) miR-941-3p in patient EV-depleted samples, (e) miR-34c-5p in patient EV samples, (f) miR-34c-5p in patient EV-depleted samples, (g) miR-208a-3p in patient EV samples, and (h) miR-208a-3p in patient EV-depleted samples. Quantitative polymerase chain reaction (qPCR) results for miR-34c-5p in patient (i) EV and (j) EV-depleted samples. qPCR values are represented as the inverse of ΔCt values (maximum number of cycles – ΔCt) to give the linear range, which is directly proportional to the concentration of each miRNA in each sample. Correlation coefficients (R2 values) and P values are reported in bottom right corners. Statistically significant (P ≤ 0.05) linear correlations are underlined.
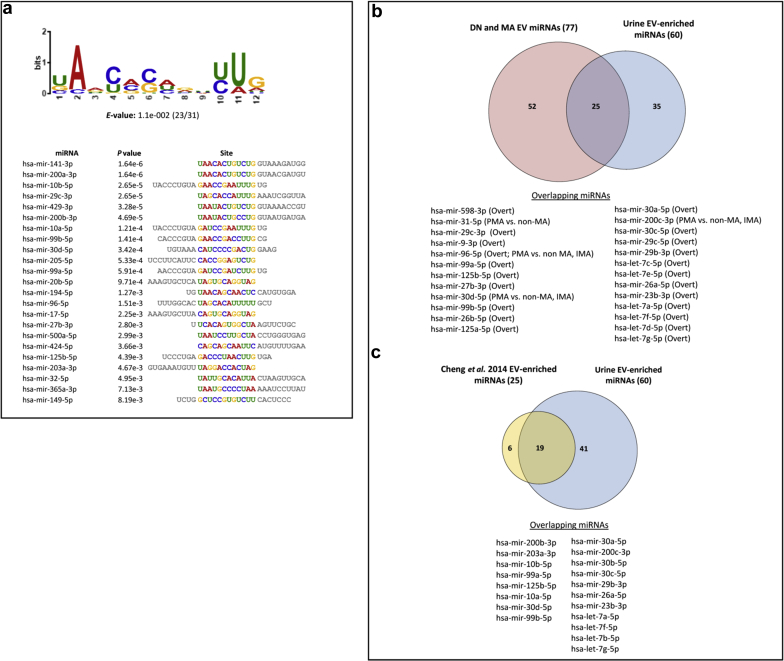
To identify miRNAs in our dataset that showed preferential enrichment in EVs, we compared miRNA profiles between EV and EV-depleted fractions across all of our patients with T1D, and identified 60 miRNAs that showed significant enrichment in EV (Supplementary Table S4). Using the MEME software suite,33 we identified an enriched motif present in the seed sequences of urinary EV-enriched miRNAs (Figure 5a), suggesting that some factors may be involved in sorting these miRNAs into EVs. Some of these EV-enriched miRNAs showed concentration differences in various comparisons (normal vs. overt, persistent non-MA vs. PMA, intermittent non-MA vs. IMA, and IMA vs. PMA) (Figure 5b).
Figure 5.
Identification of extracellular vesicle (EV)–enriched microRNAs (miRNAs) from patients with type 1 diabetes (T1D). (a) Novel sequence motif identified from the 31 urine-specific EV-enriched miRNAs (see Supplementary Table S3) with an E-value of 1.1e-002 in 23 of 31 samples. Each individual miRNA site alignment with the given P value is displayed. (b) Venn diagram showing overlap (25) between 77 miRNAs from patients with T1D who showed differential concentration changes in EVs (red) with 60 EV-enriched miRNAs (blue). (c) Venn diagram showing overlap (19) of top 25 urine EV-enriched miRNAs reported from Cheng et al. 2014180 (yellow) with urine-specific EV-enriched miRNAs identified in this work (blue).
Discussion
We report the results of the first comprehensive next generation sequencing–based analysis of the changes of miRNA profiles in urine, urinary EV, and EV-depleted urine fractions from patients with T1D with various grades of DN and MA. Urinary EVs have been proposed as a potential source of biomarkers for various forms of kidney disease, including DN.34 Although urinary EVs have been studied by various proteomics approaches,35, 36 little progress has been made in studying urinary EV-associated miRNAs during the development of T1D-associated DN. EV miRNA profiling is limited by several factors, including how the samples are collected, processed, and stored; the methods used for EV and RNA isolation; and a platform used for miRNA profiling that is both sensitive and free of technical artifacts. The Pittsburgh EDC study has allowed for urine samples to be collected and processed in a uniform manner, including optimal storage conditions, and minimal freeze-thaws. We have adapted an SEC-based method that provides consistent and reliable isolation of EVs based on their characteristic size range, minimally altering their characteristics and properties, and avoiding protein contamination. This SEC-based method performs as well if not better than ultracentrifugation and commercial kits in these regards.37, 38 We show that SEC-purified EVs contain kidney-enriched protein transcripts (AQP2, NPHS1, NPHS2), and their concentration changes are consistent with prior reports on their association with the onset of DN and ESRD.28, 29 Although SEC has many advantages over other methods, there are still some limitations that should be considered. Ultracentrifugation has been the preferred method for the isolation of EVs, due to the ability to isolate large amounts of EVs from large volumes of starting material. Current SEC columns that have been developed for EV isolation can isolate only 1 to 2 ml biofluid or cell culture media in a single use, whereas ultracentrifugation can scale up to the tens or hundreds of milliliters in a single setting. Careful considerations of the trade-offs between these methods should be considered before selecting the appropriate technique. Moreover, it has been shown that various factors that occur during RNA isolation and library preparation, including the isolation method being used in day-to-day batch effects, and RNA ligation bias can influence the miRNA profile from biofluids. Here, we have also revised and streamlined the existing small-RNA isolation and profiling method to reduce the bias that has been reported between RNA isolation and sequencing library construction kits commonly in use.39, 40
From our small-RNAseq results, we identified miRNAs that showed significant concentration changes in patients when compared with non-DN controls (for patients with overt DN) or when compared with their previous non-MA urine collection cycle (for patients with IMA or PMA). Many of these miRNAs have been previously associated with kidney function, and have roles in the pathophysiology of diabetes23, 24,41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145 (Table 5). In DN patient EV fractions, 2 of these miRNAs, miR-486-1-5p and miR-363-3p, were identified in our previous study as being predictive for patients who will eventually develop MA during DN.130 Several other miRNAs showing concentration changes in the EVs, including miR-125a-5p, miR-30c-1-5p, let-7a-1-5p, miR-26a-5p, and miR-451a-5p, also have been shown to be involved in DN in other studies.43, 60, 122, 146, 147 In addition, several elevated miRNAs (miR-26a-5p, miR-26b-5p, and miR-27b-3p) are also involved in kidney glomerular function and injury.110, 113, 148
Table 5.
miRNAs identified in this work that are associated with kidney function and/or diabetes
| miRNA | Kidney function | Diabetes function | Targeted pathways (validated) |
|---|---|---|---|
| hsa-let-7a-1-5p | LN, RCC, DN | DN | TGF-β (TGFBRI) |
| hsa-let-7c-5p | Rfib, ESRD | T1D (ESRD) | TGF-β (TGF-βRI) |
| hsa-miR-101-1-3p | AKI | – | – |
| hsa-miR-10a-5p | AKI,AKR | T2D (Glucose regulation) | cAMP (CREB1) |
| hsa-miR-10b-5p | RCC, AKR, AKI | Insulin resistance | Apoptosis (BCL2L11) |
| hsa-miR-122-5p | RCC | T2D (insulin resistance, obesity) | – |
| hsa-miR-124-1-3p | RCC | T2D (Glucagon, gastric bypass treatment) | – |
| hsa-miR-125a-5p | PCKD, DN | T2D,DN | IL-6R |
| hsa-miR-125b-1-5p | CKD, AKI | T1D (β-cell) | – |
| hsa-miR-130a-3p | DN | T1D | – |
| hsa-miR-133a-1-3p | – | T1D (β-cell) | FoxO/AMPK |
| hsa-miR-141-3p | RCC, DN | DN, Obesity-induced DM | PTEN (PTEN), TGF-β (ZEB1/2), IGF2 |
| hsa-miR-142-3p | AKI, AKR | T2D | TGF-β (TGFβRI) |
| hsa-miR-144-3p | IgA-N | T2D (Micro-vascular) | – |
| hsa-miR-148a-3p | LN-RD | T2D (β-cell) | PTEN (PTEN) |
| hsa-miR-152-3p | – | T2D (Insulin) | PTEN (PTEN) |
| hsa-miR-181b-1-5p | AKR, Nephron development | Glucose homeostasis, insulin resistance | Six2 |
| hsa-miR-182-5p | AKI, PKD | T2D (Insulin) | – |
| hsa-miR-183-5p | – | T2D (β-cell) | – |
| hsa-miR-185-5p | AKR | β-cell | Cytokine/IGF (SOCS3) |
| hsa-miR-188-5p | AKI, RIRI | – | – |
| hsa-miR-192-5p | AKI, RIRI, DN | T2D (β-cell), DN | – |
| hsa-miR-197-3p | – | Glycemic impairment | – |
| hsa-miR-200a-3p | Rfib, DN | DN, insulin | TGF-β (TGFβRI), IGF |
| hsa-miR-200c-3p | Glomerular cell function | T2D (Endo) | TGF-β (ZEB1/2) |
| hsa-miR-21-5p | Rfib, DN, IgA-N | T1D, T2D | TGF-β (SMAD7), PTEN (PTEN) |
| hsa-miR-22-3p | Rfib | T1D | TGF-β (BMP-7,6) |
| hsa-miR-23b-3p | RCC | T1D (β-cell) | PTEN (PTEN), Apoptosis (DP5) |
| hsa-miR-26a-1-5p | Podocyte injury, LN-RD, DN | T2D (Glucose/Insulin) | IGF (GSK3B), TGF-β (CTGF) |
| hsa-miR-26b-5p | AKI | T2D (β-cell- Insulin) | PTEN (PTEN) |
| hsa-miR-27b-3p | Glomerular injury | T2D (Islet) | TGF-B (gremlin 1) |
| hsa-miR-28-3p | RCC, DN | T1D (DN), T2D | – |
| hsa-miR-29b-1-3p | Rfib | T2D (Hyperglycemia) | – |
| hsa-miR-29c-3p | ESRD | T1D (ESRD) | TGF-β (targeted by) |
| hsa-miR-30a-5p | AKI, NS | T2D (Glucose, β-cell) | Notch (Notch1) |
| hsa-miR-30b-5p | LN-RD | β-cell dysfunction | – |
| hsa-miR-30c-1-5p | AKI, DN | DN | TGF-β (CTGF) |
| hsa-miR-30d-5p | AKI, PKD | T2D, β-cells (insulin) | Insulin (MAP4K4) |
| hsa-miR-31-5p | PCKD | T1D (serum), T2D (micro-vascular) | – |
| hsa-miR-335-5p | Renal senescence, DN | T1D (DN) | SOD2 |
| hsa-miR-342-3p | AKI | T1D,T2D (PBMCs) | – |
| hsa-miR-362-5p | RIRI | GDM | – |
| hsa-miR-363-3p | DN | T1D (DN) | – |
| hsa-miR-373-3p | DN | T1D (DN) | – |
| hsa-miR-451a-5p | DN | DN | – |
| hsa-miR-486-1-5p | DN, CKD, AKI, IgA-N | T2D, T1D (DN) | – |
| hsa-miR-660-5p | DN | T1D (DN) | – |
| hsa-miR-9-1-3p | – | T1D (serum) | – |
| hsa-miR-941-1-3p | – | – | Insulin (9) |
| hsa-miR-96-5p | PKD, RCC | T2D, β-cells (insulin) | IGF (synaptotagmin-like 4) |
| hsa-miR-99a-5p | AKR,RCC | Glucose/insulin regulation | mTOR |
| hsa-miR-99b-5p | AKR | T2D (IGT) | TGF-β (EMT) |
AKR, Acute Kidney Rejection/Renal Graft Rejection; AKI, acute kidney injury; CKD, chronic kidney disease; DIKD, drug induced kidney damage, DN, diabetic nephropathy; Endo, endothelial dysfunction; EMT, epithelial–mesenchymal transition; EndMT, endothelial-mesenchymal transition; ESRD, end-stage renal disease; GD, gestational diabetes mellitus; IgA-N, IgA nephropathy; IGF, insulin-like growth factor; IGT, impaired glucose tolerance; LN-RD, lupus nephritis induced renal damage; MA, microalbuminuria; miRNA, microRNA; NS, nephrotic syndrome; PCKD, poly-cystic kidney disease; PKD, progressive kidney disease; PTEN, phosphatase and tensin homolog; RCC, renal cell carcinoma; Rfib, renal fibrosis: RIRI, renal ischemia-reperfusion injury; TGF, transforming growth factor; T1D, type 1 diabetes mellitus; T2D), type 2 diabetes mellitus.
See references 23, 24,40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144.
In both urine and EVs, we found miR-31-5p to be elevated in patients with PMA. miR-31-5p has been previously shown to be upregulated in polycystic kidney disease, and elevated in the serum of patients with T1D or type 2 diabetes with microvascular complications.126, 127, 128 Additionally, miR-200c-3p, which we observed to be elevated in PMA patient EV fractions, plays an important role in glomerular cell function.94 Although both of these miRNAs showed concentration changes in urine and EVs, qPCR validation suggests that the main source of the signal in the urine is likely from the EVs that are present. Conversely, miR-200a-3p, which has a well-characterized role during renal fibrosis induced by DN,93 is also elevated in PMA urine (but not in EVs). miR-10a-5p and miR-10b-5p, shown to be enriched in kidney and involved in acute kidney injury,47, 48 are both elevated in the urine of patients with PMA compared with patients with IMA, but not the corresponding EV fractions. These results suggest that the miRNA spectra of urine and EVs are mostly unique, and the miRNA concentration changes present in urine mostly derive from non-EV miRNAs.
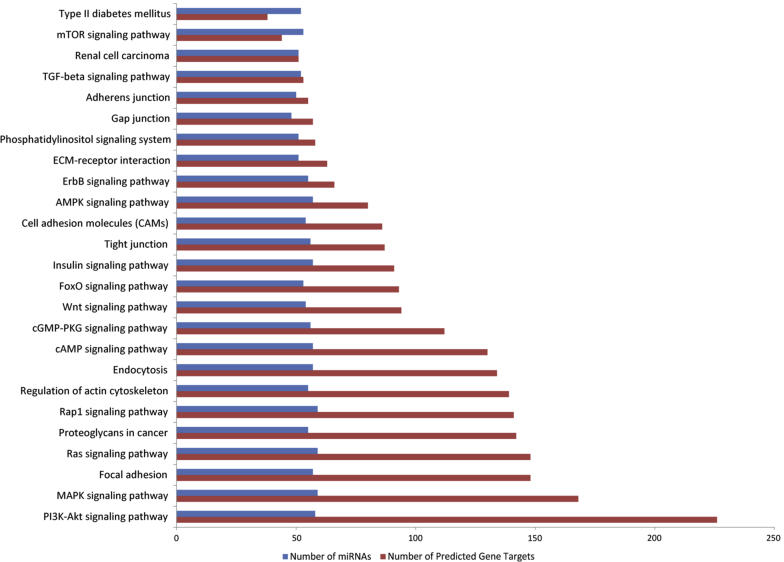
In addition, when looking at experimentally validated gene targets and pathways, many of these EV-associated miRNAs regulate pathways associated with renal fibrosis, including the transforming growth factor beta (TGF-β) signaling pathway and phosphatase and tensin homolog signaling (Table 1, Table 2, Table 3, Table 4). We used our miRNA profiling results to explore mirPath149 and identified many additional T1D- and renal fibrosis–associated pathways, including the regulation of focal and cell adhesion molecules, tight/gap/adherens junctions, and ECM-receptor interactions, among many more (Figure 6).150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164
Figure 6.
Identification of pathways associated with microRNAs (miRNAs) from patients with type 1 diabetes (T1D). Seventy-seven miRNAs from patients with T1D who showed differential concentration changes in extracellular vesicles was run through the mirPath program (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=mirpath) to identify predicted targeted pathways. Pathways associated with T1D and renal fibrosis are highlighted. Blue bars represent the number of miRNAs identified, and the red bars represent the number of predicted gene targets belonging to the associated pathway. AMPK, AMP-activated protein kinase; cGMP-PKG, cyclic GMP–protein kinase G; ECM, extracellular matrix; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; TGF, transforming growth factor.
Although many studies provide in vivo and in vitro experimental support for the intracellular roles of miRNAs in kidney diseases and function, only a few studies have analyzed circulating miRNAs in patients with T1D.20 In both plasma and urine of patients with T1D, miR-21 has been observed to be elevated in multiple cases.22, 140 We also observed such a change of miR-21 in patients with PMA. In addition, miR-148a-5p, miR-26a-5p, and miR-30a-5p have been reported to be elevated in the serum of patients with T1D.21, 140 Here we find these concentrations are also increased in overt DN patient EVs (miR-26a-5p and miR-30a-5p) and PMA patient urine (miR-148a-5p).
We compared our results with other published studies of circulating (i.e., in serum and/or plasma) miRNAs in DN. miR-21-5p, miR-29c-5p, miR-31-5p, and miR-660-5p, have been reported to be elevated in patients with DN139, 165, 166 compared with non-DN controls; we found 3 of 4 to be elevated in urinary EVs of PMA (miR-21-5p, miR-31-5p, miR-660-5p) and the fourth to be elevated in patients with overt DN (miR-29c-5p). As with serum and plasma, profiling of urinary miRNAs has been done previously in patients with T1D and DN. Our own previous work using quantitative reverse-transcriptase PCR to profile urinary miRNAs in individuals from the same patient cohort identified miR-92-5p, miR-141-3p, miR-335-5p, miR-486-5p, miR-28-3p, and miR-373-3p as being elevated in patients with DN or MA.24, 130 We found these miRNAs exhibited similar concentration changes in the data reported here, but with some being elevated exclusively in urine (miR-141-3p), or urine EVs (miR-28-3p, miR-373-3p, miR-335-5p, miR-486-5p). Limited profiling of miRNAs in urinary EVs of patients with DN or MA has also been previously reported, with miR-130a-5p and miR-192-5p levels being elevated in patients with early-stage DN.23 In our data, however, we found these miRs elevated only in the urine of patients with overt DN (miR-130a-5p) and PMA (miR-192-5p), but not in their respective EV samples. The method of EV isolation used by Barutta et al.23 (ultracentrifugation of pooled urine samples) may possibly account for the discrepancies between our results.
Indicators of diabetes progression, such as HbA1c (%) level, provide a somewhat quantitative assessment and correlate reasonably well with β-cell function. Although several miRNAs circulating in serum and plasma have been shown to have good linear correlation with HbA1c, no studies have examined correlations between urine or urinary EV miRNAs and HbA1c. The patients in this study had wide-ranging HbA1c levels (5.2% to 14.3%) that correlated well with increasing urinary EV-associated miR-941-5p, miR-34c-5p, and miR-208a-3p concentrations. Prior reports have shown that miR-34c-5p has a role in regulating glucose levels in the podocytes, where it inhibits glucose-induced apoptosis through the Notch pathway,34 and in attenuating the epithelial–mesenchymal transition required for fibrosis through the transforming growth factor-β pathway.167 Although an exact role for miR-941-3p remains elusive, it has been shown to preferentially target the insulin-signaling pathway, suggesting a possible role in regulating glucose levels.168 It is interesting that elevated circulating miR-208a-3p has been shown to be strongly associated with cardiovascular disease,169, 170, 171, 172 and elevated HbA1c is a risk factor for cardiovascular disease across various diabetic populations.173, 174, 175, 176, 177
Our analysis of global miRNA concentration changes in urine, EV, and EV-depleted urine samples revealed a set of miRNAs that show significant enrichment in the EVs (Supplementary Table S4). Of the urine EV-enriched miRNAs, many contain a conserved sequence motif that could possibly direct selective transport of miRNAs from kidney cells into urinary EVs. Previous work has identified several unique sequence motifs in miRNAs isolated from EVs derived from different cell types.178, 179 Further experimental follow-up will be needed to determine if this motif is involved in miRNA sorting of this kind. In addition, many of the miRNAs that exhibit concentration changes in patient EV fractions are also EV-enriched, suggesting that these miRNAs (Figure 5b) might be promising EV-specific biomarker candidates for DN and/or MA. A previous study used a similar small-RNAseq approach to identify miRNAs enriched in EVs from the urine of healthy individuals.180 Our cross-comparison of datasets found significant overlap of EV-enriched miRNAs between our results and this study (Figure 5c).
Although we observed several miRNAs with concentration changes similar to those reported in other prior studies, we also observed a number of miRNAs that either showed no change or showed a different direction of change. These differences are likely due at least in part to methodological differences, as these studies differ in biofluid type (serum, plasma, or urine), EV isolation methods (ultracentrifugation or commercial kits compared to SEC), approach to miRNA profiling (microarrays or quantitative reverse-transcriptase PCR compared with small-RNAseq), and by geographical and ethnic differences in patient populations, all of which can contribute to poor consistency among studies. Most circulating miRNA studies use qPCR-based techniques for measurement, which are low throughput and cannot detect novel miRNAs. Although small-RNAseq overcomes these issues, inconsistencies between qPCR and small-RNAseq–based platforms have been noted in urine samples in kidney injury,131 and this might explain the minor inconsistencies with our previously published work.24, 47 For a more detailed discussion on the issues that limit miRNA quantitation and the challenges facing standardization, please see 2 of our recent reviews.20, 181 Nonetheless, this current approach using revised EV isolation and miRNA-profiling methods has allowed us to identify a number of miRNAs showing consistent concentration changes in urine or EVs from urine, which can be validated with qPCR. These miRNAs may be further developed as biomarkers to assess the disease status of T1D-associated DN.
Disclosure
JFB and TO were paid consultants for Astute Medical and Sanofi, respectively, during the duration of this research project. The views and opinions in this research project are solely those of the contributing authors and do not necessarily reflect those of Astute Medical and Sanofi. All the other authors declared no competing interests.
Acknowledgments
We thank Inyoul Lee, Kathie Walters, and Mary Brunkow for feedback on the manuscript, and Taek-Kyun Kim, Minyoung Lee, David Baxter, and Alton Etheridge for technical advice. Funding was provided by National Institutes of Health (NIH)/DK34818 (TO), The Rossi Memorial Fund (TO, and NIH U01/RFA-RM-13-014 (DG and KW).
Footnotes
Table S1. Patient information and demographics.
Table S2. Average RNA input concentration and read counts for urine, exosome, and exosome-depleted fractions.
Table S3. Expanded microRNA profiling data for urine, and exosome fractions (≥0.6 log2FC, P ≥ 0.05).
Table S4. Extracellular vesicle–specific enrichment in urine samples of patients with type 1 diabetes mellitus.
Figure S1. Protein gels from patient urine and extracellular vesicle (EV) fractions. (A) Patient urine samples run on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis stained with Coomassie blue. (B) Corresponding EV fractions run under the same conditions. I, intermittent microalbinuria (MA) status; L, ladder; N, normal; O, overt nephrology; P, persistent MA status.
Supplementary Methods.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Patient information and demographics.
Average RNA input concentration and read counts for urine, exosome, and exosome-depleted fractions.
Expanded microRNA profiling data for urine, and exosome fractions (≥0.6 log2FC, P ≥ 0.05).
Extracellular vesicle–specific enrichment in urine samples of patients with type 1 diabetes mellitus.
Protein gels from patient urine and extracellular vesicle (EV) fractions. (A) Patient urine samples run on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis stained with Coomassie blue. (B) Corresponding EV fractions run under the same conditions. I, intermittent microalbinuria (MA) status; L, ladder; N, normal; O, overt nephrology; P, persistent MA status.
References
- 1.US Renal Data System 2016 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69:A4. doi: 10.1053/j.ajkd.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle K.R., Bakris G.L., Bilous R.W. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halimi J.-M. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab. 2012;38:291–297. doi: 10.1016/j.diabet.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Robles N.R., Villa J. Hernandez Gallego R. Non-proteinuric diabetic nephropathy. J Clin Med. 2015;4:1761–1773. doi: 10.3390/jcm4091761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Bartel D.P. MicroRNA Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tüfekci K.U., Meuwissen R.L.J., Genç S. The role of microRNAs in biological processes. Methods Mol Biol. 2014;1107:15–31. doi: 10.1007/978-1-62703-748-8_2. [DOI] [PubMed] [Google Scholar]
- 8.Erson A.E., Petty E.M. MicroRNAs in development and disease. Clin Genet. 2008;74:296–306. doi: 10.1111/j.1399-0004.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y., Shen X.J., Zou Q. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67:129–139. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen X., Ba Y., Ma L. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell P.S., Parkin R.K., Kroh E.M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valadi H., Ekström K., Bossios A. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 13.Weber J.A., Baxter D.H., Zhang S. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Li S., Li L. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denzer K., Kleijmeer M.J., Heijnen H.F. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 16.Xin H., Li Y., Buller B. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang M., Chen J., Su F. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camussi G., Deregibus M.C., Bruno S. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 19.Nassirpour R., Raj D., Townsend R. MicroRNA biomarkers in clinical renal disease: from diabetic nephropathy renal transplantation and beyond. Food Chem Toxicol. 2016;98:73–88. doi: 10.1016/j.fct.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Ghai V., Wang K. Recent progress toward the use of circulating microRNAs as clinical biomarkers. Arch Toxicol. 2016;90:2959–2978. doi: 10.1007/s00204-016-1828-2. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen L.B., Wang C., Sorenson K. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. J Diabetes Res. 2012;2012 doi: 10.1155/2012/896362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osipova J., Fischer D.-C., Dangwal S. Diabetes-associated microRNAs in pediatric patients with type 1 diabetes mellitus: a cross-sectional cohort study. J Clin Endocrinol Metab. 2014;99:E1661–E1665. doi: 10.1210/jc.2013-3868. [DOI] [PubMed] [Google Scholar]
- 23.Barutta F., Tricarico M., Corbelli A. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Argyropoulos C., Wang K., Bernardo J. Urinary microRNA profiling predicts the development of microalbuminuria in patients with type 1 diabetes. J Clin Med. 2015;4:1498–1517. doi: 10.3390/jcm4071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Théry C., Amigorena S., Raposo G. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 26.Wu X., Kim T.-K., Baxter D. sRNAnalyzer-a flexible and customizable small RNA sequencing data analysis pipeline. Nucleic Acids Res. 2017;45:12140–12151. doi: 10.1093/nar/gkx999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miranda K.C., Bond D.T., McKee M. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010;78:191–199. doi: 10.1038/ki.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato Y., Wharram B.L., Lee S.K. Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol. 2009;20:1041–1052. doi: 10.1681/ASN.2007121328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng M., Lv L.-L., Ni J. Urinary podocyte-associated mRNA profile in various stages of diabetic nephropathy. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holl R.W., Grabert M., Thon A. Urinary excretion of albumin in adolescents with type 1 diabetes: persistent versus intermittent microalbuminuria and relationship to duration of diabetes, sex, and metabolic control. Diabetes Care. 1999;22:1555–1560. doi: 10.2337/diacare.22.9.1555. [DOI] [PubMed] [Google Scholar]
- 32.Liu X.-D., Zhang L.-Y., Zhu T.-C. Overexpression of miR-34c inhibits high glucose-induced apoptosis in podocytes by targeting Notch signaling pathways. Int J Clin Exp Pathol. 2015;8:4525–4534. [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey T.L., Boden M., Buske F.A. MEME Suite: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musante L., Tataruch D.E., Holthofer H. Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Front Endocrinol (Lausanne) 2014;5:149. doi: 10.3389/fendo.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raimondo F., Corbetta S., Morosi L. Urinary exosomes and diabetic nephropathy: a proteomic approach. Mol Biosyst. 2013;9:1139–1146. doi: 10.1039/c2mb25396h. [DOI] [PubMed] [Google Scholar]
- 36.Zubiri I., Posada-Ayala M., Sanz-Maroto A. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J Proteomics. 2014;96:92–102. doi: 10.1016/j.jprot.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Baranyai T., Herczeg K., Onódi Z. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gámez-Valero A., Monguió-Tortajada M., Carreras-Planella L. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci Rep. 2016;6:33641. doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuchs R.T., Sun Z., Zhuang F. Bias in ligation-based small RNA sequencing library construction is determined by adaptor and RNA structure. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan T., Huang X., Woodcock M. Plasma extracellular RNA profiles in healthy and cancer patients. Sci Rep. 2016;6:19413. doi: 10.1038/srep19413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chafin C.B., Regna N.L., Dai R. MicroRNA-let-7a expression is increased in the mesangial cells of NZB/W mice and increases IL-6 production in vitro. Autoimmunity. 2013;46:351–362. doi: 10.3109/08916934.2013.773976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y., Yin B., Zhang C. Hsa-let-7a functions as a tumor suppressor in renal cell carcinoma cell lines by targeting c-myc. Biochem Biophys Res Commun. 2012;417:371–375. doi: 10.1016/j.bbrc.2011.11.119. [DOI] [PubMed] [Google Scholar]
- 43.Yan N., Wen L., Peng R. Naringenin ameliorated kidney injury through Let-7a/TGFBR1 signaling in diabetic nephropathy. J Diabetes Res. 2016;2016 doi: 10.1155/2016/8738760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennan E.P., Nolan K.A., Börgeson E. Lipoxins attenuate renal fibrosis by inducing let-7c and suppressing TGFβR1. J Am Soc Nephrol. 2013;24:627–637. doi: 10.1681/ASN.2012060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pezzolesi M.G., Satake E., McDonnell K.P. Circulating TGF-β1-regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes. Diabetes. 2015;64:3285–3293. doi: 10.2337/db15-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguado-Fraile E., Ramos E., Conde E. A pilot study identifying a set of microRNAs as precise diagnostic biomarkers of acute kidney injury. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang N., Zhou Y., Jiang L. Urinary microRNA-10a and microRNA-30d serve as novel, sensitive and specific biomarkers for kidney injury. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anglicheau D., Sharma V.K., Ding R. MicroRNA expression profiles predictive of human renal allograft status. Proc Natl Acad Sci U S A. 2009;106:5330–5335. doi: 10.1073/pnas.0813121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q., Xiao X., Li M. Acarbose reduces blood glucose by activating miR-10a-5p and miR-664 in diabetic rats. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shan Q., Zheng G., Zhu A. Epigenetic modification of miR-10a regulates renal damage by targeting CREB1 in type 2 diabetes mellitus. Toxicol Appl Pharmacol. 2016;306:134–143. doi: 10.1016/j.taap.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Fritz H.K.M., Lindgren D., Ljungberg B. The miR(21/10b) ratio as a prognostic marker in clear cell renal cell carcinoma. Eur J Cancer. 1990. 2014;50:1758–1765. doi: 10.1016/j.ejca.2014.03.281. [DOI] [PubMed] [Google Scholar]
- 52.Liu X., Dong C., Jiang Z. MicroRNA-10b downregulation mediates acute rejection of renal allografts by derepressing BCL2L11. Exp Cell Res. 2015;333:155–163. doi: 10.1016/j.yexcr.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Kajimoto K., Naraba H., Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA. 2006;12:1626–1632. doi: 10.1261/rna.7228806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osanto S., Qin Y., Buermans H.P. Genome-wide microRNA expression analysis of clear cell renal cell carcinoma by next generation deep sequencing. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willeit P., Skroblin P., Moschen A.R. Circulating microRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes. 2017;66:347–357. doi: 10.2337/db16-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butz H., Szabó P.M., Khella H.W.Z. miRNA-target network reveals miR-124as a key miRNA contributing to clear cell renal cell carcinoma aggressive behaviour by targeting CAV1 and FLOT1. Oncotarget. 2015;6:12543–12557. doi: 10.18632/oncotarget.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H., Liu R., Deng T. The microRNA-124-iGluR2/3 pathway regulates glucagon release from alpha cells. Oncotarget. 2016;7:24734–24743. doi: 10.18632/oncotarget.8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu Z., Yin J., Li D.C. Role of microRNAs in the treatment of type 2 diabetes mellitus with Roux-en-Y gastric bypass. Braz J Med Biol Res. 2017;50 doi: 10.1590/1414-431X20175817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piazzon N., Maisonneuve C., Guilleret I. Bicc1 links the regulation of cAMP signaling in polycystic kidneys to microRNA-induced gene silencing. J Mol Cell Biol. 2012;4:398–408. doi: 10.1093/jmcb/mjs027. [DOI] [PubMed] [Google Scholar]
- 60.Li C., Lei T. Rs12976445 polymorphism is associated with risk of diabetic nephropathy through modulating expression of microRNA-125 and interleukin-6R. Med Sci Monit. 2015;21:3490–3497. doi: 10.12659/MSM.894987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen N.X., Kiattisunthorn K., O’Neill K.D. Decreased microRNA is involved in the vascular remodeling abnormalities in chronic kidney disease (CKD) PLoS One. 2013;8 doi: 10.1371/journal.pone.0064558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Güçlü A., Koçak C., Koçak F.E. MicroRNA-125b as a new potential biomarker on diagnosis of renal ischemia–reperfusion injury. J Surg Res. 2017;207:241–248. doi: 10.1016/j.jss.2016.08.067. [DOI] [PubMed] [Google Scholar]
- 63.Klein D., Misawa R., Bravo-Egana V. MicroRNA expression in alpha and beta cells of human pancreatic islets. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danger R., Pallier A., Giral M. Upregulation of miR-142-3p in peripheral blood mononuclear cells of operationally tolerant patients with a renal transplant. J Am Soc Nephrol. 2012;23:597–606. doi: 10.1681/ASN.2011060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ortega F.J., Mercader J.M., Moreno-Navarrete J.M. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care. 2014;37:1375–1383. doi: 10.2337/dc13-1847. [DOI] [PubMed] [Google Scholar]
- 66.Pescador N., Pérez-Barba M., Ibarra J.M. Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lei Z., Xu G., Wang L. MiR-142-3p represses TGF-β-induced growth inhibition through repression of TGFβR1 in non-small cell lung cancer. FASEB J. 2014;28:2696–2704. doi: 10.1096/fj.13-247288. [DOI] [PubMed] [Google Scholar]
- 68.Zarjou A., Yang S., Abraham E. Identification of a microRNA signature in renal fibrosis: role of miR-21. Am J Physiol Renal Physiol. 2011;301:F793–F801. doi: 10.1152/ajprenal.00273.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaur K., Vig S., Srivastava R. Elevated hepatic miR-22-3p expression impairs gluconeogenesis by silencing the Wnt-responsive transcription factor Tcf7. Diabetes. 2015;64:3659–3669. doi: 10.2337/db14-1924. [DOI] [PubMed] [Google Scholar]
- 70.Tian C., Ouyang X., Lv Q. Cross-talks between microRNAs and mRNAs in pancreatic tissues of streptozotocin-induced type 1 diabetic mice. Biomed Rep. 2015;3:333–342. doi: 10.3892/br.2015.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liep J., Kilic E., Meyer H.A. Cooperative effect of miR-141-3p and miR-145-5p in the regulation of targets in clear cell renal cell carcinoma. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei J., Zhang Y., Luo Y. Aldose reductase regulates miR-200a-3p/141-3p to coordinate Keap1–Nrf2, Tgfβ1/2, and Zeb1/2 signaling in renal mesangial cells and the renal cortex of diabetic mice. Free Radic Biol Med. 2014;67:91–102. doi: 10.1016/j.freeradbiomed.2013.10.811. [DOI] [PubMed] [Google Scholar]
- 73.Ji X., Takahashi R., Hiura Y. Plasma miR-208 as a biomarker of myocardial injury. Clin Chem. 2009;55:1944–1949. doi: 10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]
- 74.Saha S., Choudhury J., Ain R. MicroRNA-141-3p and miR-200a-3p regulate insulin-like growth factor 2 during mouse placental development. Mol Cell Endocrinol. 2015;414:186–193. doi: 10.1016/j.mce.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 75.Duan Z.-Y., Cai G., Bu R. Selection of urinary sediment miRNAs as specific biomarkers of IgA nephropathy. Sci Rep. 2016;6:23498. doi: 10.1038/srep23498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karolina D.S., Armugam A., Tavintharan S. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qingjuan L., Xiaojuan F., Wei Z. miR-148a-3p overexpression contributes to glomerular cell proliferation by targeting PTEN in lupus nephritis. Am J Physiol Cell Physiol. 2016;310:C470–C478. doi: 10.1152/ajpcell.00129.2015. [DOI] [PubMed] [Google Scholar]
- 78.van de Bunt M., Gaulton K.J., Parts L. The miRNA profile of human pancreatic islets and beta-cells and relationship to type 2 diabetes pathogenesis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang S., Wang L., Dou L. MicroRNA 152 regulates hepatic glycogenesis by targeting PTEN. FEBS J. 2016;283:1935–1946. doi: 10.1111/febs.13713. [DOI] [PubMed] [Google Scholar]
- 80.Wilflingseder J., Regele H., Perco P. miRNA profiling discriminates types of rejection and injury in human renal allografts. Transplantation. 2013;95:835–841. doi: 10.1097/TP.0b013e318280b385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.lyu Z., Mao Z., Wang H. MiR-181b targets Six2 and inhibits the proliferation of metanephric mesenchymal cells in vitro. Biochem Biophys Res Commun. 2013;440:495–501. doi: 10.1016/j.bbrc.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 82.Sun X., Lin J., Zhang Y. MicroRNA-181b improves glucose homeostasis and insulin sensitivity by regulating endothelial function in white adipose tissue. Circ Res. 2016;118:810–821. doi: 10.1161/CIRCRESAHA.115.308166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilflingseder J., Jelencsics K., Bergmeister H. miR-182-5p inhibition ameliorates ischemic acute kidney injury. Am J Pathol. 2017;187:70–79. doi: 10.1016/j.ajpath.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 84.Pandey P., Qin S., Ho J. Systems biology approach to identify transcriptome reprogramming and candidate microRNA targets during the progression of polycystic kidney disease. BMC Syst Biol. 2011;5:56. doi: 10.1186/1752-0509-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maluf D.G., Dumur C.I., Suh J.L. The urine microRNA profile may help monitor post-transplant renal graft function. Kidney Int. 2014;85:439–449. doi: 10.1038/ki.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bao L., Fu X., Si M. MicroRNA-185 targets SOCS3 to inhibit beta-cell dysfunction in diabetes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun S.-Q., Zhang T., Ding D. Circulating microRNA-188, -30a, and -30e as early biomarkers for contrast-induced acute kidney injury. J Am Heart Assoc. 2016;5(8) doi: 10.1161/JAHA.116.004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu F., Lou Y.-L., Wu J. Upregulation of microRNA-210 regulates renal angiogenesis mediated by activation of VEGF signaling pathway under ischemia/perfusion injury in vivo and in vitro. Kidney Blood Press Res. 2012;35:182–191. doi: 10.1159/000331054. [DOI] [PubMed] [Google Scholar]
- 89.Serino G., Sallustio F., Cox S.N. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol. 2012;23:814–824. doi: 10.1681/ASN.2011060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J., Wang J., Li H. p53 activates miR-192-5p to mediate vancomycin induced AKI. Sci Rep. 2016;6:38868. doi: 10.1038/srep38868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zou Y.-F., Wen D., Zhao Q. Urinary microRNA-30c-5p and MicroRNA-192-5p as potential biomarkers of ischemia–reperfusion-induced kidney injury. Exp Biol Med. 2017;242:657–667. doi: 10.1177/1535370216685005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flowers E., Gadgil M., Aouizerat B.E. Circulating micrornas associated with glycemic impairment and progression in Asian Indians. Biomark Res. 2015;3:22. doi: 10.1186/s40364-015-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang B., Koh P., Winbanks C. miR-200a Prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes. 2011;60:280–287. doi: 10.2337/db10-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ji J., Qin Y., Ren J. Mitochondria-related miR-141-3p contributes to mitochondrial dysfunction in HFD-induced obesity by inhibiting PTEN. Sci Rep. 2015;5:16262. doi: 10.1038/srep16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kato M., Arce L., Wang M. A microRNA circuit mediates transforming growth factor-ß1 autoregulation in renal glomerular mesangial cells. Kidney Int. 2011;80:358–368. doi: 10.1038/ki.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang H., Liu J., Qu D. Inhibition of miR-200c restores endothelial function in diabetic mice through suppression of COX-2. Diabetes. 2016;65:1196–1207. doi: 10.2337/db15-1067. [DOI] [PubMed] [Google Scholar]
- 97.Gregory P.A., Bert A.G., Paterson E.L. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 98.Park S.-M., Gaur A.B., Lengyel E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glowacki F., Savary G., Gnemmi V. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chau B.N., Xin C., Hartner J. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003205. 121ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Loboda A., Sobczak M., Jozkowicz A. TGF- β1/Smads and miR-21 in renal fibrosis and inflammation. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McClelland A.D., Herman-Edelstein M., Komers R. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 2015;129:1237–1249. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 103.Hennino M.-F., Buob D., der Hauwaert C.V. miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Sci Rep. 2016;6:27209. doi: 10.1038/srep27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sekar D., Venugopal B., Sekar P. Role of microRNA 21 in diabetes and associated/related diseases. Gene. 2016;582:14–18. doi: 10.1016/j.gene.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 105.Long J., Badal S.S., Wang Y. MicroRNA-22 is a master regulator of bone morphogenetic protein-7/6 homeostasis in the kidney. J Biol Chem. 2013;288:36202–36214. doi: 10.1074/jbc.M113.498634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zaman M.S., Thamminana S., Shahryari V. Inhibition of PTEN gene expression by oncogenic miR-23b-3p in renal cancer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grieco F.A., Sebastiani G., Juan-Mateu J. MicroRNAs miR-23a-3p, miR-23b-3p and miR-149-5p regulate the expression of pro-apoptotic BH3-only proteins DP5 and PUMA in human pancreatic beta cells. Diabetes. 2016 doi: 10.2337/db16-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fu X., Dong B., Tian Y. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J Clin Invest. 2015;125:2497–2509. doi: 10.1172/JCI75438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng Z., Guan M., Jia Y. The coordinated roles of miR-26a and miR-30c in regulating TGFβ1-induced epithelial-to-mesenchymal transition in diabetic nephropathy. Sci Rep. 2016;6:37492. doi: 10.1038/srep37492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ichii O., Otsuka-Kanazawa S., Horino T. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu G., Ji C., Song G. MiR-26b modulates insulin sensitivity in adipocytes by interrupting the PTEN/PI3K/AKT pathway. Int J Obes. 2015;39:1523–1530. doi: 10.1038/ijo.2015.95. [DOI] [PubMed] [Google Scholar]
- 112.Petrozza V., Carbone A., Bellissimo T. Oncogenic microRNAs characterization in clear cell renal cell carcinoma. Int J Mol Sci. 2015;16:29219–29225. doi: 10.3390/ijms161226160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nassirpour R., Homer B.L., Mathur S. Identification of promising urinary microRNA biomarkers in two rat models of glomerular injury. Toxicol Sci. 2015;148:35–47. doi: 10.1093/toxsci/kfv167. [DOI] [PubMed] [Google Scholar]
- 114.Graham J.R., Williams C.M.M., Yang Z. MicroRNA-27b targets gremlin 1 to modulate fibrotic responses in pulmonary cells. J Cell Biochem. 2014;115:1539–1548. doi: 10.1002/jcb.24809. [DOI] [PubMed] [Google Scholar]
- 115.Wang C., Hu J., Lu M. A panel of five serum miRNAs as a potential diagnostic tool for early-stage renal cell carcinoma. Sci Rep. 2015;5:7610. doi: 10.1038/srep07610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zampetaki A., Kiechl S., Drozdov I. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 117.Qin W., Chung A.C.K., Huang X.R. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Silambarasan M., Tan J.R., Karolina D.S. MicroRNAs in hyperglycemia induced endothelial cell dysfunction. Int J Mol Sci. 2016;17:518. doi: 10.3390/ijms17040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gutiérrez-Escolano A., Santacruz-Vázquez E., Gómez-Pérez F. Dysregulated microRNAs involved in contrast-induced acute kidney injury in rat and human. Ren Fail. 2015;37:1498–1506. doi: 10.3109/0886022X.2015.1077322. [DOI] [PubMed] [Google Scholar]
- 120.Zhang W., Zhang C., Chen H. Evaluation of microRNAs miR-196a, miR-30a-5P, and miR-490 as biomarkers of disease activity among patients with FSGS. Clin J Am Soc Nephrol. 2014;9:1545–1552. doi: 10.2215/CJN.11561113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Y., Zhang T., Zhou Y. A Presenilin/Notch1 pathway regulated by miR-375, miR-30a, and miR-34a mediates glucotoxicity induced-pancreatic beta cell apoptosis. Sci Rep. 2016;6:36136. doi: 10.1038/srep36136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang J., Duan L., Guo T. Downregulation of miR-30c promotes renal fibrosis by target CTGF in diabetic nephropathy. J Diabetes Complications. 2016;30:406–414. doi: 10.1016/j.jdiacomp.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 123.Rudnicki M., Perco P., D Haene B. Renal microRNA- and RNA-profiles in progressive chronic kidney disease. Eur J Clin Invest. 2016;46:213–226. doi: 10.1111/eci.12585. [DOI] [PubMed] [Google Scholar]
- 124.Tang X., Muniappan L., Tang G. Identification of glucose-regulated miRNAs from pancreatic β cells reveals a role for miR-30d in insulin transcription. RNA. 2009;15:287–293. doi: 10.1261/rna.1211209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao X., Mohan R., Özcan S. MicroRNA-30d induces insulin transcription factor MafA and insulin production by targeting mitogen-activated protein 4 kinase 4 (MAP4K4) in pancreatic β-cells. J Biol Chem. 2012;287:31155–31164. doi: 10.1074/jbc.M112.362632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pandey P., Brors B., Srivastava P.K. Microarray-based approach identifies microRNAs and their target functional patterns in polycystic kidney disease. BMC Genomics. 2008;9:624. doi: 10.1186/1471-2164-9-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guay C., Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol. 2013;9:513–521. doi: 10.1038/nrendo.2013.86. [DOI] [PubMed] [Google Scholar]
- 128.Sebastiani G., Nigi L., Spagnuolo I. MicroRNA profiling in sera of patients with type 2 diabetes mellitus reveals an upregulation of miR-31 expression in subjects with microvascular complications. J Biomed Sci Eng. 2013;06:58. [Google Scholar]
- 129.Bai X.-Y., Ma Y., Ding R. miR-335 and miR-34a promote renal senescence by suppressing mitochondrial antioxidative enzymes. J Am Soc Nephrol. 2011;22:1252–1261. doi: 10.1681/ASN.2010040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Argyropoulos C., Wang K., McClarty S. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nassirpour R., Mathur S., Gosink M.M. Identification of tubular injury microRNA biomarkers in urine: comparison of next-generation sequencing and qPCR-based profiling platforms. BMC Genomics. 2014;15:485. doi: 10.1186/1471-2164-15-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Collares C.V., Evangelista A.F., Xavier D.J. Identifying common and specific microRNAs expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients. BMC Res Notes. 2013;6:491. doi: 10.1186/1756-0500-6-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei Q., Bhatt K., He H.-Z. Targeted deletion of dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21:756–761. doi: 10.1681/ASN.2009070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li J., Song L., Zhou L. A microRNA signature in gestational diabetes mellitus associated with risk of macrosomia. Cell Physiol Biochem. 2015;37:243–252. doi: 10.1159/000430349. [DOI] [PubMed] [Google Scholar]
- 135.Prabu P., Rome S., Sathishkumar C. Circulating miRNAs of ‘Asian Indian phenotype’ identified in subjects with impaired glucose tolerance and patients with type 2 diabetes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iwasaki K., Yamamoto T., Inanaga Y. MiR-142-5p and miR-486-5p as biomarkers for early detection of chronic antibody-mediated rejection in kidney transplantation. Biomarkers. 2017;22:45–54. doi: 10.1080/1354750X.2016.1204000. [DOI] [PubMed] [Google Scholar]
- 137.Viñas J.L., Burger D., Zimpelmann J. Transfer of microRNA-486-5p from human endothelial colony forming cell–derived exosomes reduces ischemic kidney injury. Kidney Int. 2016;90:1238–1250. doi: 10.1016/j.kint.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 138.Wang X., Sundquist J., Zöller B. Determination of 14 circulating microRNAs in Swedes and Iraqis with and without diabetes mellitus type 2. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bijkerk R., Duijs J.M.G.J., Khairoun M. Circulating microRNAs associate with diabetic nephropathy and systemic microvascular damage and normalize after simultaneous pancreas–kidney transplantation. Am J Transplant. 2015;15:1081–1090. doi: 10.1111/ajt.13072. [DOI] [PubMed] [Google Scholar]
- 140.Seyhan A.A., Lopez Y.O.N., Xie H. Pancreas-enriched miRNAs are altered in the circulation of subjects with diabetes: a pilot cross-sectional study. Sci Rep. 2016;6:31479. doi: 10.1038/srep31479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu N., Fu S., Liu Y. miR-96 suppresses renal cell carcinoma invasion via downregulation of Ezrin expression. J Exp Clin Cancer Res. 2015;34:107. doi: 10.1186/s13046-015-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lovis P., Gattesco S., Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biol Chem. 2008;389:305–312. doi: 10.1515/BC.2008.026. [DOI] [PubMed] [Google Scholar]
- 143.Tao J., Yang X., Han Z. Serum microRNA-99a helps detect acute rejection in renal transplantation. Transplant Proc. 2015;47:1683–1687. doi: 10.1016/j.transproceed.2015.04.094. [DOI] [PubMed] [Google Scholar]
- 144.Li W., Wang J., Chen Q.-D. Insulin promotes glucose consumption via regulation of miR-99a/mTOR/PKM2 pathway. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Turcatel G., Rubin N., El-Hashash A. MIR-99a and MIR-99b modulate TGF-β induced epithelial to mesenchymal plasticity in normal murine mammary gland cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koga K., Yokoi H., Mori K. MicroRNA-26a inhibits TGF-β-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy. Diabetologia. 2015;58:2169–2180. doi: 10.1007/s00125-015-3642-4. [DOI] [PubMed] [Google Scholar]
- 147.Mohan A., Singh R.S., Kumari M. Urinary exosomal microRNA-451-5p is a potential early biomarker of diabetic nephropathy in rats. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Long J., Wang Y., Wang W. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem. 2011;286:11837–11848. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vlachos I.S., Zagganas K., Paraskevopoulou M.D. DIANA-miRPath v3.0:deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43:W460–W466. doi: 10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chang G.-Y., Park A.S.D., Susztak K. Tracing the footsteps of glomerular insulin signaling in diabetic kidney disease. Kidney Int. 2011;79:802–804. doi: 10.1038/ki.2010.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen G., Chen H., Wang C. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cheng X., Gao W., Dang Y. Both ERK/MAPK and TGF-beta/Smad signaling pathways play a role in the kidney fibrosis of diabetic mice accelerated by blood glucose fluctuation. J Diabetes Res. 2013;2013 doi: 10.1155/2013/463740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Genovese F., Manresa A.A., Leeming D.J. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair. 2014;7:4. doi: 10.1186/1755-1536-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hills C.E., Price G.W., Squires P.E. Mind the gap: connexins and cell-cell communication in the diabetic kidney. Diabetologia. 2015;58:233–241. doi: 10.1007/s00125-014-3427-1. [DOI] [PubMed] [Google Scholar]
- 155.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lieberthal W., Levine J.S. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20:2493–2502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 158.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu F., Zhuang S. Role of receptor tyrosine kinase signaling in renal fibrosis. Int J Mol Sci. 2016;17(5) doi: 10.3390/ijms17060972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rodríguez-Peña A.B., Fuentes-Calvo I., Docherty N.G. Effect of angiotensin II and small GTPase Ras signaling pathway inhibition on early renal changes in a murine model of obstructive nephropathy. Biomed Res Int. 2014;2014 doi: 10.1155/2014/124902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sakai N., Wada T., Matsushima K. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J Hypertens. 2008;26:780–790. doi: 10.1097/HJH.0b013e3282f3e9e6. [DOI] [PubMed] [Google Scholar]
- 162.Tian W., Zhang Z., Cohen D.M. MAPK signaling and the kidney. Am J Physiol Renal Physiol. 2000;279:F593–F604. doi: 10.1152/ajprenal.2000.279.4.F593. [DOI] [PubMed] [Google Scholar]
- 163.Yu A.S.L., Kanzawa S.A., Usorov A. Tight junction composition is altered in the epithelium of polycystic kidneys. J Pathol. 2008;216:120–128. doi: 10.1002/path.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao X.-K., Cheng Y., Cheng M.L. Focal adhesion kinase regulates fibroblast migration via integrin beta-1 and plays a central role in fibrosis. Sci Rep. 2016;6:19276. doi: 10.1038/srep19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chien H.-Y., Chen C.-Y., Chiu Y.-H. Differential microRNA profiles predict diabetic nephropathy progression in Taiwan. Int J Med Sci. 2016;13:457–465. doi: 10.7150/ijms.15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang J.-Y., Gong Y.-L., Li C.-J. Circulating MiRNA biomarkers serve as a fingerprint for diabetic atherosclerosis. Am J Transl Res. 2016;8:2650–2658. [PMC free article] [PubMed] [Google Scholar]
- 167.Morizane R., Fujii S., Monkawa T. miR-34c attenuates epithelial-mesenchymal transition and kidney fibrosis with ureteral obstruction. Sci Rep. 2014;4:4578. doi: 10.1038/srep04578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hu H.Y., He L., Fominykh K. Evolution of the human-specific microRNA miR-941. Nat Commun. 2012;3:1145. doi: 10.1038/ncomms2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Glineur S.F., De Ron P., Hanon E. Paving the route to plasma miR-208a-3p as an acute cardiac injury biomarker: preclinical rat data supports its use in drug safety assessment. Toxicol Sci. 2016;149:89–97. doi: 10.1093/toxsci/kfv222. [DOI] [PubMed] [Google Scholar]
- 170.Hromadnikova I., Kotlabova K., Hympanova L. Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Navickas R., Gal D., Laucevičius A. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res. 2016;111:322–337. doi: 10.1093/cvr/cvw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Oliveira-Carvalho V., Carvalho V.O., Bocchi E.A. The emerging role of miR-208a in the heart. DNA Cell Biol. 2013;32:8–12. doi: 10.1089/dna.2012.1787. [DOI] [PubMed] [Google Scholar]
- 173.Cavero-Redondo I., Peleteiro B., Álvarez-Bueno C. Glycosylated haemoglobin as a predictor of cardiovascular events and mortality: a protocol for a systematic review and meta-analysis. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eeg-Olofsson K., Cederholm J., Nilsson P.M. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR) J Intern Med. 2010;268:471–482. doi: 10.1111/j.1365-2796.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 175.Goto A., Noda M., Matsushita Y. Hemoglobin a1c levels and the risk of cardiovascular disease in people without known diabetes: a population-based cohort study in Japan. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu L., Chan W.M., Hui Y.F. Association between HbA1c and cardiovascular disease mortality in older Hong Kong Chinese with diabetes. Diabet Med. 2012;29:393–398. doi: 10.1111/j.1464-5491.2011.03456.x. [DOI] [PubMed] [Google Scholar]
- 177.Zhao W., Katzmarzyk P.T., Horswell R. HbA1c and coronary heart disease risk among diabetic patients. Diabetes Care. 2014;37:428–435. doi: 10.2337/dc13-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Santangelo L., Giurato G., Cicchini C. The RNA-binding protein SYNCRIP Is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell Rep. 2016;17:799–808. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 179.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cheng L., Sun X., Scicluna B.J. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 2014;86:433–444. doi: 10.1038/ki.2013.502. [DOI] [PubMed] [Google Scholar]
- 181.Lee I., Baxter D., Lee M.Y. The importance of standardization on analyzing circulating RNA. Mol Diagn Ther. 2017;21:259–268. doi: 10.1007/s40291-016-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient information and demographics.
Average RNA input concentration and read counts for urine, exosome, and exosome-depleted fractions.
Expanded microRNA profiling data for urine, and exosome fractions (≥0.6 log2FC, P ≥ 0.05).
Extracellular vesicle–specific enrichment in urine samples of patients with type 1 diabetes mellitus.
Protein gels from patient urine and extracellular vesicle (EV) fractions. (A) Patient urine samples run on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis stained with Coomassie blue. (B) Corresponding EV fractions run under the same conditions. I, intermittent microalbinuria (MA) status; L, ladder; N, normal; O, overt nephrology; P, persistent MA status.