Introduction
C3 glomerulopathy (C3G) is a rare disease entity resulting from glomerular deposition of complement factors due to dysregulation of the alternative pathway of complement.1 It is characterized by bright staining for C3 along the glomerular capillary walls and is further subdivided into C3 glomerulonephritis (C3GN) and dense deposit disease (DDD) based on ultrastructural findings. Extrarenal manifestations of C3G can involve the eye with development of ocular drusen in both C3GN and DDD.2, 3, 4 Drusen is characterized by lipoproteinaceous deposition of products of alternative complement cascade between the retinal pigment endothelium and Bruch’s membrane.1 Drusen bears a morphological similarity with age-related macular degeneration. Partial lipodystrophy also is described in association with DDD.4 Involvement of other organs has not been reported in the setting of C3G. In this article, we report a case of pulmonary-renal syndrome with the kidney biopsy showing DDD, and a subsequent postmortem lung biopsy showing pulmonary capillaritis with bright C3 staining on immunofluorescence microscopy and dense osmiophilic deposits on electron microscopy (pulmonary DDD) in the setting of severe abnormalities of the alternative pathway of complement.
Case Presentation
Clinical History and Laboratory Data
A 22-year-old young man presented to the renal clinic with a history of shortness of breath and anuria for the past 5 days. He also had a history of facial puffiness and hypertension for 1 to 2 years. There was no history of oral ulcers, arthralgia, skin rashes, or other features to suggest connective tissue disorders. On examination, the patient had pallor with a blood pressure of 140/90 mm Hg (on loop diuretics). Except for the presence of crepitations in bilateral lung fields, systemic examination, including fundus examination, was not contributory. On investigation, urine examination revealed albuminuria (3+) with 5 to 10 erythrocytes per high-power field. The patient was anemic; however, there were no fragmented erythrocytes on peripheral smear examination. Renal function tests revealed a serum creatinine of 12.1 mg/dl and blood urea levels of 145 mg/dl. The laboratory findings are given in Table 1. The patient was started on dialysis and in view of normal-sized kidneys on ultrasonography and an unexplained renal failure, a kidney biopsy was performed.
Table 1.
Laboratory evaluation
| Name of assay | Values | Normal range |
|---|---|---|
| Hemoglobin | 8.4 g/dl | 12–18 g/dl |
| Total leukocyte count | 12,700/μl | 4–11 × 103/μl |
| Platelet count | 3.27 × 106/μl | 150–400 × 103/μl |
| Serum total protein/Albumin | 6.1/3.0 g/dl | 6.4–8.3/3.4–4.8 g/dl |
| Total bilirubin | 1.0 mg/dl | 0–1.0 mg/dl |
| Aspartate transferase/Alanine transferase | 17/27 U/l | 2–40/2–41 U/l |
| C3 | 0.45 g/l | 0.89–1.87 g/l |
| C4 | 0.31 g/l | 0.165–0.38 g/l |
| CFH | 82.22 μg/ml | 225–760 μg/ml |
| CFB | 112.0 mg/ml | 73–267 mg/ml |
| APFA | 0.13% (very low) | 28%–51% |
| Autoantibodies to CFH | Negative | <18 AU/ml |
| Autoantibodies to CFB | Positive | Average OD>2.06 |
| C3Nef | Positive | Ratio >1.022 |
| ANA, ANCA, and cryoglobulin | Negative | NA |
| Anti-GBM | Negative | <1.0 AI |
| HBsAg, Anti-HCV, and HIV-I/II | Negative | NA |
| SPEP, UPEP, and IFE | No “M” band | NA |
AI, activity index; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; APFA, alternate pathway functional assay; AU, arbitrary units; C, Complement; CFB, complement factor B; CFH, complement factor H; C3Nef, C3 nephritic factor; GBM, glomerular basement membrane; HBsAg, hepatitis B surface antigen; HCV, hepatitis C; IFE, immunofixation electrophoresis; NA, not applicable; SPEP, serum protein electrophoresis; UPEP, urine protein electrophoresis.
Kidney Biopsy Findings
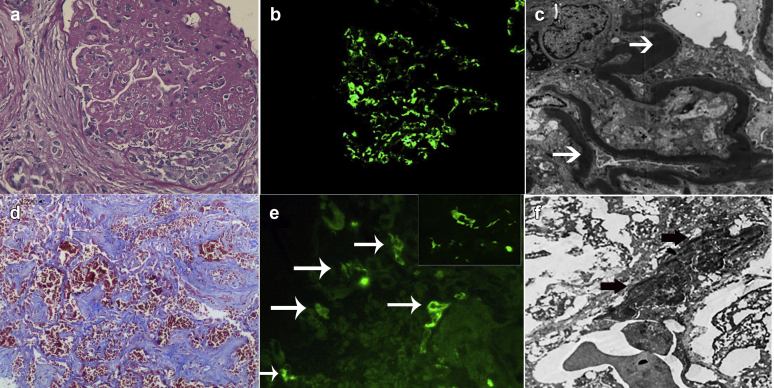
The kidney biopsy showed a membranoproliferative glomerulonephritis with fibrous/fibrocellular crescents (22%, 4-fibrocellular, 2-fibrous) and extensive focal global glomerulosclerosis. There were 27 glomeruli present, of which 13 (48%) were globally sclerosed. The glomerular basement membranes were extremely thickened on periodic acid-Schiff stains. The interstitium showed extensive (50%) tubular atrophy and interstitial fibrosis with accompanying mononuclear cell infiltrates in areas of tubular atrophy and interstitial fibrosis. Arteries and arterioles were unremarkable. Immunofluorescence microscopy showed bright granular staining for C3 (3+, scale 0–3+) in the mesangium and along glomerular capillary walls. C3 deposits also were present along the tubular basement membranes. Immunofluorescence microscopy was negative for Igs, including IgA, IgG, and IgM, and kappa and lambda light chains. Electron microscopy showed electron-dense osmiophilic intramembranous deposits in glomerular basement membranes and tubular basement membranes (Figure 1, top panel).
Figure 1.
Kidney and lung biopsy findings. Top panel shows kidney biopsy findings: (a) glomerulus shows membranoproliferative pattern of injury with markedly thickened glomerular basement membranes and a cellular crescent (hematoxylin and eosin, original magnification ×40), (b) bright staining for C3 in the mesangium and along capillary walls (original magnification ×40), and (c) osmiophilic dense intramembranous deposits (original magnification ×4000). Lower panel shows lung biopsy findings: (d) aveolar septae showing capillaritis with intra-alveolar hemorrhages (martius scarlet blue stain, original magnification ×40), (e) C3 staining along alveolar capillaries (original magnification ×20) with insert showing higher magnification of C3 staining of an alveolar capillary (original magnification ×60), and (f) sausage-shaped osmiophilic, continuous intramembranous electron-dense deposits in an alveolar capillary basement membrane (electron microscopy, original magnification ×4000). Arrows point to dense sausage-shaped osmiophilic deposits.
Diagnosis
Dense deposit disease
Additional Investigations and Follow-up
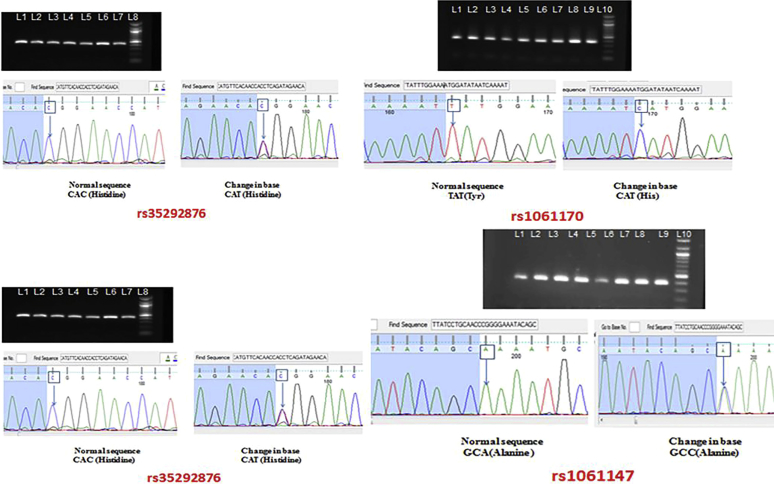
Serology for hepatitis B and C, HIV-1/2, antinuclear antibody/antineutrophil cytoplasmic antibody, anti-glomerular basement membrane, and cryoglobulins was negative. Serological workup showed markedly low C3 (0.45 g/l, normal range 0.89–1.87 g/l), low alternate complement pathway functional assay (0.13%, normal range 28%–51%), low complement factor H (CFH) (82.22 mg/ml), normal C4, and normal complement factor B (CFB) (112.0 g/ml). These results confirmed activation of the alternative pathway of complement. Autoantibodies against C3 convertase (C3Nef), CFH (aCFH), and CFB (aCFB) were then tested by enzyme-linked immunosorbent assay (ELISA) methods. Both aCFB and C3Nef were positive in our patient, whereas aCFH was negative (confirmed twice). In addition to serological workup, genetic analysis (all exons of CFH and CFHR5 gene) was carried out and 4 variants of the CFH gene (rs1061147, rs1061170, rs2274700, and rs35292876) (Figure 2) were detected.
Figure 2.
Sequencing chromatograms showing 4 single-nucleotide polymorphisms of the CFH gene rs1061170 (p.His402Tyr), rs2274700 (p.Ala473=), rs35292876 (p.His878=), and rs1061147 (p.Ala 243=). All 4 polymorphisms were heterozygous.
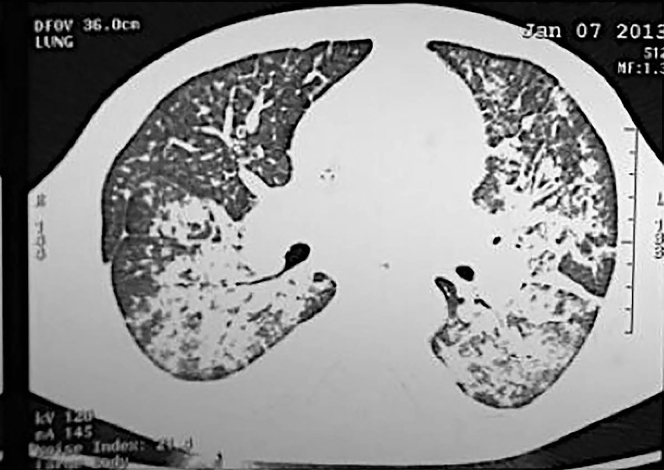
During the hospital stay, the patient developed hemoptysis and a subsequent fall in hemoglobin (6 mg/dl), and the computerized tomography of the chest was suggestive of pulmonary hemorrhage (Figure 3). With a diagnosis of pulmonary-renal syndrome, plasma exchange (40 ml/kg per session and replacement with fresh frozen plasma) was commenced (13 sessions over 2 weeks) to remove the pathogenic autoantibodies and replace the deficient factors. In addition, pulse methyl prednisolone (1 g/d for 3 days) followed by oral prednisolone (1 mg/kg per day) was started. During the course of the disease and treatment the patient succumbed to his illness.
Figure 3.
Computerized tomography scan of the chest shows ground-glass opacities suggestive of an alveolar hemorrhage.
Lung Biopsy Findings
After informed consent by the family, a postmortem lung biopsy was performed within a half an hour of the patient’s demise. The lung biopsy showed pulmonary hemorrhages and intra-alveolar macrophages, and alveolar capillaries with thickened capillary walls. There was edema and fibrin exudation into the alveoli. There was no evidence of infection or viral inclusions. Immunofluorescence microscopy showed bright C3 staining (3+, scale 0–3+) along the alveolar capillary walls. The C3 staining was circumferential, with some capillaries showing brighter staining than others. In addition, accentuation of C3 along portions of the capillaries was present; this likely represented the wide ribbon-shaped deposits seen on electron microscopy. The alveolar capillaries were negative for IgG, IgM, IgA, and C1q. As control, immunofluorescence studies were also performed on autopsy lung tissue with sepsis, diffuse alveolar damage due to adult respiratory syndrome, and IgA nephropathy with pulmonary hemorrhages. In all cases, immunofluorescence studies did not reveal deposits of C3 along the alveolar capillaries (data not shown). Electron microscopy showed dense ribbon-shaped osmiophilic deposits along the capillary walls, similar to those seen along the glomerular basement membranes, surrounded by alveolar space (Figure 1, lower panel). However, the alveolar capillaries are collapsed by the air and hemorrhage in the alveolar spaces, and as such the dense deposits along the capillary walls appear to be pushed together with minimal or no capillary lumen. To determine whether the C3 staining and dense deposits were present in other organs, we also performed immunofluorescence studies and electron microscopy on splenic and liver tissues. The splenic and liver needle biopsies were taken at the same time as lung biopsy. Both splenic and liver tissues were negative for C3 staining and showed no dense deposits along capillaries on electron microscopy (data not shown).
Diagnosis
Dense deposit disease involving alveolar capillaries
Materials and Methods
Complement 3 and 4
Complement (C) 3 and C4 was done using MININEPHPLUS, a small, semiautomated nephelometer and commercially available kits for C3 and C4 estimation (Binding Site, San Diego, CA) using standard protocols suggested by the manufacturer. The results were interpreted after taking normal cutoff in our laboratory (n = 45).
Alternate Pathway Functional Assay
Alternate pathway functional assay was performed using the Wieslab ELISA kit (Malmö, Sweden) as per manufacturer instructions and methods. Patient serum was diluted using alternate pathway diluent supplied with the kit and was incubated in ELISA wells for 1 hour at 37°C. After incubation, the wells were washed, and C5b-9, if present in the tested sample, gets attached to labeled antibody. Further washing steps remove the excess of antibody, and remaining antigen-antibody complexes were detected using alkaline phosphatase substrate-tagged antibody. The color produced was measured at 405 nm using the ELISA reader. Samples were tested in duplicate along with diluent, and positive and negative controls. The final value was calculated as a percentage of healthy controls and expressed in terms of normal, decreased, or increased functional activity.
Complement Factors H and B
CFH and CFB levels were estimated using Assay Max, human CFH, and CFB ELISA Kits (Assaypro, St. Charles, MO). Serum stored at −80 °C was used to check these serological levels in patients. Mean was calculated from duplicate values obtained in ELISA for each standard and samples. To generate a standard curve, a graph was plotted using standard concentrations on the x-axis and the corresponding absorbance (optical density) on the y-axis. The concentration of unknown samples was determined from the standard curve. Final values were expressed after multiplying the final value with dilution factor. Average ±2 SD value was used to define range of CFH in our patients.
C3 Nephritic Factor
The presence of C3Nef was analyzed by ELISA as previously described.1 After obtaining purified human C3b, factor B, and factor D from Complement Technology Inc. (Tyler, TX), a manual ELISA was performed on 96-well flat-bottom detachable microtiter ELISA plates. Plates were pretreated with glutaraldehyde and coated with C3b 5 μg/ml (overnight) at 4 °C. After washing 3 times with phosphate-buffered saline (Sigma-Aldrich, St. Louis, MO), 1% bovine serum albumin was added for 1 hour at 37 °C to block the free reactive sites. To form convertase, factor B (5 μg/ml) and factor D (0.25 μg/ml), prepared in gelatin veronal buffer supplemented with 2 mM NiCl2, were added into wells and incubated for 30 minutes at 37 °C. Each sample was tested in triplicate with a nonconvertase well with the omission of this step. Patient serum was diluted (1:100) and was added in triplicate for 25 minutes at 37 °C. The ELISA plate was washed with wash buffer (phosphate-buffered saline [Sigma-Aldrich], containing 0.5% Triton-X). Final incubation was done with horseradish peroxidase–labeled goat anti-human IgG antibody for 30 minutes at 37 °C. Enzymatic activity was measured using 3,3′,5,5′-tetramethylbenzidine. Optical density was taken at 450 nm.
Anti-CFH and CFB
Autoantibodies to CFH and CFB were tested using ELISA protocols as described previously.19 Overnight coating was done at 4 °C using purified CFH or CFB proteins as described previously for C3Nef, and 1% bovine serum albumin was used for blocking free sites. Serum sample was added after dilution and incubated for 1 hour at 37 °C. ELISA wells were washed as described previously and incubated for 1 hour with horseradish peroxidase–labeled goat anti-human IgG antibody specific for the γ chain. After final washing, enzymatic activity was measured using 3,3′,5,5′-tetramethylbenzidine, and the optical density was taken at 450 nm. An optical density 2 SD above normal (based on 45 healthy controls) was considered positive.
Genetic Testing
Genomic DNA was extracted from blood (EDTA), using a DNA extraction kit (#51104; Qiagen, Hilden, Germany) following standard protocols for DNA isolation from blood. CFH (NG_007259.1) and CFHR5 (NG_016365.1) gene primers were designed covering exon and adjoining intronic regions. All primers were checked using primer designing tool software at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Polymerase chain reaction was done for all the exons of CFH and CFHR5 genes. For sequencing, the amplified products were electrophoresed on agarose gel to check the size and quality of amplified polymerase chain reaction product and corresponding bands from agarose gel were excised to do polymerase chain reaction product purification using a gel purification kit (#28704; Qiagen). Bidirectional sequencing was done for both strands using the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). Sequencing reactions were analyzed on an ABI 3130 Genetic Analyzer (Applied Biosystems). The amplified sequence data were compared with the normal sequence in the GenBank database. DNA of 52 healthy individuals was isolated and all variants were tested in control populations as per methods described previously.
Discussion
Pulmonary-renal syndrome is a life-threatening condition that is characterized by rapidly progressive glomerulonephritis and diffuse pulmonary hemorrhage. Accurate and early diagnosis is of paramount importance to prevent renal failure and death. The most common causes of pulmonary-renal syndrome are anti–neutrophil antibody–associated glomerulonephritis and anti–glomerular basement membrane glomerulonephritis, which account for approximately 90% of the cases. Less common causes include antiphospholipid syndrome, lupus, IgA vasculitis, and mixed connective tissue disorders.5 In this report, we described a case of pulmonary-renal syndrome in a patient with DDD that involved both the kidney and lungs. To the best of our knowledge, this is the first report of pulmonary-renal syndrome due to DDD involving both the glomerular and pulmonary capillaries. Kidney and lung biopsies were keys to the diagnosis of renal and pulmonary DDD.
C3G is a recently described group of disorders that are characterized by bright glomerular C3 staining and are associated with acquired and genetic defects of the alternative pathway of complement.1, 6 C3G is divided into DDD and C3GN based on electron microscopy. DDD is characterized by osmiophilic electron-dense deposits within the glomerular basement membranes and rounded deposits in the mesangium. In comparison, C3GN is characterized by subendothelial and mesangial deposits, although intramembranous and subepithelial deposits also may be present.2, 3 Most patients with C3G present with hematuria and proteinuria, although a few patients may present with a rapidly progressive glomerulonephritis. On kidney biopsy, the most common pattern of injury is that of membranoproliferative glomerulonephritis,7 followed by a mesangial proliferative glomerulonephritis. In a small subset of C3G, a crescentic glomerulonephritis also may be present.8, 9, 10, 11 However, despite the presence of a severe crescentic glomerulonephritis, pulmonary involvement has not been reported in any of these patients. Conceptually, deposition of complement factors in the glomerular capillaries with ensuing C3G development should also result in complement factor accumulation in the pulmonary capillaries with ensuing capillaritis, because both the kidneys and lungs represent organs of high blood perfusion. Furthermore, the alveolar basement membrane has structural homology to the glomerular basement membrane,12 and it could be postulated that the accumulation of complement factors in the alveolar capillaries in the setting of severe complement abnormalities could result in alveolar capillaritis and pulmonary hemorrhage. Yet, pulmonary-renal syndrome is uncommon in C3G, even in patients with a severe crescentic C3G. This may be explained by the better clearance of complement factors in the pulmonary capillaries compared with glomerular capillaries. It is also possible that a low-grade pulmonary component may be present in some cases, but pulmonary DDD involvement has not been proven due to lack of lung biopsy in such cases. Indeed, in our experience at the Mayo Clinic, we have recently seen a 24-year-old young woman with C3 glomerulonephritis presenting with renal failure and diffuse alveolar hemorrhage. Despite immunosuppression and plasma exchange, the patient developed end-stage kidney disease and is currently on dialysis. The lungs were not biopsied.
Extrarenal DDD and C3GN include acquired partial lipodystrophy and ocular drusen. Partial lipodystrophy is seen in 5% to 17% of the patients with DDD and may precede renal involvement. Approximately 83% of patients with partial lipodystrophy have low C3 levels or presence of C3Nef.13 Fundal findings are reported in up to 85% of patients with DDD.14 Ocular drusen is characterized by the presence of lipoproteinaceous deposits (complement debris) between ocular Bruch’s membrane and the retinal pigment epithelium and is seen in 70% of the patients with DDD over 3 decades.1 To the best of our knowledge, other organ involvement has not been described.
Several unique findings are pertinent to our case. First, our patient presented with renal failure and hemoptysis qualifying for pulmonary-renal syndrome. Kidney biopsy confirmed the diagnosis of DDD. Only postmortem lung biopsy revealed DDD involvement of the pulmonary capillaries. This is an unusual presentation for DDD. In our patient, we were able to document the pulmonary DDD only because we were able to obtain a postmortem lung biopsy. Second, there was severe activation of the alternative pathway of complement with markedly low C3 levels and alternate complement pathway functional assay. Further evaluation revealed antibodies to C3 convertase (C3Nef) and antibodies to CFB (aCFB). Although C3Nef is common in DDD,4 recent studies also have shown C3G associated with abnormalities in CFB, both genetic and acquired.15, 16 aCFB is similar to C3Nef, binds and stabilizes C3bBb convertase (solid phase), and prevents decay, resulting in accelerated C3 consumption.17, 18 Presence of both C3Nef and aCFB is reported in 9% of patients with C3G.19 Finally, genetic evaluation revealed 4 genetic variants in the CFH gene, of which 2 (rs1061170 and rs2274700) are previously described in DDD, another (rs35292876) is reported as a silent polymorphism in patients with atypical hemolytic uremic syndrome, and the last (rs1061147) is associated with ocular drusen and age-related macular degeneration.20, 21, 22, 23 Taken together, the multiple abnormalities of the alternative pathway of complement in our patient encompassing both genetic and acquired components likely resulted in severe activation of alternative pathway of complement with ensuing renal and pulmonary DDD. A limitation of the report is that complete genetic evaluation of other complement-regulating proteins was not performed. Third, the involvement of the kidneys and lungs appears to represent an extremely aggressive form of DDD, with renal DDD likely preceding development of lung DDD.
It is interesting to note that although the kidney biopsy showed mostly sclerosing lesions and markedly thickened glomerular basement membranes, the alveolar capillaries showed acute lesions with red blood cells and fibrin exudation in the absence of any features suggestive of an acute infection. The findings suggest that the lung lesions were of more recent onset than the renal lesions. Other disease associations noted with DDD, such as partial lipodystrophy and age-related macular degeneration, also occur at different time periods of the disease process.
To summarize, we describe a patient presenting with a pulmonary-renal syndrome with DDD involvement of both kidneys and lungs in the setting of severe dysregulation of the alternative pathway of complement. DDD is a rare cause of pulmonary-renal syndrome; early recognition, evaluation, and treatment are required to prevent development of end-stage kidney disease and other complications. We propose that DDD also should be included in the differential diagnosis of pulmonary-renal syndrome in the setting of severe activation of alternative pathway of complement.
Disclosure
All the authors declared no competing interests.
References
- 1.Pickering M.C., D'Agati V.D., Nester C.M. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalvin L.A., Fervenza F.C., Sethi S., Pulido J.S. Manifestations of complement-mediated and immune complex-mediated membranoproliferative glomerulonephritis: a comparative consecutive series. Ophthalmology. 2016;123:1588–1594. doi: 10.1016/j.ophtha.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Dalvin L.A., Fervenza F.C., Sethi S., Pulido J.S. Shedding light on fundus drusen associated with membranoproliferative glomerulonephritis: breaking stereotypes I, II, and III. Retin Cases Brief Rep. 2016;10:72–78. doi: 10.1097/ICB.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 4.Appel G.B., Cook H.T., Hageman G. Membranoproliferative glomerulonephritis type ii (dense deposit disease): an update. J Am Soc Nephrol. 2005;16:1392–1403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- 5.West S.C., Arulkumaran N., Ind P.W., Pusey C.D. Pulmonary-renal syndrome: a life threatening but treatable condition. Postgrad Med J. 2013;89:274–283. doi: 10.1136/postgradmedj-2012-131416. [DOI] [PubMed] [Google Scholar]
- 6.Servais A., Noël L.H., Roumenina L.T. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 7.Sethi S., Fervenza F.C. Membranoproliferative glomerulonephritis: a new look at an old entity. N Engl J Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 8.Nasr S.H., Valeri A.M., Appel G.B. Dense deposit disease: clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol. 2009;4:22–32. doi: 10.2215/CJN.03480708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fervenza F.C., Smith R.J.H., Sethi S. Association of a novel complement factor H mutation with severe crescentic and necrotizing glomerulonephritis. Am J Kidney Dis. 2012;60:126–132. doi: 10.1053/j.ajkd.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi S., Sahani M., Oei L.S., Rao R. Crescentic glomerulonephritis and dense deposit disease in a woman with breast carcinoma on immunosuppressive chemotherapy. Am J Kidney Dis. 2004;44:e33–e37. [PubMed] [Google Scholar]
- 11.Ravindran A, Fervenza FC, Smith RJ, Sethi S. C3 glomerulonephritis with a severe crescentic phenotype [e-pub ahead of print]. Pediatr Nephrol. https://doi.org/10.1007/s00467-017-3702-8. Accessed June 7, 2017. [DOI] [PubMed]
- 12.Vaccaro C.A., Brody J.S. Structural features of alveolar wall basement membrane in the adult rat lung. J Cell Biol. 1981;91:427–437. doi: 10.1083/jcb.91.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith R.J.H., Alexander J., Barlow P.N. New approaches to the treatment of dense deposit disease. J Am Soc Nephrol. 2007;18:2447–2456. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAvoy C., Silvestri G. Retinal changes associated with type 2 glomerulonephritis. Eye. 2005;19:985–989. doi: 10.1038/sj.eye.6701697. [DOI] [PubMed] [Google Scholar]
- 15.Imamura H., Konomoto T., Tanaka E. Familial C3 glomerulonephritis associated with mutations in the gene for complement factor B. Nephrol Dial Transplant. 2015;30:862–864. doi: 10.1093/ndt/gfv054. [DOI] [PubMed] [Google Scholar]
- 16.Sethi S., Smith R.J.H., Dillon J.J., Fervenza F.C. C3 Glomerulonephritis associated with complement factor B mutation. Am J Kidney Dis. 2015;65:520–521. doi: 10.1053/j.ajkd.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Marinozzi M.C., Roumenina L.T., Chauvet S. Anti-factor B and anti-C3b autoantibodies in C3 glomerulopathy and Ig-associated membranoproliferative GN. J Am Soc Nephrol. 2017;28:1603–1613. doi: 10.1681/ASN.2016030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strobel S., Zimmering M., Papp K. Anti-factor B autoantibody in dense deposit disease. Mol Immunol. 2010;47:1476. doi: 10.1016/j.molimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Meyer N.C., Wang K. Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol. 2012;7:265–274. doi: 10.2215/CJN.07900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrera-Abeleda M.A., Nishimura C., Frees K. Allelic variants of complement genes associated with dense deposit disease. J Am Soc Nephrol. 2011;22:1551–1559. doi: 10.1681/ASN.2010080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrera-Abeleda M.A., Nishimura C., Smith J.L.H. Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease) J Med Genet. 2006;43:582–589. doi: 10.1136/jmg.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karkhane R., Ahmadraji A., Riazi Esfahani M. Complement factor H and LOC387715/ARMS2/HTRA1 variant's frequencies and phenotypic associations in neovascular age-related macular degeneration, a pilot study. J Curr Ophthalmol. 2016;28:32–36. doi: 10.1016/j.joco.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bu F., Maga T., Meyer N.C. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2014;25:55–64. doi: 10.1681/ASN.2013050453. [DOI] [PMC free article] [PubMed] [Google Scholar]