Acute kidney injury (AKI) is a common multifactorial syndrome affecting critically ill patients across the entire span of human age, from preterm neonates,1 through childhood, adolescence/young adult years,2, 3 and older adulthood.4 These epidemiological studies demonstrate that AKI has incremental associations with mortality, prolonged mechanical ventilation (MV), and intensive care unit and hospital length of stay (LOS). AKI survivors are at increased risk for chronic kidney disease (CKD), accelerated progression to end-stage kidney disease, and rehospitalization.5, 6 AKI has been studied in nearly 50 million adults in the past decade, reflecting its public health importance and focus for clinical and translational research.7 Large-scale initiatives have been launched to raise awareness and to improve the quality of care for patients with AKI.8, 9
Currently, we are able to provide supportive care to critically ill patients with AKI in only the form of fluid vigilance, nephrotoxic medication avoidance, and, in severe cases, initiation of renal replacement therapy. Pharmacological and device-oriented therapeutic trials to date have largely failed to improve outcomes in patients with AKI, largely because of the reliance of serum creatinine as the diagnostic biomarker for AKI in the clinical setting. Over the past 20 years, novel AKI biomarkers have been discovered to detect AKI ahead of the functional change manifested by an increase in serum creatinine.10 Although these biomarkers have performed well to predict AKI in the clinical research setting, widespread use of novel biomarkers to assist in clinical decision support has been limited and, in some cases, not helpful.11, 12 Recently, use of biomarkers in the cardiac surgery setting to direct an AKI avoidance bundle has shown some promising results to decrease post−cardiac surgery−associated AKI rates.13
However, the timing of insult leading to AKI is often not known outside the surgical setting. Thus, one would expect AKI biomarkers to demonstrate diminished predictive ability in this heterogeneous setting. The work undertaken to identify and to validate novel AKI biomarkers has been likened to the search for a renal troponin I. Yet, an essential component for a successful translation of the comparison of AKI biomarkers to troponin is their integration into a diagnostic pathway that mirrors that of troponin’s use in the acute coronary syndrome. Outside of the context of demographic factors (e.g., diabetes, obesity) and symptomatology (radiating chest pain) that raise suspicion for myocardial ischemia, elevated troponin levels are common, and do not reflect myocardial infarction.14 Thus, a stratification system that identifies AKI risk based on clinical context and signs of decreased kidney function will similarly be essential to direct biomarker assessment in only the patients who are truly at risk.
Dr. Lakhmir Chawla and I proposed a conceptual model, termed “renal angina,” to identify critically ill patients at risk for AKI at the time of intensive care unit (ICU) admission, with a call to operationalize this model in children and adults.15 My team and others have tested a renal angina index in multiple critically ill pediatric cohorts and demonstrated that an RAI of ≥8 in the first 12 hours of ICU admission exhibits very high sensitivity and negative predictive value for AKI development or persistence at 72 hours of ICU admission in children.16, 17 Furthermore, the addition of novel AKI biomarkers to the RAI improves AKI prediction significantly,18, 19 thereby recapitulating the cardiac angina−troponin relationship.
In this issue of Kidney International Reports, Matsuura and colleagues20 assess an adult modification of the RAI to predict AKI in a cohort of critically ill Asian adult patients combined from 3 separate prospective studies. The authors modified the pediatric RAI by inclusion of sepsis and diabetes in the high-risk group. In addition, the authors only used serum creatinine change and did not integrate fluid overload into their RAI model. Their primary outcome was severe AKI, defined as the Kidney Disease Improving Global Outcomes (KDIGO) Stage 2 or 3,21 that persisted for 48 hours. Only 263 of 851 (30.9%) of the initial cohort met the inclusion criteria, and 24 of these patients developed severe AKI (9.1%). In the authors’ initial analysis, their RAI had only a fair predictive ability (are under the curve [AUC] of 0.63), with a very high percentage of patients with severe AKI who had an RAI of <10, thus showing less than acceptable sensitivity and negative predictive value. The predictive power of the RAI improved (AUC of 0.73) when assessed only in patients who were admitted to the ICU from the general wards, an improvement that was generated by increased sensitivity and negative predictive value. Finally, integration of the novel biomarker liver-type fatty acid binding protein (L-FABP) into the RAI model improved severe AKI prediction performance further (AUC of 0.79 to 0.82). Thus, these investigators have validated our concept of context-driven AKI biomarker assessment to predict severe AKI in the adult population.
However, a number of issues exist with respect to the current study. Only about one-third of the original combined patient cohort was retained in the analysis, and the severe AKI rate observed is far less than the 20% to 30% reported in other large epidemiological studies of AKI in adults. In addition, even the most optimal performance of the integrated RAI−LFABP model does not outperform some novel AKI biomarkers reported in isolation in other studies.10, 22 The fact that the RAI did not perform as well as what we have seen in pediatric studies (AUC 0.76−0.82 with NPV of 92−99), likely stems from the relatively higher prevalence of multiple comorbidities seen in adults compared to children. In other words, it may not be surprising that a kidney-specific prediction model is negatively impacted by other comorbidities external to the kidney. The relatively low AKI rate and poor sensitivity of the authors’ RAI model suggest that that they were not dealing with a very high-risk population.
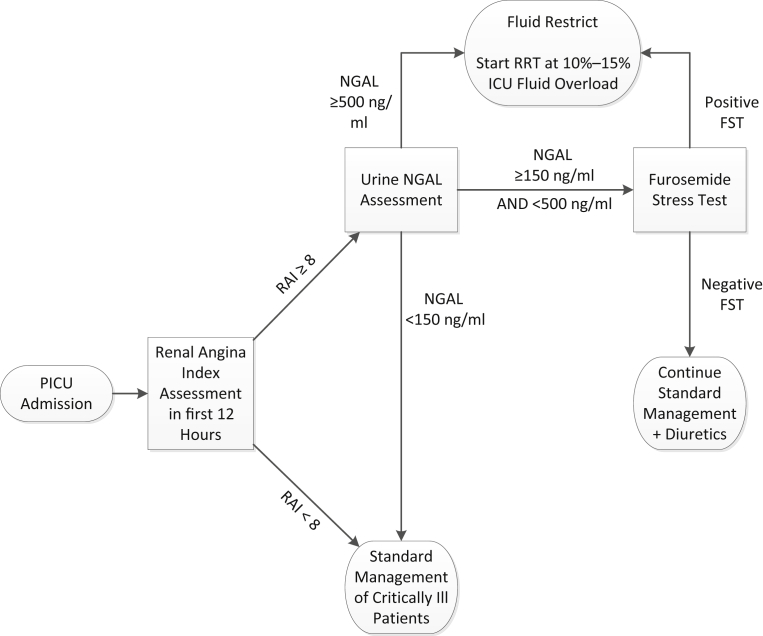
Nonetheless, combining data from 3 separate adult studies and centers, and the improvement in AKI prediction using context-driven biomarker assessment, represent a major step forward toward the development of a feasible and actionable AKI bundle. By placing a premium on sensitivity and negative predictive value, the renal angina risk stratification concept can focus the clinician on only those patients who are truly at risk when they make a decision. In this context, the RAI−biomarker risk assessment can provide real-time clinical decision support with respect to fluid resuscitation algorithms, nephrotoxic medication avoidance, and, potentially, more confidence in escalating care to include renal replacement therapy. We are currently developing a real-time RAI assessment program in our electronic health record to lead to an automatic biomarkers assessment at 12 hours of ICU admission in patients with an RAI of ≥8 (Figure 1). Based on the integration of these factors, clinicians will be directed along a particular pathway with respect to fluid management, diuretic challenge (the furosemide stress test)23 and renal replacement therapy initiation. Although the pediatric AKI research community has led the way with respect to development and validation of a renal angina index model, the initial foray into this concept by Matsuura and colleagues20 should stimulate intense prospective research into calibrating their RAI model, with AKI biomarker integration, in the critically ill adult population, which still suffers an unacceptably high rate of morbidity and mortality from this deadly disease.
Figure 1.
Use of the renal angina index and a biomarker to assist with clinical decision support. FST, furosemide stress test; ICU, intensive care unit; NGAL, neutrophil gelatinase-associated lipocalin; PICU, pediatric intensive care unit; RAI, renal angina index; RRT, renal replacement therapy.
Disclosure
SLG is a consultant for and receives grant funding from Bioporto, Incorporated.
Footnotes
See Clinical Research on Page 677
References
- 1.Jetton J.G., Boohaker L.J., Sethi S.K. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–194. doi: 10.1016/S2352-4642(17)30069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddourah A., Basu R.K., Bagshaw S.M. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuhrman D.Y., Kane-Gill S., Goldstein S.L. Acute kidney injury epidemiology, risk factors, and outcomes in critically ill patients 16-25 years of age treated in an adult intensive care unit. Ann Intensive Care. 2018;8:26. doi: 10.1186/s13613-018-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoste E.A., Bagshaw S.M., Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 5.Koulouridis I., Price L.L., Madias N.E. Hospital-acquired acute kidney injury and hospital readmission: a cohort study. Am J Kidney Dis. 2015;65:275–282. doi: 10.1053/j.ajkd.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla L.S., Amdur R.L., Amodeo S. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susantitaphong P., Cruz D.N., Cerda J. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta R.L., Cerda J., Burdmann E.A. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 9.Koyner J.L., Cerda J., Goldstein S.L. The daily burden of acute kidney injury: a survey of U.S. nephrologists on World Kidney Day. Am J Kidney Dis. 2014;64:394–401. doi: 10.1053/j.ajkd.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Alge J.L., Arthur J.M. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10:147–155. doi: 10.2215/CJN.12191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varnell C.D., Jr., Goldstein S.L., Devarajan P. Impact of near real-time urine neutrophil gelatinase-associated lipocalin assessment on clinical practice. Kidney Int Rep. 2017;2:1243–1249. doi: 10.1016/j.ekir.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wald R., Adhikari N.K., Smith O.M. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88:897–904. doi: 10.1038/ki.2015.184. [DOI] [PubMed] [Google Scholar]
- 13.Meersch M., Schmidt C., Hoffmeier A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agewall S., Giannitsis E., Jernberg T. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2011;32:404–411. doi: 10.1093/eurheartj/ehq456. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein S.L., Chawla L.S. Renal angina. Clin J Am Soc Nephrol. 2010;5:943–949. doi: 10.2215/CJN.07201009. [DOI] [PubMed] [Google Scholar]
- 16.Basu R.K., Zappitelli M., Brunner L. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85:659–667. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu R.K., Kaddourah A., Goldstein S.L. Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: a multicentre, multinational, prospective observational study. Lancet Child Adolesc Health. 2017;2:112–120. doi: 10.1016/S2352-4642(17)30181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon S., Goldstein S.L., Mottes T. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant. 2016;31:586–594. doi: 10.1093/ndt/gfv457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu R.K., Wang Y., Wong H.R. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol. 2014;9:654–662. doi: 10.2215/CJN.09720913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuura M., Srisawat N., Claure-Del Granado R. Use of the renal angina index in determining acute kidney injury. Kidney Int Rep. 2018;3:677–683. doi: 10.1016/j.ekir.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group—KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;(suppl):1–138. [Google Scholar]
- 22.Kashani K., Al-Khafaji A., Ardiles T. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chawla L.S., Davison D.L., Brasha-Mitchell E. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207. doi: 10.1186/cc13015. [DOI] [PMC free article] [PubMed] [Google Scholar]