Abstract
Objectives
The objective of this study was to map the global risk of the major arboviral diseases transmitted by Aedes aegypti and Aedes albopictus by identifying areas where the diseases are reported, either through active transmission or travel-related outbreaks, as well as areas where the diseases are not currently reported but are nonetheless suitable for the vector.
Methods
Data relating to five arboviral diseases (Zika, dengue fever, chikungunya, yellow fever, and Rift Valley fever (RVF)) were extracted from some of the largest contemporary databases and paired with data on the known distribution of their vectors, A. aegypti and A. albopictus. The disease occurrence data for the selected diseases were compiled from literature dating as far back as 1952 to as recent as 2017. The resulting datasets were aggregated at the country level, except in the case of the USA, where state-level data were used. Spatial analysis was used to process the data and to develop risk maps.
Results
Out of the 250 countries/territories considered, 215 (86%) are potentially suitable for the survival and establishment of A. aegypti and/or A. albopictus. A. albopictus has suitability foci in 197 countries/territories, while there are 188 that are suitable for A. aegypti. There is considerable variation in the suitability range among countries/territories, but many of the tropical regions of the world provide high suitability over extensive areas. Globally, 146 (58.4%) countries/territories reported at least one arboviral disease, while 123 (49.2%) reported more than one of the above diseases. The overall numbers of countries/territories reporting autochthonous vector-borne occurrences of Zika, dengue, chikungunya, yellow fever, and RVF, were 85, 111, 106, 43, and 39, respectively.
Conclusions
With 215 countries/territories potentially suitable for the most important arboviral disease vectors and more than half of these reporting cases, arboviral diseases are indeed a global public health threat. The increasing proportion of reports that include multiple arboviral diseases highlights the expanding range of their common transmission vectors. The shared features of these arboviral diseases should motivate efforts to combine interventions against these diseases.
Keywords: Aedes aegypti, Aedes albopictus, Arboviral diseases, Chikungunya, Dengue, RVF, Yellow fever, Zika
Introduction
Every year more than one billion people are infected with and more than one million people die from vector-borne diseases, of which mosquito-borne diseases make up a significant proportion (World Health Organization (WHO), 2014). Aedes aegypti and Aedes albopictus have received a great deal of attention worldwide, since both species are efficient vectors for human arboviral diseases such as Zika (Thangamani et al., 2016; Marchette et al., 1969; Gardner et al., 2016; Marcondes and de FF de Ximenes, 2016), dengue (Jansen and Beebe, 2010; Rosen et al., 1983), chikungunya (Paupy et al., 2010; Burt et al., 2012), and yellow fever (Aitken et al., 1979) A. aegypti is also a vector for zoonotic diseases such as Rift Valley fever (RVF) (Mweya et al., 2013), which is considered to be one of the more serious emerging zoonotic diseases (Pepin et al., 2010).
The arboviral diseases mentioned above are increasingly becoming a global health concern due to their rapid geographical spread and high disease burden. Especially over the past 30 years, the distribution and public health impact of these arboviruses have increased dramatically (Carlson et al., 2016; Hafiz et al., 2016; Bhatt et al., 2013; Charrel et al., 2014; Messina et al., 2016; Jentes et al., 2011; Nanyingi et al., 2015), due to the widespread distribution of their vectors paired with increases in trade and travel (Kraemer et al 2015c). A considerable number of studies have mapped the global or regional distribution of A. aegypti and A. albopictus, determined their ecological requirements, and described their habitats (Khormi and Kumar, 2014; Brady et al., 2013; Cianci et al., 2015; Rao et al., 1973; David et al., 2016; Brady et al., 2014; Li et al., 2014). Recently, Kraemer et al. (2015a) mapped the global distribution of A. aegypti and A. albopictus and found that the habitat suitability range for these species is at its widest ever, occurring in all continents including North America and Europe.
Studies have shown strong associations between disease occurrence and the distribution of vectors transmitting them (Carlson et al., 2016; Messina et al., 2016). A considerable number of studies have used these associations to developed risk maps for arboviral diseases (Carlson et al., 2016; Bhatt et al., 2013; Charrel et al., 2014; Messina et al., 2016; Jentes et al., 2011; Rogers et al., 2006; Clements et al., 2007; Brady et al., 2012). Risk maps support decision-making by helping to target disease interventions geographically. Combining interventions known to be effective against multiple diseases transmitted by the same vector offers the most cost-effective and sustainable strategy for the reduction of disease burden.
In this context, the habitat suitability of A. aegypti and A. albopictus was combined with the occurrence of five diseases, namely Zika, dengue, yellow fever, chikungunya, and RVF, by compiling a comprehensive disease occurrence dataset from the published literature and reports from different organizations (such as WHO, CDC, ECDC, …). The diseases focused on were chosen based on (1) their widespread occurrence, (2) emergence and re-emergence issues, and (3) the availability of data and literature. As a result, Zika, dengue, yellow fever, and chikungunya, the major human arboviral diseases, and RVF, a major emerging zoonotic arboviral disease, were selected. The objective of this study was to map the global risk by identifying areas where the diseases are reported, either through active transmission or travel-related outbreaks, as well as areas where the diseases are not currently reported but are nonetheless suitable for the vector.
Materials and methods
Vector occurrence data
The global occurrences of A. aegypti and A. albopictus have been compiled and published by Kraemer et al. (2015a,b). These previous publications contain data on the known global occurrences of A. aegypti and A. albopictus between 1960 and 2014. The dataset contains 19 930 spatially unique occurrence records for A. aegypti and 22 137 for A. albopictus. We used outputs from species distribution models (SDM) developed and applied by Kraemer et al. (2015a) in GeoTiff raster format for use in the present study. Kraemer and colleagues developed probabilistic global environmental suitability maps for both A. aegypti and A. albopictus using boosted regression trees (BRT) modelling and environmental covariates at a spatial resolution of 5 km. Details regarding the modelling approach, the environmental covariates used, and the predictive performance of these models can be found in the original publication (Kraemer et al., 2015a).
Disease occurrence data
A thorough review was performed based on the global occurrence of diseases transmitted by A. aegypti and/or A. albopictus. The data sources used in this study for each of the five diseases are shown in the Supplementary Material (Annex 1). In addition to the sources indicated in Supplementary Material Annex 1, occurrence records for Zika compiled by Carlson et al. (2016) and those for dengue com piled by Messina et al. (2014) were used. The disease occurrence data for the selected diseases were compiled from literature dating as far back as 1952 to as recent as 2017. In the present study the occurrences of the diseases under consideration were defined and classified as follows. Zika, dengue, chikungunya, and yellow fever occurrences were classified into four categories: (1) no known disease occurrence and no risk of the disease, (2) no known disease occurrence but at risk (countries/territories having a suitability range for A. aegypti and/or A. albopictus), (3) autochthonous vector-borne transmission, and (4) travel-related occurrence. Five categories of occurrence were developed for RVF: (1) no known previous occurrence, (2) countries/territories at risk (countries/territories having a suitability range for A. aegypti and/or A. albopictus), (3) endemic or previously known serious outbreaks, (4) periodic occurrence of cases or serological evidence, and (5) travel-related occurrence.
Geoprocessing and risk mapping
Country-level disease occurrence records extracted from the various sources noted were gathered into one dataset (Supplementary Material Annexes 2 and 3). To assess the number of countries/territories at risk, the habitat suitability models of A. aegypti and A. albopictus were compared with disease occurrence. In order to do so, a global suitability map was created based on the following four classes: areas suitable for both A. aegypti and A. albopictus, areas suitable for A. aegypti, areas suitable for A. albopictus, and areas suitable for neither A. aegypti nor A. albopictus. As the suitability maps developed by Kraemer et al. (2015a) featured suitability values ranging from 0 (not suitable) to 1 (highly suitable), all areas where the suitability was higher than 0.5 were considered suitable (Liu et al., 2005). In a first step, the entire country was counted as suitable if any part of that country showed suitability for a vector at a probability greater than 0.5. In a second step, the proportion of suitable areas versus the entire surface area of the country was computed. This proportion is referred to as ‘the suitability range’ in the remainder of this article.
The following classifications were used to map the global risk of the selected diseases: (1) countries having no know previous diseases occurrence, (2) countries having known previous autochthonous vector-borne transmission, (3) countries having previous known travel-related occurrence, (4) countries having no risk of transmission (countries/territories having no suitability range for either of the vectors), (5) countries at risk (countries/territories having at most 50% suitability range), and (6) countries at high risk (countries/territories having at least 50% suitability range). The risk maps were developed at the country level, except for the USA, where disease reporting was available at the state level. The geoprocessing and risk mapping were performed using QGIS version 2.18.0 (QGIS Development Team, 2009).
Results
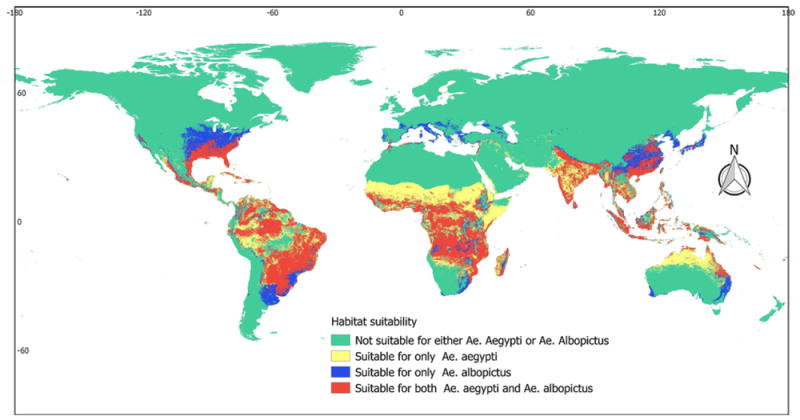
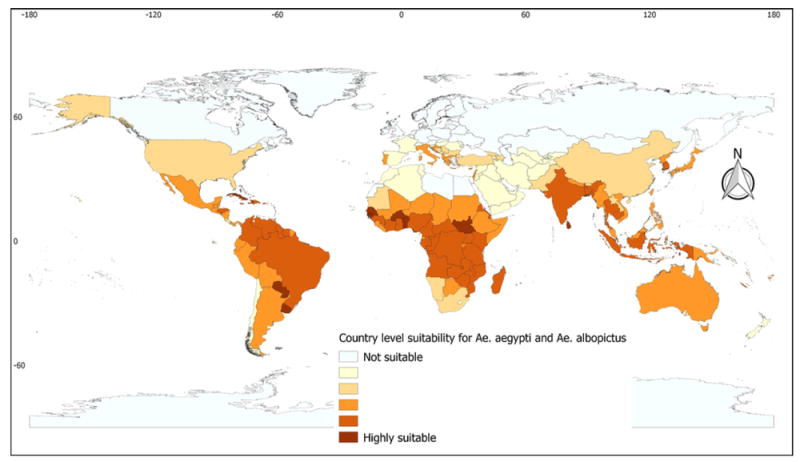
Habitat suitability for Aedes aegypti and Aedes albopictus
The habitat suitability model indicated that of the 250 countries/territories considered, 215 (86%) are potentially suitable for the existence and development of A. aegypti and/or A. albopictus (Table 1 and Supplementary Material Annex 2). The suitability of A. albopictus was found to be most widespread, presenting suitability foci in 197 countries/territories, compared with A. aegypti, which demonstrated suitability foci in 188 countries/territories. As shown in Figure 1, highly suitable areas for both A. aegypti and A. albopictus were identified in the southern USA, Caribbean, South America, Sub-Saharan Africa, Indian subcontinent, Southeast Asia, and some Pacific countries. Patchy foci of suitable areas were found in countries of Southern Europe and North Africa along the Mediterranean coast. Moreover, considerable suitable foci were identified in Israel, the Palestinian Authority, and areas along the Euphrates and Tigris rivers. The coastal parts of northern Australia also show considerable suitability. While for A. aegypti, suitable ranges were found to be concentrated in the tropical and sub-tropical parts of the world, the ranges for A. albopictus were found to extend into the temperate part of the world as well, especially in Southern Europe and the central USA.
Table 1.
Number of countries/territories suitable for the vectors and number of countries/territories affected by the diseases, by region.
| Region | Number of countries/territories | Number of countries/territories suitable for | Number of countries/territories affected by | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Aedes aegypti | Aedes albopictus | Either vector | Zika | Dengue fever | Yellow fever | Chikungunya fever | RVF | ||
| Africa | 58 | 56 | 56 | 57 | 14 | 36 | 30 | 26 | 36 |
| Americasa | 56 | 52 | 44 | 52 | 48 | 46 | 13 | 46 | 0 |
| Asia | 52 | 45 | 43 | 49 | 11 | 15 | 0 | 20 | 3 |
| Europe | 56 | 12 | 32 | 32 | 0 | 3 | 0 | 3 | 0 |
| Oceaniab | 28 | 23 | 22 | 25 | 12 | 11 | 0 | 11 | 0 |
| Overall number of countries/territories | 250 | 188 | 197 | 215 | 85 | 111 | 43 | 106 | 39 |
RVF, Rift Valley fever.
Includes Central America, North America, the Caribbean, and South America.
Includes Australia and the Pacific islands.
Figure 1.
Global predicted habitat suitability of Aedes aegypti and Aedes albopictus.
The tropical and sub-tropical parts of the world manifested high suitability ranges for A. aegypti and/or A. albopictus, with percentages varying considerably among countries. Countries of Sub-Saharan Africa, the Caribbean, and Oceania were found to present a large suitability range, while most European, North American, and Northern Asian countries were seen to manifest a limited or no suitability range. Within Europe, a wide suitability range was found in Italy, Greece, and Croatia. Countries such as Brazil, Colombia, and Venezuela in South America, as well as others in the Indian sub continent, also presented considerably wider suitability (Figure 2 and Supplementary Material Annex 2).
Figure 2.
Country-level suitability range for Aedes aegypti and/or Aedes albopictus: suitability ranges from 0 (white) to 100% (deep red). The percentage suitability was computed based on all grid cells that manifested suitability levels higher than 0.5.
Arboviral disease occurrences
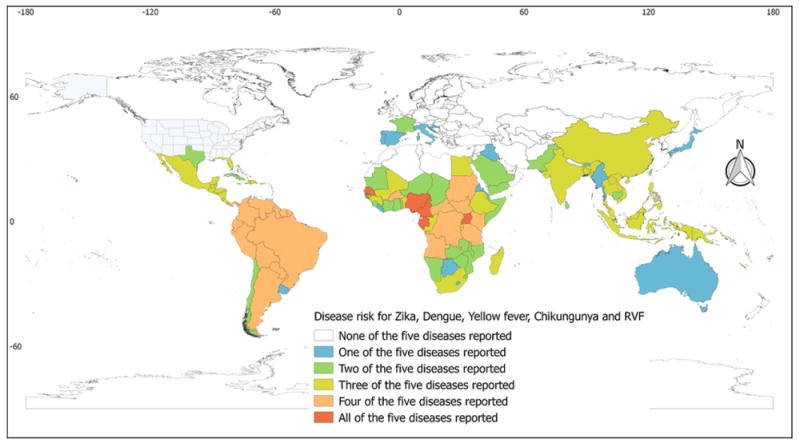
The occurrence of at least one of the arboviral diseases considered was reported from 146 (58.4%) countries/territories, of which 123 (49.2%) reported multiple diseases. The overall number of countries/territories reporting autochthonous vector-borne occurrences was 85 for Zika, 111 for dengue, 106 for chikungunya, 43 for yellow fever, and 39 for RVF. Most of these countries are located in tropical and sub-tropical parts of the globe (Table 1 and Figure 3).
Figure 3.
Global country-level occurrences of the selected arboviral diseases. The map depicts the occurrences of selected arboviral diseases from no occurrence, shown in white, to the occurrence of all of the selected arboviral diseases, shown in red.
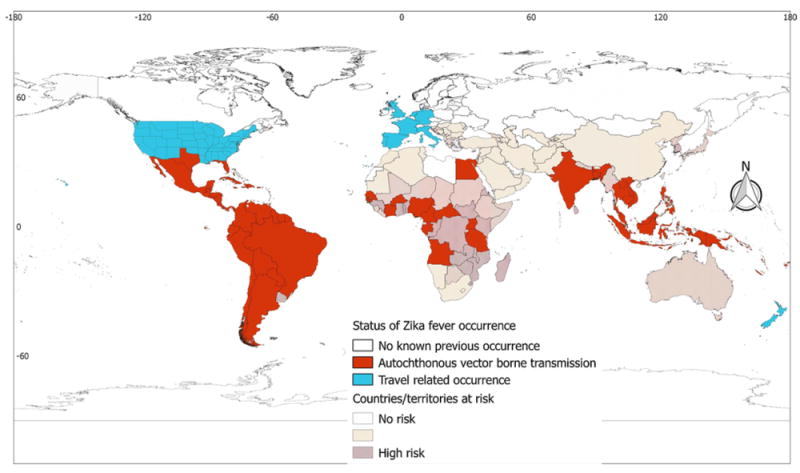
Zika fever
The disease has been reported from 85 countries/territories, although 215 countries/territories are potentially suitable for the vectors involved (Table 1). Analysis in regional blocks showed that autochthonous vector-borne transmissions of Zika cases have been reported from all regions except Europe. In contrast to the wide vector suitability of almost all Sub-Saharan African countries and the specific foci of suitability present in North African countries, the disease has so far been reported from only 14 African countries. In the case of the Americas, with the exception of the northern part of the USA and Canada, almost all areas were found to be potentially suitable for the vector, and cases of the disease have been reported from most of the countries in this region. In Asia, suitable areas were found in the Middle East along the Euphrates and Tigris rivers, but there has been no report of autochthonous vector-borne transmission of the disease from this sub-region. The Indian subcontinent and most of the countries of Southeast Asia were identified as suitable for the vectors, and the disease has been reported from some of these countries. Southern China and Myanmar are suitable for the vector, but no cases of the disease have been reported from these countries to date. Most of the Pacific (Oceania) countries are suitable for the vector and a considerable number of cases and even serious outbreaks of the disease have been reported from this region (Table 1 and Figure 4). Travel-associated cases of Zika have been reported from eight European countries (Supplementary Material Annex 2) and 48 states of the USA (Supplementary Material Annex 3).
Figure 4.
Global Zika fever occurrence. The global distribution of Zika fever corresponds well with the global Zika risk. Discrepancies are apparent in Sub-Saharan Africa, where there is a high risk of Zika fever but few occurrence reports. It is emphasized that displaying occurrences at the country level overstates the distribution of the virus, especially in countries such as Argentina and Chile.
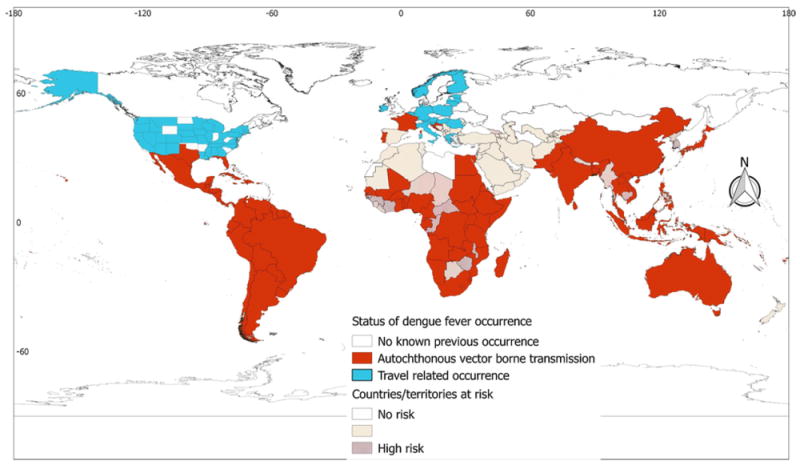
Dengue fever
Dengue fever was found to be the most widely distributed disease among the five arboviral diseases considered in this study. Autochthonous vector-borne transmission of the disease has been reported from 111 countries/territories and all regional blocks. Three European countries, namely Croatia, France, and Portugal, have reported autochthonous vector-borne transmission of dengue. All Sub-Saharan African countries were found to be suitable for the vectors, and the disease is widespread in Africa, reported so far from 36 countries/territories. The disease has also been reported from some North African countries, namely Egypt, Mali, and Sudan. Furthermore, cases of the disease have been reported from most countries in the Americas, including the USA. In the Americas, autochthonous vector-borne transmission of the disease has been reported from 46 countries/territories of the 52 that were found to be suitable for the vector. Cases of the disease have also been reported from a considerable number of countries/territories in Asia and Oceania (Table 1 and Figure 5). Travel-associated cases of dengue have been reported from 16 European countries (Supplementary Material Annex 2) and 40 states of the USA (Supplementary Material Annex 3).
Figure 5.
Global dengue fever occurrence. The global distribution of dengue fever corresponds well with the global dengue risk. The distribution of dengue fever extends to the temperate part of the world, with some European countries reporting its occurrence. It is emphasized that displaying occurrences at the country level overstates the distribution of the virus, especially in China, Argentina, and Chile.
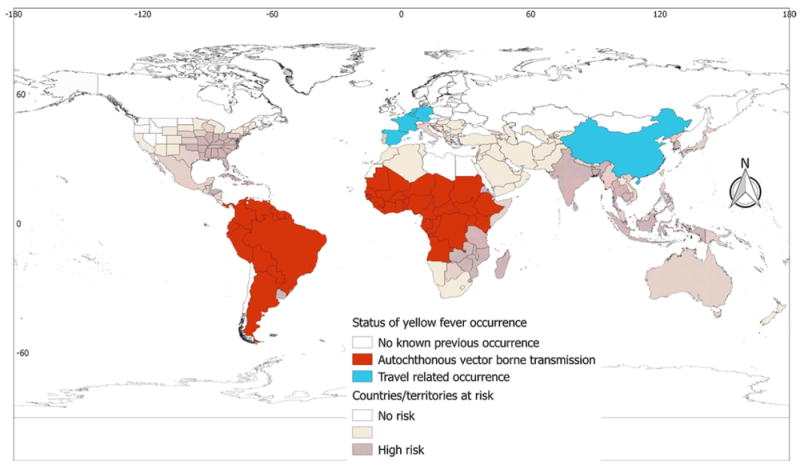
Yellow fever
Autochthonous vector-borne transmission of yellow fever has been reported from two regions (Africa and the Americas), while travel-associated cases have been reported from Europe and Asia. Autochthonous vector-borne transmission of the disease has never been reported from Europe, Asia, or Oceania, although Southern European countries and most of Oceania were identified as suitable for the vector(s). Yellow fever is principally a problem in African countries; the disease has been reported from 30 African countries. Countries of Southern Africa were found to be suitable for the vector, but cases of the disease have not been reported from this region. With regard to the Americas, disease cases have been reported from 13 countries of South America. No autochthonous vector-borne transmission of the disease has been reported from countries of North America. In Asia, China has reported travel-associated cases, although most of the southern and south-eastern Asian countries/territories were found to be suitable for the vector(s) (Table 1 and Figure 6).
Figure 6.
Global yellow fever occurrence. There are discrepancies between the global yellow fever risk and yellow fever occurrence. The discrepancies are apparent in the southern USA, Mexico, Caribbean countries, Southern Africa, Southern Europe, the Indian subcontinent, and Southeast Asian countries, as well as Oceania. It is emphasized that displaying occurrences at the country level overstates the distribution of the virus in Argentina.
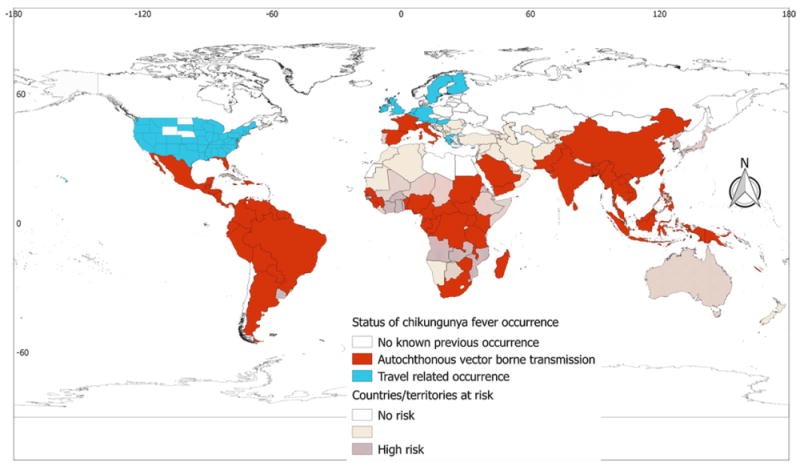
Chikungunya fever
Chikungunya fever is the second most widely distributed arboviral disease after dengue fever. In total, 106 countries/territories have reported autochthonous vector-borne transmission of the disease (Table 1). The disease has been reported from all regions. Three European countries (France, Italy, and Spain) have reported autochthonous vector-borne transmission of the disease. All countries of Sub-Saharan Africa have been found to be suitable for the vector and the disease has already established autochthonous vector-borne transmission in 26 countries/territories. The Horn of Africa (Djibouti, Eritrea, Ethiopia, and Somalia) and countries of south-western Africa were identified as suitable for the disease, but there have been no reports of the disease from this region. The disease is widespread in most of the Americas: of the 52 countries/territories found suitable for the vectors, 46 have reported autochthonous vector-borne transmission of the disease. Of the American countries/territories at risk, Cuba, Chile, and Uruguay are the only countries that have not reported the disease and the other three are relatively small territories. Gulf countries (Yemen and Saudi Arabia), India, China, and most Southeast Asian countries have reported cases of the disease. Most of the Pacific/Oceania countries have also reported the disease. Although there are suitable areas in coastal Australia and Japan, no reports of the disease have been made so far from those countries (Table 1 and Figure 7).
Figure 7.
Global chikungunya fever occurrence. The global distribution of chikungunya fever corresponds well with the global chikungunya risk, with minor discrepancies in countries of Sub-Saharan Africa. The distribution of chikungunya fever extends to the temperate part of the world, with some European countries reporting its occurrence. It is emphasized that displaying occurrences at the country level overstates the distribution of the virus in Argentina.
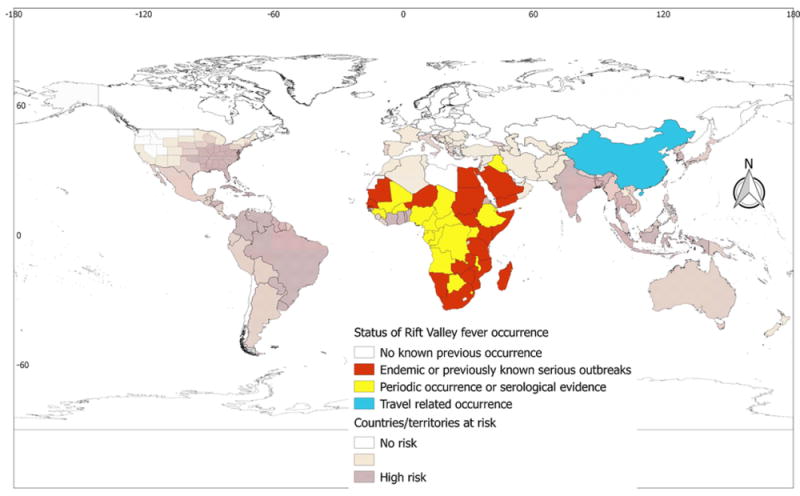
Rift Valley fever (RVF)
Unlike other arboviral diseases, cases of RVF have been reported only from countries of Africa and Asia. Specifically, RVF is widespread in Africa, with 36 African countries having reported cases of the disease (Table 1 and Figure 8). Iraq, Saudi Arabia, and Yemen are the only countries that have reported cases of RVF outside of Africa (Supplementary Material Annex 2). Despite the extensive suitability ranges for the potential vector of RVF, A. aegypti, in the Americas, Pacific/Oceania, and Southeast Asian countries, no reports of RVF have been made from these regions.
Figure 8.
Global Rift Valley fever (RVF) occurrence. There are discrepancies between the global RVF risk and RVF occurrence. The discrepancies are apparent in all regional blocks except Africa.
Discussion
Arboviruses present an ongoing challenge to public health, international travel, trade, and food safety and security (Gubler, 2002). In the past decades, arboviral diseases have emerged or re-emerged, with Zika, dengue, yellow fever, chikungunya, and RVF topping the list (Marcondes and de FF de Ximenes, 2016; Burt et al., 2012; Carlson et al., 2016; Bhatt et al., 2013; Charrel et al., 2014; Messina et al., 2016; Jentes et al., 2011; Nanyingi et al., 2015; Rogers et al., 2006). The epidemiology and host–vector dynamics of arboviral diseases are complex. The dynamics of arboviruses are manifest in their transmission and prevalence in mosquitoes, humans, and other reservoirs, in addition to the vectorial capacity of these mosquitoes. A cross-reference of the habitat suitability model of A. aegypti and A. albopictus (the two most likely globally cosmopolitan arboviral vectors) with five important diseases that they transmit was performed in this study.
Zika, dengue, and yellow fever are arboviral diseases caused by closely related viruses in the genus Flavivirus (Demir and Kilic, 2016). Since its first detection in humans in Uganda and Tanzania in 1952 and subsequently in Nigeria in 1954, Zika virus has travelled throughout Africa and tropical Asia causing minor outbreaks (Mlacker et al., 2016; Dick et al., 1952). The epidemiology of Zika appears to have changed significantly since 2007, after the first large Zika outbreak on the Pacific Island of Yap in the Federated States of Micronesia (Duffy et al., 2009; Hayes, 2009). Previously, Zika virus was only known to cause mild sporadic infections in humans, but the 2015 outbreak in Brazil was associated with severe symptoms such as neurological complications (Lover, 2016; Millichap, 2016; Johansson et al., 2016). In this respect, the World Health Organization (WHO) declared Zika to be a global public health emergency in February 2016 (WHO, 2016a), and since then, Zika virus has received a great deal of media attention and has become a topic of public concern for researchers and policy-makers. Since 2007, outbreaks of Zika virus infection have occurred in Africa, Southeast Asia, and the Pacific Islands, and outbreaks have been ongoing in the Americas, the Caribbean, Oceania/Pacific Islands, and Africa. The burden Zika poses on public health is serious; in the months prior to submitting this study (September 2016 to August 2017), over 70 countries reported confirmed autochthonous vector-borne transmission of Zika virus infection (ECDC, 2017).
Despite the presence of suitable habitats, vectors, and circulating pathogens, no large-scale outbreak of Zika has been observed in Africa since 2007. The reasons why Zika virus continues to escape its transmission cycle in this ‘suitable’ part of the world remain open for investigation. A partial explanation may be the complex interaction between the vectors and pathogens. For example, recent studies have illustrated that infection of A. aegypti by Wolbachia restricts infection and transmission of Zika virus (Caragata et al., 2016; Dutra et al., 2016). In areas such as Sub-Saharan Africa, where A. aegypti and A. albopictus co-exist, competition could also alter the epidemiology of Zika and other arboviral diseases. Previous studies have explored the likely competition between these two vectors and have discussed scenarios that result in stable coexistence or competitive displacement (Juliano et al., 2004; Murrell and Juliano, 2008). Alto and Lounibos (2013) stated that, “competition can enhance susceptibility of infection to arboviruses”; however, the net effect of this type of interaction on vector competence is yet to be fully investigated.
The present study indicated the global burden of dengue to be extensive. According to the WHO, the incidence of dengue has increased 30-fold over the last five decades, with up to 100 million infections now estimated to occur globally each year; this places almost half of the world’s population at risk (WHO, 2017a). Previous estimates by Brady et al. (2012) and Bhatt et al. (2013) put this figure even higher. According to Brady et al. (2012), 3.97 billion people in 128 countries are at risk of contracting dengue, while Bhatt et al. (2013) indicated that 390 million dengue infections occur every year, of which only 24% manifest clinically (Bhatt et al., 2013). Given the widespread occurrence of the competent vectors (Kraemer et al., 2015a), it seems unlikely that these values are overestimated. The fact that many countries report only laboratory-confirmed cases, which represent only a small proportion of the burden, could explain the discrepancy between these dengue burden estimates and the dengue burden notified to the WHO. The establishment of autochthonous vector-borne transmission in three European countries (Croatia, France, and Portugal) indicates the likely future spread of dengue virus. Being supported by the wider suitability range of A. albopictus, as well as travel-associated dengue cases, the virus could potentially establish at least limited autochthonous vector-borne transmission in other temperate regions, with countries of southern Europe bordering the Mediterranean Sea appearing to have an especially elevated risk.
Yellow fever is a re-emerging haemorrhagic viral disease with a high case fatality rate. It is an old disease, having caused major epidemics in the past centuries. Yellow fever was effectively controlled in the mid-1900s through vaccination and vector control. Over the past two decades, however, there has been a resurgence of yellow fever in Africa and Latin America (Gubler, 2004). Since 2000, outbreaks of yellow fever have been reported from four Latin American countries and 19 African countries. A travel-related outbreak of yellow fever occurred in China, with workers returning from Angola being the likely source of the 2016 outbreak. The recent (December 2015 to October 2016) outbreak in Angola resulted in 4347 suspected cases and 377 deaths (WHO, 2016c).
The resurgence of yellow fever has not been as dramatic as dengue or Zika. The presence of an effective, safe, and economic vaccine for yellow fever is thought to have significantly limited the distribution and burden of this disease (Monath and Vasconcelos, 2015). The disease poses a significant hazard to unvaccinated travellers to Africa and Latin America and unprotected individuals in these areas. The recent expansion in the distribution of A. aegypti and A. albopictus and a rise in air travel, have increased the risk of the introduction and spread of yellow fever to North and Central America, the Caribbean, Southern Europe, and many Asian countries (Monath and Vasconcelos, 2015; Ortiz-Martínez et al., 2017). The USA has suitable conditions in areas such as the south of Florida and Texas, where A. albopictus is present and has been linked to the transmission of dengue, chikungunya, and Zika. The ongoing yellow fever outbreak in Brazil could serve as a source of infection for the USA and other yellow fever-free countries in the region. Due to the presence of competent vectors and the presence of imported cases of yellow fever (Supplementary Material Annex 2), it appears likely that yellow fever may establish autochthonous vector-borne transmission in southern European countries.
Chikungunya is the second most widespread arboviral disease in the group, after dengue, and has been reported from 106 countries/territories. Chikungunya is endemic throughout Africa, and over the past decade it has also spread throughout the countries of the Indian Ocean, Asia, South Pacific, Southern Europe, Caribbean, and Central America. The rapid emergence of the virus has been linked to the geographical expansion of its vectors, A. aegypti and A. albopictus (Horwood and Buchy, 2015). Human infections in Africa have been at relatively low levels for a number of years, but in 1999 and 2000 there was a large outbreak in the Democratic Republic of the Congo, and in 2007 there was an outbreak in Gabon (WHO, 2017b). In 2005, a major outbreak of chikungunya occurred on some of the islands in the Indian Ocean. Since 2005, India, Indonesia, the Maldives, Myanmar, and Thailand have reported large numbers of cases. In 2007, transmission was reported for the first time in Europe from north-eastern Italy. In October 2014, France confirmed four cases of locally acquired chikungunya infection in Montpellier, France (WHO, 2017b; Grandadam et al., 2011). Due to the presence of the competent vector, A. albopictus, in countries of Southern Europe, the disease could establish autochthonous vector-borne transmission in countries such as Portugal, Greece, Croatia, Montenegro, Albania, and Slovenia. The first documented outbreak of chikungunya with autochthonous vector-borne transmission in the Americas was reported in 2013. Since 2013, more than one million chikungunya cases have been recorded from the Caribbean islands and Latin American countries, with Colombia, Brazil, and Bolivia having the largest burden.
RVF virus belongs to the genus Phlebovirus of the family Bunyaviridae (Pepin et al., 2010) and causes a severe zoonotic disease in animals and humans (Clements et al., 2007). RVF was first reported in the 1930s from Kenya and there have since been several epizootics in South Africa, West Africa, Madagascar, North Africa (Egypt), and most recently (2006–2007) East Africa (mainly Kenya, Tanzania, and Somalia). The disease is believed to be endemic in most African countries. In 2000, an outbreak of RVF in animals was reported on the western Saudi Arabia–Yemen border (Centers for Disease Control and Prevention (CDC), 2000). This outbreak was the first time that cases of RVF had been reported outside of Africa. RVF is a serious zoonotic disease that can result in human deaths; Mohamed et al. (2010) recorded a human fatality rate of 28% during the 2007 outbreak in Tanzania, while Al-Hazmi et al. (2003) recorded a fatality rate of 34% in humans during the 2000 RVF outbreak in Saudi Arabia. Since 2000, severe outbreaks of RVF have occurred in Niger, Mauritania, South Africa, Madagascar, Sudan, Kenya, Somalia, Tanzania, Yemen, and Saudi Arabia (WHO, 2016b).
RVF has a high potential to spread to other parts of the world via the transportation of infected livestock, humans, or mosquitoes, or by an act of bioterrorism. For example, trade in infected animals was responsible for the outbreaks that occurred in Egypt, Yemen, and Saudi Arabia (WHO, 2016b). Due to the presence of suitable habitats for the competent vector along the Euphrates and Tigris rivers, the present study infers a considerable probability of RVF introduction into countries of East and Southeast Asia and Oceania. Furthermore, due to the presence of the competent vector in the Mediterranean basin (Moutailler et al., 2008), European countries along the Mediterranean Sea have a significant risk of RVF virus introduction.
The establishment of arboviral disease is governed by complex interactions among vector, pathogen, and environment. The presence of a competent vector and pathogen, together with a suitable habitat, are required for sustained autochthonous vector-borne transmission of these arboviral diseases. Thus, suitability for the competent arboviral vectors only does not necessarily imply the future presence of these arboviral diseases in all of the 215 countries/territories identified. However, supported by international travel and trade, the global spread of these arboviral diseases and their vectors will likely expand over time. It is important to note that the susceptibility of mosquitoes to viral pathogens varies spatially, with climatic variables such as temperature and relative humidity having an influence on vector competence (Kilpatrick et al., 2008). For example, Jupille et al. (2016) indicated that A. albopictus and A. aegypti from Europe were not particularly susceptible to the Zika virus (Asian genotype). Thus, the probability that Zika will establish autochthonous vector-borne transmission in the temperate parts of the world is minimal. Over and above vector competence, temperature is also known to affect mosquito physiology, development, survival, reproduction, and biting rate, which will affect the epidemiology of vector-borne diseases. The complexity of interactions among mosquito vectors, arboviral pathogens, and environmental drivers has been discussed by Shragai et al. (2017), as well as by Alto and Lounibos (2013).
It is fully acknowledged that the present study is not without limitations. A number of other vectors in the genus Aedes as well as the genera Anopheles and Culex are known to transmit a variety of arboviral diseases. These vectors were not included in this study in order to maintain a focus on the most likely globally cosmopolitan arboviral vectors. However, some species not considered in this study could be important in regional patterns of arboviral transmission and establishment. In addition, there are considerable differences in disease reporting and vector surveillance capacity among different countries, with robust disease reporting and vector surveillance being more widely practiced in developed countries compared to countries with developing economies. If it were not for this limited capacity for disease reporting and vector surveillance in the global south, the burden and distribution of these arboviral diseases may well have been higher than documented here, as many cases of arboviral disease may go undetected. In addition, with the exception of the USA, risk maps are illustrated at the country level. This tends to mask the high degree of heterogeneity within countries with a wide geographical range; for example, northern China, southern Argentina, and southern Chile would be at low to no risk for these arboviruses.
In conclusion, this study reaffirms the importance of arboviral diseases and the need to combine interventions against them. These arboviral diseases have common vectors, and the high percentage of countries reporting multiple arboviral diseases reinforces their com mon transmission features. Thus, it is important to combine interventions against these diseases to achieve cost-effective control and prevention.
Supplementary Material
Acknowledgments
The habitat suitability models of Aedes aegypti and Aedes albopictus were provided by Moritz U.G. Kraemer. We would like to extend our gratitude to Moritz U.G. Kraemer and his team for sharing their models.
Funding
No funding was available for this study.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2017.11.026.
Conflict of interest
We declare no competing interests.
Ethics committee approval
Not applicable.
References
- Aitken THG, Tesh RB, Beaty BJ, Rosen L. Transovarial transmission of yellow fever virus by mosquitoes (Aedes aegypti) Am J Trop Med Hyg. 1979;28:119–121. doi: 10.4269/ajtmh.1979.28.119. [DOI] [PubMed] [Google Scholar]
- Al-Hazmi M, Ayoola EA, Abdurahman M, et al. Epidemic Rift Valley fever in Saudi Arabia: a clinical study of severe illness in humans. Clin Infect Dis. 2003;36:245–252. doi: 10.1086/345671. [DOI] [PubMed] [Google Scholar]
- Alto BW, Lounibos LP. Vector competence for arboviruses in relation to the larval environment of mosquitoes. In: Takken W, Koenraadt CJM, editors. Ecology of parasite-vector interactions. Wageningen Academic Publishers; Wageningen: 2013. pp. 81–102. [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6 doi: 10.1371/journal.pntd.0001760. https://doi.org/10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OJ, Johansson MA, Guerra CA, et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field condition. Parasit Vectors. 2013;6:351. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OJ, Golding N, Pigott DM, et al. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit Vectors. 2014;7:338. doi: 10.1186/1756-3305-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- Caragata E, Dutra H, Moreira L. Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microb Cell. 2016;3:293–295. doi: 10.15698/mic2016.07.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C, Dougherty E, Getz W. An ecological assessment of the pandemic threat of Zika virus. PLoS Negl Trop Dis. 2016;10(8):e000. doi: 10.1371/journal.pntd.0004968. https://doi.org/10.1101/040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Outbreak of Rift Valley fever—Saudi Arabia, August–October, 2000. MMWR Morb Mortal Wkly Rep. 2000;49:905–908. [PubMed] [Google Scholar]
- Charrel RN, Leparc-Goffart I, Gallian P, de Lamballerie X. Globalization of chikungunya: 10 years to invade the world. Clin Microbiol Infect. 2014;20:662–663. doi: 10.1111/1469-0691.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianci D, Hartemink N, Zeimes CB, Vanwambeke SO, Ienco A, Caputo B. High resolution spatial analysis of habitat preference of Aedes Albopictus (Diptera: Culicidae) in an urban environment. J Med Entomol. 2015;52:329–335. doi: 10.1093/jme/tjv026. [DOI] [PubMed] [Google Scholar]
- Clements ACA, Pfeiffer DU, Martin V, Otte MJ. A Rift Valley fever atlas for Africa. Prev Vet Med. 2007;82:72–82. doi: 10.1016/j.prevetmed.2007.05.006. [DOI] [PubMed] [Google Scholar]
- David MR, dos Santos LMB, Vicente ACP, Maciel-de-Freitas R. Effects of environment, dietary regime and ageing on the dengue vector microbiota: evidence of a core microbiota throughout Aedes aegypti lifespan. Mem Inst Oswaldo Cruz. 2016:1–11. doi: 10.1590/0074-02760160238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir T, Kilic S. Zika virus: a new arboviral public health problem. Folia Microbiol (Praha) 2016;61:523–527. doi: 10.1007/s12223-016-0467-6. [DOI] [PubMed] [Google Scholar]
- Dick G, Kitchen S, Haddow A. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Duffy MR, Chen T-H, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Dutra HLC, Rocha MN, Dias FBS, Mansur SB, Caragata EP, Moreira LA. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC. Current Zika transmission. [22 May 2017];2017 http://ecdc.europa.eu/en/healthtopics/zika_virus_infection/zika-outbreak/Pages/Zika-countries-with-transmission.aspx.
- Gardner LM, Chen N, Sarkar S. Global risk of Zika virus depends critically on vector status of Aedes albopictus. Lancet Infect Dis. 2016;16:522–523. doi: 10.1016/S1473-3099(16)00176-6. [DOI] [PubMed] [Google Scholar]
- Grandadam M, Caro V, Plumet S. Chikungunya virus, Southeastern France. Emerg Infect Dis. 2011;17:910–913. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comp Immunol Microbiol Infect Dis. 2004;27:319–330. doi: 10.1016/j.cimid.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hafiz MY, Mahmood SU, Shoaib M, Yusuf FH. Concern over Zika virus outbreak: another alarming global threat. Infect Drug Resist. 2016;9:149–151. doi: 10.2147/IDR.S108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15:1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood PF, Buchy P. Chikungunya. Rev Sci Tech. 2015;34:479–489. doi: 10.20506/rst.34.2.2373. [DOI] [PubMed] [Google Scholar]
- Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next. Microbes Infect. 2010;12:272–279. doi: 10.1016/j.micinf.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Jentes ES, Poumerol G, Gershman MD, et al. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the informal WHO working group on geographic risk for yellow fever. Lancet Infect Dis. 2011;11:622–632. doi: 10.1016/S1473-3099(11)70147-5. [DOI] [PubMed] [Google Scholar]
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med. 2016;375:1–4. doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupille H, Seixas G, Mousson L, Sousa C, Failloux A-B. Zika virus, a new threat for Europe? PLoS Negl Trop Dis. 2016;10(8):e00. doi: 10.1371/journal.pntd.0004901. https://doi.org/10.1371/journal.pntd.0004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khormi HM, Kumar L. Climate change and the potential global distribution of Aedes aegypti: spatial modelling using geographical information system and CLIMEX. Geospat Health. 2014;8:405–415. doi: 10.4081/gh.2014.29. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000092. https://doi.org/10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer MUG, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife. 2015;4:1–18. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer MUG, Sinka ME, Duda, et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data. 2015;2 doi: 10.1038/sdata.2015.35. https://doi.org/10.1038/sdata.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kamara F, Zhou G, et al. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003301. https://doi.org/10.1371/journal.pntd.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Berry PM, Dawson TP, Pearson RG. Selecting thresholds of occurrence in the prediction of species distributions. Ecography (Cop) 2005;28:385–393. [Google Scholar]
- Lover AA. Zika virus and microcephaly. Lancet Infect Dis. 2016;16:1331–1332. doi: 10.1016/S1473-3099(16)30462-5. [DOI] [PubMed] [Google Scholar]
- Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18:411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- Marcondes CB, de FF de Ximenes M. Zika virus in Brazil and the danger of infestation by aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. 2016;49:4–10. doi: 10.1590/0037-8682-0220-2015. [DOI] [PubMed] [Google Scholar]
- Messina JP, Pigott DM, Duda KA, et al. A global compendium of human dengue virus occurrence. Sci Data. 2014;1 doi: 10.1038/sdata.2014.4. https://doi.org/10.1038/sdata.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina JP, Kraemer MU, Brady OJ, et al. Mapping global environmental suitability for Zika virus. Elife. 2016;5:e15272. doi: 10.7554/eLife.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millichap JG. Zika virus infection and microcephaly. Pediatr Neurol Briefs. 2016;30:8. doi: 10.15844/pedneurbriefs-30-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlacker S, Shafa G, Aldahan AS, Shah VV, Samarkandy S, Nouri K. Origin of the Zika virus revealed: a historical journey across the world. Int J Dermatol. 2016;55:1369–1372. doi: 10.1111/ijd.13399. [DOI] [PubMed] [Google Scholar]
- Mohamed M, Mosha F, Mghamba J, et al. Epidemiologic and clinical aspects of a Rift Valley fever outbreak in humans in Tanzania, 2007. Am J Trop Med Hyg. 2010;83:22–27. doi: 10.4269/ajtmh.2010.09-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP, Vasconcelos PFC. Yellow fever. J Clin Virol. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Moutailler S, Krida G, Schaffner F, Vazeille M, Failloux A-B. Potential vectors of Rift Valley fever virus in the Mediterranean region. Vector Borne Zoonotic Dis. 2008;8:749–753. doi: 10.1089/vbz.2008.0009. [DOI] [PubMed] [Google Scholar]
- Murrell EG, Juliano Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mweya CN, Kimera SI, Kija JB, Mboera LEG. Predicting distribution of Aedes aegypti and Culex pipiens complex, potential vectors of Rift Valley fever virus in relation to disease epidemics in East Africa. Infect Ecol Epidemiol. 2013;3 doi: 10.3402/iee.v3i0.21748. https://doi.org/10.3402/iee.v3i0.21748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanyingi MO, Munyua P, Kiama SG, et al. A systematic review of Rift Valley fever epidemiology 1931–2014. Infect Ecol Epidemiol. 2015;5:1–12. doi: 10.3402/iee.v5.28024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Martínez Y, Patiño-Barbosa AM, Rodriguez-Morales AJ. Yellow fever in the Americas: the growing concern about new epidemics. F1000Res. 2017;6:398. doi: 10.12688/f1000research.11280.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupy C, Ollomo B, Kamgang B, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in central Africa. Vector Borne Zoonotic Dis. 2010;10:259–266. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41 doi: 10.1051/vetres/2010033. https://doi.org/10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. http://qgis.osgeo.org.
- Rao TR, Trpis M, Gillett JD, Teesdale C, Tonn RJ. Breeding places and seasonal incidence of Aedes aegypti, as assessed by the single-larva survey method. Bull World Health Organ. 1973;48:615–622. [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, Wilson AJ, Hay SI, Graham AJ. The global distribution of yellow fever and dengue. Adv Parasitol. 2006;62:181–220. doi: 10.1016/S0065-308X(05)62006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen L, Shroyer DA, Tesh RB, Freier JE, Lien JC. Transovarial transmission of dengue viruses by mosquitoes: Aedes albopictus and Aedes aegypti. Am J Trop Med Hyg. 1983;32:1108–1119. doi: 10.4269/ajtmh.1983.32.1108. [DOI] [PubMed] [Google Scholar]
- Shragai T, Tesla B, Murdock C, Harrington LC. Zika and chikungunya: mosquito-borne viruses in a changing world. Ann N Y Acad Sci. 2017 doi: 10.1111/nyas.13306. https://doi.org/10.1111/nyas.13306. [DOI] [PubMed]
- Thangamani S, Huang J, Hart CE, Guzman H, Tesh RB. Vertical transmission of Zika virus in Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2016;95:1169–1173. doi: 10.4269/ajtmh.16-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. WHO statement on the first meeting of the International Health Regulations Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. [30 November 2016];2016 http://www.who.int/mediacentre/news/statements/2016/%0A1st-emergency-committee-zika/en/
- WHO. Rift Valley fever: fact sheet. [5 June 2017];2016 http://www.who.int/mediacentre/factsheets/fs207/en/
- WHO. Yellow fever: situation report. [26 May 2017];2016 http://apps.who.int/iris/bitstream/10665/250661/1/yellowfeversitrep28Oct16-eng.pdf?ua=1.
- WHO. What is dengue? [23 May 2017];2017 http://www.who.int/denguecontrol/disease/en/
- WHO. Chikungunya: factsheet. [26 May 2017];2017 http://www.who.int/mediacentre/factsheets/fs327/en/
- World Health Organization (WHO) A global brief on vector-borne diseases. 2014 http://apps.who.int/iris/bitstream/10665/111008/1/WHO_DCO_WHD_2014.1_eng.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


