Abstract
Introduction
In the general population, medication nonadherence contributes to poorer outcomes. However, little is known about medication adherence among adults with chronic kidney disease (CKD). We evaluated the association of self-reported medication adherence with CKD progression and all-cause death in patients with CKD.
Methods
In this prospective observational study of 3305 adults with mild-to-moderate CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study, the baseline self-reported medication adherence was assessed by responses to 3 questions and categorized as high, medium, and low. CKD progression (50% decline in eGFR or incident end-stage renal disease) and all-cause death were measured using multivariable Cox proportional hazards.
Results
Of the patients, 68% were categorized as high adherence, 17% medium adherence, and 15% low adherence. Over a median follow-up of 6 years, there were 969 CKD progression events and 675 deaths. Compared with the high-adherence group, the low-adherence group experienced increased risk for CKD progression (hazard ratio = 1.27, 95% confidence interval = 1.05, 1.54) after adjustment for sociodemographic and clinical factors, cardiovascular medications, number of medication types, and depressive symptoms. A similar association existed between low adherence and all-cause death, but did not reach standard statistical significance (hazard ratio = 1.14 95% confidence interval = 0.88, 1.47).
Conclusion
Baseline self-reported low medication adherence was associated with an increased risk for CKD progression. Future work is needed to better understand the mechanisms underlying this association and to develop interventions to improve adherence.
Keywords: CKD, death, medication adherence, progression
Medication nonadherence has been recognized as a major barrier to disease treatment and contributes to disease progression, death, and increased health care costs in the United States.1 Medication nonadherence in patients with chronic conditions is common, with rates across studies averaging 50% and higher.2, 3, 4, 5 Moreover, an association between low medication adherence and increased risk of adverse clinical outcomes has been reported among patients with diabetes mellitus, hypertension, coronary artery disease, heart failure, and HIV.6, 7, 8, 9, 10
It is estimated that more than 26 million individuals in the United States have chronic kidney disease (CKD).11 The progression of CKD to end-stage renal disease (ESRD) is associated with high rates of morbidity and mortality and increased health care costs.12 Despite the magnitude of this problem and evidence suggesting that risk factor control is an important determinant of CKD progression,13 very little is known regarding the impact of medication adherence on CKD progression. To address this knowledge gap, we used data from the prospective Chronic Renal Insufficiency Cohort (CRIC) Study to evaluate the association of self-reported medication adherence with CKD progression and all-cause death. We hypothesized that low medication adherence would be associated with higher risk for CKD progression among persons with CKD.
Methods
Study Participants
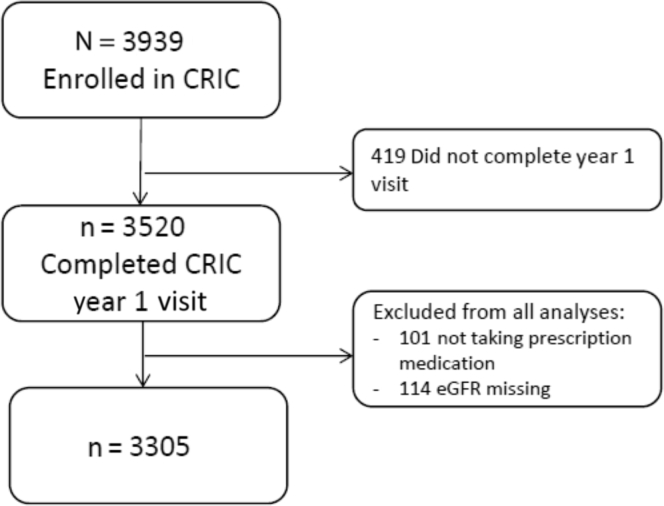
The CRIC Study is an ongoing multicenter, prospective, observational study of risk factors for CKD and cardiovascular disease progression. The design, methods and baseline characteristics of study participants have been previously described.14, 15, 16 From 2003 to 2008, the study recruited 3939 adult participants aged 21 to 74 years with an estimated glomerular filtration rate (eGFR) of 20 to 70 ml/min per 1.73 m2 at 7 clinical centers in the United States. Exclusion criteria included individuals unable to provide consent, institutionalized, pregnant, and with certain severe chronic conditions.14 Self-reported medication adherence was evaluated at the year-1 study visit, which was considered to be the baseline for this study. Figure 1 delineates the derivation of the study population. The study protocol adhered to the Declaration of Helsinki and was approved by the institutional review boards of participating centers. All participants provided written informed consent.
Figure 1.
Analytic cohort flowchart. CRIC, Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate.
Measurements
Information about sociodemographic variables, medical history, psychosocial factors, and medications were obtained by self-report. Height, weight, and waist circumference were measured.15 Body mass index was calculated as weight in kilograms divided by height in meters squared. At each annual clinic visit, 3 seated blood pressure (BP) measurements were obtained using a Tycos Classic Hand Aneroid cuff and sphygmomanometer (Welch Allyn, Skaneateles Falls, NY) following a standardized protocol. The mean of all BP measurements was used as the BP value for that visit. Hypertension was defined as mean BP ≥ 140/90 mm Hg or use of antihypertensive medication. Hemoglobin A1c (HbA1c) was measured using high-performance liquid chromatography (BioRad, Hercules, CA). Diabetes was defined as fasting plasma glucose of ≥126 mg/dl or use of insulin or oral hypoglycemic medications. Depressive symptoms were assessed using the Beck Depression Inventory–II (BDI), and a score of ≥11 was considered to be indicative of clinically meaningful depressive symptoms based on a recent study in CKD patients.17 GFR was estimated annually using a CRIC-specific equation.18 A 24-hour urine sample collected at study entry was used to measure urine protein and creatinine.
Predictor
Medication adherence was assessed based on the responses to 3 questions as follows: (i) “In the past week, how many days did you forget to take a pill?” (ii) “In the past week, how many days did you not take a pill on purpose?” (iii) “In the past week, how many days did you add an extra pill?”19, 20 The 3 possible responses to each question were: 0 days, 1 day, and 2 days or more. These 3 items were used in a study by Choo et al.19 Because this is not a validated questionnaire and there is no scoring system for these items, we derived our own approach to scoring. We ranked forgetting to take a pill as the least significant form of nonadherence, and purposefully not taking a medicine or adding a medication as the most significant forms of nonadherence, based on a prior study that used a modified version of these questions.20 We divided the score into 3 adherence categories: high, medium, and low. Individuals with a response of “0 days” to all 3 items were categorized as high adherence; individuals reporting only forgetting a pill at least 1 day in the past week were categorized as medium adherence; and individuals who reported purposefully adding or missing a pill 1 day or more in the past week were categorized as low adherence.
Outcome Assessment
The primary outcomes for this study were (i) CKD progression, defined as 50% decline in eGFR from baseline or occurrence of ESRD (i.e., receipt of long-term dialysis therapy or kidney transplantation); and (ii) death from any cause. As secondary outcomes we also evaluated the following: (i) eGFR slope; (ii) baseline systolic BP > 140 or diastolic BP > 90 mm Hg; and (iii) baseline HbA1c > 7%. Ascertainment of ESRD was by self-report every 6 months and supplemented by cross-linkage with the US Renal Data System. The outcome of all-cause death was ascertained from reports of next of kin, death certificates, hospital records, and linkage with the Social Security Death Master File. Participants were followed up until the occurrence of death, withdrawal from study, or March 2016, when the database was locked for analysis.
Statistical Analysis
Descriptive statistics were used to summarize baseline participant characteristics using mean ± SD or median and interquartile range (IQR) for continuous variables, and frequency distribution and percentage for categorical variables. Baseline characteristics were compared between groups using a t test or analysis of variance accordingly. A 2-sided P value of less than 0.05 was considered statistically significant. There were 432 participants who had missing data for at least 1 covariate (260 urine protein, 129 health insurance, 37 BDI, and 6 other). Therefore, missing data were imputed using multiple imputation with 10 iterations. Cox proportional hazard regression analyses were used to estimate hazard ratios for CKD progression and all-cause death with corresponding 95% confidence intervals (CI) by adherence status. The high adherence group served as the reference category. We fitted models that adjusted sequentially for potential explanatory variables for the outcome. Model 1 adjusted for clinical center and sociodemographic factors (age, gender, race/ethnicity, education, marital status, and health insurance). Model 2 adjusted for the variables in model 1 and clinical factors (diabetes, hypertension, cardiovascular disease, body mass index, eGFR, and proteinuria), cardiovascular medications (antiplatelet, angiotensin-converting enzyme [ACE] inhibitor, or angiotensin receptor blocker [ARB], β-blocker, and statin), number of medications types per day, and Beck Depression Inventory−II score. Multivariable logistic regression was used to evaluate the cross-sectional association between adherence and baseline BP > 140/90 and HbA1c. In addition, we evaluated the association between level of adherence and eGFR slope using linear mixed-effects regression models. All analyses were performed using SAS version 9.3 software (SAS Institute, Cary, NC).
Results
Baseline Characteristics by Adherence Category
The mean age of the participants was 59 years; 45% were women; 49% had diabetes; the mean eGFR was 42 ml/min per 1.73 m2; the median proteinuria was 140 mg/d; and participants reported taking a mean of 9.4 different medications. Of the 3305 participants, 2258 (68%) were categorized as having high adherence, 570 (17%) as having medium adherence, and 477 (15%) as having low adherence (Table 1). The distribution of responses for each adherence question is detailed in Supplementary Table S1. As compared to the high-adherence group, individuals categorized as having low adherence were significantly more likely to be younger, female, non-Hispanic black or Hispanic, single, and with lower socioeconomic status. In addition, the low-adherence group was more likely to have diabetes, BP ≥ 140/90, higher body mass index, lower eGFR, higher proteinuria, higher Beck Depression Inventory scores, and to be taking a greater number of medication types. Overall, a similar pattern was observed when comparing the medium-adherence group to the high-adherence group.
Table 1.
Baseline characteristics and medication adherence status of chronic kidney disease patients
| Variable | Medication adherence |
P value | ||
|---|---|---|---|---|
| High | Medium | Low | ||
| N | 2258 | 570 | 477 | |
| Age | 60.1 (10) | 57.1 (11) | 57.4 (11) | <0.001 |
| Male | 1292 (57) | 288 (50) | 238 (50) | <0.001 |
| Non-Hispanic white | 1034 (45) | 243 (43) | 157 (33) | <0.001 |
| Non-Hispanic black | 915 (41) | 234 (41) | 237 (49) | 0.001 |
| Hispanic | 228 (10) | 68 (12) | 66 (14) | 0.042 |
| Other | 86 (4) | 25 (4) | 20 (4) | 0.788 |
| Annual income < $20,000 | 630 (28) | 172 (30) | 181 (38) | <0.001 |
| Less than high school education | 414 (18) | 108 (19) | 113 (24) | 0.026 |
| Currently married | 1314 (58) | 292 (51) | 226 (48) | <0.001 |
| Health insurance | 2032 (93) | 499 (91) | 407 (89) | 0.003 |
| Current smoking | 263 (12) | 81 (14) | 69 (14) | 0.095 |
| Diabetes | 1122 (50) | 261 (46) | 271 (57) | 0.002 |
| BP > 140/90 | 525 (23) | 139 (24) | 133 (28) | 0.098 |
| Body mass index, kg/m2 | 31.8 (7.4) | 32.4 (7.7) | 34.08 (9.1) | <0.001 |
| Cardiovascular disease | 801 (35) | 202 (35) | 194 (41) | 0.091 |
| Number of medications per day | 9.39 (4.4) | 9.05 (4.5) | 9.82 (5) | <0.001 |
| Number of antihypertensive agents per day | 2.7 (1.5) | 2.6 (1.5) | 2.8 (1.5) | <0.001 |
| ACE-I/ARB | 1625 (72) | 398 (70) | 329 (69) | 0.381 |
| Antiplatelet agent | 1197 (53) | 269 (47) | 209 (44) | <0.001 |
| Statin | 1408 (62) | 326 (58) | 264 (56) | 0.005 |
| BDI ≥ 11 | 482 (22) | 183 (33) | 183 (39) | <0.001 |
| Hemoglobin (g/dl) | 12.82 (2) | 12.83 (2) | 12.55 (2) | <0.001 |
| Albumin (g/dl) | 4.1 (0.4) | 4.0 (0.4) | 4.0 (0.5) | <0.001 |
| Calcium | 9.32 (0.50) | 9.31 (0.545) | 9.21 (0.53) | <0.001 |
| Phosphate | 3.68 (0.63) | 3.74 (0.713) | 3.77 (0.67) | <0.001 |
| Total cholesterol | 178.15 (42) | 187.77 (45) | 187.41 (46) | <0.001 |
| LDL cholesterol | 96.57 (33) | 104.04 (37) | 104.74 (37) | <0.001 |
| Hemoglobin A1c | 6.51 (1.4) | 6.53 (1.5) | 6.69 (1.6) | <0.001 |
| eGFR | 42.3 (15.3) | 42.6 (16) | 40.04 (16.4) | <0.001 |
| Urine protein, g/d, median (IQR) | 0.12 (0.05, 0.55) | 0.15 (0.05, 0.86) | 0.21 (0.05, 1.28) | |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BDI, Beck Depression Inventory–II; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LDL, low-density lipoprotein.
Data are given as mean SD or as number (percentage) unless otherwise noted.
Outcomes
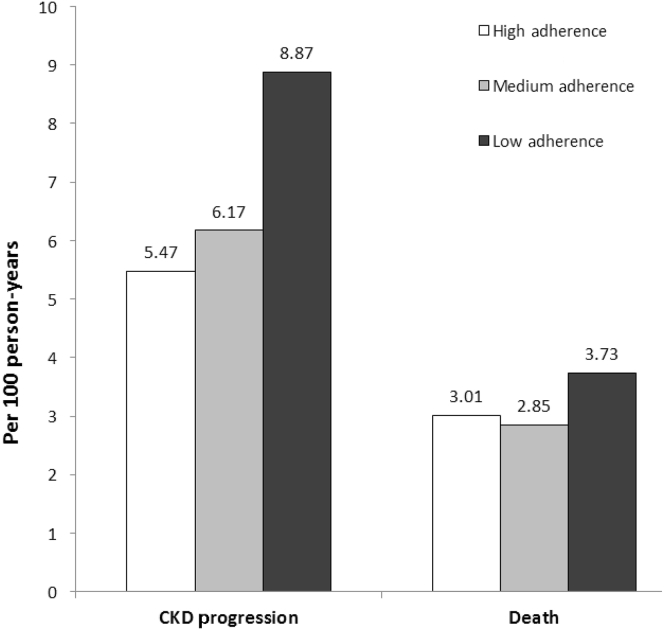
Over a median follow-up of 6 years, participants experienced 969 CKD progression events, and there were 675 deaths. Of note, there was a graded increase in event rates for CKD progression across the categories of adherence from high to low (5.82, 6.48, and 9.52 per 100 person-years, respectively) (Figure 2). The corresponding rates of all-cause death were 3.08, 2.97, and 3.99 per 100 person-years, respectively.
Figure 2.
Rates of chronic kidney disease (CKD) progression and all-cause death, by level of medication adherence.
As compared to the high-adherence group, the low-adherence group experienced a significant increased risk for CKD progression (adjusted hazard ratio [HR] = 1.27, 95% confidence interval [CI] = 1.05, 1.54) (Table 2). The risk for adverse outcomes was not significantly increased for the medium-adherence group. In multivariable analysis, compared to high adherence, low adherence was associated with a higher rate of eGFR decline over time (mean difference of −0.43 ml/min per 1.73m2 per year, P < 0.001) and the medium-adherence group with a similar rate (0.04 ml/min per 1.73 m2 per year, P = 0.56). There was a nonsignificant trend for higher risk of death in the low-adherence group (adjusted HR = 1.14, 95% CI = 0.88, 1.47). On cross-sectional multivariable regression analysis, compared to high adherence, neither medium nor low adherence was associated with baseline BP > 140/90 (odds ratio [OR] = 1.11, 95% CI = 0.79, 1.55, and OR = 1.08, 95% CI = 0.82, 1.41, respectively) or HbA1c > 7% (OR = 1.21, 95% CI = 0.82, 1.79, and OR = 0.88, 95% CI 0.65, 1.20, respectively).
Table 2.
Association of medication adherence status with chronic kidney disease (CKD) progression and all-cause death (n = 3305)
| Outcome | Predictor | Model 1 | Model 2 |
|---|---|---|---|
| CKD progression | High adherence (reference) | 1.00 | 1.00 |
| Medium adherence | 1.00 (0.83, 1.20) | 1.08 (0.89, 1.31) | |
| Low adherence | 1.43 (1.20, 1.70) | 1.27 (1.05, 1.54) | |
| Death | High adherence (reference) | 1.00 | 1.00 |
| Medium adherence | 1.05 (0.83, 1.32) | 0.98 (0.76,1.28) | |
| Low adherence | 1.34 (1.07,1.68) | 1.14 (0.88, 1.47) |
Data are adjusted hazard ratios with 95% confidence intervals in parentheses.
Model 1: clinical center and sociodemographic factors (age, gender, race/ethnicity, education, health insurance, and marital status).
Model 2: Model 1 plus clinical factors (diabetes, hypertension, cardiovascular disease, body mass index, estimated glomerular filtration rate, proteinuria), cardiovascular medications (angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, β-blocker, statin), number of types of medications per day, and Beck Depression Inventory–II score.
Discussion
In the largest study to examine the association of medication adherence with CKD progression in the United States, we found that approximately one-third of individuals reported suboptimal medication adherence. Moreover, low medication adherence was associated with an increased risk for CKD progression. These findings suggest that low medication adherence is an underrecognized but important risk factor for CKD progression.
It has been well established that low medication adherence is associated with increased risk of adverse clinical outcomes among non-CKD patients with other chronic diseases such as diabetes mellitus, hypertension, coronary artery disease, heart failure, and HIV.6, 7, 8, 9, 10, 21 For example, Gehi et al. found self-reported medication nonadherence to be associated with more than a 2-fold increased risk for cardiovascular events among patients with stable coronary artery disease.21 However, the impact of medication adherence on outcomes has not been comprehensively evaluated in the CKD population, despite the fact that CKD patients have multiple comorbidities requiring complex medication regimens.22, 23, 24
Others have reported a similar prevalence of nonadherence in CKD populations. In the Reasons for Geographic and Racial Differences in Stroke Study (REGARDS), self-reported medication nonadherence was present in 27.7% of individuals with CKD and was associated with increased odds for uncontrolled hypertension.25 In another recently published cross-sectional analysis of CKD patients, Hsu et al. found lower medication adherence in approximately 50% of participants, which was associated with a higher prevalence of adverse safety events and potential medication-related dosing problems.20 Furthermore, we found that individuals with low adherence were more likely to be racial/ethnic minorities, have more co-morbidities, and have more symptoms of depression, suggesting that clinicians may need to be more vigilant in the assessment of adherence in patients with these characteristics.
There have been very few studies evaluating the impact of medication adherence on outcomes in CKD patients. In a large population-based study of more than 180,000 hypertensive patients living in Quebec, Roy et al. found that medication nonadherence assessed using pharmacy records was associated with an increased risk of ESRD, but in sensitivity analyses this association was not significant in CKD patients.26 In contrast, a recent study that included 332 Stage 3 to 4 CKD patients in Thailand found that low adherence was associated with increased risk for CKD progression.27 Our results reinforce the findings of this study in a larger diverse U.S. cohort.
In contrast to our findings regarding CKD progression, we observed a trend for higher risk of death in the low-adherence group that was not significant. The reasons for this null finding are unclear. A previous meta-analysis by Simpson et al. in non-CKD populations included several studies which similarly reported nonsignificant associations between low adherence and mortality.7, 28, 29, 30 However, the combined data used for the meta-analysis revealed a significant association to be present, suggesting a lack of power in the individual studies.28
Our findings suggest that the effects of low medication adherence observed in other populations can be extended to the CKD population. CKD patients have multiple comorbidities that require pharmacologic management for control. In particular, hypertension control and use of renin−angiotensin−aldosterone system blockers have a major impact on slowing CKD progression.13 In addition to the findings from the REGARDS study discussed earlier,25 a recently published study using data from the African American Study of Kidney Disease and Hypertension (AASK) reported that lower medication adherence was associated with higher systolic blood pressure visit-to-visit variability,31 a factor that is associated with increased risk for cardiovascular events and death.32 In addition to the association of adherence with disease control, it has also been suggested that individuals who report adherence to medications may engage in healthier behaviors.28 This concept has been termed the “healthy adherer” effect and is supported by the consistent association between nonadherence to placebo and adverse outcomes in clinical trials.33
We found that individuals with intentional nonadherence (purposefully missing or adding a pill) were particularly at increased risk for CKD progression. Others have also found a strong association between intentional nonadherence and adverse outcomes. As described earlier, Hsu et al. found that this type of nonadherence was associated with an increased risk for adverse safety events and potential medication-related dosing problems.20 Similarly, in a population of Korean Americans with hypertension, intentional nonadherence was more strongly associated with uncontrolled hypertension than unintentional nonadherence.34 Distinguishing between intentional and nonintentional nonadherence may help in the clinical approach to nonadherence.35, 36 Intentional nonadherence is proposed to be influenced by the balance of an individual’s reasons for and against taking medication.35 To effectively address this type of nonadherence, one must take into account an individual’s perceptions (e.g., regarding medication side effects), beliefs, and knowledge.37
Our study has several strengths, including the large and diverse sample, prospective design, long-term follow-up, and detailed characterization of a wide range of patient features. However, some limitations must be considered. First, the measurement of medication adherence was assessed by self-report, which is known to have low sensitivity and to underestimate nonadherence.2, 38 However, currently there is not a gold standard to assess medication adherence, and even objective measures of adherence have significant limitations.1, 39 Furthermore, self-report has been found to have a high negative predictive value, performs as well as other objective measures of adherence, and is practical, less intrusive, and cost-effective.1, 2, 38, 39 Another significant limitation was that the study did not use a validated adherence instrument. For this reason, we had to derive our own approach to scoring. In addition, several factors that may influence medication adherence including self-efficacy, disease knowledge, and medication side effects were not assessed. Finally, our findings are subject to residual confounding and bias that may occur in observational studies.
In conclusion, in this study, self-reported medication nonadherence was highly prevalent and more common in racial/ethnic minorities and individuals with greater comorbidity burden. Furthermore, we found that low medication adherence was an independent predictor of CKD progression. Our findings suggest that medication nonadherence may represent a modifiable risk factor for CKD progression. Future work is needed to understand and to develop effective interventions to improve adherence in the CKD population.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Research idea and study design: JPL, EAC, ACR, CD; data acquisition: EAC, ACR, MF, MKW, EL, AO, JS, CD, JPL; data analysis/interpretation: EAC, ACR, JC, MF, MKW, JWK, SL, EL, AO, ACP, LKS, JS, CD, JPL; statistical analysis: J.C.; supervision or mentorship: JPL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. EAC and JPL take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
Funding for the CRIC Study was obtained under a cooperative agreement from the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente Northern California NIH/NCRR UCSF-CTSI UL1 RR-024131. JPL is funded by the NIDDK K24DK092290. EAC-C is funded by a Research Supplement to Promote Diversity in Health-Related Research U01-DK060980. ACC is funded by NIDDK K23DK094829.
Footnotes
Table S1. Baseline characteristics by adherence status.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Contributor Information
Esteban A. Cedillo-Couvert, Email: ecedillo@uic.edu.
CRIC Study Investigators:
Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, John W. Kusek, James P. Lash, Mahboob Rahman, Panduranga S. Rao, and Raymond R. Townsend
Supplementary Material
Baseline characteristics by adherence status.
References
- 1.Osterberg L., Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2.Haynes R.B., McDonald H.P., Garg A.X. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288:2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.K., Grace K.A., Taylor A.J. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 4.Jackevicius C.A., Mamdani M., Tu J.V. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; Geneva, Switzerland: 2003. Adherence to long-term therapies: evidence for action. [Google Scholar]
- 6.Ho P.M., Rumsfeld J.S., Masoudi F.A. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz R.I., Viscoli C.M., Berkman L. Treatment adherence and risk of death after a myocardial infarction. Lancet. 1990;336:542–545. doi: 10.1016/0140-6736(90)92095-y. [DOI] [PubMed] [Google Scholar]
- 8.Granger B.B., Swedberg K., Ekman I. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 9.Chesney M. Adherence to HAART regimens. AIDS Patient Care STDs. 2003;17:169–177. doi: 10.1089/108729103321619773. [DOI] [PubMed] [Google Scholar]
- 10.Krousel-Wood M., Holt E., Joyce C. Differences in cardiovascular disease risk when antihypertensive medication adherence is assessed by pharmacy fill versus self-report: the Cohort Study of Medication Adherence among Older Adults (CoSMO) J Hypertens. 2015;33:412–420. doi: 10.1097/HJH.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 12.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2015. 2015 USRDS annual data report: epidemiology of kidney disease in the United States. [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 14.Feldman H.I., Appel L.J., Chertow G.M. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 15.Lash J.P., Go A.S., Appel L.J. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer M.J., Go A.S., Lora C.M. CKD in Hispanics: baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedayati S.S., Minhajuddin A.T., Toto R.D. Validation of depression screening scales in patients with CKD. Am J Kidney Dis. 2009;54:433–439. doi: 10.1053/j.ajkd.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson A.H., Yang W., Hsu C. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choo P.W., Rand C.S., Inui T.S. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hsu K.L., Fink J.C., Ginsberg J.S. Self-reported medication adherence and adverse patient safety events in CKD. Am J Kidney Dis. 2015;66:621–629. doi: 10.1053/j.ajkd.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gehi A.K., Ali S., Na B. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. Arch Intern Med. 2007;167:1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKillop G., Joy J. Polypharmacy and nonadherence to prescribed medicines in CKD. Br J Ren Med. 2013;18:9–11. [Google Scholar]
- 23.Burnier M., Pruijm M., Wuerzner G. Drug adherence in chronic kidney diseases and dialysis. Nephrol Dial Transplant. 2015;30:39–44. doi: 10.1093/ndt/gfu015. [DOI] [PubMed] [Google Scholar]
- 24.Magacho E.J.C., Ribeiro L.C., Chaoubah A. Adherence to drug therapy in kidney disease. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol. 2011;44:258–262. doi: 10.1590/s0100-879x2011007500013. [DOI] [PubMed] [Google Scholar]
- 25.Muntner P., Judd S.E., Krousel-Wood M. Low medication adherence and hypertension control among adults with CKD: data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2010;56:447–457. doi: 10.1053/j.ajkd.2010.02.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy L., White-Guay B., Dorais M. Adherence to antihypertensive agents improves risk reduction of end-stage renal disease. Kidney Int. 2013;84:570–577. doi: 10.1038/ki.2013.103. [DOI] [PubMed] [Google Scholar]
- 27.Tangkiatkumjai M., Walker D.-M., Praditpornsilpa K. Association between medication adherence and clinical outcomes in patients with chronic kidney disease: a prospective cohort study. Clin Exp Nephrol. 2016;21:504–512. doi: 10.1007/s10157-016-1312-6. [DOI] [PubMed] [Google Scholar]
- 28.Simpson S.H., Eurich D.T., Majumdar S.R. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallagher E.J., Viscoli C.M., Horwitz R.I. The relationship of treatment adherence to the risk of death after myocardial infarction in women. JAMA. 1993;270:742–744. [PubMed] [Google Scholar]
- 30.Compliance and adverse event withdrawal: their impact on the West of Scotland Coronary Prevention Study. Eur Heart J. 1997;18:1718–1724. doi: 10.1093/oxfordjournals.eurheartj.a015165. [DOI] [PubMed] [Google Scholar]
- 31.Hong K., Muntner P., Kronish I. Medication adherence and visit-to-visit variability of systolic blood pressure in African Americans with chronic kidney disease in the AASK Trial. J Hum Hypertens. 2016;30:73–78. doi: 10.1038/jhh.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothwell P.M., Howard S.C., Dolan E. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 33.Irvine J., Baker B., Smith J. Poor adherence to placebo or amiodarone therapy predicts mortality: results from the CAMIAT study. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial. Psychosom Med. 1999;61:566–575. doi: 10.1097/00006842-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Kim E.-Y., Han H.-R., Jeong S. Does knowledge matter? Intentional medication nonadherence among middle-aged Korean Americans with high blood pressure. J Cardiovasc Nurs. 2007;22:397–404. doi: 10.1097/01.JCN.0000287038.23186.bd. [DOI] [PubMed] [Google Scholar]
- 35.Wroe A.L. Intentional and unintentional nonadherence: a study of decision making. J Behav Med. 2002;25:355–372. doi: 10.1023/a:1015866415552. [DOI] [PubMed] [Google Scholar]
- 36.Lehane E., McCarthy G. Intentional and unintentional medication non-adherence: a comprehensive framework for clinical research and practice? A discussion paper. Int J Nurs Stud. 2007;44:1468–1477. doi: 10.1016/j.ijnurstu.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Rosenstock I.M. The health belief model and preventive health behavior. Health Educ Monogr. 1974;2:354–386. [Google Scholar]
- 38.Rolley J.X., Davidson P.M., Dennison C.R. Medication adherence self-report instruments: implications for practice and research. J Cardiovasc Nurs. 2008;23:497–505. doi: 10.1097/01.JCN.0000338931.96834.16. [DOI] [PubMed] [Google Scholar]
- 39.Inui T.S., Carter W.B., Pecoraro R.E. Screening for noncompliance among patients with hypertension: is self-report the best available measure? Med Care. 1981;19:1061–1064. doi: 10.1097/00005650-198110000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics by adherence status.