Abstract
Introduction
Markers of oxidative stress increase with age and are prevalent with chronic kidney disease. However, the role of oxidative stress markers as predictors for kidney function decline in the general population is unclear.
Methods
We investigated whether a baseline urinary excretion of oxidative DNA damage (8-oxo-7,8-dihydro-2′-deoxyguanosine [8-oxodG]) and oxidative RNA damage (8-oxo-7,8-dihydroguanosine [8-oxoGuo]) was associated with the age-related glomerular filtration rate (GFR) decline or incident low-grade albuminuria during a median of 5.6 years of follow-up. In the Renal Iohexol Clearance Survey in the Sixth Tromsø Study, we measured GFR using iohexol clearance in 1591 participants without renal disease, diabetes, or cardiovascular disease. Low-grade albuminuria was defined as an albumin-creatinine ratio >1.13 mg/mmol.
Results
The mean (SD) annual GFR change was −0.84 (2.00) ml/min per 1.73 m2 per year. In linear mixed models, urinary 8-oxodG and 8-oxoGuo levels were not associated with the GFR change rate. In a multivariable adjusted logistic regression model, a baseline urinary 8-oxoGuo in the highest quartile was associated with an increased risk of low-grade albuminuria at follow-up (odds ratio: 2.64; 95% confidence interval: 1.50–4.65). When the highest quartile of urinary 8-oxoGuo was added to the baseline model, the area under the receiver operating characteristics curve for predicting low-grade albuminuria at follow-up improved from 0.67 to 0.71 (P = 0.002).
Conclusion
Oxidative stress measured as urinary 8-oxoGuo excretion was independently associated with incident low-grade albuminuria, but neither 8-oxoGuo nor 8-oxodG predicted an accelerated age-related GFR decline in a cohort representative of the middle-aged general population during almost 6 years of follow-up.
Keywords: aging, albuminuria, epidemiology, GFR decline, oxidative stress, kidney function decline
Age-related glomerular filtration rate (GFR) decline is an important cause of the high prevalence of chronic kidney disease (CKD) found in old age.1, 2 CKD is a strong risk factor for cardiovascular disease, end-stage renal disease, and mortality3; however, there is a considerable interindividual variation in the age-related GFR decline, even after accounting for traditional CKD risk factors, such as hypertension and diabetes.4, 5 Identifying novel risk factors for early kidney dysfunction, such as accelerated GFR decline or low-grade albuminuria, may suggest underlying pathologic mechanisms and prompt the development of an early and targeted treatment for the prevention of CKD.6
Oxidative stress is defined as a condition in which the generation of reactive species exceeds the antioxidant repair and defense system, leading to tissue damage. It increases with age and is associated with the pathogenesis of several age-related chronic diseases.7 Experimental studies show that oxidative stress induces inflammation, endothelial dysfunction, mitochondrial dysfunction, fibrosis, and accelerated kidney function decline in animal kidneys.8, 9, 10 In humans, cross-sectional studies show that higher levels of oxidative stress markers are associated with an increased severity of CKD.11, 12, 13, 14, 15, 16 Particularly, oxidative damage to mitochondrial DNA, expressed by the formation of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), is proposed to promote age-related degenerative processes in the kidneys.10, 17, 18 The proximal tubular cells in the kidneys are particularly susceptible to oxidative DNA damage, due to their large number of mitochondria. Urinary levels of 8-oxodG and 8-oxo-7,8-dihydroguanosine (8-oxoGuo) are established markers of oxidative DNA and RNA damage, respectively, as well as total systemic oxidative stress.19, 20 Prospective studies of the association of urinary 8-oxodG and 8-oxoGuo excretion with kidney dysfunction are scarce and have not been conducted in the general population. We measured GFR as iohexol clearance at baseline and follow-up in the Renal Iohexol Clearance Survey Follow-Up Study (RENIS-FU), which is the largest longitudinal population-based cohort with accurate measurements of GFR.21 The aim of this study was to investigate whether higher urinary levels of 8-oxodG and 8-oxoGuo predicted a faster age-related GFR decline or low-grade albuminuria during 6 years of follow-up.
Materials and Methods
Study Participants
The Renal Iohexol Clearance Survey in the Sixth Tromsø Study (RENIS-T6) is a substudy of the population-based Sixth Tromsø Study and was performed between October 2007 and June 2009. It included a representative sample from the general population of 1627 persons aged 50 to 62 years without self-reported cardiovascular disease, diabetes, or kidney disease. There were 826 women and 801 men. A detailed description of the study is published elsewhere.22 All of the participants in the RENIS-T6 were invited to the RENIS-FU, except for 23 participants who had died and 7 who had a possible delayed allergic reaction to iohexol.
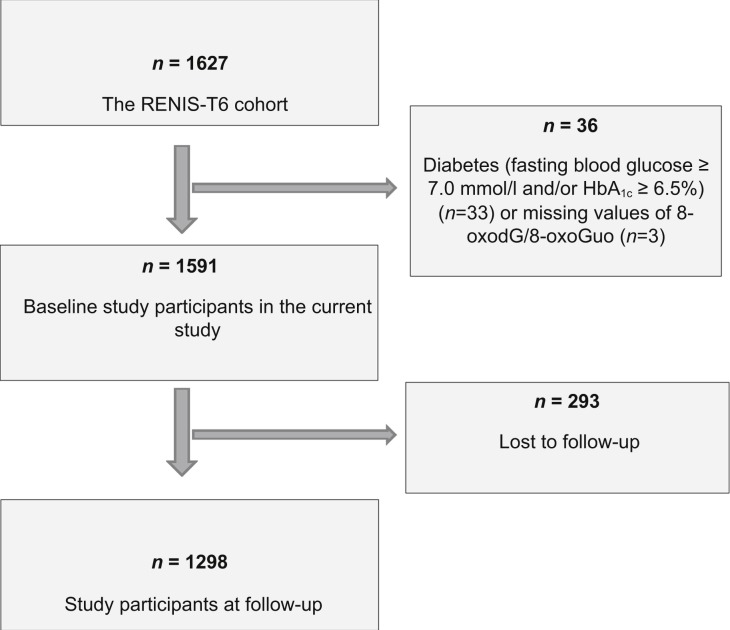
The RENIS-FU was performed between September 2013 and January 2015. Of the 1597 subjects invited, 1368 gave a positive response. Five participants were excluded because of problems with the cannulation of the antecubital vein, and 39 participants did not meet at their appointments. We excluded 33 participants who were diagnosed with diabetes at baseline (fasting glucose ≥ 7.0 mmol/l and/or hemoglobin A1c [HbA1c] ≥6.5%) and an additional 3 participants with missing 8-oxodG and 8-oxoGuo values, leaving 1298 (81%) participants with a follow-up measurement in the current study (Figure 1).
Figure 1.
Flowchart of the study participants in the Renal Iohexol Clearance Survey in the Sixth Tromsø Study (RENIS-T6) and the RENIS Follow-Up Study.
The study adhered to the Declaration of Helsinki, and the Regional Ethics Committee of Northern Norway approved it. All participants gave informed written consent.
Data
The measurements in RENIS-T6 and RENIS-FU were performed at the Clinical Research Unit at the University Hospital of North Norway. The participants met between 8 and 10 AM. They were instructed to avoid large meals with meat and to avoid taking nonsteroidal anti-inflammatory drugs during the preceding 48 hours, in addition to fast and abstain from smoking from midnight before the examination. Participants with an acute illness were rescheduled to another appointment. All participants answered a questionnaire about their current alcohol, tobacco, and medication use. Tobacco use was dichotomized as current smoker or as a continuous variable (the number of cigarettes currently smoked) in the regression model analyses. Alcohol use was dichotomized as the use of alcohol more than once weekly.
Measurements
The urinary levels of 8-oxodG and 8-oxoGuo were measured by liquid chromatography tandem mass spectrometry. The Supplemental Material provides a more detailed description of the analysis. A second void morning spot urine after an overnight fast was collected at baseline in RENIS-T6. The samples were centrifuged, and the supernatant was immediately put on ice and frozen at −80°C within a few hours. The frozen urine samples were thawed and mixed before the liquid chromatography tandem mass spectrometry analysis. The 8-oxodG and 8-oxoGuo were obtained from Cayman Chemical Co. (Ann Arbor, MI). The internal standard 8-oxodG (15N5, 98%) was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). Water was obtained from a Millipore Advantage Milli-Q system (Millipore SAS, Molsheim, France). Synthetic urine was prepared according to the method of Kark et al.23 with some modifications. The samples were prepared by adding 50 μl of a 50-nM aqueous internal standard (8-oxodG, 15N5, 98%) to 60 μl urine in a 1-ml collection plate (Waters, Milford, MA). To each of the wells, 500 μl 0.1% formic acid was then added and mixed. The samples were analyzed by liquid chromatography tandem mass spectrometry using the Waters Acquity UPLC I-class system with an autosampler and a binary solvent delivery system (Waters, Milford, MA) interfaced to the Waters Xevo TQ-S benchtop tandem quadrupole mass spectrometer (Waters, Manchester, UK).
The concentrations of 8-oxodG and 8-oxoGuo were normalized for urinary creatinine (8-oxodGUCR and 8-oxoGuoUCR). The between-day coefficients of variation (CVs) for 8-oxodGUCR and 8-oxoGuoUCR were ≤5.7% at 21 different days and were calculated using the quality controls (QCs). The concentrations for the high and low QCs were 50 nmol/l and 5 nmol/l, respectively. The intraday precision values were calculated by assaying 6 different urine samples 6 times on the same day. The CVs for both markers were <5.5%. The interassay CVs for 8-oxodGUCR and 8-oxoGuoUCR during the measurements were 4.7% and 3.8% for the lowest QC and 4.1% and 5.1% for the highest QC, respectively. The intra-assay CVs for 8-oxodGUCR and 8-oxoGuoUCR were 3.6% and 3.7% for the lowest QC and 2.4% and 3.3% for the highest QC, respectively.
The GFR was measured using a single-sample clearance of plasma iohexol at both baseline and follow-up. The single-sample iohexol clearance was validated against gold standard methods for measuring the GFR.24 A detailed description of the GFR measurement is published elsewhere.22 The CV for the analysis was 3.0% at baseline and 3.1% at follow-up. There was a mean difference of 2.28 ml/min per 1.73 m2 between the baseline GFR measurements in RENIS-T6 and the repeated baseline measurements of the thawed samples. Accordingly, all of the baseline GFR measurements were corrected by adding this difference to the baseline values, as described previously.21 A random sample of 5.5% of the subjects in the follow-up study had an additional GFR measurement after 2 weeks and within 2 months to investigate intra-individual variation in the measurements. The mean CV (95% confidence interval) for the intra-individual (day-to-day) variation of the GFR was 4.2% (3.4%–4.9%).21In the RENIS-T6 and RENIS-FU, 3 samples of the first-void morning spot urine were collected on separate days. The urinary excretion of albumin and creatinine were measured in unfrozen urine samples. The urine albumin to creatinine ratio (UACR) was calculated for each of the urine samples, and the mean values were used in the analysis. Low-grade albuminuria was defined as a UACR >1.13 mg/mmol (>10 mg/g), according to the “high-normal albuminuria” cutoff proposed by the CKD Prognosis Consortium.25, 26 Blood pressure, fasting serum glucose, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and hemoglobin A1c were measured with standard methods as previously described.27
Statistical Analyses
The baseline and follow-up characteristics are presented as the means (SD), medians (interquartile range) for skewed variables, and numbers (percent). Differences between the participants included in the follow-up study and those lost to follow-up were tested with the 2 independent samples t-test, Wilcoxon-Mann-Whitney test, and χ2 or Fisher exact test, as appropriate. A linear trend over the increasing quartiles of 8-oxodG and 8-oxoGuo was tested with linear and median regression for continuous variables and with logistic regression for dichotomous variables. For the mixed model and the logistic regression analyses, we used continuous and quartiles of 8-oxodGUCR and 8-oxoGuoUCR levels as independent variables in separate models. Quartile 1 was used as a reference. In addition, we compared persons with the highest quartile of 8-oxodGUCR/8-oxoGuoUCR with the 3 lowest quartiles.
The associations of the oxidative stress markers with the annual GFR change were examined in a linear mixed regression model with a random intercept and slope.28 GFR, standardized to body surface area (ml/min per 1.73 m2), was used as the dependent variable. The time from baseline to follow-up was used as the time variable. The associations of the oxidative stress markers with the GFR decline rate were modeled as interactions between the oxidative stress markers and the time variable. All of the 1591 subjects at baseline, as well as the extra follow-up measurements of the GFR for 5.5% of the participants, were included in the model because a linear mixed regression model allows missing observations at 1 or more points in time.29 We included 3 models that were adjusted as follows: model 1 was unadjusted; model 2 was adjusted for sex, baseline age, body weight, and height; and model 3 was additionally adjusted for baseline diastolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting triglycerides, HbA1c, high-sensitivity C-reactive protein (hs-CRP), a dichotomous variable for the weekly use of alcohol, the number of cigarettes currently smoked, UACR, and the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
The odds ratio for incident low-grade albuminuria was analyzed using logistic regression models with the same adjustment variables described previously, except that the UACR was replaced with baseline GFR in model 3. In these analyses, persons with baseline low-grade albuminuria were excluded (n = 122). To evaluate the classification power of 8-oxoGuoUCR for predicting low-grade albuminuria at follow-up, we compared the area under the receiver operating characteristics curve for the logistic regression models, with and without 8-oxoGuoUCR, using the likelihood test.
Statistical significance was defined as P < 0.05. The analyses were performed with STATA 14 (www.stata.com) (StataCorp, College Station, TX).
Results
The RENIS-T6 Study consisted of 1627 subjects aged 50 to 62 years without self-reported kidney disease, diabetes mellitus, or cardiovascular disease. We excluded 33 persons who fulfilled diagnostic criteria for diabetes mellitus at baseline (fasting glucose ≥7.0 mmol/l or HbA1c ≥6.5%) and 3 persons with missing 8-oxodG/8-oxoGuo values, leaving 1591 persons at baseline in the current study. After a median (interquartile range) of 5.6 (5.2–6.0) years, 1298 persons were included in the RENIS-FU Study (Figure 1).
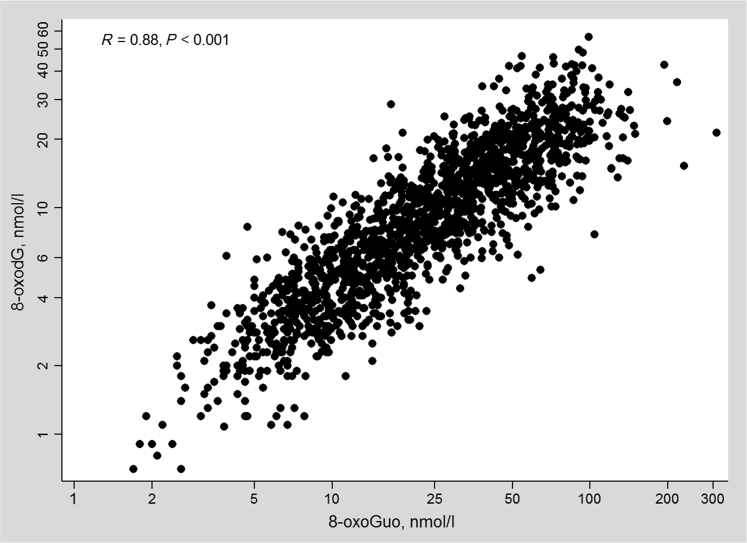
The median (interquartile range) concentrations of 8-oxodGUCR and 8-oxoGuoUCR were 1.36 (1.04–1.74) nmol/mmol and 3.45 (2.68–4.44) nmol/mmol, respectively. The urinary levels of 8-oxodG and 8-oxoGuo were higher in men compared with women, but were lower in men after correction for urinary creatinine. Thus, we sex-adjusted the baseline characteristics of the study population according to the quartiles of 8-oxodGUCR and 8-oxoGuoUCR, as shown in Tables 1 and 2. Higher quartiles of both markers were associated with a higher age and with current smoking. There were no statistically significant cross-sectional associations of 8-oxodGUCR and 8-oxoGuoUCR with either GFR or UACR at baseline. The study participants with higher quartiles of 8-oxoGuoUCR had an unfavorable metabolic profile (higher weight, body mass index, fasting glucose, hs-CRP, and lower high-density lipoprotein cholesterol). There was a significant positive correlation between 8-oxodG and 8-oxoGuo (R = 0.75, P < 0.001; Figure 2), which was attenuated when using 8-oxodGUCR and 8-oxoGuoUCR (R = 0.39, P < 0.001). There were small differences between those included in the follow-up study and those lost to follow-up (Supplementary Table S1), except for the current smoking status (19% vs. 28%, P < 0.001) and a higher 8-oxoGuoUCR level in the group lost to follow-up (3.62 nmol/mmol vs. 3.42 nmol/mmol, P = 0.02).
Table 1.
The baseline study population characteristics according to the quartile of urinary 8-oxodG: The Renal Iohexol Clearance Survey Follow-Up Study
| Baseline characteristics | Quartile of 8-oxodG, range (nmol/mmol creatinine) |
P for linear trenda | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 (n = 397) | Quartile 2 (n = 398) | Quartile 3 (n = 398) | Quartile 4 (n = 398) | ||||||
| (0.20–1.04) | (1.05–1.36) | (1.37–1.73) | (1.74–5.15) | ||||||
| Men, % | 65.7 | 56.0 | 43.7 | 30.4 | <0.001 | ||||
| Age baseline, yr | 57.2 | (3.9) | 58.1 | (3.8) | 58.3 | (3.8) | 58.6 | (3.9) | <0.001 |
| Body mass index, kg/m2 | 27.4 | (4.0) | 27.5 | (3.9) | 27.1 | (3.9) | 26.8 | (4.0) | 0.10 |
| GFR,b ml/min per 1.73 m2 | 93.5 | (14.0) | 93.1 | (13.8) | 94.7 | (13.8) | 93.9 | (14.0) | 0.40 |
| Systolic blood pressure, mm Hg | 128.9 | (17.3) | 129.6 | (17.1) | 130.0 | (17.0) | 129.2 | (17.3) | 0.80 |
| Diastolic blood pressure, mm Hg | 83.8 | (9.5) | 83.9 | (9.4) | 83.2 | (9.4) | 82.6 | (9.6) | 0.23 |
| Fasting glucose, mmol/l | 5.32 | (0.47) | 5.33 | (0.46) | 5.34 | (0.46) | 5.28 | (0.47) | 0.26 |
| Hemoglobin A1c, % | 5.54 | (0.34) | 5.54 | (0.33) | 5.54 | (0.33) | 5.52 | (0.34) | 0.74 |
| HDL-cholesterol, mmol/l | 1.53 | (0.40) | 1.53 | (0.40) | 1.55 | (0.40) | 1.55 | (0.40) | 0.79 |
| LDL-cholesterol, mmol/l | 3.76 | (0.87) | 3.64 | (0.86) | 3.68 | (0.86) | 3.58 | (0.87) | 0.04 |
| High-sensitivity C-reactive protein, mg/l | 1.20 | (1.07–1.34) | 1.16 | (1.03–1.30) | 1.19 | (1.06–1.33) | 1.17 | (1.04–1.31) | 0.98 |
| Triglycerides, mmol/l | 1.00 | (0.70–1.40) | 1.00 | (0.70–1.50) | 1.10 | (0.80–1.40) | 1.10 | (0.80–1.50) | 0.09 |
| Urinary albumin-creatinine ratio, mg/mmol | 0.22 | (0.15–0.28) | 0.25 | (0.19–0.31) | 0.20 | (0.14–0.27) | 0.26 | (0.19–0.32) | 0.59 |
| ACEi, % | 2.9 | 2.9 | 4.9 | 3.9 | 0.46 | ||||
| ARB, % | 13.6 | 16.2 | 18.3 | 13.5 | 0.28 | ||||
| Current smoker, % | 14.0 | 15.5 | 23.4 | 28.8 | <0.001 | ||||
| Alcohol consumption, % | 29.9 | 36.1 | 23.7 | 33.3 | 0.42 | ||||
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density.
lipoprotein; 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine.
Numbers were adjusted for sex and are presented as mean (SD), median (interquartile range), and percent.
Linear trend over increasing quartiles of urinary 8-oxodG was adjusted for gender.
GFR was measured by iohexol clearance.
Table 2.
The baseline study population characteristics according to the quartile of urinary 8-oxoGuo: The Renal Iohexol Clearance Survey Follow-Up Study
| Baseline characteristics | Quartile of 8-oxoGuo, range (nmol/mmol creatinine) |
P for linear trenda | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 (n = 397) | Quartile 2 (n = 398) | Quartile 3 (n = 398) | Quartile 4 (n = 398) | ||||||
| (0.73–2.68) | (2.69–3.45) | (3.46–4.44) | (4.45–13.4) | ||||||
| Men, % | 59.4 | 52.8 | 42.5 | 42.2 | <0.001 | ||||
| Age baseline, yr | 57.0 | (3.8) | 58.1 | (3.8) | 58.5 | (3.8) | 58.6 | (3.8) | <0.001 |
| Body mass index, kg/m2 | 26.5 | (3.9) | 27.0 | (3.9) | 27.6 | (3.9) | 27.7 | (3.9) | <0.001 |
| GFR,b ml/min per 1.73 m2 | 94.4 | (13.9) | 93.4 | (13.8) | 92.8 | (13.8) | 94.5 | (13.8) | 0.23 |
| Systolic blood pressure, mm Hg | 129.2 | (17.1) | 129.8 | (17.0) | 130.0 | (17.1) | 128.7 | (17.1) | 0.69 |
| Diastolic blood pressure, mm Hg | 83.5 | (9.4) | 83.4 | (9.4) | 83.5 | (9.4) | 83.0 | (9.4) | 0.89 |
| Fasting glucose, mmol/l | 5.25 | (0.46) | 5.30 | (0.46) | 5.34 | (0.46) | 5.39 | (0.46) | <0.001 |
| Hemoglobin A1c, % | 5.51 | (0.33) | 5.52 | (0.33) | 5.57 | (0.33) | 5.55 | (0.33) | 0.05 |
| HDL-cholesterol, mmol/l | 1.57 | (0.40) | 1.56 | (0.39) | 1.52 | (0.40) | 1.49 | (0.40) | 0.03 |
| LDL-cholesterol, mmol/l | 3.57 | (0.86) | 3.68 | (0.86) | 3.69 | (0.86) | 3.72 | (0.86) | 0.07 |
| High-sensitivity C-reactive protein, mg/l | 1.03 | (0.90–1.17) | 1.16 | (1.03–1.30) | 1.17 | (1.04–1.31) | 1.39 | (1.25–1.53) | 0.003 |
| Triglycerides, mmol/l | 1.00 | (0.90–1.10) | 1.00 | (0.90–1.10) | 1.10 | (1.00–1.20) | 1.10 | (1.00–1.20) | 0.29 |
| Urinary albumin-creatinine ratio, mg/mmol | 0.16 | (0.10–0.22) | 0.25 | (0.19–0.31) | 0.26 | (0.20–0.31) | 0.22 | (0.16–0.28) | 0.11 |
| ACEi, % | 3.4 | 4.9 | 2.8 | 3.5 | 0.56 | ||||
| ARB, % | 12.3 | 17.6 | 15.8 | 16.1 | 0.25 | ||||
| Current smoker, % | 12.2 | 22.2 | 19.3 | 28.0 | <0.001 | ||||
| Alcohol consumption, % | 36.7 | 33.5 | 33.5 | 27.9 | 0.12 | ||||
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density.
lipoprotein; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine.
Numbers were adjusted for sex and are presented as mean (SD), median (interquartile range), and percent.
Linear trend over increasing quartiles of urinary 8-oxoGuo was adjusted for gender.
GFR was measured by iohexol clearance.
Figure 2.
Scatterplot of the correlation between the urinary log 8-oxoGuo and log 8-oxodG.
The unadjusted mean (SD) GFR change rate during the study period was −0.84 (2.00) ml/min per 1.73 m2 per year. We examined the associations of the oxidative stress markers with the annual GFR change rate in 3 separate linear mixed regression models, as shown in Table 3. The levels of urinary 8-oxodGUCR and 8-oxoGuoUCR were not associated with the annual GFR decline. We did not find any significant nonlinear associations when the same models as described previously were analyzed with fractional polynomial transformations. We then examined the risk of a rapid GFR decline, defined as an annual GFR loss >3.0 ml/min per 1.73 m2 (n = 133), and the risk of low GFR, defined as GFR <60 ml/min per 1.73 m2 at follow-up (n = 26), as dichotomous outcomes in separate logistic regression models with an adjustment for the same variables as in the linear mixed models. Neither 8-oxodGUCR nor 8-oxoGuoUCR was associated with an increased risk of rapid GFR decline or low GFR (data not shown).
Table 3.
The association of urinary 8-oxodG and 8-oxoGuo with measured GFR change rates in mixed models: The Renal Iohexol Clearance Survey Follow-Up Study
| Risk factora | Model 1 unadjusted |
Model 2 adjusted for sex, age, weight, and height |
Model 3 fully adjustedb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (ml/min per 1.73 m2 per year) | (95% CI) | P | Estimate (ml/min per 1.73 m2 per year) | (95% CI) | P | Estimate (ml/min per 1.73 m2 per year) | (95% CI) | P | |
| Urinary 8-oxodG | |||||||||
| Per 1 nmol/mmol | −0.12 | (−0.29 to 0.06) | 0.19 | −0.09 | (−0.27 to 0.09) | 0.35 | −0.02 | (−0.20 to 0.16) | 0.82 |
| Quartile 1 | 0.00 | (ref) | 0.00 | (ref) | 0.00 | (ref) | |||
| Quartile 2 | −0.12 | (−0.42 to 0.18) | −0.11 | (−0.40 to 0.19) | −0.08 | (−0.37 to 0.21) | |||
| Quartile 3 | −0.16 | (−0.46 to 0.13) | −0.14 | (−0.44 to 0.16) | −0.09 | (−0.39 to 0.21) | |||
| Quartile 4 | −0.24 | (−0.54 to 0.05) | −0.20 | (−0.51 to 0.11) | −0.08 | (−0.38 to 0.23) | |||
| (0.45c) | (0.64c) | (0.88c) | |||||||
| Q4 versus Q1–Q3d | −0.15 | (−0.39 to 0.09) | 0.23 | −0.11 | (−0.36 to 0.14) | 0.39 | −0.02 | (−0.26 to 0.23) | 0.89 |
| Urinary 8-oxoGuo | |||||||||
| Per 1 nmol/mmol | −0.04 | (−0.11 to 0.03) | 0.28 | −0.02 | (−0.10 to 0.05) | 0.52 | 0.0003 | (−0.07 to 0.08) | 0.99 |
| Quartile 1 | 0.00 | (ref) | 0.00 | (ref) | 0.00 | (ref) | |||
| Quartile 2 | −0.09 | (−0.39 to 0.20) | −0.06 | (−0.35 to 0.24) | −0.02 | (−0.32 to 0.27) | |||
| Quartile 3 | 0.05 | (−0.24 to 0.35) | 0.10 | (−0.19 to 0.40) | 0.17 | (−0.12 to 0.47) | |||
| Quartile 4 | −0.21 | (−0.51 to 0.09) | −0.15 | (−0.45 to 0.15) | −0.02 | (−0.32 to 0.29) | |||
| (0.34c) | (0.39c) | (0.56c) | |||||||
| Q4 versus Q1–Q3d | −0.20 | (−0.44 to 0.05) | 0.12 | −0.17 | (−0.41 to 0.08) | 0.18 | −0.07 | (−0.32 to 0.17) | 0.56 |
CI, confidence interval; 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine.
8-oxodG and 8-oxoGuo were normalized for urinary creatinine.
Adjusted for sex, baseline age, height, body weight, diastolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting triglycerides, hemoglobin A1c, high-sensitivity C-reactive protein; a dichotomous variable for the weekly use of alcohol; number of cigarettes currently smoked; log urine albumin-to-creatinine ratio; and the use of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker.
Wald test for linear trend was applied.
Persons with 8-oxodG or 8-oxoGuo in the highest quartile compared with the lowest quartiles.
Table 4 shows results of the unadjusted and multivariable adjusted logistic regression analyses for the risk of incident low-grade albuminuria. In total, 46 persons developed incident low-grade albuminuria defined as a UACR between 1.13 and 3.4 mg/mmol and 8 developed a UACR level between 3.4 and 19.0 mg/mmol (lower range of albuminuria) at follow-up. These were analyzed together as 1 low-grade albuminuria group. There was a higher odds ratio for low-grade albuminuria when 8-oxodGUCR was used as a continuous variable in the unadjusted analyses, but after an adjustment for age, gender, weight, and height, this association was not statistically significant. For 8-oxoGuoUCR, there was a statistically significant trend for higher odds ratios with increasing quartiles in all models (P < 0.02). When comparing the highest quartile of 8-oxoGuoUCR with the lowest 3 quartiles, the odds ratio was 2.64 (95% confidence interval: 1.50–4.65) in the unadjusted analyses. After multivariable adjustments, including baseline GFR, the odds ratio remained relatively unchanged (P < 0.001). The area under the receiver operating characteristics curve for predicting low-grade albuminuria at follow-up increased from 0.67 (95% confidence interval: 0.60–0.75) to 0.71 (95% confidence interval: 0.63–0.78) by including 8-oxoGuoUCR (quartile 4 compared with quartiles 1–3) in addition to all of the other variables in model 3, resulting in a statistically improved model as evaluated by the likelihood test (P = 0.002). There were no interactions of age, gender, fasting glucose, HbA1c, or hs-CRP with either 8-oxodG or 8-oxoGuo in the models presented in Table 4.
Table 4.
The association of urinary 8-oxodG and 8-oxoGuo with the risk of low-grade albuminuria in logistic regression models: The Renal Iohexol Clearance Survey Follow-Up Study
| Low-grade albuminuriaa | Unadjusted |
Adjusted for sex, and baseline age, weight, and height |
Fully adjustedb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | (95% CI) | P | Odds ratio | (95% CI) | P | Odds ratio | (95% CI) | P | |
| Urinary 8-oxodG | |||||||||
| Per 1 nmol/mmol | 1.53 | (1.04–2.26) | 0.03 | 1.33 | (0.88–2.01) | 0.18 | 1.24 | (0.80–1.90) | 0.34 |
| Quartile 1 | (ref) | (ref) | (ref) | ||||||
| Quartile 2 | 0.88 | (0.37–2.11) | 0.81 | (0.34–1.95) | 0.75 | (0.31–1.81) | |||
| Quartile 3 | 1.28 | (0.57–2.87) | 1.08 | (0.48–2.46) | 0.92 | (0.40–2.14) | |||
| Quartile 4 | 1.65 | (0.76–3.58) | 1.26 | (0.56–2.84) | 1.07 | (0.46–2.48) | |||
| (0.41c) | (0.76c) | (0.54c) | |||||||
| Q4 versus Q1–Q3d | 1.57 | (0.86–2.84) | 0.14 | 1.30 | (0.71–2.41) | 0.40 | 1.22 | (0.65–2.29) | 0.54 |
| Urinary 8-oxoGuo | |||||||||
| Per 1 nmol/mmol | 1.19 | (1.02–1.39) | 0.03 | 1.18 | (1.00–1.39) | 0.05 | 1.16 | (0.98–1.37) | 0.09 |
| Quartile 1 | (ref) | (ref) | (ref) | ||||||
| Quartile 2 | 1.18 | (0.47–2.94) | 1.12 | (0.45–2.82) | 1.07 | (0.42–2.72) | |||
| Quartile 3 | 1.22 | (0.49–3.05) | 1.16 | (0.46–2.93) | 1.14 | (0.45–2.93) | |||
| Quartile 4 | 2.99 | (1.36–6.57) | 2.87 | (1.28–6.44) | 2.84 | (1.23–6.54) | |||
| (0.009c) | (0.01c) | (0.02c) | |||||||
| Q4 versus Q1–Q3d | 2.64 | (1.50–4.65) | 0.001 | 2.62 | (1.48–4.65) | 0.001 | 2.64 | (1.46–4.80) | 0.001 |
CI, confidence interval; 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine.
8-oxodG and 8-oxoGuo were normalized for urinary creatinine.
Low-grade albuminuria was defined as a urinary albumine-creatinine ratio >1.13 mg/mmol (>10 mg/g).
Adjusted for sex, baseline age, height, body weight, diastolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting triglycerides, hemogloin A1c, high-sensitivity C-reactive protein; a dichotomous variable for the weekly use of alcohol; number of cigarettes currently smoked; the use of an angiotensin-converting enzyme inhibitor and angiotensin receptor blocker; and baseline measured glomerular filtration rate.
Wald test for linear trend was applied.
Persons with 8-oxodG or 8-oxoGuo in the highest quartile compared with the lowest quartiles.
Discussion
To the best of our knowledge, this is the first study to describe the relationship between urinary markers of oxidative stress and renal function decline using measured GFR in a cohort from the general population. The age-related GFR decline of 0.84 ml/min per 1.73 m2 per year found in our study is comparable with previous studies using estimated GFR.30 None of the markers were associated with a change in the GFR decline during the observation period, but urinary 8-oxoGuo excretion was associated with an increased risk of low-grade albuminuria.
Previous studies on the relationship between oxidative stress markers and kidney function have been mainly cross-sectional and predominantly in patients with established CKD.11, 12, 13, 14, 15, 16 The plasma level of F2-isoprostane, a marker of lipid peroxidation, has been correlated with CKD stages and has been higher in patients with CKD compared with healthy controls,11, 12, 14 but only 1 of these studies found that plasma F2-isoprostane correlated with the estimated GFR.11 Another study reported that serum 8-oxodG levels inversely correlated with creatinine clearance in patients with CKD.16
We are aware of only 1 longitudinal study that examined 8-oxodG or 8-oxoGuo as predictors of kidney outcomes. Hinokio et al.31 found that 24-hour urinary 8-oxodG excretion was associated with an increased risk of the progression of diabetic nephropathy, defined as albuminuria, after 5 years of follow-up. Interestingly, 8-oxodG was the strongest independent predictor among several well-known CKD risk factors, such as blood pressure, HbA1c, and duration of diabetes.
The mitochondrial and nuclear DNA and RNA in cellular pools are common targets of oxidative damage. Guanine is the nucleic acid base most susceptible to oxidation. In a steady state condition, the amount of excreted 8-oxodG and 8-oxoGuo in the urine represents the guanine oxidation rate from DNA and RNA, respectively.19 In diabetic mice models, mitochondrial oxidative stress is associated with increased 8-oxodG levels from mitochondrial DNA, as measured both in urine and in the kidneys.32, 33 Moreover, the accumulation of 8-oxodG in the mitochondria of the glomeruli and an increased urinary 8-oxodG excretion were associated with glomerular and tubular injury in mice during Dox treatment.10 In kidney biopsies from patients with focal segmental glomerular sclerosis, similar results of accumulation of 8-oxodG in endothelial cells were found, but not in kidney biopsies from healthy controls.10 Our population was relatively healthy and we excluded persons with diabetes, cardiovascular disease, and renal disease at entry of the study. It is possible that the underlying mechanisms that accelerate the age-related GFR decline are different from what has been reported in specific forms of kidney disease.10, 32, 33
We found that urinary excretion of the oxidatively damaged RNA marker 8-oxoGuo independently predicted incident low-grade albuminuria, even after a multivariable adjustment, including smoking status and baseline GFR. This finding is noteworthy because low-grade albuminuria, defined as a UACR >1.13 mg/mmol, predicts cardiovascular disease, CKD, and mortality in the general population.25, 26 Interventions with an antioxidant treatment or diet may reduce oxidative stress. In a randomized controlled trial, olive oil consumption reduced urinary 8-oxodG excretion significantly.34 The same study reported a considerable difference in the urinary excretion of 8-oxodG and 8-oxoGuo between countries in Europe, suggesting a positive effect of diet on oxidative stress levels.34 Moreover, in a large study with elderly Danish twins, genetic factors had little influence on the interindividual variability of urinary 8-oxodG and 8-oxoGuo excretion,35 which further suggests that oxidative stress is a modifiable risk factor and a possible target for preventive measures. However, it remains unclear whether antioxidants can prevent GFR decline, CKD, or CKD progression.
Urinary 8-oxoGuo excretion was not associated with the age-related GFR decline, but it is possible that low-grade albuminuria may precede an acceleration of GFR decline. However, although low-grade albuminuria is a risk factor for cardiovascular disease, it is not established as an independent risk factor for age-related GFR decline or incident CKD. Even longer follow-up and repeated GFR measurements are needed to fully assess the long-term effect of urinary 8-oxoGuo excretion on the age-related GFR decline. Unlike 8-oxoGuo, urinary 8-oxodG excretion was not associated with incident low-grade albuminuria in the multivariable adjusted analyses. The reason for this discrepancy is not clear. Urinary 8-oxodG levels were associated with age and smoking at baseline, whereas urinary 8-oxoGuo levels were associated with several other CKD risk factors at baseline, including a higher hs-CRP as a marker of inflammation. Increased levels of inflammation are correlated with oxidative stress in CKD patients,12, 14 and we recently found that a higher serum hs-CRP concentration was associated with a more rapid GFR decline.36 However, the association between urinary 8-oxoGuo excretion and low-grade albuminuria remained unchanged after a multivariable adjustment, including hs-CRP. RNA may be more susceptible to oxidative damage than DNA due to its single-stranded structure, fewer protective proteins, and its intracellular location close to the mitochondria, which produces the reactive oxygen species.20
The primary strength of this study was the use of GFR measurements in a large cohort from the general population rather than an estimated GFR based on creatinine or cystatin C. Iohexol clearance is accurate compared with gold standard methods,24, 37, 38 and the intra-individual variation in our study was lower than in previous studies that measured GFR.38 The UACR was measured in unfrozen urine samples. We measured urinary levels of 8-oxodG and 8-oxoGuo using liquid chromatography tandem mass spectrometry, which is more reliable than enzyme-linked immunosorbent assay methods.39 In addition, all participants were investigated after an overnight fast, including the withdrawal of tobacco use, which is known to influence the day-to-day variation in 8-oxodG excretion.40 This study also has some weaknesses. The generalizability is limited to relatively healthy, middle-aged white persons and there were higher urinary levels of 8-oxoGuo among those lost to follow-up compared with those included in the follow-up study. We used spot urine samples instead of the gold standard 24-hour urine samples to measure the concentrations of 8-oxodG and 8-oxoGuo. We corrected the levels of 8-oxodG and 8-oxoGuo for urinary creatinine, which reduces the intra-individual variation and is shown to correlate strongly with 24-hour urine samples.39
We conclude that higher urinary excretion of oxidatively damaged RNA was associated with an increased risk of incident low-grade albuminuria, but the urinary excretion of oxidatively damaged DNA and RNA were not associated with the age-related GFR decline during almost 6 years of follow-up. Studies with an even longer follow-up period and repeated GFR measurements are needed to fully evaluate the long-term effect of oxidative stress on the GFR decline in the general population.
Disclosure
All of the authors declared no competing interests.
Acknowledgments
This study was funded by the North Norwegian Regional Health Authority, UiT The Arctic University of Norway, and a grant from Boehringer-Ingelheim. We thank Britt-Ann Winther Eilertsen, Bjørg Skog Høgseth, Saskia van Heusden, and the rest of the staff at the Clinical Research Unit (University Hospital of North Norway) for assistance in planning the study, performing the procedures, and collecting data according to the Good Clinical Practice standard. We also thank Harald Strand and the staff at the Department of Medical Biochemistry (University Hospital of North Norway) for the high-performance liquid chromatography analyses of iohexol, and Inger Sperstad and Ingrid Dorthea Sandstad at the Clinical Research Center (University Hospital of North Norway) for the database support. Most of all, we thank all participants in the Renal Iohexol Clearance Survey in the Sixth Tromsø Study cohort for making this study possible. Parts of this study were presented in abstract form at the American Society of Nephrology annual meeting in Chicago, Illinois, USA, 15–20 November 2016.
Footnotes
Table S1. The baseline characteristics of participants included in the Renal Iohexol Clearance Survey Follow-Up Study and those lost to follow-up.
Supplemental Material. The measurement of oxidative stress markers.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
The baseline characteristics of participants included in the Renal Iohexol Clearance Survey Follow-Up Study and those lost to follow-up
The measurement of oxidative stress markers
References
- 1.Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Stevens L.A., Coresh J., Levey A.S. CKD in the elderly—old questions and new challenges: World Kidney Day 2008. Am J Kidney Dis. 2008;51:353–357. doi: 10.1053/j.ajkd.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Shlipak M.G., Matsushita K., Ärnlöv J. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muntner P. Longitudinal measurements of renal function. Semin Nephrol. 2009;29:650–657. doi: 10.1016/j.semnephrol.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Kronborg J., Solbu M., Njolstad I. Predictors of change in estimated GFR: a population-based 7-year follow-up from the Tromso study. Nephrol Dial Transplant. 2008;23:2818–2826. doi: 10.1093/ndt/gfn148. [DOI] [PubMed] [Google Scholar]
- 6.Webster A.C., Nagler E.V., Morton R.L. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 7.Engelfriet P.M., Jansen E.H., Picavet H.S. Biochemical markers of aging for longitudinal studies in humans. Epidemiol Rev. 2013;35:132–151. doi: 10.1093/epirev/mxs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison D., Griendling K.K., Landmesser U. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7a–11a. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz S., Pergola P.E., Zager R.A. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daehn I., Casalena G., Zhang T. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014;124:1608–1621. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dounousi E., Papavasiliou E., Makedou A. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48:752–760. doi: 10.1053/j.ajkd.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Oberg B.P., McMenamin E., Lucas F.L. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 13.Himmelfarb J., McMonagle E., McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58:2571–2578. doi: 10.1046/j.1523-1755.2000.00443.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramos L.F., Shintani A., Ikizler T.A. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol. 2008;19:593–599. doi: 10.1681/ASN.2007030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamouzis I., Sarafidis P.A., Karamouzis M. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol. 2008;28:397–404. doi: 10.1159/000112413. [DOI] [PubMed] [Google Scholar]
- 16.Akagi S., Nagake Y., Kasahara J. Significance of 8-hydroxy-2′-deoxyguanosine levels in patients with chronic renal failure. Nephrology (Carlton) 2003;8:192–195. doi: 10.1046/j.1440-1797.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 17.Conti S., Cassis P., Benigni A. Aging and the renin-angiotensin system. Hypertension. 2012;60:878–883. doi: 10.1161/HYPERTENSIONAHA.110.155895. [DOI] [PubMed] [Google Scholar]
- 18.Sastre J., Pallardo F.V., Vina J. The role of mitochondrial oxidative stress in aging. Free Radic Biol Med. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 19.Poulsen H.E., Nadal L.L., Broedbaek K. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochim Biophys Acta. 2014;1840:801–808. doi: 10.1016/j.bbagen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Poulsen H.E., Specht E., Broedbaek K. RNA modifications by oxidation: a novel disease mechanism? Free Radic Biol Med. 2012;52:1353–1361. doi: 10.1016/j.freeradbiomed.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Eriksen B.O., Stefansson V.T., Jenssen T.G. Elevated blood pressure is not associated with accelerated glomerular filtration rate decline in the general non-diabetic middle-aged population. Kidney Int. 2016;90:404–410. doi: 10.1016/j.kint.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Eriksen B.O., Mathisen U.D., Melsom T. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int. 2010;78:1305–1311. doi: 10.1038/ki.2010.321. [DOI] [PubMed] [Google Scholar]
- 23.Kark R.M., Lawrence J.R., Muehrcke R.C. 2nd ed. Hoeber Medical Division, Harper and Row; New York, NY: 1964. Primer of Urinalysis. [Google Scholar]
- 24.Delanaye P., Ebert N., Melsom T. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research:a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin Kidney J. 2016;9:682–699. doi: 10.1093/ckj/sfw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey A.S., de Jong P.E., Coresh J. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita K., van der Velde M., Astor B.C. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathisen U.D., Melsom T., Ingebretsen O.C. Ambulatory blood pressure is associated with measured glomerular filtration rate in the general middle-aged population. J Hypertens. 2012;30:497–504. doi: 10.1097/HJH.0b013e32834f973a. [DOI] [PubMed] [Google Scholar]
- 28.Boucquemont J., Heinze G., Jager K.J. Regression methods for investigating risk factors of chronic kidney disease outcomes: the state of the art. BMC Nephrol. 2014;15:45. doi: 10.1186/1471-2369-15-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leffondre K., Boucquemont J., Tripepi G. Analysis of risk factors associated with renal function trajectory over time: a comparison of different statistical approaches. Nephrol Dial Transplant. 2015;30:1237–1243. doi: 10.1093/ndt/gfu320. [DOI] [PubMed] [Google Scholar]
- 30.Lindeman R.D., Tobin J., Shock N.W. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 31.Hinokio Y., Suzuki S., Hirai M. Urinary excretion of 8-oxo-7, 8-dihydro-2′-deoxyguanosine as a predictor of the development of diabetic nephropathy. Diabetologia. 2002;45:877–882. doi: 10.1007/s00125-002-0831-8. [DOI] [PubMed] [Google Scholar]
- 32.Kakimoto M., Inoguchi T., Sonta T. Accumulation of 8-hydroxy-2′-deoxyguanosine and mitochondrial DNA deletion in kidney of diabetic rats. Diabetes. 2002;51:1588–1595. doi: 10.2337/diabetes.51.5.1588. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakar S., Starnes J., Shi S. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol. 2007;18:2945–2952. doi: 10.1681/ASN.2006080895. [DOI] [PubMed] [Google Scholar]
- 34.Machowetz A., Poulsen H.E., Gruendel S. Effect of olive oils on biomarkers of oxidative DNA stress in Northern and Southern Europeans. FASEB J. 2007;21:45–52. doi: 10.1096/fj.06-6328com. [DOI] [PubMed] [Google Scholar]
- 35.Broedbaek K., Ribel-Madsen R., Henriksen T. Genetic and environmental influences on oxidative damage assessed in elderly Danish twins. Free Radic Biol Med. 2011;50:1488–1491. doi: 10.1016/j.freeradbiomed.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schei J., Stefansson V.T., Eriksen B.O. Association of TNF receptor 2 and CRP with GFR decline in the general nondiabetic population. Clin J Am Soc Nephrol. 2017;12:624–634. doi: 10.2215/CJN.09280916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bird N.J., Peters C., Michell A.R. Comparison of GFR measurements assessed from single versus multiple samples. Am J Kidney Dis. 2009;54:278–288. doi: 10.1053/j.ajkd.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Stevens L.A., Levey A.S. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20:2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 39.Barregard L., Moller P., Henriksen T. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Antiox Redox Signal. 2013;18:2377–2391. doi: 10.1089/ars.2012.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanolin M.E., Girardi P., Degan P. Measurement of a urinary marker (8-hydroxydeoxy-guanosine, 8-OHdG) of DNA oxidative stress in epidemiological surveys: a pilot study. Int J Biol Markers. 2015;30:e341–e345. doi: 10.5301/jbm.5000129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The baseline characteristics of participants included in the Renal Iohexol Clearance Survey Follow-Up Study and those lost to follow-up
The measurement of oxidative stress markers