Abstract
Background
To evaluate oral anticoagulant (OAC) utilization in patients with atrial fibrillation after the changes in the health insurance coverage policy in July 2015.
Methods
We used the Health Insurance Review and Assessment Service-National Patient Samples (HIRA-NPS) between 2014 and 2016. The HIRA-NPS, including approximately 1.4 million individuals, is a stratified random sample of 3% of the entire Korean population using 16 age groups and 2 sex groups. The HIRA-NPS comprises personal and medical information such as surgical or medical treatment provided, diagnoses, age, sex, region of medical institution, and clinician characteristics. The studied drugs included non-vitamin K antagonist OACs (NOACs) such as apixaban, dabigatran, edoxaban, and rivaroxaban, and were compared with warfarin. We analyzed drug utilization pattern under three aspects: person, time, and place.
Results
The number of patients with atrial fibrillation who were prescribed OACs was 3,114, 3,954, and 4,828; and the proportions of prescribed NOACs to total OACs were 5.1%, 36.2%, and 60.8% in 2014, 2015, and 2016, respectively. The growth rate of OACs prescription increased from 61.4 patients/quarter before June 2015 to 147.7 patients/quarter thereafter. These changes were predominantly in elderly individuals aged more than 70 years. The proportion of NOACs to OACs showed significant regional difference.
Conclusion
The change of health insurance coverage policy substantially influenced OACs prescription pattern in whole Korean region. But the impact has been significantly different among regions and age groups, which provides the evidence for developing standard clinical practice guideline on OACs use.
Keywords: Atrial Fibrillation, Anticoagulants, Drug Utilization Evaluation, Non-Vitamin K Antagonist Oral Anticoagulants, Health Insurance Coverage Policy
Graphical Abstract
INTRODUCTION
Atrial fibrillation (Afib) is the most common cardiac arrhythmia.1 It is well documented that the presence of Afib is increasing the risk of ischemic stroke by five times, contributing to approximately 20% of all cases.2 The prevalence of Afib increases rapidly with age; thus, a wide range of 0.5%–2.0% has been reported depending on the study population.3,4 In Korea, the overall prevalence of Afib was 0.5% based on the national health insurance claims data; however, it was potentially underestimated.5 According to community surveys, the prevalence of Afib in individuals aged over 80 years was 7%, which was relatively higher than the reported 3% using national health insurance claims data.5,6 Since Korea is the most rapidly aging country in the world, it will be an important public health issue to prevent stroke from increasing the prevalence of Afib.
Patients with Afib are prescribed oral anticoagulants (OACs) when they have risk factor of stroke according to CHA2DS2-VASc score. Generally, two or more points of CHA2DS2-VASc score are used as criteria for determining whether to use OACs. Warfarin, a traditional OAC, is used to prevent stroke in patients with Afib. Despite the preventive effect of warfarin on stroke, it was difficult to use owing to problems such as interactions with other drugs and foods, necessity for periodic international normalized ratio (INR) tests, and individualized dose setting for each patient. In recent decades, non-vitamin K antagonist OACs (NOACs) have been developed and marketed. Several post-approval observational studies have been conducted on the safety and efficacy of NOACs.7,8,9,10,11 In these observational studies, apixaban, dabigatran, and rivaroxaban showed similar or better safety and efficacy than warfarin. Similar results were shown in a study using health claims data in Korea.11
Several studies have been performed regarding the changes in OAC prescription patterns following NOAC approval.12,13,14,15 With the approval of NOACs, the prescription of NOACs increased rapidly, as reported in previous studies, while the prescription of warfarin showed a relative decrease. In Korea, NOACs were marketed in the early part of 2010; however, the coverage of the national health insurance was limited to patients with a high risk of stroke whose conditions were not managed by warfarin. In July 2015, the health insurance coverage policy for NOACs was revised to allow their use regardless of whether warfarin was used. The purpose of our study was to evaluate the drug prescription pattern of OACs according to factors of person, time, and place aspects owing to the change in the insurance coverage policy.
METHODS
Data source
We used the Health Insurance Review and Assessment Service-National Patient Samples (HIRA-NPS) for evaluating utilization of OACs between 2014 and 2016. The Health Insurance Review and Assessment Service (HIRA) received claims made by all medical institutions in Korea from 2000. The HIRA database includes personal and medical information such as surgical or medical treatment provided, diagnoses, age, and sex of approximately 50 million Koreans, and characteristics of medical institutions and clinicians. The diagnosis was coded in accordance with the International Classification of Disease, Tenth Revision (ICD-10). The generic drug names were coded in accordance with the Korean national code system. The HIRA-NPS is a stratified random sample of 3% of the population in the HIRA data using 16 age groups and 2 sex groups.16 These data include approximately 1.4 million individuals (48.7%, men; 51.3%, women). Each year, the data are newly extracted; thus, the data of the individual patients could not be continuously followed up.
Study design
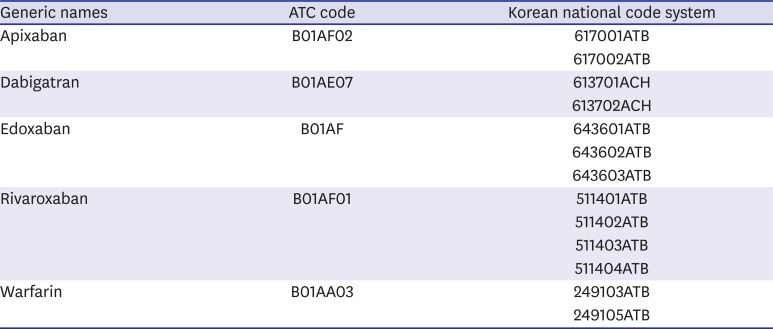
The studied drugs included NOACs, such as apixaban, dabigatran, edoxaban, and rivaroxaban, which were compared with warfarin, which is a traditional vitamin K antagonist OAC. The doses of the individual drugs marketed in Korea were as follows: apixaban, 2.5 mg and 5 mg; dabigatran, 110 mg and 150 mg; edoxaban, 15 mg, 30 mg, and 60 mg; rivaroxaban, 2.5 mg, 10 mg, 15 mg, and 20 mg; and warfarin, 2 mg and 5 mg (drug code, Appendix 1). We only included OACs prescribed to out-patients for this study. We defined reduced doses as prescribed one time doses of 2.5 mg, 110 mg, 30 mg, and 15 mg or less of apixaban, dabigatran, edoxaban, and rivaroxaban, respectively.
In this study, we included individuals with a concurrent diagnosis of Afib (ICD-10: I48) at the time of OAC prescription. I48 code included not only Afib but also atrial flutter. Since difficulty of differential diagnosis and similar management plan of Afib and atrial flutter, we did not classify these 2 diagnoses. We only used primary or secondary diagnosis of each hospital visit in database.
We analyzed drug utilization in three aspects: person, time, and place. We analyzed the characteristics of the patients and clinicians for evaluating the person factors, such as age and sex. We evaluated the specialty of the clinicians, type of institutes, and prescription dose and duration for analyzing the clinician characteristics. We also performed a time series analysis on OAC prescription on a quarterly basis for evaluating the time factor. A subgroup analysis was performed for evaluating the difference owing to the specialty of the clinicians. The region of medical facilities was divided into 17 provinces based on the administrative district of Korea. The data of Sejong were not sufficient to evaluate the prescription pattern because of the relatively small amount of OACs prescribed during the study period.
Since HIRA-NPS was constructed from the claims database, the basic unit of data was prescription. When patient had multiple prescriptions, identification number of the patients can be used to identify whom the prescription was for. Patient-based analyses were conducted for the analysis of patient characteristics of person factors such as sex and age. Prescription-based analyses were performed to analyze prescription patterns by physician characteristics of person factors and time factors.
Statistical analysis
We employed the χ2 test for categorical variables and the t-test and analysis of variance for continuous variables. Distributions according to priory describing the person aspects were evaluated. For evaluating the time aspects, we performed time series analyses according to the entire OAC prescription, type of institute, and region of institute. The time series analyses added the year 2013 to evaluate more clearly the changes in the OAC prescription over time. The unit of the time series analysis was set as a quarter because approximately 60% of the prescription duration for 1 month or more and approximately 80% of the prescription duration for 3 months or less were used. We performed the Durbin-Watson test for assumption of serial autocorrelation, and our results showed no temporal correlation of the quarterly amount of OAC prescription. A segmented regression analysis of the interrupted time series method was used to estimate the effect of insurance coverage change on the prescription of OACs at the third quarter of 2015.17 We presented the results of simple linear regression analyses before and after July 2015. The regional difference in the OAC prescription was evaluated by visual inspection owing to the drawing of the map according to the proportion of the prescribed NOACs and OACs using the QGIS software. All statistical analyses were performed using SAS 9.4 (SAS institute Inc., Cary, NC, USA).
Ethics statement
Because this study was performed using a de-identified secondary database, it was exempted from review by the Institutional Review Board (IRB) of Seoul National University College of Medicine and Seoul National University Hospital (IRB No. 1711-017-897).
RESULTS
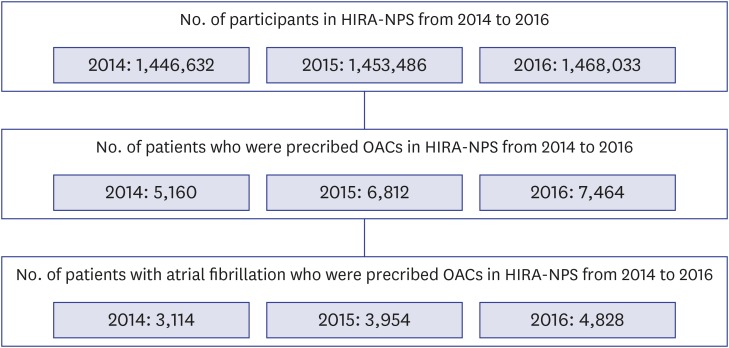
In the HIRA-NPS, the number of patients who were prescribed OACs was 5,160, 6,812, and 7,464 in 2014, 2015, and 2016, respectively (Fig. 1). When the indication of OAC prescription was restricted to Afib, the number of patients who were prescribed OACs was reduced to 3,114, 3,954, and 4,828 in 2014, 2015, and 2016, respectively. The age and sex of the patients who were prescribed OACs for each drug from 2014 to 2016 were evaluated (Table 1). Clinicians prescribed NOACs more frequently than warfarin to the elderly patients in 2016 than in 2014. Based on the number of OAC prescriptions, the prescription of warfarin decreased, and that of NOACs increased over time (Table 2). Most OACs were prescribed by clinicians of internal medicine. The prescriptions of warfarin were relatively frequent by thoracic surgeons and nephrologists who treat mechanical valve disease and kidney disease, respectively, which are contraindications of NOAC use. Compared with the prescriptions in tertiary and secondary hospitals, prescriptions in primary clinics were relatively few. All kinds of OACs were prescribed at an average duration of approximately 60 days (Table 3). The NOACs were prescribed in a reduced dose at approximately 50%, and the proportion of dabigatran prescription at a reduced dose was higher than those of the other NOAC prescriptions.
Fig. 1. Flow chart for selecting study population in HIRA-NPS.
HIRA-NPS = Health Insurance Review and Assessment Service-National Patient Samples, OACs = oral anticoagulants.
Table 1. Age and sex distribution of patients with atrial fibrillation who were prescribed OACs in HIRA-NPS between 2014 and 2016.
| Characteristics | Warfarin (%) | Apixaban (%) | Dabigatran (%) | Edoxaban (%) | Rivaroxaban (%) | Total (%) | ||
|---|---|---|---|---|---|---|---|---|
| 2014 | 3,013 (96.8) | 17 (0.5) | 87 (2.8) | 0 (0.0) | 58 (1.9) | 3,114 (100.0) | ||
| Sex | ||||||||
| Male | 1,639 (96.9) | 13 (0.8) | 45 (2.7) | 0 (0.0) | 29 (1.7) | 1,691 (100.0) | ||
| Female | 1,374 (96.6) | 4 (0.3) | 42 (3.0) | 0 (0.0) | 29 (2.0) | 1,423 (100.0) | ||
| Age (mean ± SD) | 69.3 ± 11.0 | 66.8 ± 11.9 | 73.5 ± 9.9 | - | 69.8 ± 11.3 | 69.4 ± 11.0 | ||
| < 60 | 571 (97.4) | 4 (0.7) | 9 (1.5) | 0 (0.0) | 12 (2.0) | 586 (100.0) | ||
| 60–69 | 735 (97.0) | 6 (0.8) | 16 (2.1) | 0 (0.0) | 12 (1.6) | 758 (100.0) | ||
| 70–79 | 1,216 (96.9) | 5 (0.4) | 37 (2.9) | 0 (0.0) | 21 (1.7) | 1,255 (100.0) | ||
| ≥ 80 | 491 (95.3) | 2 (0.4) | 25 (4.9) | 0 (0.0) | 13 (2.5) | 515 (100.0) | ||
| 2015 | 3,062 (77.4) | 446 (11.3) | 625 (15.8) | 0 (0.0) | 664 (16.8) | 3,954 (100.0) | ||
| Sex | ||||||||
| Male | 1,697 (77.5) | 246 (11.2) | 359 (16.4) | 0 (0.0) | 339 (15.5) | 2,190 (100.0) | ||
| Female | 1,365 (77.4) | 200 (11.3) | 266 (15.1) | 0 (0.0) | 325 (18.4) | 1,764 (100.0) | ||
| Age (mean ± SD) | 69.1 ± 11.1 | 72.7 ± 9.5 | 71.9 ± 9.1 | - | 72.3 ± 10.0 | 69.9 ± 10.8 | ||
| < 60 | 576 (87.1) | 42 (6.3) | 58 (8.8) | 0 (0.0) | 70 (10.6) | 661 (100.0) | ||
| 60–69 | 811 (79.3) | 106 (10.4) | 162 (15.8) | 0 (0.0) | 161 (15.7) | 1,023 (100.0) | ||
| 70–79 | 1,187 (75.6) | 190 (12.1) | 273 (17.4) | 0 (0.0) | 276 (17.6) | 1,570 (100.0) | ||
| ≥ 80 | 488 (69.7) | 108 (15.4) | 132 (18.9) | 0 (0.0) | 157 (22.4) | 700 (100.0) | ||
| 2016 | 2,134 (44.2) | 780 (16.2) | 826 (17.1) | 350 (7.2) | 1,352 (28.0) | 4,828 (100.0) | ||
| Sex | ||||||||
| Male | 1,179 (44.1) | 424 (15.9) | 494 (18.5) | 185 (6.9) | 733 (27.4) | 2,674 (100.0) | ||
| Female | 955 (44.3) | 356 (16.5) | 332 (15.4) | 165 (7.7) | 619 (28.7) | 2,154 (100.0) | ||
| Age (mean ± SD) | 68.5 ± 11.4 | 73.1 ± 9.3 | 71.7 ± 9.4 | 72.7 ± 8.7 | 73.0 ± 9.2 | 70.7 ± 10.5 | ||
| < 60 | 460 (63.4) | 70 (9.6) | 89 (12.3) | 28 (3.9) | 138 (19.0) | 726 (100.0) | ||
| 60–69 | 552 (46.8) | 169 (14.3) | 207 (17.5) | 88 (7.5) | 321 (27.2) | 1,180 (100.0) | ||
| 70–79 | 791 (40.0) | 336 (17.0) | 368 (18.6) | 161 (8.1) | 598 (30.3) | 1,976 (100.0) | ||
| ≥ 80 | 331 (35.0) | 205 (21.7) | 162 (17.1) | 73 (7.7) | 295 (31.2) | 946 (100.0) | ||
OACs = oral anticoagulants, HIRA-NPS = Health Insurance Review and Assessment Service-National Patient Samples, SD = standard deviation.
Table 2. Prescription pattern of OACs in patients with atrial fibrillation by specialty of clinicians and type of institutes from HIRA-NPS between 2014 and 2016.
| Characteristics | Warfarin (%) | Apixaban (%) | Dabigatran (%) | Edoxaban (%) | Rivaroxaban (%) | Total (%) | P value | |
|---|---|---|---|---|---|---|---|---|
| Year | < 0.001 | |||||||
| 2014 | 15,234 (96.3) | 48 (0.3) | 361 (2.3) | 0 (0.0) | 173 (1.1) | 15,816 (100.0) | ||
| 2015 | 14,267 (76.4) | 1,011 (5.4) | 1,630 (8.7) | 0 (0.0) | 1,758 (9.4) | 18,666 (100.0) | ||
| 2016 | 10,011 (44.3) | 2,828 (12.5) | 3,436 (15.2) | 873 (3.9) | 5,455 (24.1) | 22,603 (100.0) | ||
| Specialty of clinicians | < 0.001 | |||||||
| Internal medicine | 30,247 (70.2) | 2,836 (6.6) | 3,627 (8.4) | 750 (1.7) | 5,652 (13.1) | 43,112 (100.0) | ||
| Neurology | 6,443 (61.4) | 968 (9.2) | 1,619 (15.4) | 95 (0.9) | 1,372 (13.1) | 10,497 (100.0) | ||
| Thoracic surgery | 1,441 (97.2) | 9 (0.6) | 1 (0.1) | 8 (0.5) | 23 (1.6) | 1,482 (100.0) | ||
| Others | 1,381 (69.3) | 74 (3.7) | 180 (9.0) | 20 (1.0) | 339 (17.0) | 1,994 (100.0) | ||
| Type of institutes | < 0.001 | |||||||
| Tertiary hospital | 15,937 (69.8) | 2,373 (10.4) | 1,916 (8.4) | 318 (1.4) | 2,304 (10.1) | 22,848 (100.0) | ||
| Secondary hospital | 17,942 (66.9) | 1,289 (4.8) | 2,854 (10.6) | 480 (1.8) | 4,263 (15.9) | 26,828 (100.0) | ||
| Primary clinics | 5,633 (76.0) | 225 (3.0) | 657 (8.9) | 75 (1.0) | 819 (11.1) | 7,409 (100.0) | ||
| Total prescription | 39,512 (69.2) | 3,887 (6.8) | 5,427 (9.5) | 873 (1.5) | 7,386 (12.9) | 57,085 (100.0) | ||
OACs = oral anticoagulants, HIRA-NPS = Health Insurance Review and Assessment Service-National Patient Samples.
Table 3. Prescription pattern of OACs in patients with atrial fibrillation by prescription dose and duration from HIRA-NPS between 2014 and 2016.
| Characteristics | Warfarin (%) | Apixaban (%) | Dabigatran (%) | Edoxaban (%) | Rivaroxaban (%) | P value | |
|---|---|---|---|---|---|---|---|
| Prescription duration (mean ± SD; day) | 62.6 ± 45.1 | 66.8 ± 37.0 | 57.8 ± 34.9 | 54.1 ± 33.1 | 58.3 ± 37.3 | < 0.001 | |
| ≤ 7 | 1,186 (3.0) | 78 (2.0) | 99 (1.8) | 22 (2.5) | 163 (2.2) | ||
| 8–30 | 12,498 (31.6) | 850 (21.9) | 1,844 (34.0) | 337 (38.6) | 2,754 (37.3) | ||
| 31–60 | 10,483 (26.5) | 936 (24.1) | 1,427 (26.3) | 227 (26.0) | 1,737 (23.5) | ||
| 61–90 | 7,284 (18.4) | 1,064 (27.4) | 1,126 (20.8) | 167 (19.1) | 1,333 (18.1) | ||
| > 90 | 8,061 (20.4) | 959 (24.7) | 931 (17.2) | 120 (13.8) | 1,399 (18.9) | ||
| Average one time dose (mean ± SD; mg) | 2.9 ± 1.2 | 3.8 ± 1.3 | 124.3 ± 19.2 | 43.0 ± 15.0 | 16.8 ± 3.3 | < 0.001 | |
| Reduced dosea | - | 1,924 (49.5) | 3,477 (64.1) | 492 (56.4) | 3,937 (53.3)) | ||
| Total prescription | 39,512 (100.0) | 3,887 (100.0) | 5,427 (100.0)) | 873 (100.0) | 7,386 (100.0) | ||
OACs = oral anticoagulants, HIRA-NPS = Health Insurance Review and Assessment Service-National Patient Samples, SD = standard deviation.
aReduced dose defined as prescribed 2.5 mg, 110 mg, 30 mg, and 15 mg or less of apixaban, dabigatran, edoxaban, and rivaroxaban, respectively.
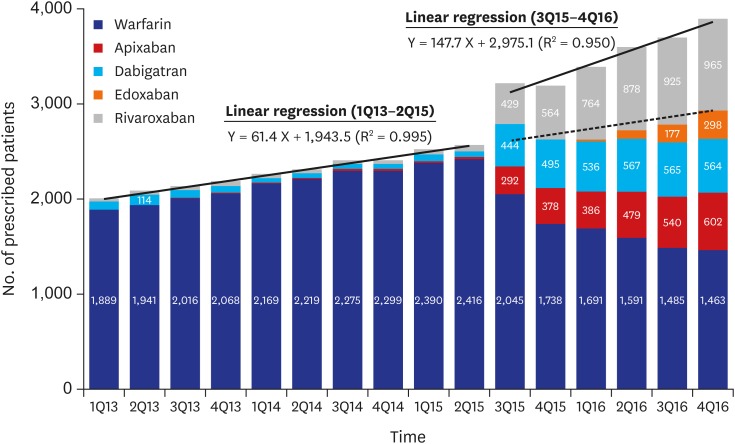
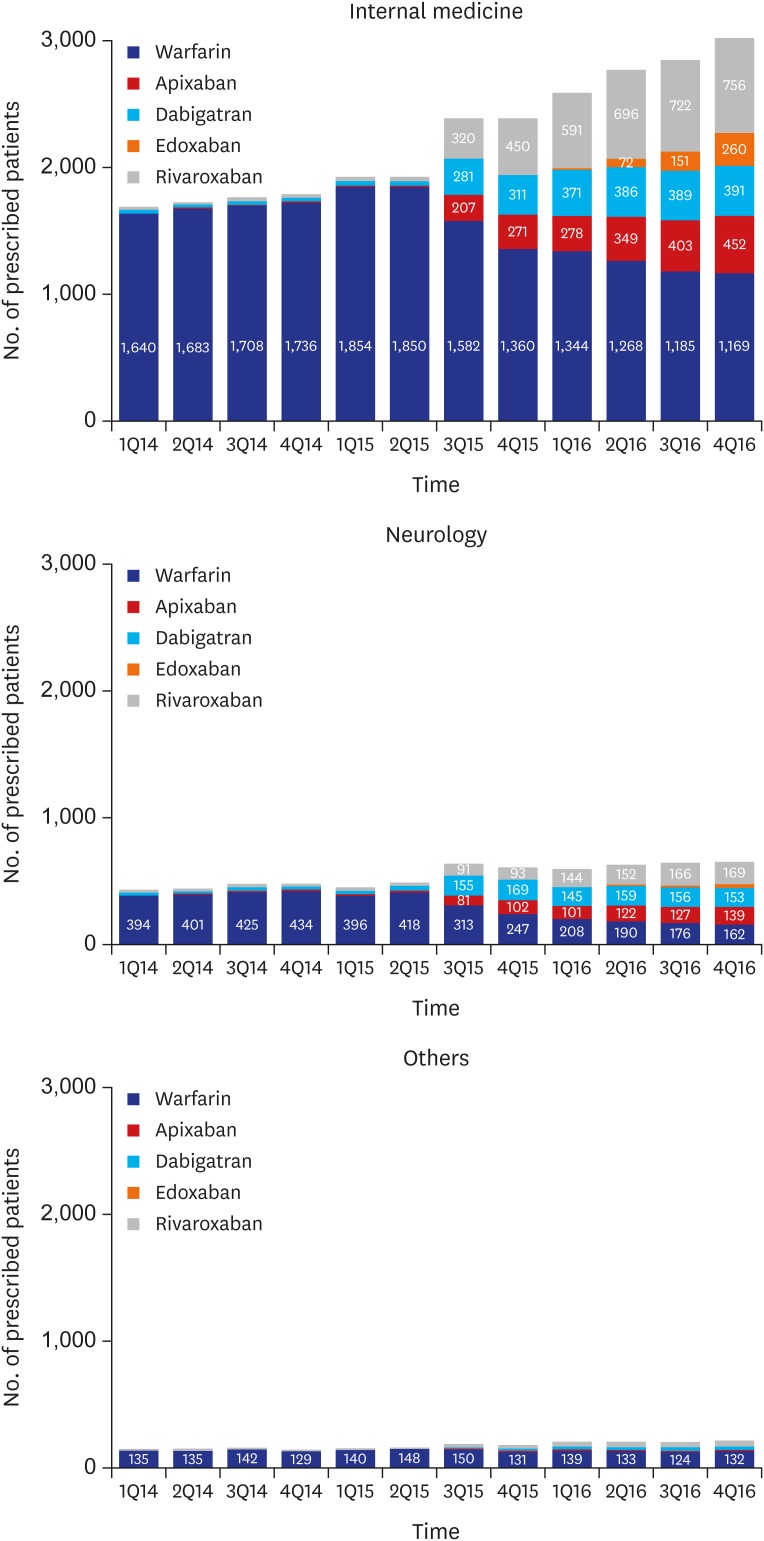
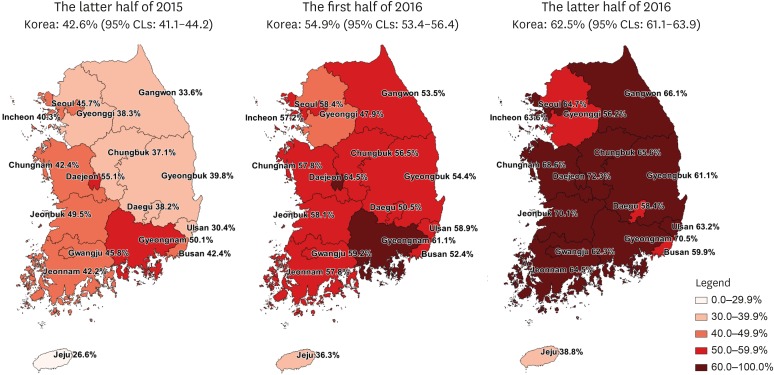
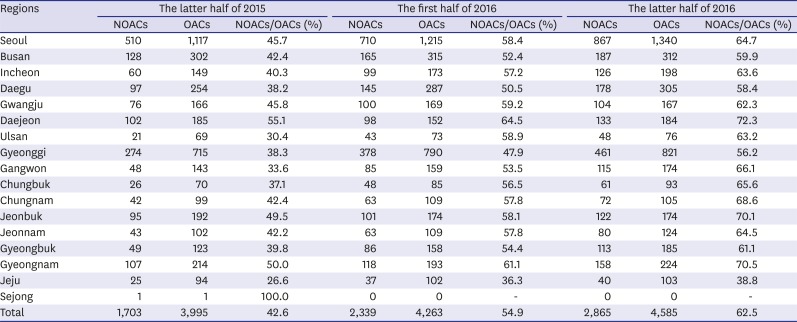
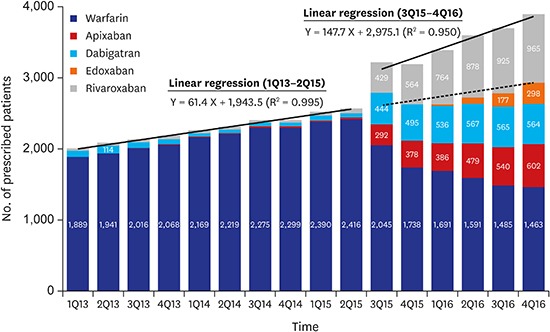
We identified the patients who were prescribed OACs on a quarterly basis for identifying changes in the prescription patterns of OACs over time (Fig. 2). Since the third quarter of 2015 when the coverage of NOACs has changed, 417.8 patients per quarter were prescribed OACs compared with the previous trends (P < 0.001). The growth rate also increased from a rate of 61.4 patients per quarter before the second quarter of 2015 to 147.7 patients per quarter thereafter (P < 0.001). According to the prescription trends by specialty of clinicians, the number of OAC prescription by internists most rapidly increased (Fig. 3). According to the prescription proportions between NOACs and OACs by regions of institute, most areas increased at similar rates; however, the increase in Jeju-do was not significant (Fig. 4, Appendix 2). At the latter half of 2016, the proportion of NOACs to OACs in most areas was approximately 60%, but was less than 40% in Jeju-do.
Fig. 2. Quarterly trend of OAC prescription among patients with atrial fibrillation from HIRA-NPS between 2013 and 2016. Solid line = results of segmented regression analysis of interrupted time series method, dotted line = expected number of patients who were prescribed OACs, estimated by the trend between the first quarter of 2013 and the second quarter of 2015.
OACs = oral anticoagulants, HIRA-NPS = Health Insurance Review and Assessment Service-National Patient Samples.
Fig. 3. Quarterly trend of OAC prescription among patients with atrial fibrillation according to specialty of clinicians from HIRA-NPS between 2013 and 2016.
OACs = oral anticoagulants, HIRA-NPS = Health Insurance Review and Assessment Service-National Patient Samples.
Fig. 4. Proportion of patients who were prescribed NOACs versus OACs according to region of institutes from HIRA-NPS between the latter half of 2015 and 2016.
95% CLs = 95% confidence limits, NOACs = non-vitamin K antagonist oral anticoagulants, OACs = oral anticoagulants, HIRA-NPS = Health Insurance Review and Assessment Service-National Patient Samples.
DISCUSSION
We evaluated how the prescription of OACs changed in Korea between 2014 and 2016. The biggest change occurred in mid-2015 when the insurance coverage policy was changed. Prior to that, the rate of prescription growth was 61.4 patients per quarter, and this trend remained constant. In July 2015, the change in OAC prescriptions due to policy change was 417.8, via an interrupted time series analysis. It was extrapolated to approximately 14,000 patients in the total Korean population. The prescription growth rate also increased more than twice to 147.7 patients. These changes were relatively steep compared to the precedents of other countries.12,13 However, conservatively considering the prevalence of Afib to be 0.5% of population, this OAC prescription among patients with Afib was still insufficient.5
Most of the prescription increases occurred in the patients aged over 70 years. Compared with the age-specific prescription rates in 2015 and 2016, the growth rate in those aged over 80 years was approximately 35.1%, and that in those aged over 70 years was approximately 28.7%. The proportion of NOACs to the total OACs also increased significantly in the old age group. More than 60% of the patients aged over 70 years received NOACs instead of warfarin in 2016. A previous meta-analysis suggested that NOAC use did not increase the bleeding risks and that NOACs had an equal or greater efficacy than warfarin in elderly patients.18 One hypothesis that could explain our results was that the prescription of NOACs increased because there are difficulties in handling adequate doses of warfarin in elderly patients.
Based on our results, we found differences in the trends of OAC prescription according to region, type of institute, and specialty of clinicians. The proportion of the prescriptions for NOACs compared with that for the total OACs for all 17 provinces in Korea had increased overall, with the exception of Jeju-do. This change occurred more rapidly in Daejeon and Gyeongsangnam-do than in other regions; however, it was not clinically significant. However, this change was found to be significantly slow in Jeju-do compared with that in the inland areas. There may be various reasons, such as preference of clinicians, baseline medical condition of patients, and economic status of patients owing to higher costs of NOACs relative to warfarin. It is not desirable that the prescription is significantly different among regions. Effective clinical practice guidelines for prescribing warfarin and NOACs based patients' baseline condition and characteristics of Koreans are then needed.
One of the reasons for the difficulty in administering warfarin was that the INR should be checked periodically.19 Therefore, it was not easy to prescribe warfarin at primary clinics. Unlike warfarin, NOACs generally do not require periodic checks of the INR; thus, the prescription of NOACs at primary clinics was expected to increase. However, our results showed that the increase in the prescription at primary clinics was relatively smaller than that at secondary or tertiary hospitals. It is necessary to consider how to manage patients with Afib as well as the consensus that hypertension and diabetes should be managed mainly at primary clinics.20
The prescriptions of OACs since 2015 was mainly increased by internists. Patients with Afib was generally cared by internists, mainly cardiologists. Therefore, it is reasonable that the prescriptions for newly discovered patients with Afib or additional prescriptions for previously cared patients without OACs prescription was done by internists. OACs were mainly prescribed by neurologists to prevent recurrence in patients who had previously been diagnosed with stroke as observed in fewer new cases. The prescription of NOACs compared with that of warfarin did not increase among thoracic surgeons and nephrologists, who mainly treated patients with heart valve diseases and severe renal diseases, which were contraindications of NOAC use.
This study has several strengths. First, our study represents the entire Korean population because of the use of the HIRA-NPS sampled from the HIRA database.16 The HIRA database contained 98% of Koreans' health insurance claim data. Second, we quantified changes in the trends of prescription owing to changes in the insurance coverage policy in July 2015. The R2 value of the segmented regression analysis of the interrupted time series method for the prescription pattern was 0.95, indicating a significant explanatory power. Therefore, the present prescription level can predict future prescription patterns.
This study also has some limitations. First, the data we used were separately extracted every year and were not available for continuous follow-up of individual patients. Therefore, it was impossible to analyze the patterns of prescription for new users or switchers. According to the trend of drug use, at least 20% of the patients have been newly prescribed with OACs since the second half of 2015. It was estimated that approximately half of the existing warfarin users have switched to NOACs by the end of 2016. Second, because of the use of the claims data, accurate diagnosis of Afib via echocardiogram could not be confirmed. In previous studies, the validity of the diagnostic codes in the HIRA database was evaluated, and the result showed an overall validity of approximately 70%.21,22 Third, medical histories were not evaluated because of the short follow-up of the individual patients. Further studies are needed to determine the difference in the OAC selection according to the CHA2DS2-VASc score.
This study identified the changes in the use of OACs in response to the changes in the national health insurance coverage policy. Differences in the drug use patterns were identified by region and type of institute. Further studies are needed to determine whether regional differences in drug use patterns are linked to actual clinical outcomes. A method to activate OAC prescription at primary clinics is needed. In addition, as the use of NOACs in Korea increases rapidly, further studies are needed to assess their efficacy and safety among Korean patients with cancer and the prescription tendency according to the presence of stroke or bleeding risk among patients. The findings of this study can be used as baseline data for researchers evaluating OACs in patients with Afib.
ACKNOWLEDGMENTS
The authors greatly appreciate the Health Insurance Review and Assessment Service (HIRA) for providing Health Insurance Review and Assessment Service-National Patient Samples (HIRA-NPS) for this study (Serial number of data: HIRA-NPS-2013-0046, HIRA-NPS-2014-0123, HIRA-NPS-2015-0109, and HIRA-NPS-2016-0051).
Appendix 1
Drug code of OACs in Korea
OACs = oral anticoagulants, ATC = anatomical therapeutic chemical.
Appendix 2
Proportion of patients who were prescribed NOACs versus OACs according to region of institutes from HIRA-NPS between the latter half of 2015 and 2016
NOACs = non-vitamin K antagonist oral anticoagulants, OACs = oral anticoagulants, HIRA-NPS = Health Insurance Review and Assessment Service-National Patient Samples.
Footnotes
Funding: This research was supported by Korea Medical Institute (KMI) Grant Program in 2017.
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Ko YJ, Kim M, Park BJ. Formal analysis: Ko YJ. Methodology: Ko YJ, Kim S, Park K, Lee J, Yang BR, Kim MS, Park BJ. Validation: Kim S. Writing - original draft: Ko YJ. Writing - review & editing: Kim S, Park K, Kim M, Lee J, Yang BR, Kim MS, Park BJ.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15(4):486–493. doi: 10.1093/europace/eus333. [DOI] [PubMed] [Google Scholar]
- 4.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639–654. doi: 10.1038/nrcardio.2014.118. [DOI] [PubMed] [Google Scholar]
- 5.Lee SR, Choi EK, Han KD, Cha MJ, Oh S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int J Cardiol. 2017;236:226–231. doi: 10.1016/j.ijcard.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Eun JN, Cho JG, Kim SS, Park HW, Lee KH, Yoon NS, et al. Prevalence of atrial fibrillation in the elderly in Korea. J Korean Geriatr Soc. 2016;20(1):29–35. [Korean] [Google Scholar]
- 7.Potpara TS, Lip GY. Postapproval observational studies of non-vitamin K antagonist oral anticoagulants in atrial fibrillation. JAMA. 2017;317(11):1115–1116. doi: 10.1001/jama.2017.1152. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GY, Larsen TB. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2017;356:j510. doi: 10.1136/bmj.j510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman CI, Peacock WF, Bunz TJ, Alberts MJ. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and previous stroke or transient ischemic attack. Stroke. 2017;48(8):2142–2149. doi: 10.1161/STROKEAHA.117.017474. [DOI] [PubMed] [Google Scholar]
- 10.Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. doi: 10.1136/bmj.i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha MJ, Choi EK, Han KD, Lee SR, Lim WH, Oh S, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2017;48(11):3040–3048. doi: 10.1161/STROKEAHA.117.018773. [DOI] [PubMed] [Google Scholar]
- 12.Kjerpeseth LJ, Ellekjær H, Selmer R, Ariansen I, Furu K, Skovlund E. Trends in use of warfarin and direct oral anticoagulants in atrial fibrillation in Norway, 2010 to 2015. Eur J Clin Pharmacol. 2017;73(11):1417–1425. doi: 10.1007/s00228-017-2296-1. [DOI] [PubMed] [Google Scholar]
- 13.Weitz JI, Semchuk W, Turpie AG, Fisher WD, Kong C, Ciaccia A, et al. Trends in prescribing oral anticoagulants in Canada, 2008–2014. Clin Ther. 2015;37(11):2506–2514.e4. doi: 10.1016/j.clinthera.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Komen J, Forslund T, Hjemdahl P, Andersen M, Wettermark B. Effects of policy interventions on the introduction of novel oral anticoagulants in Stockholm: an interrupted time series analysis. Br J Clin Pharmacol. 2017;83(3):642–652. doi: 10.1111/bcp.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staerk L, Fosbøl EL, Gadsbøll K, Sindet-Pedersen C, Pallisgaard JL, Lamberts M, et al. Non-vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: Temporal trends 2011–2015 in Denmark. Sci Rep. 2016;6(1):31477. doi: 10.1038/srep31477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim L, Kim JA, Kim S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol Health. 2014;36:e2014008. doi: 10.4178/epih/e2014008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 18.Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62(5):857–864. doi: 10.1111/jgs.12799. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Song YB, Shin DH, Kim JS, Choi JO, On YK, et al. How well does the target INR level maintain in warfarin-treated patients with non-valvular atrial fibrillation? Yonsei Med J. 2009;50(1):83–88. doi: 10.3349/ymj.2009.50.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das M, Panter L, Wynn GJ, Taylor RM, Connor N, Mills JD, et al. Primary Care Atrial Fibrillation Service: outcomes from consultant-led anticoagulation assessment clinics in the primary care setting in the UK. BMJ Open. 2015;5(12):e009267. doi: 10.1136/bmjopen-2015-009267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimm H, Yun JE, Lee SH, Jang Y, Jee SH. Validity of the diagnosis of acute myocardial infarction in Korean national medical health insurance claims data: the Korean heart study (1) Korean Circ J. 2012;42(1):10–15. doi: 10.4070/kcj.2012.42.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park BJ, Sung JH, Park KD, Seo SW, Kim SW. Studying on improving diagnosis codes in national health insurance claims data. Seoul, Korea: Seoul National University; 2003. pp. 19–52. [Google Scholar]