Abstract
Background
The optimal endoscopic screening interval for early gastric cancer (EGC) detection still remains controversial. Thus, we performed this prospective study to clarify the optimal interval between endoscopic examinations for EGC detection.
Methods
A questionnaire survey for penultimate endoscopy and gastric cancer (GC) diagnosis interval was used; the findings were then analyzed. The patients were divided into two groups according to GC type and endoscopic examinations intervals.
Results
A total of 843 patients were enrolled. The endoscopic GC detection interval (P < 0.001), tumor location (P < 0.001), tumor size (P < 0.001), histology (P < 0.001), tumor stage (P < 0.001), and treatment modality (P < 0.001) showed significant differences in the univariate analysis between EGC and advanced gastric cancer (AGC). Endoscopic examination intervals below 2 years and 3 years were associated with higher proportions of EGC detection (adjusted odds ratio, 2.458 and 3.022, respectively) (P < 0.001). The patients with endoscopic examination to GC diagnosis interval of < 2 years showed significant differences in tumor size (P < 0.001), tumor stage (P < 0.001), and treatment modality (P < 0.001) compared to those with intervals of > 2 years and without screening. Similar results were observed in those with < 3-year intervals.
Conclusion
Triennial endoscopic screening might be as effective as biennial screening in increasing the detection rate of EGC and the risk of subsequent curable endoscopic resections.
Keywords: Gastric Cancer, Optimal Interval, Endoscopic Screening
Graphical Abstract
INTRODUCTION
The incidence rate of gastric cancer (GC) is known to be high, with more than 1 million new patients affected in 2012. Although its incidence rate is declining, GC is still the second most common cancer-related cause of death worldwide.1,2 GC is most prevalent in East Asia, including Korea and Japan by region.3 The prognosis of patients with GC is highly correlated with the stage of cancer diagnosis. The 5-year survival rate of patients with early gastric cancer (EGC) is known to be over 90%.4 This suggests that early detection and treatment, especially endoscopic resection, can decrease the morbidity and mortality rates of patients with GC.5
In Korea, the National Cancer Screening Program recommends a GC screening interval of 2 years for men and women aged over 40 years.6 The optimal interval of endoscopic screening for the early detection of GC has been investigated in many studies. A previous study has shown that a barium meal study and subsequent endoscopic examinations at 1.5-year intervals were beneficial for the early detection of GC.7 Since then, an interval of 2 or 3 years was regarded as optimal in many reports.8,9,10,11 There were also several studies conducted on the optimal interval for high-risk groups, and one study in Korea has reported that a 1-year interval is beneficial for patients with severe intestinal metaplasia.5 However, only few studies exist, and such studies report variable results.
Therefore, to clarify the optimal interval of endoscopic examination for the early detection of GC, we analyzed the clinicopathological characteristics according to the type of GC and endoscopic interval. We also confirmed the detection rate of EGC and the stage of GC according to the extent of endoscopic examination interval.
METHODS
Study design
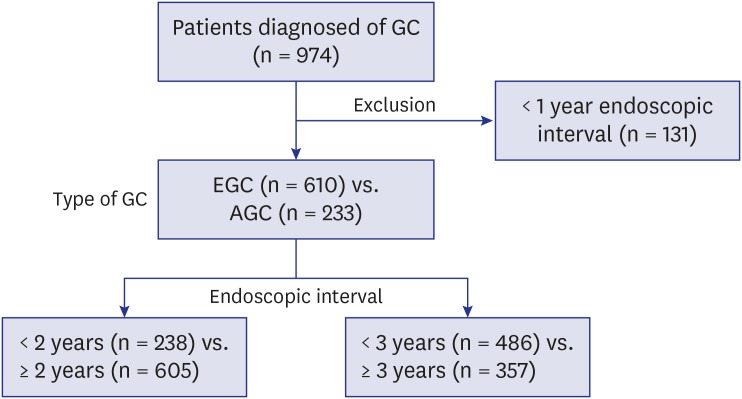
We investigated patients diagnosed with and treated accordingly for GC at Kyungpook National University Chilgok Hospital between February 2014 and July 2016. The enrolled patients' demographic and clinicopathological data were obtained prospectively using a questionnaire survey on their first visit to the outpatient clinic. Patients who underwent endoscopy within 1 year before the diagnosis of GC were excluded because of the possibility that they could have undergone endoscopy for follow-ups of uncertain diagnosis of GC or GC-related symptoms. The remaining enrolled patients were divided into two groups according to the type of GC (EGC vs. advanced gastric cancer [AGC]) and the interval between the time of penultimate endoscopy and diagnosis of GC (standard of 2 years and 3 years; Fig. 1). EGC was defined as the presence of tumors limited to the mucosa and submucosa regardless of size or regional lymph node metastases; AGC was defined as the presence of tumors invading the submucosa.9,12 The clinical factors included sex, age, endoscopic interval, and family history of GC. A family history of GC was defined as the presence of a first-degree relative with GC. The characteristics of the tumors, including location, size, stage, and histology, were also compared. Tumor location was confirmed using endoscopic examination. The “upper 1/3” portion included the cardia, fundus, and upper body; the “mid 1/3” portion included the mid-body; and the “lower 1/3” portion included the lower body, angle, and antrum. GC was staged in accordance with the 7th edition of the American Joint Committee on Cancer guidelines.13 Tumor histology was classified according to the World Health Organization classification of GC.14 Additionally, when analyzed according to the endoscopic intervals, it was classified as histologically differentiated types (including well- and moderately differentiated tubular adenocarcinoma and papillary adenocarcinoma) and undifferentiated types (including poorly differentiated tubular adenocarcinoma, poorly cohesive carcinoma including signet ring cell carcinoma, mucinous adenocarcinoma, mixed adenocarcinoma and others).15,16
Fig. 1. Study design.
GC = gastric cancer, EGC = early gastric cancer, AGC = advanced gastric cancer.
The treatment modalities of GC composed of endoscopic resection (endoscopic submucosal dissection or endoscopic mucosal resection), surgery, and palliative chemotherapy. We also calculated the number of patients who were transferred or lost to follow-up.
Statistical analysis
The Student's t-test was used to analyze continuous variables and the χ2 test or Fisher's exact test to analyze categorical variables. To adjust for possible confounding variables, the logistic regression analysis was used. Statistical analyses and data collection were performed using the SPSS ver. 20.0 (SPSS Inc., Chicago IL, USA). A P value of < 0.05 was considered statistically significant.
Ethics statement
Written informed consent was obtained from all enrolled patients. Further, the Institutional Review Board (IRB) of Kyungpook National University Chilgok Hospital approved this study (IRB No. 2012-12-152).
RESULTS
Baseline characteristics according to the type of GC
Among a total of 974 patients, 131 were excluded owing to endoscopic interval of less than a year. Thus, 843 patients were included in the analysis. Table 1 shows the baseline characteristics including the demographics of the enrolled patients, between the EGC and AGC groups. There were no differences in sex, age, and family history of GC between the two groups. The percentage of endoscopic interval of < 3 years was significantly higher in the EGC group than in the AGC group (65.2% vs. 37.8%; P < 0.001), which was in contrast to that of > 5 years or no screening (25.9% vs. 57.5%; P < 0.001).
Table 1. Baseline characteristics according to the type of GC.
| Characteristics | EGC (n = 610) | AGC (n = 233) | P value | |
|---|---|---|---|---|
| Sex | 0.823 | |||
| Male | 428 (70.2) | 166 (71.2) | ||
| Female | 182 (29.8) | 67 (28.8) | ||
| Age (mean ± SD), yr | 63.6 ± 11.1 | 64.8 ± 12.6 | 0.194 | |
| Endoscopic interval, mon | < 0.001 | |||
| 12–24 | 200 (32.8) | 39 (16.7) | ||
| 24–36 | 198 (32.5) | 49 (21.0) | ||
| 36–60 | 54 (8.9) | 11 (4.7) | ||
| > 60 | 49 (8.0) | 37 (15.9) | ||
| Never | 109 (17.9) | 97 (41.6) | ||
| Family history of GC | 115 (18.9) | 38 (16.3) | 0.449 | |
| Location | < 0.001 | |||
| Upper 1/3 | 77 (12.6) | 62 (26.7) | ||
| Mid 1/3 | 55 (9.0) | 39 (16.8) | ||
| Lower 1/3 | 477 (78.3) | 131 (56.5) | ||
| Size, cm | < 0.001 | |||
| ≤ 2 | 419 (68.7) | 21 (9.0) | ||
| 2–3 | 117 (19.2) | 48 (20.6) | ||
| 3–5 | 66 (10.8) | 84 (36.1) | ||
| > 5 | 8 (1.3) | 80 (34.3) | ||
| Stage | < 0.001 | |||
| I | 605 (99.2) | 34 (14.6) | ||
| II | 5 (0.8) | 47 (20.2) | ||
| III | 0 (0.0) | 90 (38.6) | ||
| IV | 0 (0.0) | 62 (26.6) | ||
| Histology | < 0.001 | |||
| Tubular adenocarcinoma | 460 (75.4) | 130 (55.8) | ||
| Papillary adenocarcinoma | 7 (1.1) | 3 (1.3) | ||
| Mucinous adenocarcinoma | 0 (0.0) | 2 (0.9) | ||
| Poorly cohesive carcinoma | 110 (18.0) | 74 (31.8) | ||
| Mixed | 27 (4.4) | 19 (8.2) | ||
| Others | 6 (1.0) | 5 (2.1) | ||
| Treatment | < 0.001 | |||
| ESD or EMR | 305 (50.0) | 0 (0.0) | ||
| Surgery | 250 (41.0) | 139 (59.7) | ||
| Palliative CTx | 0 (0.0) | 39 (16.7) | ||
| Transfer or f/u loss | 55 (9.0) | 55 (23.6) | ||
EGC = early gastric cancer, AGC = advanced gastric cancer, SD = standard deviation, GC = gastric cancer, ESD = endoscopic submucosal dissection, EMR = endoscopic mucosal resection, CTx = chemotherapy, f/u = follow up.
Shown in Table 1, the tumors were mainly located in the lower 1/3rd portion in the EGC group (78.3%) and percentage of location in the upper 1/3rd portion was comparatively higher in the AGC group (EGC vs. AGC, 12.6% vs. 26.7%; P < 0.001). The proportion of small-sized tumors (≤ 2 cm) was higher in the EGC group (EGC vs. AGC, 68.7% vs. 9.0%; P < 0.001) and that of > 3 cm-sized tumors was higher in the AGC group (EGC vs. AGC, 12.1% vs. 70.4%; P < 0.001). The proportions of differentiated type histology and endoscopic resection were significantly higher in the EGC group than in the AGC group (all P < 0.001).
Clinical characteristics according to the vigilance of endoscopic interval
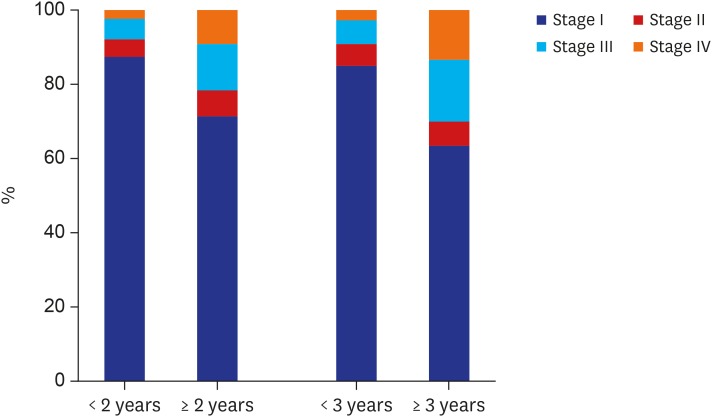
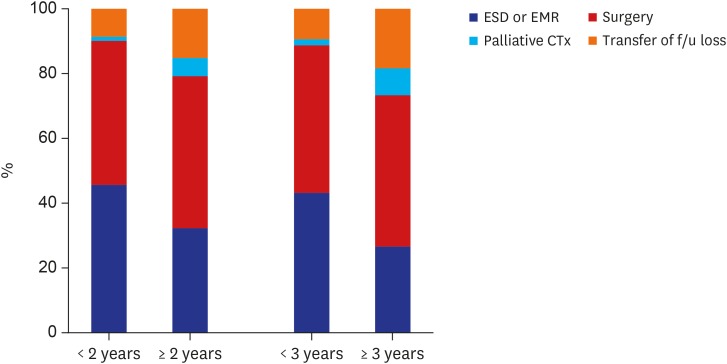
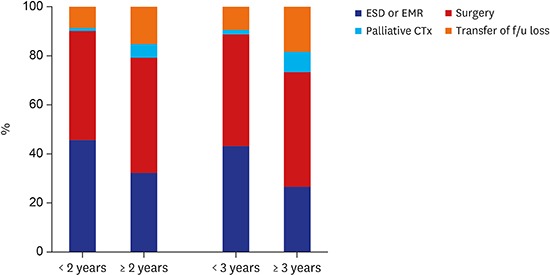
Meanwhile, the clinicopathological characteristics according to the vigilance of endoscopic interval were analyzed. The group of patients, who underwent upper gastrointestinal endoscopic examination in < 2 years before being diagnosed with GC showed significant differences in several variables compared with the groups of patients who underwent screening beyond 2 years and who did not undergo screening at all (Table 2). The tumor size tended to be small (≤ 2 cm) (< 2 years vs. ≥ 2 years, 63.9% vs. 47.6%; P < 0.001); the rate of tumor stage I was significantly higher (< 2 years vs. ≥ 2 years, 87.4% vs. 71.2%; P < 0.001); the proportion of endoscopic management increased (< 2 years vs. ≥ 2 years, 45.8% vs. 32.4; P < 0.001) (Figs. 2 and 3).
Table 2. Clinical characteristics according to the vigilance of endoscopic interval (2 years).
| Characteristics | < 2 yr (n = 238) | ≥ 2 yr (n = 605) | P value | |
|---|---|---|---|---|
| Sex | 0.591 | |||
| Male | 164 (68.6) | 430 (71.1) | ||
| Female | 74 (31.1) | 175 (28.9) | ||
| Age (mean ± SD), yr | 63.5 ± 11.6 | 64.1 ± 11.5 | 0.503 | |
| Family history of GC | 0.593 | |||
| Yes | 40 (16.8) | 113 (18.7) | ||
| No | 198 (83.2) | 492 (81.3) | ||
| Tumor location | 0.596 | |||
| Upper 1/3 | 36 (15.1) | 103 (17.1) | ||
| Mid 1/3 | 24 (10.1) | 70 (11.6) | ||
| Lower 1/3 | 178 (74.8) | 430 (71.3) | ||
| Tumor size, cm | < 0.001 | |||
| ≤ 2 | 152 (63.9) | 288 (47.6) | ||
| 2–3 | 41 (17.2) | 124 (20.5) | ||
| 3–5 | 35 (14.7) | 115 (19.0) | ||
| > 5 | 10 (4.2) | 78 (12.9) | ||
| Tumor stage | < 0.001 | |||
| Stage I | 208 (87.4) | 431 (71.2) | ||
| Stage II | 11 (4.6) | 41 (6.8) | ||
| Stage III | 13 (5.5) | 77 (12.7) | ||
| Stage IV | 6 (2.5) | 56 (9.3) | ||
| Histology | 0.199 | |||
| Differentiated | 146 (61.3) | 340 (56.2) | ||
| Undifferentiated | 92 (38.7) | 265 (43.8) | ||
| Treatment | < 0.001 | |||
| ESD or EMR | 109 (45.8) | 196 (32.4) | ||
| Surgery | 106 (44.5) | 283 (46.8) | ||
| Palliative CTx | 3 (1.3) | 36 (6.0) | ||
| Transfer or f/u loss | 20 (8.4) | 90 (14.9) | ||
SD = standard deviation, GC = gastric cancer, ESD = endoscopic submucosal dissection, EMR = endoscopic mucosal resection, CTx = chemotherapy, f/u = follow up.
Fig. 2. Tumor stage according to the vigilance of endoscopic interval.
Fig. 3. Treatment modalities according to the vigilance of endoscopic interval.
ESD = endoscopic submucosal dissection, EMR = endoscopic mucosal resection, CTx = chemotherapy, f/u = follow up.
Additionally, the clinicopathological characteristics were analyzed on the basis of the 3-year endoscopic interval (Table 3). The proportions of small-sized tumor (< 3 years vs. ≥ 3 years, 61.5% vs. 39.5%; P < 0.001), tumor stage I (< 3 years vs. ≥ 3 years, 85.0% vs. 63.3%; P < 0.001), and endoscopic management (< 3 years vs. ≥ 3 years, 43.2% vs. 26.6%; P < 0.001) were significantly higher in the group with a < 3-year interval, which was similar to that of the group with < 2-year interval (Figs. 2 and 3).
Table 3. Clinical characteristics according to the vigilance of endoscopic interval (3 years).
| Characteristics | < 3 yr (n = 486) | ≥ 3 yr (n = 357) | P value | |
|---|---|---|---|---|
| Sex | 0.872 | |||
| Male | 344 (70.8) | 250 (70.0) | ||
| Female | 142 (29.2) | 107 (30.0) | ||
| Age (mean ± SD), yr | 63.9 ± 11.0 | 64.0 ± 12.2 | 0.925 | |
| Family history of GC | 0.678 | |||
| Yes | 91 (18.7) | 62 (17.4) | ||
| No | 395 (81.3) | 295 (82.6) | ||
| Tumor location | 0.426 | |||
| Upper 1/3 | 75 (15.5) | 64 (18.0) | ||
| Mid 1/3 | 51 (10.5) | 43 (12.1) | ||
| Lower 1/3 | 359 (74.0) | 249 (69.9) | ||
| Tumor size, cm | < 0.001 | |||
| ≤ 2 | 299 (61.5) | 141 (39.5) | ||
| 2–3 | 85 (17.5) | 80 (22.4) | ||
| 3–5 | 79 (16.3) | 71 (19.9) | ||
| > 5 | 23 (4.7) | 65 (18.2) | ||
| Tumor stage | < 0.001 | |||
| Stage I | 413 (85.0) | 226 (63.3) | ||
| Stage II | 29 (6.0) | 23 (6.4) | ||
| Stage III | 30 (6.2) | 60 (16.8) | ||
| Stage IV | 14 (2.9) | 48 (13.4) | ||
| Histology | 0.189 | |||
| Differentiated | 290 (59.7) | 196 (54.9) | ||
| Undifferentiated | 196 (40.3) | 161 (45.1) | ||
| Treatment | < 0.001 | |||
| ESD or EMR | 210 (43.2) | 95 (26.6) | ||
| Surgery | 222 (45.7) | 167 (46.8) | ||
| Palliative CTx | 9 (1.9) | 30 (8.4) | ||
| Transfer or f/u loss | 45 (9.3) | 65 (18.2) | ||
SD = standard deviation, GC = gastric cancer, ESD = endoscopic submucosal dissection, EMR = endoscopic mucosal resection, CTx = chemotherapy, f/u = follow up.
Detection rate of EGC and early stage of GC according to the endoscopic examination interval
A logistic regression analysis was used to identify the detection rate of EGC according to the interval of endoscopic examination (Table 4). Possible confounding variables, including sex, age, family history of GC, and histology of GC at diagnosis, were adjusted for. The intervals between endoscopic examinations below 2 years and 3 years were associated with a higher proportion of detection of EGC (adjusted odds ratio [aOR], 2.458 and 3.022; 95% confidence interval [CI], 1.656–3.648 and 2.189–4.170, respectively; P < 0.001). We also analyzed the detection of early stage (stage I) of GC according to the interval in the same way (Table 5), the possibility of early stage of GC was found to be significantly higher in below 2 and 3 years (aOR, 2.748 and 3.176; 95% CI, 1.792–4.215 and 2.270–4.444, respectively; P < 0.001).
Table 4. The odds ratios according to the endoscopic examination intervals with the type of GC (EGC vs. AGC) as the dependent variable.
| Endoscopic examination intervals, mon | aOR | 95% CI | P value |
|---|---|---|---|
| < 24 | 2.458 | 1.656–3.648 | < 0.001 |
| ≥ 24 | 1 | ||
| < 36 | 3.022 | 2.189–4.170 | < 0.001 |
| ≥ 36 | 1 | ||
| Screened | 3.144 | 2.225–4.442 | < 0.001 |
| Never been screened | 1 |
Possible confounding variables (i.e., age, sex, family history of GC and histology of GC) at diagnosis are adjusted in this analysis.
GC = gastric cancer, EGC = early gastric cancer, AGC = advanced gastric cancer, aOR = adjusted odds ratio, CI = confidence interval.
Table 5. The ORs according to the endoscopic examination intervals with the stage of GC (stage I vs. stage II–IV) as the dependent variable.
| Endoscopic examination intervals, mon | aOR | 95% CI | P value |
|---|---|---|---|
| < 24 | 2.748 | 1.792–4.215 | < 0.001 |
| ≥ 24 | 1 | ||
| < 36 | 3.176 | 2.270–4.444 | < 0.001 |
| ≥ 36 | 1 | ||
| Screened | 2.763 | 1.944–3.929 | < 0.001 |
| Never been screened | 1 |
Possible confounding variables (i.e., age, sex, family history of GC and histology of GC) at diagnosis are adjusted in this analysis.
OR = odds ratio, GC = gastric cancer, aOR = adjusted odds ratio, CI = confidence interval.
DISCUSSION
GC is still one of the most common malignancies worldwide. In 2012, nearly half of all GC cases developed in Eastern Asia.17 The prognosis of GC is related to the stage at the time of diagnosis.10,18 The 5-year survival rate of patients with EGC exceeds 90%.19 The mortality rate of patients with GC in Korea has been steadily declining for the past 30 years, probably owing to the early detection and treatment of the disease after screening was introduced.20 We compared EGC- and AGC-diagnosed patients using a univariate analysis. The proportions of < 2 cm-size tumors, more differentiated histology, curative management, and < 3-year endoscopic interval were significantly higher in the EGC group than those in the AGC group.
Several studies on the optimal interval of endoscopic examination have been conducted. The study on Chinese populations did not show a decrease in mortality with endoscopic screening every 5 years.21 However, previous studies in Japan suggested that intervals of < 1.5 or 2 years are appropriate for the early diagnosis and curative resection of GC.7,8 In Korea, several studies showed that the effective interval to detect EGC is < 3 years.22,23 Other studies have suggested that biennial endoscopic screening can increase the rate of early detection of EGC and reduce the number of cases of progression to AGC.10,24 In one cross-sectional study of these reports, the authors analyzed GC patients except the group of endoscopic resection as a treatment of GC. As a result, the 5-year GC-specific survival rates of < 2 years interval group were significantly higher than > 2 years interval group and the risk of AGC decreased in < 2 years interval group.24 In addition, a 1-year GC screening interval is beneficial for patients with severe intestinal metaplasia in another study.5 As mentioned above, our study showed that both endoscopic intervals of < 2 years and 3 years are appropriate for EGC detection.
Meanwhile, one study showed significant results when a family history of GC, one of the important risk factors of GC, was present. In this study, the interval of < 3 years was effective for the group of patients who had a positive family history of GC (odds ratio [OR], 1.62; 95% CI, 1.10–2.37; P = 0.014).11 Conversely, when the relationship between family history of GC and endoscopic interval was analyzed in our study, no statistically significant results were obtained. We think that further research and evaluation are needed on this subject.
In addition, a study from Korea reported that 1-year intervals of GC screening would be effective for patients with severe intestinal metaplasia.5 Another study showed that a shorter endoscopic screening interval improves the optimal detection of EGC and survival.23 However, we believe that a considerable portion of the GCs with < 1-year intervals are likely to be missing cancers. One meta-analysis reported that missing cancers occur in one out of ten GCs (9.4%; 95% CI, 5.7–13.1).25 Another previous meta-analysis showed 11.3% of GCs were missing cancers within 3 years before upper gastrointestinal cancer diagnosis.26 Based on these findings, we excluded patients diagnosed with GC with a 1-year interval in our study.
In our study, we performed both univariate and multivariable analyses using logistic regression by dividing the patients into two groups according to the intervals of 2 years and 3 years. In the univariate analysis, the proportions of EGC (< 2-cm size, stage I) and endoscopic resection were significantly higher in both < 2-year and < 3-year intervals. Further, the OR for the detection of EGC or early stage of GC in the multivariable analysis was significantly higher in both the < 2-year and < 3-year interval groups. The P value between the two ORs in both cases was less than 1. In other words, although the OR was higher in the < 3-year interval group than in the < 2-year interval group, it is difficult to conclude that the 3-year interval was better than the 2-year interval for EGC detection. Moreover, there was no statistically significant difference between the two groups. One study mentioned that the OR for AGC detection did not increase in the 2-year (19–30 months) (OR, 1.11) and 3-year (31–42 months) interval groups (OR, 1.21), but increased in the 4-year (43–54 months) (OR, 2.53) and 5-year (55–66 months) interval groups (OR, 2.16). Thus, such a study reported that an endoscopic examination interval of ≤ 3 years was associated with an earlier stage of GC; therefore, changing the interval of GC screening to 3 years should be considered.11 Another report suggested that 3-year intervals were adequate for endoscopic treatment, but did not yield significant results in reducing the rate found in AGC.10 Our findings are also consistent with such results in part. When taken together, we suggest that 3-year examination intervals are as efficient as 2-year intervals for the early detection of EGC, with a high probability of curative treatment.
Our study has several limitations. First, this study was performed in a single center; thus, we cannot conclude that the enrolled patients can represent all patients with GC. However, the total number of enrolled patients was up to 1,000, and we think that this complements such a limitation. Second, although we enrolled the patients prospectively using a questionnaire, we relied on each patient's memory in several parts, such as the date of penultimate endoscopy and family history of GC, which can lead to a recall bias. Third, we did not include patients diagnosed with gastric adenoma. In the hypothesis of gastric carcinogenesis called Correa's cascade, chronic inflammation can develop into atrophic gastritis, which in turn can progress to intestinal metaplasia, gastric adenoma and gastric adenocarcinoma.27 Because gastric adenoma is a premalignant lesion, early detection and appropriate treatment will help prevent GC, particularly EGC.28,29 In addition, a study has been conducted on the optimal endoscopic interval including gastric adenoma.10 Fourth, Helicobacter pylori infection, one of the major risk factors for GC, was not analyzed because not all patients were tested for infection with this pathogen, and this could affect the outcome of the analysis. Fifth, this study was conducted in areas with high prevalence rate of GC; therefore, our results may not be suitable for use in areas with low prevalence rates, including Western regions. Finally, we did not analyze the survival rate. In another study, the cutoff interval to increase the risk of AGC and detect EGC was 3 years; this screening interval had a similar effect on the disease-free survival rate.23 But survival rate of GC is well correlated to the tumor stage and we analyzed the stage of the GC to replace the survival rate. If we could add a direct analysis of the survival rate, it would largely support our opinion.
In conclusion, triennial endoscopic screening might be as effective as biennial screening in increasing the detection rate of EGC and rate of curative endoscopic management and in sequentially reducing the number of lesions that could progress to AGC.
Footnotes
Funding: This study was supported by a grant from the National R & D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1631100).
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Jin S, Jeon SW, Kwon Y. Data curation: Jin S, Yeo SJ, Kwon SH, Lee SJ. Formal analysis: Jin S. Writing - original draft: Jin S. Writing - review & editing: Jeon SW, Kwon Y, Nam SY.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Khanderia E, Markar SR, Acharya A, Kim Y, Kim YW, Hanna GB. The influence of gastric cancer screening on the stage at diagnosis and survival: a meta-analysis of comparative studies in the far east. J Clin Gastroenterol. 2016;50(3):190–197. doi: 10.1097/MCG.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.1, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 4.Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50(3):378–381. doi: 10.1136/gut.50.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon H, Kim N, Lee HS, Shin CM, Park YS, Lee DH, et al. Effect of endoscopic screening at 1-year intervals on the clinicopathologic characteristics and treatment of gastric cancer in South Korea. J Gastroenterol Hepatol. 2012;27(5):928–934. doi: 10.1111/j.1440-1746.2011.07038.x. [DOI] [PubMed] [Google Scholar]
- 6.National cancer control programs in Korea. J Korean Med Sci. 2007;22(Suppl):S3–S4. doi: 10.3346/jkms.2007.22.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiratori Y, Nakagawa S, Kikuchi A, Ishii M, Ueno M, Miyashita T, et al. Significance of a gastric mass screening survey. Am J Gastroenterol. 1985;80(11):831–834. [PubMed] [Google Scholar]
- 8.Mori Y, Arita T, Shimoda K, Yasuda K, Yoshida T, Kitano S. Effect of periodic endoscopy for gastric cancer on early detection and improvement of survival. Gastric Cancer. 2001;4(3):132–136. doi: 10.1007/pl00011735. [DOI] [PubMed] [Google Scholar]
- 9.Nam SY, Choi IJ, Park KW, Kim CG, Lee JY, Kook MC, et al. Effect of repeated endoscopic screening on the incidence and treatment of gastric cancer in health screenees. Eur J Gastroenterol Hepatol. 2009;21(8):855–860. doi: 10.1097/MEG.0b013e328318ed42. [DOI] [PubMed] [Google Scholar]
- 10.Park CH, Kim EH, Chung H, Lee H, Park JC, Shin SK, et al. The optimal endoscopic screening interval for detecting early gastric neoplasms. Gastrointest Endosc. 2014;80(2):253–259. doi: 10.1016/j.gie.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Nam JH, Choi IJ, Cho SJ, Kim CG, Jun JK, Choi KS, et al. Association of the interval between endoscopies with gastric cancer stage at diagnosis in a region of high prevalence. Cancer. 2012;118(20):4953–4960. doi: 10.1002/cncr.27495. [DOI] [PubMed] [Google Scholar]
- 12.Murakami T. Pathomorphological diagnosis. Definition and gross classification of early gastric cancer. Jpn J Cancer Res. 1971;11:53–55. [Google Scholar]
- 13.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 14.Fléjou JF. WHO Classification of digestive tumors: the fourth edition. Ann Pathol. 2011;31(5 Suppl):S27–S31. doi: 10.1016/j.annpat.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc. 2015;81(2):333–341.e1. doi: 10.1016/j.gie.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 16.Kim JM, Sohn JH, Cho MY, Kim WH, Chang HK, Jung ES, et al. Pre- and post-ESD discrepancies in clinicopathologic criteria in early gastric cancer: the NECA-Korea ESD for Early Gastric Cancer Prospective Study (N-Keep) Gastric Cancer. 2016;19(4):1104–1113. doi: 10.1007/s10120-015-0570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi IJ. Endoscopic gastric cancer screening and surveillance in high-risk groups. Clin Endosc. 2014;47(6):497–503. doi: 10.5946/ce.2014.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Yang HK, Ahn YO. Gastric cancer in Korea. Gastric Cancer. 2002;5(3):177–182. doi: 10.1007/s101200200031. [DOI] [PubMed] [Google Scholar]
- 19.Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Takahashi M, et al. Significant prognostic factors in patients with early gastric cancer. Int Surg. 2000;85(4):286–290. [PubMed] [Google Scholar]
- 20.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014;46(2):109–123. doi: 10.4143/crt.2014.46.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riecken B, Pfeiffer R, Ma JL, Jin ML, Li JY, Liu WD, et al. No impact of repeated endoscopic screens on gastric cancer mortality in a prospectively followed Chinese population at high risk. Prev Med. 2002;34(1):22–28. doi: 10.1006/pmed.2001.0925. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS, Park HA, Kim BS, Yook JH, Lee MS. Efficacy of screening for gastric cancer in a Korean adult population: a case-control study. J Korean Med Sci. 2000;15(5):510–515. doi: 10.3346/jkms.2000.15.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H, Min BH, Lee JH, Son HJ, Kim JJ, Rhee JC, et al. Survival outcome associated with the screening interval for gastric cancer in Korea. Digestion. 2011;84(2):142–148. doi: 10.1159/000326857. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Kim SM, Ha MH, Seo JE, Choi MG, Lee JH, et al. Does the interval of screening endoscopy affect survival in gastric cancer patients?: a cross-sectional study. Medicine (Baltimore) 2016;95(49):e5490. doi: 10.1097/MD.0000000000005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pimenta-Melo AR, Monteiro-Soares M, Libânio D, Dinis-Ribeiro M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28(9):1041–1049. doi: 10.1097/MEG.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 26.Menon S, Trudgill N. How commonly is upper gastrointestinal cancer missed at endoscopy? A meta-analysis. Endosc Int Open. 2014;2(2):E46–E50. doi: 10.1055/s-0034-1365524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735–6740. [PubMed] [Google Scholar]
- 28.Lansdown M, Quirke P, Dixon MF, Axon AT, Johnston D. High grade dysplasia of the gastric mucosa: a marker for gastric carcinoma. Gut. 1990;31(9):977–983. doi: 10.1136/gut.31.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134(4):945–952. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]