Abstract
The objective of this study was to investigate and summarize the levels of incidence of Salmonella spp., Listeria monocytogenes, Staphylococcus aureus and Campylobacter spp. in poultry meat commercialized in Europe. After systematic review, incidence data and study characteristics were extracted from 78 studies conducted in 21 European countries. Pooled prevalence values from 203 extracted observations were estimated from random-effects meta-analysis models adjusted by pathogen, poultry type, sampling stage, cold preservation type, meat cutting type and packaging status. The results suggest that S. aureus is the main pathogen detected in poultry meat (38.5%; 95% CI: 25.4–53.4), followed by Campylobacter spp. (33.3%; 95% CI: 22.3–46.4%), while L. monocytogenes and Salmonella spp. present lower prevalence (19.3%; 95% CI: 14.4–25.3% and 7.10%; 95% CI: 4.60–10.8%, respectively). Despite the differences in prevalence, all pathogens were found in chicken and other poultry meats, at both end-processing step and retail level, in packed and unpacked products and in several meat cutting types. Prevalence data on cold preservation products also revealed that chilling and freezing can reduce the proliferation of pathogens but might not be able to inactivate them. The results of this meta-analysis highlight that further risk management strategies are needed to reduce pathogen incidence in poultry meat throughout the entire food chain across Europe, in particular for S. aureus and Campylobacter spp.
Keywords: chicken, Listeria monocytogenes, Campylobacter, Salmonella, Staphylococcus aureus
1. Introduction
Among the group of infectious bacteria, Salmonella spp., Listeria monocytogenes, enterotoxigenic Staphylococcus aureus and Campylobacter spp. are the main contaminants in food due to their high occurrence worldwide and for being major causes of gastroenteritis in humans [1,2].
The food poisoning symptoms caused by Salmonella spp. and Campylobacter spp. are usually abdominal cramps, fever, vomiting and diarrhea, which is often bloody in the case of the latter pathogen [3]. Listeriosis is the foodborne disease more likely to lead to hospitalization and mortality, and symptoms can include headache, stiff neck, confusion, loss of balance and convulsions in addition to fever and muscle aches. In pregnant women, listeriosis is particularly dangerous as it can lead to miscarriage, stillbirth, premature delivery, or life-threatening infection of the new-born [3]. S. aureus food poisoning symptoms include diarrhea, nausea, stomach cramps and vomiting [3]. Although it is not regarded as a disease as severe as listeriosis, for instance, in some rare cases, acute staphylococcal enterotoxicosis can cause death due to complications [4].
Epidemiological studies have suggested that products of poultry origin are among the most common vehicles for transmission of Salmonella spp. and Campylobacter spp. [5,6]. According to the European Food Safety Authority (EFSA), there is ample evidence that Campylobacter spp. is a foodborne hazard related to poultry meat, by cross-contamination from contaminated broiler meat to ready-to-eat foods [7]. It is estimated that 20% to 30% of human cases of campylobacteriosis are caused by handling, preparation, and consumption of broiler meat, while 50% to 80% may be attributed to the chicken reservoir as a whole [8]. EFSA also points Salmonella spp. as a high priority pathogen regarding poultry meat inspection [8], as poultry meat and poultry products are common sources of sporadic and outbreak-related cases of salmonellosis [9].
Listeriosis is usually associated with ready-to-eat products, including products made of poultry meat, in which contamination has occurred before or during processing, followed by growth during prolonged refrigerated storage [8]. With regard to S. aureus, it is considered that the risk of disease is more related with improper hygiene and storage throughout the food chain than its occurrence in raw meat, as this bacterium can be found naturally in poultry meats but also in the environment [8], facilitating cross-contamination between surrounding areas and poultry products.
It is important to perform research on pathogens’ detection in food to assess the main zoonotic disease carriers and to combine this information with other available in literature, through meta-analysis. By doing this, it is possible to address a wide range of food safety questions, such as the incidence of diseases and the risks imposed to public health [10]. Hence, the objective of this study was to conduct a systematic review of the incidence of Salmonella spp., L. monocytogenes, S. aureus and Campylobacter spp. in raw poultry meat at the end of the processing stage and sold at European retail establishments. In addition, meta-analysis was applied to quantitatively combine and compare the incidence of each pathogen: (i) in poultry meat as a whole; (ii) in chicken vs. other poultry meats; (iii) in poultry by different sampling stages; (iv) in chicken or other poultry meats according to distinct types of cut; (v) in chicken or other poultry meats by packaging status; and (vi) in poultry by cold preservation type.
2. Materials and Methods
As a statistical analysis of a large collection of results from published studies, meta-analysis aims to integrate and interpret the findings to achieve comprehensive conclusions that the individual studies alone would not demonstrate clearly [11]. In this study, the population is defined as raw poultry meat surveyed at the end of the processing stage or at retail establishments in Europe, while the measured outcome is the detection of pathogens. Electronic literature search was carried out in Scopus and Scielo databases to find articles and official reports published since 2000 summarizing the incidence of microbiological hazards in chicken meat produced and commercialized in Europe. The search was done systematically and aimed to find quality studies validated by the scientific community. Grey literature was not procured for two reasons; on the one hand, to avoid data validity concerns; and, on the other hand, to avoid data duplication, since it is probable that high-quality theses and reports be also published in peer-reviewed journals.
The bibliographic searches were undertaken using a formula that combined terms regarding the existence (prevalence, incidence, occurrence, quality, contamination, survey, sampling) of pathogens (Campylobacter, Listeria monocytogenes, Salmonella, Staphylococcus aureus) in the selected products (chicken, turkey, broiler, poultry), while excluding other meta-analysis studies, systematic reviews and articles regarding feed and where products were artificially inoculated (artificial, inocul*, spiked). All the terms were combined by properly applying the AND, OR and AND NOT logical connectors.
After assessing all the information from the recovered publications, seventy-eight primary studies [6,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] published from 2000 until April 2017 were considered appropriate for inclusion as they satisfied the following criteria: (i) reported outcomes from European processing units or retail establishments; (ii) use of approved microbiological methods; and (iii) presenting sufficient and extractable data (in presence/absence format).
As the incidence of microbial hazards in poultry meat is a binary trait (i.e., a sample tests either positive or negative for the pathogen), the parameter used to measure the effect size was the raw proportion p (calculated as the number of successes or positive samples, s, divided by the total sample size, n). Thus, from each primary study, the number of positive samples s and the total number n were extracted, and p was calculated. Additionally, study characteristics, such as country of origin, survey’s year, sample weight (in g), poultry class (chicken or other poultry), type of meat cut (i.e., whole carcass, pre-cut, minced meat or giblets), sampling stage (i.e., end of processing or retail), packaging status (i.e., unpacked or packed in modified atmosphere packaging—MAP,) and cold preservation type (i.e., chilled or frozen) were annotated.
Several multilevel meta-analysis models were fitted to appropriate data subsets in order to estimate overall or pooled prevalence values for: (i) each pathogen in poultry meat as a whole; (ii) each pathogen in chicken and in other poultry meats; (iii) each pathogen in poultry meat disaggregated by sampling stage; (iv) each pathogen in chicken and in other poultry meats by type of cut; (v) each pathogen in chicken and in other poultry meats by packaging status; and (vi) each pathogen in poultry meat by different cold preservation types. For a detailed explanation on the calculation of study’s weight (precision) and multilevel meta-analysis modelling for incidence data, refer to Xavier et al. [11] and Viechtbauer et al. [89]. Meta-analysis models, and Galbraith and forest plots were built in RStudio version 1.0.136 (RStudio, Boston, MA, USA) using the ‘metafor’ [89] and ‘sqldf’ [90] packages.
3. Results
Following study quality checking, a total of 203 observations of positive and negative results of incidence of foodborne pathogens in poultry meats were retrieved.
Most of the observations excerpted from the primary studies were regarding L. monocytogenes (n = 75), followed by Salmonella spp. (n = 51) and Campylobacter spp. (n = 50). S. aureus was the pathogen with the fewest observations retrieved (n = 27). It is worth mentioning that the meta-analysis results represent a synthesis of 21 European countries; namely, Austria, Bulgaria, Croatia, Denmark, Estonia, Finland, Germany, Greece, Hungary, Ireland, Italy, the Netherlands, Poland, Portugal, Romania, Serbia, Spain, Sweden, Switzerland, UK, and Turkey. Therefore, the pooled prevalence estimates, to be presented as follows, cannot be generalized to other European countries.
3.1. Incidence of Pathogens in Poultry Meat
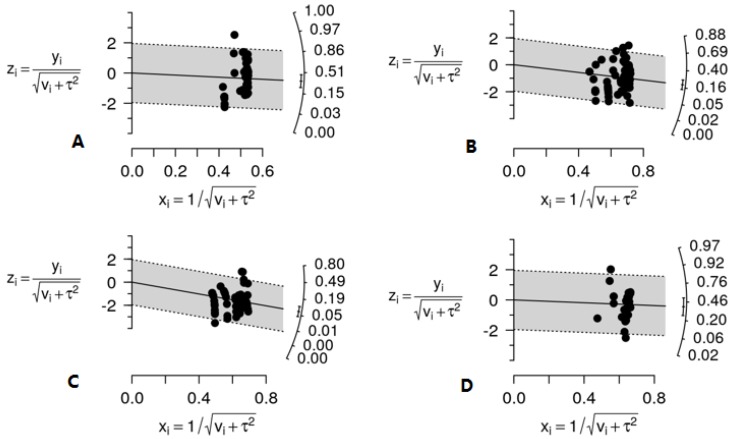
The overall prevalence of the four pathogens in poultry meat can be read from the Galbraith plots presented in Figure 1. In a meta-analytical Galbraith plot, the standardized logit transformation of the incidence value (y-axis) taken from a study is plotted against its precision (x-axis), and the meta-analysis solution is shown on the prevalence scale to the right. None of the plots showed evidence of outliers, yet all of them suggest the presence of heterogeneity among the observations extracted from the literature, fact also corroborated by the intra-class correlation I2 values, which in most cases were higher than 0.25 (Table 1). In meta-analysis, between-study variability can be considered as significant when it represents at least 25% of the total variability in the outcome measure.
Figure 1.
Galbraith plots of the meta-analyses of the incidence of the four pathogens in poultry meat surveyed at the end-processing stage and at retail establishments in Europe; Campylobacter spp. overall prevalence 33.3%; 95% CI: 22.3–46.4% (A); L. monocytogenes overall prevalence 19.3%; 95% CI: 14.4–25.3% (B); Salmonella spp. overall prevalence 7.10%; 95% CI: 4.60–10.8% (C); S. aureus overall prevalence 38.5%; 95% CI: 25.4–53.4% (D).For each observation i, yi denotes the logit-transformed incidence; vi the sampling variance; τ2 the between-study variance; xi the incidence value’s precision; and zi, the standardized logit-transformed incidence
Table 1.
Meta-analysis of the incidence of pathogens in poultry meat by type of cut. Heterogeneity analysis comprises between-study variability to total variability ratio (I2) and proportion of between-study variability explained by type of meat cut (R2).
| Microorganism | Product (n) | Pooled Prevalence | 95% CI Pooled Prevalence | Heterogeneity |
|---|---|---|---|---|
| Campylobacter spp. | Chicken (30) | 0.486 | (0.281–0.696) |
I2 = 0.419 R2 = 0.251 |
| Pre-cut (17) | 0.305 | (0.115–0.595) | ||
| Whole carcass (11) | 0.719 | (0.432–0.896) | ||
| Other Poultry (20) | 0.230 | (0.090–0.472) |
I2 = 0.211 R2 = 0.204 |
|
| Pre-cut (10) | 0.248 | (0.103–0.487) | ||
| Whole carcass (8) | 0.311 | (0.138–0.559) | ||
| L. monocytogenes | Chicken (45) | 0.210 | (0.141–0.301) |
I2 = 0.330 R2 = 0.046 |
| Pre-cut (33) | 0.202 | (0.125–0.311) | ||
| Whole carcass (9) | 0.246 | (0.131–0.414) | ||
| Other Poultry (30) | 0.129 | (0.078–0.204) |
I2 = 0.408 R2 = 0.012 |
|
| Pre-cut (16) | 0.117 | (0.063–0.207) | ||
| Whole carcass (9) | 0.143 | (0.058–0.310) | ||
| Mince (5) | 0.190 | (0.073–0.409) | ||
| Salmonella spp. | Chicken (26) | 0.032 | (0.016–0.064) |
I2 = 0.455 R2 = 0.154 |
| Pre-cut (18) | 0.058 | (0.027–0.122) | ||
| Whole carcass (5) | 0.023 | (0.007–0.068) | ||
| Giblets (3) | 0.015 | (0.003–0.059) | ||
| Other Poultry (25) | 0.057 | (0.027–0.119) |
I2 = 0.285 R2 = 0.176 |
|
| Pre-cut (17) | 0.058 | (0.026–0.125) | ||
| Whole carcass (4) | 0.141 | (0.049–0.343) | ||
| Mince (4) | 0.119 | (0.032–0.357) | ||
| S. aureus | Chicken (16) | 0.399 | (0.198–0.640) |
I2 = 0.483 R2 = 0.246 |
| Pre-cut (7) | 0.283 | (0.090–0.612) | ||
| Whole carcass (3) | 0.726 | (0.386–0.918) | ||
| Giblets (6) | 0.430 | (0.169–0.736) | ||
| Other Poultry (11) | 0.431 | (0.204–0.692) |
I2 = 0.313 R2 = 0.157 |
|
| Pre-cut (6) | 0.298 | (0.151–0.502) | ||
| Whole carcass (5) | 0.413 | (0.189–0.679) |
Overall, S. aureus bears the highest pooled prevalence in poultry meat (38.5%; 95% CI: 25.4–53.4%), followed by Campylobacter spp. (33.3%; 95% CI: 22.3–46.4%) and L. monocytogenes (19.3%; 95% CI: 14.4–25.3%), while Salmonella spp. was the pathogen of lowest prevalence (7.10%; 95% CI: 4.60–10.8%).
3.2. Incidence of Pathogens by Poultry Type: Chicken Vs. Other Poultry Meats
The meta-analysis results on the prevalence of pathogens in poultry meat by poultry class are presented in Table 1. In chicken meat, Campylobacter spp. presented the highest prevalence (48.6%; 95% CI: 28.1–69.6%), followed by S. aureus (39.9%; 95% CI: 19.8–64.0%). For other poultry meats, such as turkey and duck, the microorganism of highest prevalence was S. aureus (43.1%; 95% CI: 20.4–69.2%), followed by Campylobacter spp. (23.0%; 95% CI: 9.00–47.2%). Despite the outcomes of this meta-analysis are, in principle, not directly comparable to the official incidence figures from the latest EFSA report [91]—since the former involves a precision-weighted average of results spanning from 2000 to 2017, while the latter compiles non-weighted averages from 2016—the EFSA’s high mean incidence of Campylobacter in broiler meat (36.7%) and turkey meat (11.0%) [91] supported our findings. The pooled prevalence estimates for Salmonella spp. in chicken meat (3.2%; 95% CI: 1.6–6.4%) and other poultry meats (5.7%; 95% CI: 2.7–11.9%) were lower than the mean incidence values of Salmonella spp. in fresh broiler meat (6.4%) and fresh turkey meat (7.7%) from the 2016 EFSA report [91], although such values were still within the confidence intervals of the meta-analytical estimates. The pooled prevalence estimates of S. aureus in chicken/other poultry and of L. monocytogenes in chicken (21.0%; 95% CI: 14.1–30.1%) and other poultry meats (12.9%; 95% CI: 7.80–20.4%) could not be contrasted against EU official figures since neither L. monocytogenes nor S. aureus in raw meats or meat preparations are of compulsory notification to EFSA.
3.3. Incidence of Pathogens by Type of Cut
For different types of cuts commercialized (pre-cut, whole carcass, giblets, mince), it was observed that, in whole carcasses, S. aureus is the pathogen of greatest concern as it has the highest prevalence (72.6%; 95% CI: 38.6–91.8%) while in pre-cut meat, Campylobacter spp. was the pathogen of greatest prevalence (30.5%; 95% CI: 11.5–59.5%). Observations regarding giblets and minced meat were only found for two pathogens each (Salmonella spp. and S. aureus in giblets; and L. monocytogenes and Salmonella spp. in minced meat). S. aureus had the highest prevalence in giblets (43.0%; 95% CI: 16.9–73.6%) while L. monocytogenes was the pathogen most frequently recovered in minced meat (19.0%; 95% CI: 7.30–40.9%).
This data partition, by type of cut, allows some insight into the sources of heterogeneity between studies. Overall, for Salmonella spp., S. aureus and Campylobacter spp., the different types of meat cuts were able to explain 15 to 25% of this variability (R2). However, for L. monocytogenes, this moderator, cut type, is seemingly not a source of between-study variability.
3.4. Incidence of Pathogens by Sampling Stage
The meta-analysis results on the incidence of pathogens by sampling stage are shown in Table 2. S. aureus presented the highest prevalence in poultry meat surveyed at retail level (51.6%; 95% CI: 31.8–70.9%) and at the end-processing stage (38.1%; 95% CI: 14.2–69.7%), followed by Campylobacter spp. (44.3%; 95% CI: 28.1–61.8% at retail level; 30.7%; 95% CL: 11.8–59.4% at end-processing). Salmonella spp. had the lowest prevalence both at the end-processing stage (5.40%; 95% CI: 1.60–16.1%) and at retail level (10.4%; 95% CI: 5.30–19.3%). Except for L. monocytogenes, the recovery of pathogens in poultry meat at retail (i.e., sampled from supermarkets and butcher’s) is more frequent than when sampled in factories at the end of processing (Table 2).
Table 2.
Meta-analysis of the incidence of pathogens in poultry meat surveyed in Europe by sampling stage.
| Pathogen | n | Pooled Prevalence | 95% CI Pooled Prevalence |
|---|---|---|---|
| Campylobacter spp. | |||
| End-processing | 9 | 0.307 | (0.118–0.594) |
| Retail | 41 | 0.443 | (0.281–0.618) |
| L. monocytogenes | |||
| End-processing | 14 | 0.217 | (0.084–0.458) |
| Retail | 61 | 0.171 | (0.111–0.255) |
| Salmonella spp. | |||
| End-processing | 16 | 0.054 | (0.016–0.161) |
| Retail | 35 | 0.104 | (0.053–0.193) |
| S. aureus | |||
| End-processing | 5 | 0.381 | (0.142–0.697) |
| Retail | 22 | 0.516 | (0.318–0.709) |
3.5. Incidence of Pathogens by Packaging Status
The results of the meta-analysis performed on the incidence of pathogens by packaging status are presented in Table 3. Overall, Campylobacter spp. was the pathogen of greatest prevalence in either packed or unpacked poultry meats, with the highest prevalence occurring in chicken (47.2%; 95% CI: 19.4–76.9% in packed chicken; 47.1%; 95% CI: 13.1–84.1% in unpacked chicken). Further, it can be observed that, for the other three bacteria, all unpacked products revealed higher prevalence of pathogens than the packed ones, which can be explained by the fact that packed products have a physical barrier which helps to decrease and slow down the meat deterioration processes [92].
Table 3.
Meta-analysis of the incidence of pathogens in chicken and other poultry meat by packaging status. Heterogeneity analysis comprises between-study variability to total variability ratio (I2) and proportion of between-study variability explained by packaging status (R2).
| Microorganism | Product (n) | Pooled Prevalence | 95% CI Pooled Prevalence | Heterogeneity |
|---|---|---|---|---|
| Campylobacter spp. | Chicken |
I2 = 0.419 R2 = 0.018 |
||
| Packed (20) | 0.472 | (0.194–0.769) | ||
| Unpacked (9) | 0.471 | (0.131–0.841) | ||
| Other Poultry |
I2 = 0.211 R2 = NA * |
|||
| Packed (18) | 0.311 | (0.195–0.456) | ||
| L. monocytogenes | Chicken |
I2 = 0.330 R2 = 0.034 |
||
| Packed (37) | 0.185 | (0.114–0.287) | ||
| Unpacked (8) | 0.307 | (0.114–0.605) | ||
| Other Poultry |
I2 = 0.408 R2 = 0.047 |
|||
| Packed (18) | 0.125 | (0.074–0.201) | ||
| Unpacked (12) | 0.148 | (0.079–0.260) | ||
| Salmonella spp. | Chicken |
I2 = 0.455 R2 = 0.007 |
||
| Packed (17) | 0.031 | (0.010–0.097) | ||
| Unpacked (9) | 0.048 | (0.021–0.108) | ||
| Other Poultry |
I2 = 0.258 R2 = NA |
|||
| Packed (25) | 0.079 | (0.039–0.150) | ||
| S. aureus | Chicken |
I2 = 0.483 R2 = NA |
||
| Packed (15) | 0.408 | (0.165–0.708) | ||
| Other Poultry |
I2 = 0.313 R2 = 0.157 |
|||
| Packed (6) | 0.298 | (0.152–0.502) | ||
| Unpacked (5) | 0.413 | (0.189–0.679) |
(*) Not applicable.
Contrarily to the moderator “type of cut”, this moderator “packaging status” explained only marginally the between-study variability present in the data.
3.6. Incidence of Pathogens by Cold Preservation Type
The results of the meta-analysis on the incidence of pathogens by cold preservation type are shown in Table 4. In chilled poultry meat, S. aureus was the pathogen of highest prevalence (46.9%; 95% CI: 30.8–63.7%), followed by Campylobacter spp. (43.9%; 95% CI: 24.2–65.7%). Salmonella spp. shows the lowest prevalence (7.10%; 95% CI: 4.10–12.0%) in chilled meat.
Table 4.
Meta-analysis of the incidence of pathogens in poultry meat by cold preservation type.
| Stage | Microorganism | n | Pooled Prevalence | 95% CI Pooled Prevalence |
|---|---|---|---|---|
| Chilled | Campylobacter spp. | 45 | 0.439 | (0.242–0.657) |
| L. monocytogenes | 74 | 0.177 | (0.126–0.243) | |
| Salmonella spp. | 48 | 0.071 | (0.041–0.121) | |
| S. aureus | 26 | 0.469 | (0.308–0.637) | |
| Frozen | Campylobacter spp. | 5 | 0.098 | (0.032–0.263) |
As few data available were available for freezing preservation, conclusions regarding contaminated frozen poultry meat can only be drawn for one pathogen, Campylobacter spp. From the prevalence values obtained in chilled and frozen meats, it can be stated that freezing affects the growth and recovery of Campylobacter spp., thus ensuring lower prevalence of this pathogen in poultry meats (9.80%; 95% CI: 3.20–26.3%) and improving food safety.
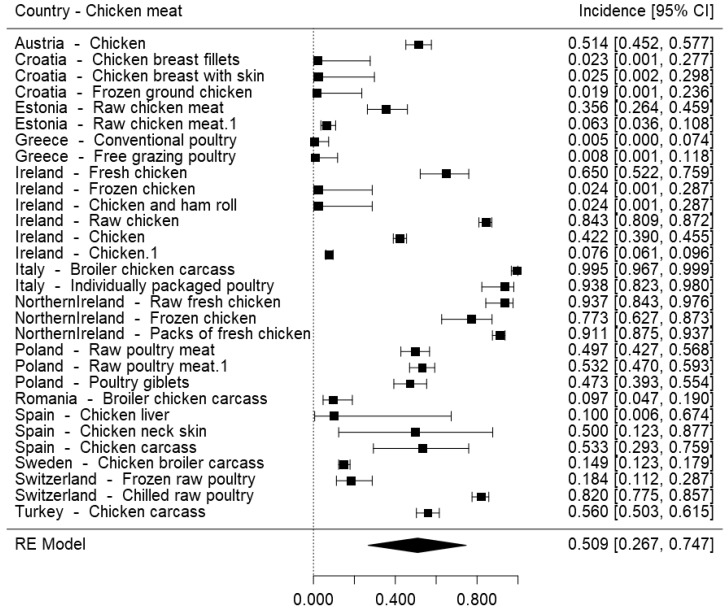
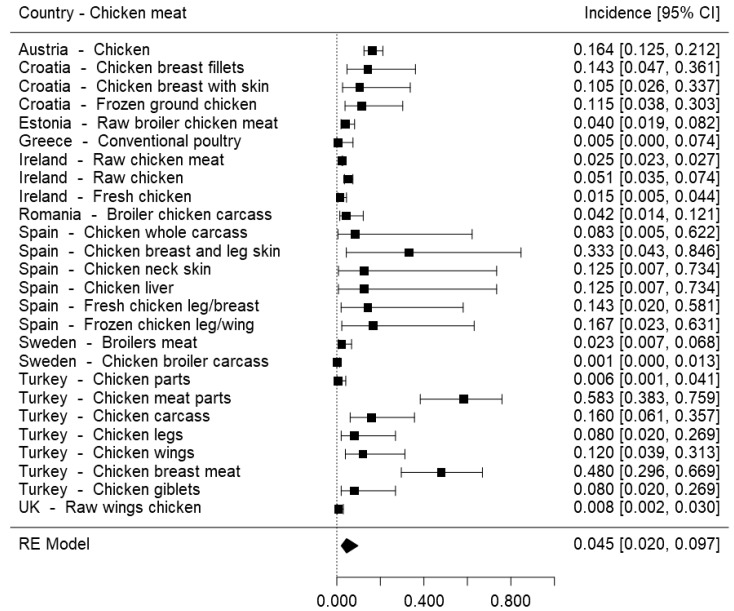
To gather the incidence measures retrieved from primary studies for Campylobacter spp. and Salmonella spp. in chicken meat, forest plots were built (Figure 2 and Figure 3). These pathogens were selected to assess their incidence per European country as they are both great concerns in terms of food safety, but one has currently very high incidence in this type of product, with more control still being needed, while the other is nowadays better controlled, after the implementation of various control processes specific to Salmonella spp.
Figure 2.
Forest plot of the incidence of Campylobacter spp. in chicken meat surveyed in European establishments.
Figure 3.
Forest plot of the incidence of Salmonella spp. in chicken meat surveyed in European establishments.
Figure 2 reveals that the highest overall frequencies of Campylobacter spp. in chicken meat were reported in Italy (99.5%; 95% CI: 96.7–99.9%) and Northern Ireland (93.7%; 95% CI: 84.3–97.6%). Regarding Salmonella spp., Figure 3 shows that Turkey and Spain reported the highest overall frequencies of this pathogen in chicken meat, with prevalence values as high as 58.3% (95% CI: 38.3–75.9%) and 33.3% (95% CI: 4.30–84.6%), respectively.
Conclusions should be carefully taken from these plots, as the results might reflect more than just values of prevalence: for example, in Spain, coverage of the surveillance system for campylobacteriosis has improved and the number of reported confirmed cases has almost doubled since 2012 [91]. In any case, it can be stated that these forest plots consolidate the fact that Salmonella spp. is currently better controlled (from the incidence point of view but also the quality of the surveillance system) throughout the entire food chain in comparison to Campylobacter spp., as the prevalence of the latter is significantly higher than the first. It also emphasizes the pressing need of establishing control processes for Campylobacter spp. as effective as the ones in place for Salmonella spp., and that might not be the same in different countries, as previously stated by Skarp et al. (2015) [93].
4. Discussion
4.1. Incidence of S. aureus
The general meta-analysis, that is, with all data collected without any partitions, reveals that S. aureus is the pathogen of highest prevalence in overall poultry meats. According to Pepe et al. (2006) [94], staphylococci is one of the most common bacteria present in poultry slaughtering and processing environments, which is corroborated by the results of the sampling stage meta-analysis, in which S. aureus presented the highest prevalence in chicken meat surveyed at the end of processing (38.1%; 95% CI: 14.2–69.7%), but also at retail level (51.6%; 95% CI: 31.8–70.9%). The high prevalence at the end-processing stage is likely to be a consequence of the lack of control steps or the inefficiency to avoid contamination by this pathogen from the beginning of the process, at the slaughterhouses, where the skin and mucous membranes of animals are often contaminated, whether it is naturally or due to cross-contamination with infected carcasses. At retail level, there is not always a strict temperature control, making the products susceptible to this pathogen through various routes of contamination. As the vegetative form of S. aureus requires temperatures above those used for refrigeration to grow to levels of concentration of public health relevance [8], this is a relatively easy microorganism to control if the cold chain is secured throughout production, in comparison to L. monocytogenes, for instance, which grows even at refrigeration temperatures. In this sense, it is easier to reduce the health risks associated with this pathogen, which can explain the currently reduced effects on public health and low number of diseased.
Regarding the different cutting types, whole carcasses revealed to be the product with highest prevalence of pathogens, in particularly S. aureus, the one of greatest incidence. The trend observed among all analyzed microorganisms, that greater incidence occurs in whole carcasses in comparison with cut/minced meat and giblets, may be associated with the microbiological detection method used. In whole carcasses, microbiological analysis is preceded by homogenization of the entire carcass [54], meaning that the entire surface is sampled. On the contrary, analysis of pre-cut or minced meat and giblets are carried out by sampling 25 g of the product or swabbing 100 cm2 of the surface [75]. As it would be expected, sampling of whole carcasses is a much more sensitive technique in terms of pathogen detection in comparison to smaller samples of 25 g or 100 cm2 swabs, hence the higher prevalence detected.
For a mesophilic bacterium, S. aureus has a relatively high heat resistance [95], which can explain why some of the control steps implemented are not suitable for its inactivation. In particular, packaging was found insufficient to reduce the presence of this pathogen, as packed poultry meats revealed considerable prevalence of S. aureus, which may be caused by inefficient sterilization of the packaging material. Besides having a physical barrier to microorganisms, packed products are also subjected to a specific gas composition. Although the gas composition certainly impacts the spoilage of poultry meats, it is necessary to optimize the gas mixture that will affect bacterial growth the most [96].
Overall, special attention must be paid to S. aureus in undercooked meats and in chicken that will be used to produce transformed products such as sausages, as it is possible for S. aureus to survive and proliferate during further meat processing.
4.2. Incidence of Campylobacter spp.
The general meta-analysis showed that Campylobacter spp. is a pathogen of high prevalence in European poultry meats, which agrees with the statements of Humphrey et al. (2007), where poultry was pointed as an important source of campylobacteriosis, mostly because this bacterium is often carried in the intestinal tract of such animals [97].
Campylobacter spp. has a great colonization capacity, higher levels of intestinal carriage at slaughter than other bacterium (for instance Salmonella spp.) [98], and it is highly resistant to procedures such as scalding, washing and cooling, which generally reduce the incidence of other microorganisms to acceptable levels [97]. The reason for this survival may be the unique ability to attach to poultry tissues during carcass processing [98]. The incidence of Campylobacter spp. in the many chilled poultry meat samples surveyed across Europe confirms that, while cooling may be able to reduce the fast proliferation that generally occurs during the slaughter process, yet, it is insufficient to inactivate this pathogen [98,99]. The significantly lower prevalence estimated for frozen poultry (~10%), as opposed to the ~44% pooled prevalence in chilled poultry, was expected since freezing is known to decrease the number of campylobacters in time. In a fate study [100], it was found that C. jejuni inoculated in poultry meat samples decreased from 7.5 log CFU/g to 3.8 log CFU/g after only 30 min storage at −20 °C.
Regardless of the type of cut or packaging status, in chicken and other poultry meats, high incidence of this pathogen was observed. Furthermore, this meta-analysis also revealed high prevalence of Campylobacter spp. at the end of processing and retail level. Recently, studies are showing that campylobacters may be more robust than previously thought and represent a superior challenge to food safety [95], reason as to why the implemented safety control procedures during meat processing might be scarce or inadequate. With farms being the preliminary site of Campylobacter entry into production, the major intervention strategies should be targeted at farm level, enhancing biosecurity and implementing better monitoring, as interventions at slaughter process are less efficient [93].
There are plenty of opportunities for improvement along the food chain when it comes to the overall goal of reducing campylobacteriosis, with new and effective measures needing to be quickly implemented as Campylobacter has been the most commonly reported gastrointestinal bacterial pathogen in humans in the EU since 2005 [91]. In particular, C. jejuni and C. coli have been reported as the main causes of human campylobacteriosis [91,93].
4.3. Incidence of L. monocytogenes
Considered by EFSA as the second leading cause of food poisoning outbreaks [91], Listeria monocytogenes was found to be, in this meta-analysis, the third most incident pathogen in chicken and other poultry meats.
L. monocytogenes can be found naturally in the environment (soil, sewage, animal feces and water) [99,101] and in foods. In particular, poultry can be asymptomatic carriers of this pathogen and introduce contamination in slaughterhouses [102,103]. This is a great concern at the industrial level because this pathogen has the ability to withstand and adapt to various environmental stresses: it can multiply at low temperatures and form biofilms on food-processing equipment and food-contact surfaces, causing cross-contamination in chicken meat and its derivatives [102] and making a wide range of foodstuffs susceptible to contamination, which is particularly concerning for RTE foods that are not cooked or re-heated. The formation of these biofilms can be the cause for the high incidence values of L. monocytogenes found at the end-processing stage and retail level.
Commonly found in bird feces [104], L. monocytogenes high incidence in carcasses is likely to arise from cross-contamination during processing. At the end of the process, cooling of meat is intended to inhibit the multiplication of pathogens, but it was observed that in chilled poultry meats, the prevalence of L. monocytogenes was still quite relevant, confirming that refrigeration is not enough to inhibit the proliferation of this cold-resistant pathogen.
Following what was already observed for other pathogens, the occurrence of L. monocytogenes is higher in broiler meat commercialized without packaging than in packed ones. Packaging can stop further cross-contamination after the products are packed, but if the product is already contaminated with high levels of pathogens, if the wrapper is not properly sterilized, or if the inactivation processes used after packaging are not adequate, presence of pathogens can still occur.
4.4. Incidence of Salmonella spp.
In this work, we did not verify high levels of incidence of Salmonella spp. in poultry meat when compared to the other three pathogens under study. The highest prevalence found was in whole carcasses of poultry meat other than chicken, which is probably a consequence of the microbiological detection method, as previously stated.
It is evident that the low incidence of Salmonella spp. observed in this work is due to the high investments in zoonoses control in the past years. Since 2003, EU Member States have the responsibility to implement Salmonella national control programs and report the results to the European Commission and EFSA, as part of the annual EU zoonoses monitoring. These programs aim to reduce Salmonella prevalence in certain animal populations, particularly in breeding flocks of Gallus gallus, laying hens, broilers and breeding and fattening turkeys [91].
Currently, the establishment of strict biosecurity measures at farm level (including Salmonella-free poultry feed and water), vaccination programs in the parent flocks, and testing/removal of positive flocks from production have been used as control measures [104]. Additionally, the use of feed additives and acidified food and water have been encouraged, as the pH reduction is expected to have a protective effect on the feed, milling and feeding equipment and on the general environment [104].
This large reduction of salmonellosis and Salmonella prevalence in food products in the past years should be taken as a motivation for the control of other pathogens, as the effective implementation of control programs demonstrate that it is possible to produce safer food if the proper, adequate measures are implemented. However, it is crucial to keep in mind that the efficacy of such interventions depends on the level of bacterial contamination and that the control steps must be specific for each microorganism, as they have their own characteristics and different resistance to diverse conditions.
Despite the declining trend of salmonellosis in the EU that can give an increased sense of security, continuous research is mandatory to solve new difficulties, such as the increasing antimicrobial resistance in non-typhoid Salmonella species that has become a serious problem for public health worldwide [104]. Moreover, aiming for the reduction of one specific serovar or serotype is not sufficient, as the currently predominant serotypes in poultry flocks are likely to change over time [104].
5. Conclusions
It is a great concern that chicken meat has been considered one of the main causes of food poisoning while the poultry industry is simultaneously one of the most important sources of animal protein for the world’s population [102]. Pathogenic contamination of poultry can occur at any level: in the initial production environment, through vertical transmission (via egg, triggering the birth of infected chicks) or horizontal transmission (caused by contaminated environment or feed); or in the slaughtering process [6,47]. With gastrointestinal tract of birds and slaughtering facilities identified as the main reservoirs of poultry meat contaminants [96], it is necessary to implement preventive and corrective measures in several stages, but mainly on farm to reduce the initial levels of contamination that enter the process. As every stage of poultry meat production and processing systems has its own unique challenges regarding pathogen contamination and control, a multi-hurdle approach is likely to be the best strategy for pathogen reduction and elimination [105].
This meta-analysis on incidence data from European surveys indicated that S. aureus is currently the main contaminating pathogen of poultry meat, followed closely by Campylobacter spp. The establishment of control processes specific to these pathogens, as it was done in Salmonella spp. control programs, will certainly have a great impact on their current values of incidence, enabling the production and provision of safer meat products to consumers.
Adequate interventions at the processing stages can be assessed through challenge tests and predictive microbiology. In particular, growth and inactivation models can take into account factors such as the levels of contamination when carcasses leave the processing plant, storage time in retail stores, transport time, storage times in homes and the temperatures carcasses were exposed to during each of these periods [104], making this a unique tool for researchers and food companies to increase food safety and prevent new outbreaks.
Despite industries’ responsibility to take action on these matters, consumers should be further educated and encouraged to take preventive measures to ensure their health and well-being, such as: sanitization of hands, surfaces and utensils before and after handling poultry meat; separation of raw poultry meat from other foods (especially cooked) to avoid cross-contamination; proper storage of products, ensuring refrigeration temperatures under 5 °C; consumption of properly cooked, non-washed, poultry meat, as washing can spread bacteria and contaminate kitchen surfaces.
Acknowledgments
Gonzales-Barron wishes to acknowledge the financial support provided by the Portuguese Foundation for Science and Technology (FCT) through the award of a five-year Investigator Fellowship (IF) in the mode of Development Grants (IF/00570).
Author Contributions
V.R. performed the bibliographic searches and extracted meta-analytical data; A.G.-T. double-checked extracted data, adjusted meta-analysis models and wrote 20% of the article; B.N.S. wrote 60% of the article and revised it; U.G.-B. and V.C. conceived and designed the work, coded data analysis scripts, produced meta-analytical graphs, wrote 20% of the article, revised it and proofread it.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Van Nierop W., Dusé A.G., Marais E., Aithma N., Thothobolo N., Kassel M., Stewart R., Potgieter A., Fernandes B., Galpin J.S., et al. Contamination of chicken carcasses in Gauteng, South Africa, by Salmonella, Listeria monocytogenes and Campylobacter. Int. J. Food Microbiol. 2005;99:1–6. doi: 10.1016/j.ijfoodmicro.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Hennekinne J.-A., De Buyser M.-L., Dragacci S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012;36:815–836. doi: 10.1111/j.1574-6976.2011.00311.x. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Food Safety: Symptoms and Sources of Food Poisoning. [(accessed on 23 April 2018)];2018 Available online: http://www.cdc.gov/foodsafety/symptoms.html.
- 4.Balaban N., Rasooly A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000;61:1–10. doi: 10.1016/S0168-1605(00)00377-9. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen F., Bailey R., Williams S., Henderson P., Wareing D.R.A., Bolton F.J., Ward L., Humphrey T.J. Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. Int. J. Food Microbiol. 2002;76:151–164. doi: 10.1016/S0168-1605(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M., Palmu L., Bjorkroth K., Korkeala H. Prevalence of Listeria monocytogenes in broilers at the abattoir, processing plant, and retail level. J. Food Prot. 2001;64:994–999. doi: 10.4315/0362-028X-64.7.994. [DOI] [PubMed] [Google Scholar]
- 7.EFSA Panel on Biological Hazards (BIOHAZ) Scientific Opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010;8:1437. [Google Scholar]
- 8.EFSA Panel on Biological Hazards (BIOHAZ) EFSA Panel on Contaminants in the Food Chain (CONTAM) EFSA Panel on Animal Health and Welfare (AHAW) Scientific Opinion on the public health hazards to be covered by inspection of meat (poultry) EFSA J. 2012;10:2741. [Google Scholar]
- 9.Pires S., de Knegt L., Hald T. Estimation of the Relative Contribution of Different Food and Animal Sources to Human Salmonella Infections in the European Union. EFSA Supporting Publications; Parma, Italy: 2011. Question No EFSA-Q-2010-00685. [Google Scholar]
- 10.Gonzales-Barron U., Butler F. The use of meta-analytical tools in risk assessment for food safety. Food Microbiol. 2011;28:823–827. doi: 10.1016/j.fm.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Xavier C., Gonzales-Barron U., Paula V., Estevinho L., Cadavez V. Meta-analysis of the incidence of foodborne pathogens in Portuguese meats and their products. Food Res. Int. 2014;55:311–323. doi: 10.1016/j.foodres.2013.11.024. [DOI] [Google Scholar]
- 12.Akpolat N., Elci S., Atmaca S., Gül K. Listeria monocytogenes in products of animal origin in Turkey. Vet. Res. Commun. 2004;28:561–567. doi: 10.1023/B:VERC.0000042872.07616.18. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Hernando A., Prieto M., García-Fernández C., Alonso-Calleja C., Capita R. Increase over time in the prevalence of multiple antibiotic resistance among isolates of Listeria monocytogenes from poultry in Spain. Food Control. 2012;23:37–41. doi: 10.1016/j.foodcont.2011.06.006. [DOI] [Google Scholar]
- 14.Álvarez-Astorga M., Capita R., Alonso-Calleja C., Moreno B., García-Fernández M. Microbiological quality of retail chicken by-products in Spain. Meat. Sci. 2002;62:45–50. doi: 10.1016/S0309-1740(01)00225-X. [DOI] [PubMed] [Google Scholar]
- 15.Angelidis A., Koutsoumanis K. Prevalence and concentration of Listeria monocytogenes in sliced ready-to-eat meat products in the Hellenic retail market. J. Food Prot. 2006;69:938–942. doi: 10.4315/0362-028X-69.4.938. [DOI] [PubMed] [Google Scholar]
- 16.Antunes P., Reu C., Sousa J., Pestana N., Peixe L. Incidence and susceptibility to antimicrobial agents of Listeria spp. and Listeria monocytogenes isolated from poultry carcasses in Porto, Portugal. J. Food Prot. 2002;65:1888–1893. doi: 10.4315/0362-028X-65.12.1888. [DOI] [PubMed] [Google Scholar]
- 17.Aras Z., Ardic M. Occurrence and Antibiotic Susceptibility of Listeria Species in Turkey Meats. Korean J. Food Sci. Anim. Resour. 2015;35:669–673. doi: 10.5851/kosfa.2015.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayaz N.D., Erol I. Rapid Detection of Listeria monocytogenes in ground turkey by immunomagnetic separation and polymerase chain reaction. J. Rapid Meth. Aut. Microbiol. 2009;17:214–222. doi: 10.1111/j.1745-4581.2009.00172.x. [DOI] [Google Scholar]
- 19.Aydin F., Gumussoy K.S., Ica T., Sumerkan B., Esel D., Akan M., Ozdemir A. The prevalence of Campylobacter jejuni in various sources in Kayseri, Turkey, and molecular analysis of isolated strains by PCR-RFLP. Turk. J. Vet. Anim. Sci. 2007;31:13–19. [Google Scholar]
- 20.Beninati C., Reich F., Muscolino D., Giarratana F., Panebianco A., Klein G., Atanassova V. ESBL-producing bacteria and MRSA isolated from poultry and turkey products imported from Italy. Czech J. Food Sci. 2015;33:97–102. doi: 10.17221/428/2014-CJFS. [DOI] [Google Scholar]
- 21.Boer E., Zwartkruis-Nahuis J., Wit B., Huijsdens X., Neeling A., Bosch T., van Oosterom R., Vila A., Heuvelink A. Prevalence of methicillin-resistant Staphylococcus aureus in meat. Int. J. Food Microbiol. 2009;134:52–56. doi: 10.1016/j.ijfoodmicro.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Busani L., Cigliano A., Taioli E., Caligiuri V., Chiavacci L., Di Bella C., Battisti A., Duranti A., Gianfranceschi M., Nardella M.C., et al. Prevalence of Salmonella enterica and Listeria monocytogenes contamination in foods of animal origin in Italy. J. Food Prot. 2005;68:1729–1733. doi: 10.4315/0362-028X-68.8.1729. [DOI] [PubMed] [Google Scholar]
- 23.Capita R., Alonso-Calleja C., Del Camino García-Fernández M., Moreno B. Microbiological quality of retail poultry carcasses in Spain. J. Food Prot. 2001;64:1961–1966. doi: 10.4315/0362-028X-64.12.1961. [DOI] [PubMed] [Google Scholar]
- 24.Capita R., Alonso-Calleja C., Moreno B., García-Fernández M. Assessment of Baird-Parker Agar as Screening Test for Determination of Staphylococcus aureus in Poultry Meat. J. Microbiol. 2001;39:321–325. [Google Scholar]
- 25.Capita R., Alonso-Calleja C., Moreno B., García-Fernández M. Occurrence of Listeria species in retail poultry meat and comparison of a cultural/immunoassay for their detection. Int. J. Food Microbiol. 2001;65:75–82. doi: 10.1016/S0168-1605(00)00497-9. [DOI] [PubMed] [Google Scholar]
- 26.Capita R., Alonso-Calleja C., Prieto M., Del Camino García-Fernández M., Moreno B. Comparison of PALCAM and modified oxford plating media for isolation of Listeria species in poultry meat following UVM II or fraser secondary enrichment broths. Food Microbiol. 2001;18:555–563. doi: 10.1006/fmic.2001.0446. [DOI] [Google Scholar]
- 27.Carp-Carare C., Vlad-Sabie A., Floristean V.-C. Detection and serotyping of Listeria monocytogenes in some food products from North-East of Romania. Rom. Rev. Lab. Med. 2013;21:285–292. doi: 10.2478/rrlm-2013-0025. [DOI] [Google Scholar]
- 28.Cetinkaya F., Cibik R., Soyutemiz G.E., Ozakin C., Kayali R., Levent B. Shigella and Salmonella contamination in various foodstuffs in Turkey. Food Control. 2008;19:1059–1063. doi: 10.1016/j.foodcont.2007.11.004. [DOI] [Google Scholar]
- 29.Cetinkaya F., Mus T.E., Yibar A., Guclu N., Tavsanli H., Cibik R. Prevalence, serotype identification by multiplex polymerase chain reaction and antimicrobial resistance patterns of Listeria monocytogenes isolated from retail foods. J. Food Saf. 2014;34:42–49. doi: 10.1111/jfs.12093. [DOI] [Google Scholar]
- 30.Ceylan Z.G., Demirkaya A.K., Adiguzel G. Incidence of Listeria monocytogenes in retail chicken meat and establishing relationship with some bacteria by logistic regression. J. Food Qual. 2008;31:121–130. doi: 10.1111/j.1745-4557.2007.00188.x. [DOI] [Google Scholar]
- 31.Cloak O., Duffy G., Sheridan J., Blair I., McDowell D. A survey on the incidence of Campylobacter spp. and the development of a surface adhesion polymerase chain reaction (SA-PCR) assay for the detection of Campylobacter jejuni in retail meat products. Food Microbiol. 2001;18:287–298. doi: 10.1006/fmic.2001.0400. [DOI] [Google Scholar]
- 32.Cui Y., Guran H.S., Harrison M.A., Hofacre C.L., Alali W.Q. Salmonella Levels in Turkey Neck Skins, Drumstick Bones, and Spleens in Relation to Ground Turkey. J. Food Prot. 2015;78:1945–1953. doi: 10.4315/0362-028X.JFP-15-240. [DOI] [PubMed] [Google Scholar]
- 33.Dan S., Tăbăran A., Mihaiu L., Mihaiu M. Antibiotic susceptibility and prevalence of foodborne pathogens in poultry meat in Romania. J. Infect. Dev. Coun. 2015;9:035–041. doi: 10.3855/jidc.4958. [DOI] [PubMed] [Google Scholar]
- 34.Duggan S., Jordan E., Gutierrez M., Barrett G., O’Brien T., Hand D., Kenny K., Fanning J., Leonard N., Egan J. Salmonella in meats, water, fruit and vegetables as disclosed from testing undertaken by Food Business Operators in Ireland from 2005 to 2009. Irish Vet. J. 2012;65:17. doi: 10.1186/2046-0481-65-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durul B., Acar S., Bulut E., Kyere E.O., Soyer Y. Subtyping of Salmonella Food Isolates Suggests the Geographic Clustering of Serotype Telaviv. Foodborne Pathog. Dis. 2015;12:958–965. doi: 10.1089/fpd.2015.1995. [DOI] [PubMed] [Google Scholar]
- 36.Egervarn M., Borjesson S., Byfors S., Finn M., Kaipe C., Englund S., Lindblad M. Escherichia coli with extended-spectrum beta-lactamases or transferable AmpC beta-lactamases and Salmonella on meat imported into Sweden. Int. J. Food Microbiol. 2014;171:8–14. doi: 10.1016/j.ijfoodmicro.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Elmali M., Can H.Y., Yaman H. Prevalence of Listeria monocytogenes in poultry meat. Food Sci. Technol. 2015;35:672–675. doi: 10.1590/1678-457X.6808. [DOI] [Google Scholar]
- 38.Filiousis G., Johansson A., Frey J., Perreten V. Prevalence, genetic diversity and antimicrobial susceptibility of Listeria monocytogenes isolated from open-air food markets in Greece. Food Control. 2009;20:314–317. doi: 10.1016/j.foodcont.2008.05.018. [DOI] [Google Scholar]
- 39.Fox E.M., Wall P.G., Fanning S. Control of Listeria species food safety at a poultry food production facility. Food Microbiol. 2015;51:81–86. doi: 10.1016/j.fm.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Gundogan N., Citak S., Yucel N., Devren A. A note on the incidence and antibiotic resistance of Staphylococcus aureus isolated from meat and chicken samples. Meat Sci. 2005;69:807–810. doi: 10.1016/j.meatsci.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Guven K., Mutlu M., Gulbandilar A., Cakir P. Occurrence and characterization of Staphylococcus aureus isolated from meat and dairy products consumed in Turkey. J. Food Saf. 2010;30:196–212. doi: 10.1111/j.1745-4565.2009.00200.x. [DOI] [Google Scholar]
- 42.Iseri O., Erol I. Incidence and antibiotic resistance of Salmonella spp. in ground turkey meat. Br. Poult. Sci. 2010;51:60–66. doi: 10.1080/00071660903395379. [DOI] [PubMed] [Google Scholar]
- 43.Kalender H. Prevalence of Listeria Species in Ground Beef and Chicken Meat Sold in Eastern Turkey. Pak. Vet. J. 2012;32:456–458. [Google Scholar]
- 44.Kilic S., Kuplulu O. Detection the enterotoxin producing capacity of coagulase positive Staphylococcus by EIA (Enzyme Immuno Assay) isolated from turkey meat. Ankara Univ. Vet. Fak. Derg. 2009;56:183–186. [Google Scholar]
- 45.Koluman A., Unlu T., Dikici A., Tezel A., Akcelik E.N., Burkan Z.T. Presence of Staphylococcus aureus and Staphylococcal Enterotoxins in Different Foods. Kafkas Univ. Vet. Fak. Derg. 2011;17:S55–S60. [Google Scholar]
- 46.Kosek-Paszkowska K., Bania J., Bystron J., Molenda J., Czerw M. Occurrence of Listeria sp. in raw poultry meat and poultry meat products. Bull. Vet. Inst. Pulawy. 2005;49:219–222. [PubMed] [Google Scholar]
- 47.Kozačinski L., Fleck Z., Kozačinski Z., Filipovic I., Mitak M., Bratulic M., Mikuš T. Evaluation of shelf life of pre-packed cut poultry meat. Vet. Arh. 2012;82:47–58. [Google Scholar]
- 48.Kozačinski L., Hadžiosmanović M., Zdolec N. Microbiological quality of poultry meat on the Croatian market. Vet. Arh. 2006;76:305–313. [Google Scholar]
- 49.Kramarenko T., Nurmoja I., Kaerssin A., Meremaee K., Horman A., Roasto M. The prevalence and serovar diversity of Salmonella in various food products in Estonia. Food Control. 2014;42:43–47. doi: 10.1016/j.foodcont.2014.01.032. [DOI] [Google Scholar]
- 50.Kramarenko T., Roasto M., Meremaee K., Kuningas M., Poltsama P., Elias T. Listeria monocytogenes prevalence and serotype diversity in various foods. Food Control. 2013;30:24–29. doi: 10.1016/j.foodcont.2012.06.047. [DOI] [Google Scholar]
- 51.Lambertz S.T., Nilsson C., Bradenmark A., Sylven S., Johansson A., Jansson L.-M., Lindblad M. Prevalence and level of Listeria monocytogenes in ready-to-eat foods in Sweden 2010. Int. J. Food Microbiol. 2012;160:24–31. doi: 10.1016/j.ijfoodmicro.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Latorre L., Parisi A., Fraccalvieri R., Normanno G., La Porta M.C.N., Goffredo E., Palazzo L., Ciccarese G., Addante N., Santagada G. Low prevalence of Listeria monocytogenes in foods from Italy. J. Food Prot. 2007;70:1507–1512. doi: 10.4315/0362-028X-70.6.1507. [DOI] [PubMed] [Google Scholar]
- 53.Ledergerber U., Regula G., Stephan R., Danuser J., Bissig B., Stark K. Risk factors for antibiotic resistance in Campylobacter spp. isolated from raw poultry meat in Switzerland. BMC Public Health. 2003;3:39. doi: 10.1186/1471-2458-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindblad M., Lindmark H., Lambertz S.T., Lindqvist R. Microbiological baseline study of broiler chickens at Swedish slaughterhouses. J. Food Prot. 2006;69:2875–2882. doi: 10.4315/0362-028X-69.12.2875. [DOI] [PubMed] [Google Scholar]
- 55.Little C.L., Sagoo S.K., Gillespie I.A., Grant K., McLauchlin J. Prevalence and Level of Listeria monocytogenes and Other Listeria Species in Selected Retail Ready-to-Eat Foods in the United Kingdom. J. Food Prot. 2009;72:1869–1877. doi: 10.4315/0362-028X-72.9.1869. [DOI] [PubMed] [Google Scholar]
- 56.Mackiw E., Rzewuska K., Stos K., Jarosz M., Korsak D. Occurrence of Campylobacter spp. in Poultry and Poultry Products for Sale on the Polish Retail Market. J. Food Prot. 2011;74:986–989. doi: 10.4315/0362-028X.JFP-10-503. [DOI] [PubMed] [Google Scholar]
- 57.Madden R.H., Moran L., Scates P., McBride J., Kelly C. Prevalence of Campylobacter and Salmonella in Raw Chicken on Retail Sale in the Republic of Ireland. J. Food Prot. 2011;74:1912–1916. doi: 10.4315/0362-028X.JFP-11-104. [DOI] [PubMed] [Google Scholar]
- 58.Malpass M., Williams A., Jones D., Omed H. Microbiological quality of chicken wings damaged on the farm or in the processing plant. Food Microbiol. 2010;27:521–525. doi: 10.1016/j.fm.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Manfreda G., De Cesare A., Bondioli V., Stern N., Franchini A. Enumeration and identity of Campylobacter spp. in Italian broilers. Poult. Sci. 2006;85:556–562. doi: 10.1093/ps/85.3.556. [DOI] [PubMed] [Google Scholar]
- 60.Manios S.G., Grivokostopoulos N.C., Bikouli V.C., Doultsos D.A., Zilelidou E.A., Gialitaki M.A., Skandamis P.N. A 3-year hygiene and safety monitoring of a meat processing plant which uses raw materials of global origin. Int. J. Food Microbiol. 2015;209:60–69. doi: 10.1016/j.ijfoodmicro.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 61.Mayrhofer S., Paulsen P., Smulders F., Hilbert F. Antimicrobial resistance profile of five major food-borne pathogens isolated from beef, pork and poultry. Int. J. Food Microbiol. 2004;97:23–29. doi: 10.1016/j.ijfoodmicro.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Meldrum R.J., Charles D., Mannion P., Ellis P. Variation in the microbiological quality of commercially produced vacuum-packed cooked sliced meat between production and the end of shelf-life. Int. J. Environ. Res. 2014;24:269–277. doi: 10.1080/09603123.2013.809704. [DOI] [PubMed] [Google Scholar]
- 63.Mena C., Almeida G., Carneiro L., Teixeira P., Hogg T., Gibbs P. Incidence of Listeria monocytogenes in different food products commercialized in Portugal. Food Microbiol. 2004;21:213–216. doi: 10.1016/S0740-0020(03)00057-1. [DOI] [Google Scholar]
- 64.Miranda J.N., Vazquez B.I., Fente C.A., Calo-Mata P., Cepeda A., Franco C.M. Comparison of Antimicrobial Resistance in Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes Strains Isolated from Organic and Conventional Poultry Meat. J. Food Prot. 2008;71:2537–2542. doi: 10.4315/0362-028X-71.12.2537. [DOI] [PubMed] [Google Scholar]
- 65.Moore J., Wilson T., Wareing D., Humphrey T., Murphy P. Prevalence of thermophilic Campylobacter spp. in ready-to-eat foods and raw poultry in Northern Ireland. J. Food Prot. 2002;65:1326–1328. doi: 10.4315/0362-028X-65.8.1326. [DOI] [PubMed] [Google Scholar]
- 66.Moran L., Scates P., Madden R.H. Prevalence of Campylobacter spp. in Raw Retail Poultry on Sale in Northern Ireland. J. Food Prot. 2009;72:1830–1835. doi: 10.4315/0362-028X-72.9.1830. [DOI] [PubMed] [Google Scholar]
- 67.Navas J., Ortiz S., López P., López V., Martínez-Suárez J. Different enrichment procedures for recovery of Listeria monocytogenes from raw chicken samples can affect the results of detection (by chromogenic plating or real-time PCR) and lineage or strain identification. J. Food Prot. 2007;70:2851–2854. doi: 10.4315/0362-028X-70.12.2851. [DOI] [PubMed] [Google Scholar]
- 68.Ojeniyi B., Christensen J., Bisgaard M. Comparative investigations of Listeria monocytogenes isolated from a turkey processing plant, turkey products, and from human cases of listeriosis in Denmark. Epidemiol. Infect. 2000;125:303–308. doi: 10.1017/S0950268899004483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ormanci F.S.B., Erol I., Ayaz N.D., Iseri O., Sariguzel D. Immunomagnetic separation and PCR detection of Listeria monocytogenes in turkey meat and antibiotic resistance of the isolates. Br. Poult. Sci. 2008;49:560–565. doi: 10.1080/00071660802298328. [DOI] [PubMed] [Google Scholar]
- 70.Özdemir H., Keyvan E. Isolation and characterisation of Staphylococcus aureus strains isolated from beef, sheep and chicken meat. Ankara Univ Vet. Fak. Derg. 2016;63:333–338. [Google Scholar]
- 71.Paludi D., Vergara A., Festino A., Di Ciccio P., Costanzo C., Conter M., Zanardi E., Ghidini S., Ianieri A. Antimicrobial resistance pattern of methicillin-resistant Staphylococcus aureus in the food industry. J. Biol. Regul. Homeost. Agents. 2011;25:671–677. [PubMed] [Google Scholar]
- 72.Praakle-Amin K., Hanninen M., Korkeala H. Prevalence and genetic characterization of Listeria monocytogenes in retail broiler meat in Estonia. J. Food Prot. 2006;69:436–440. doi: 10.4315/0362-028X-69.2.436. [DOI] [PubMed] [Google Scholar]
- 73.Rantsiou K., Lamberti C., Cocolin L. Survey of Campylobacter jejuni in retail chicken meat products by application of a quantitative PCR protocol. Int. J. Food Microbiol. 2010;141:S75–S79. doi: 10.1016/j.ijfoodmicro.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Reiter M.G., Lopez C., Jordano R., Medina L.M. Comparative Study of Alternative Methods for Food Safety Control in Poultry Slaughterhouses. Food Anal. Method. 2010;3:253–260. doi: 10.1007/s12161-010-9129-5. [DOI] [Google Scholar]
- 75.Roasto M., Praakle K., Korkeala H., Elias P., Hanninen M. Prevalence of Campylobacter in raw chicken meat of Estonian origin. Arch. Lebensmittelhyg. 2005;56:61–62. [Google Scholar]
- 76.Siriken B., Ayaz N.D., Erol I. Listeria monocytogenes in retailed raw chicken meat in Turkey. Berl. Munch. Tierarztl. Wochenschr. 2014;127:43–49. [PubMed] [Google Scholar]
- 77.Soultos N., Koidis P., Madden R. Presence of Listeria and Salmonella spp. in retail chicken in Northern Ireland. Lett. Appl. Microbiol. 2003;37:421–423. doi: 10.1046/j.1472-765X.2003.01423.x. [DOI] [PubMed] [Google Scholar]
- 78.Stella S., Soncini G., Ziino G., Panebianco A., Pedonese F., Nuvoloni R., Di Giannatale E., Colavita G., Alberghini L., Giaccone V. Prevalence and quantification of thermophilic Campylobacter spp. in Italian retail poultry meat: Analysis of influencing factors. Food Microbiol. 2017;62:232–238. doi: 10.1016/j.fm.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 79.Stoyanchev T., Vashin I., Ring C., Atanassova V. Prevalence of Campylobacter spp. in poultry and poultry products for sale on the Bulgarian retail market. Anton Leeuwenhoek. 2007;92:285–288. doi: 10.1007/s10482-007-9154-6. [DOI] [PubMed] [Google Scholar]
- 80.Trajković-Pavlović L., Popović M., Novaković B., Gusman-Pasterko V., Jevtić M., Mirilov J. Occurrence of Campylobacter, Salmonella, Yersinia enterocolitica and Listeria monocytogenes in some retail food products in Novi Sad. Cent. Eur. J. Public Health. 2007;15:167–171. doi: 10.21101/cejph.a3432. [DOI] [PubMed] [Google Scholar]
- 81.Trajković-Pavlović L., Novaković B., Martinov-Cvejin M., Gusman V., Bijelović S., Dragnić N., Balać D. How a routine checking of Escherichia coli in retailed food of animal origin can protect consumers against exposition to Campylobacter spp. and Listeria monocytogenes? Vojnosanit. Pregl. 2010;67:627–633. doi: 10.2298/VSP1008627T. [DOI] [PubMed] [Google Scholar]
- 82.Vitas A., Aguado V., Garcia-Jalon I. Occurrence of Listeria monocytogenes in fresh and processed foods in Navarra (Spain) Int. J. Food Microbiol. 2004;90:349–356. doi: 10.1016/S0168-1605(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 83.Voidarou C., Vassos D., Rozos G., Alexopoulos A., Plessas S., Tsinas A., Skoufou M., Stavropoulou E., Bezirtzoglou E. Microbial challenges of poultry meat production. Anaerobe. 2011;17:341–343. doi: 10.1016/j.anaerobe.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 84.Vossenkuhl B., Brandt J., Fetsch A., Käsbohrer A., Kraushaar B., Alt K., Tenhagen B.-A. Comparison of spa Types, SCCmec types and antimicrobial resistance profiles of MRSA isolated from Turkeys at farm, slaughter and from retail meat indicates transmission along the production chain. PLoS ONE. 2014;9:e96308. doi: 10.1371/journal.pone.0096308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vural A., Erkan M., Yeşilmen S. Microbiological quality of retail chicken carcasses and their products in Turkey. Med. Weter. 2006;62:1371–1374. [Google Scholar]
- 86.Whyte P., McGill K., Cowley D., Madden R., Moran L., Scates P., Carroll C., O’Leary A., Fanning S., Collins J., et al. Occurrence of Campylobacter in retail foods in Ireland. Int. J. Food Microbiol. 2004;95:111–118. doi: 10.1016/j.ijfoodmicro.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 87.Yucel N., Citak S., Onder M. Prevalence and antibiotic resistance of Listeria species in meat products in Ankara, Turkey. Food Microbiol. 2005;22:241–245. doi: 10.1016/j.fm.2004.03.007. [DOI] [Google Scholar]
- 88.Zadernowska A., Chajecka-Wierzchowska W. Prevalence, biofilm formation and virulence markers of Salmonella sp and Yersinia enterocolitica in food of animal origin in Poland. LWT-Food Sci. Technol. 2017;75:552–556. doi: 10.1016/j.lwt.2016.10.007. [DOI] [Google Scholar]
- 89.Viechtbauer W. Conducting Meta-Analyses in R with the metaphor Package. J. Stat. Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 90.Grothendieck G. Sqldf: Manipulate R Data Frames Using SQL. [(accessed on 6 April 2018)];2017 R package version 0.4-11. Available online: https://CRAN.R-project.org/package=sqldf.
- 91.EFSA The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15:e05077. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Narasimha Rao D., Sachindra N.M. Modified atmosphere and vacuum packaging of meat and poultry products. Food Rev. Int. 2002;18:263–293. doi: 10.1081/FRI-120016206. [DOI] [Google Scholar]
- 93.Skarp C.P.A., Hänninen M.L., Rautelin H.I.K. Campylobacteriosis: The role of poultry meat. Clin. Microbiol. Infect. 2016;22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 94.Pepe O., Blaiotta G., Bucci F., Anastasio M., Aponte M., Villani F. Staphylococcus aureus and staphylococcal enterotoxin A in breaded chicken products: Detection and behavior during the cooking process. Appl. Environ. Microbiol. 2006;72:7057–7062. doi: 10.1128/AEM.00198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stewart C.M. Chapter 12: Staphylococcus aureus and staphylococcal enterotoxins. In: Hocking A.D., editor. Foodborne Microorganisms of Public Health Significance. 6th ed. Australian Institute of Food Science and Technology (NSW Branch); Sydney, Australia: 2003. pp. 359–380. [Google Scholar]
- 96.Rouger A., Tresse O., Zagorec M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms. 2017;5:50. doi: 10.3390/microorganisms5030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Humphrey T., O’Brien S., Madsen M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007;117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 98.Mead G.C. Microbiological quality of poultry meat: A review. Rev. Bras. Cienc. Avic. 2004;6:135–142. doi: 10.1590/S1516-635X2004000300001. [DOI] [Google Scholar]
- 99.Goh S.G., Kuan C.H., Loo Y.Y., Chang W.S., Lye Y.L., Soopna P., Tang J.Y., Nakaguchi Y., Nishibuchi M., Afsah-Hejri L., et al. Listeria monocytogenes in retailed raw chicken meat in Malaysia. Poult. Sci. 2012;91:2686–2690. doi: 10.3382/ps.2012-02349. [DOI] [PubMed] [Google Scholar]
- 100.Ivic-Kolevska S., Miljkovic-Selimovic B., Kocic B. Survival of Campylobacter jejuni in chicken meat at frozen storage temperatures. Acta Microbiol. Immun. Hung. 2012;59:185–198. doi: 10.1556/AMicr.59.2012.2.4. [DOI] [PubMed] [Google Scholar]
- 101.Milillo S., Stout J., Hanning I., Clement A., Fortes E., Den Bakker H., Wiedmann M., Ricke S.C. Listeria monocytogenes and hemolytic Listeria innocua in poultry. Poult. Sci. 2012;91:2158–2163. doi: 10.3382/ps.2012-02292. [DOI] [PubMed] [Google Scholar]
- 102.Moura G.F., Sigarini C.O., Figueiredo E.E.S. Listeria monocytogenes in Chicken Meat. J. Food Nutr. Res. 2016;4:436–441. [Google Scholar]
- 103.Mbata T.I. Poultry meat pathogens and its Control. Int. J. Food Saf. 2017;7:20–28. [Google Scholar]
- 104.Mouttotou N., Ahmad S., Kamran Z., Koutoulis K.C. Chapter 12: Prevalence, Risks and Antibiotic Resistance of Salmonella in Poultry Production Chain. In: Mares M., editor. Current Topics in Salmonella and Salmonellosis. InTech; Rijeka, Croatia: 2017. pp. 215–234. [Google Scholar]
- 105.Hiett K.L. Tracing pathogens in poultry and egg production at the abattoir. In: McMeekin T.A., editor. Tracing Pathogens in the Food Chain. Woodhead Publishing Limited; Cambridge, UK: 2011. pp. 465–502. [Google Scholar]