Abstract
Recent genetic studies have suggested that many genes contribute to differences between closely related species that prevent gene exchange, particularly hybrid male sterility and female species preferences. We have examined the genetic basis of hybrid sterility and female species preferences in Drosophila pseudoobscura and Drosophila persimilis, two occasionally hybridizing North American species. Contrary to findings in other species groups, very few regions of the genome were associated with these characters, and these regions are associated also with fixed arrangement differences (inversions) between these species. From our results, we propose a preliminary genic model whereby inversions may contribute to the speciation process, thereby explaining the abundance of arrangement differences between closely related species that co-occur geographically. We suggest that inversions create linkage groups that cause sterility to persist between hybridizing taxa. The maintenance of this sterility allows the species to persist in the face of gene flow longer than without such inversions, and natural selection will have a greater opportunity to decrease the frequency of interspecies matings.
A fundamental goal in speciation research has been to determine the number of genes that contribute to barriers to gene exchange (or reproductive isolating mechanisms) between closely related species. Recent studies of two particular barriers to gene exchange, hybrid male sterility and female species preferences, have found that these characters are often highly polygenic, with many regions of the genome being associated with at least some effect (for reviews see refs. 1–4). Hybrid male sterility appears particularly highly polygenic when segments of one species are experimentally introgressed into the genetic background of another and made homozygous (e.g., refs. 5 and 6). Perhaps the most extensively studied groups for these characters have been the two races of Drosophila melanogaster and the three species of the Drosophila simulans clade; D. simulans, D. mauritiana, and D. sechellia, although many other species (including non-Drosophila) have also been investigated. However, these species may be atypical of Drosophila species in particular or species in general, because all the hybridizations studied involved homosequential taxa: taxa not differing in their gene arrangements.
Chromosomal rearrangements are thought to be important in speciation because sometimes they can disrupt meiosis in hybrids, thereby causing sterility (7–9). Drosophila species have been studied extensively with regard to chromosomal rearrangements and their fitness consequences, both in the context of direct effects of inversions (10–12) and effects associated with allelic differences between genes contained within them (e.g., refs. 13 and 14). One of the most common types of rearrangement, the paracentric inversion, is not considered to play a role in speciation, because hybrids of parents differing in these gene arrangements are fully fertile and viable. Recombination is prevented effectively between different arrangements, although it still occurs readily across uninverted regions of these chromosomes.
Here we examine the genetic basis of hybrid male sterility as evaluated by introgressions and female species preferences in the North American fruit flies Drosophila pseudoobscura and Drosophila persimilis. These species differ by several inversions (see Materials and Methods). The two species hybridize, albeit rarely, in nature, and gene flow has been detected at the sequence level (15, 16). Natural selection seems to have strengthened the mate discrimination exercised by these females to prevent maladaptive hybridization (17), because hybrid male offspring are sterile. These species seem to have separated initially before the split between the D. melanogaster races or the split between D. simulans and D. mauritiana; thus genetic divergence between them should be greater on average in the absence of introgression.
Previous studies of these species have identified the genetic basis of their differences in cuticular hydrocarbon profile (18) and courtship wing vibration (19). The genetic bases of hybrid male sterility, male mating success, hybrid inviability, and a hybrid courtship dysfunction also have been determined by using backcross hybrid offspring of these species (20, 21). Finally, Tan (22) examined the genetic basis of female preferences in these species, but a lack of available phenotypic markers limited this analysis. All of these varied studies reached the same conclusion: all traits map primarily or exclusively to the inverted regions on the X chromosome and second chromosome of these species (see Fig. 1). We evaluate whether this pattern is observed in characters thought to be highly polygenic in their differences between species.
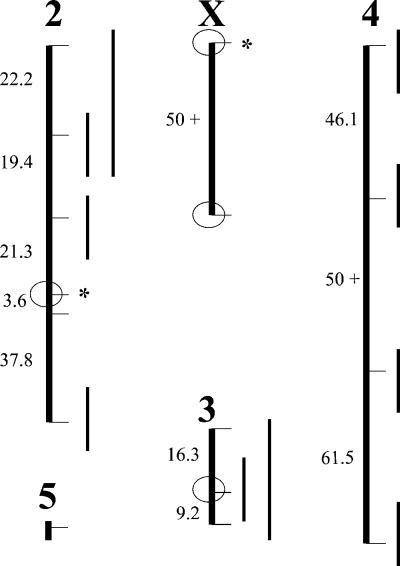
Figure 1.
Recombinational distances (in Kosambi centimorgans) between genetic markers in hybrids of D. pseudoobscura and D. persimilis. The circles indicate positions of large inversions that differentiate the strains used. Also indicated are positions of genomic segments introgressed from D. persimilis into D. pseudoobscura (bars right of the chromosomes) and the positions of loci associated with species discrimination by backcross females (asterisks right of the chromosomes).
Materials and Methods
Fly Strains.
D. pseudoobscura and D. persimilis have a metacentric X and four telocentric autosomes, with a total of ≈100 cytological “bands” across their genomes. These species are separated completely by paracentric inversions along two chromosomes: six cytological bands on the left arm of the X and five cytological bands in the center of the second chromosome (23). These species are usually separated by a paracentric inversion along the right arm of the X (11 cytological bands), although the D. pseudoobscura arrangement is found in a small number of D. persimilis individuals and is associated with strong meiotic drive. Finally, the third chromosomes of both these species are highly polymorphic for inversions, and one abundant arrangement is shared between the two species. These inversions prevent the formation of offspring recombinant for regions within them, but recombination still occurs readily across the uninverted regions of these chromosomes (24–26).
Two strains were used in the mapping experiment: D. pseudoobscura Flagstaff 1993 (3rd chromosome arrangement “Arrowhead”) and D. persimilis Mt. St. Helena 1993 (3rd chromosome arrangement “Standard”). These chromosome arrangements differ by a single inversion along the middle of this chromosome spanning seven cytological bands, and they seem to be common within these species. Both strains have been maintained in the laboratory for several years and used previously in various other studies (e.g., refs. 21 and 27). All crosses were carried out at 20 ± 1°C, 85% relative humidity, on standard sugar/yeast/agar medium.
Genetics of Female Species Preferences.
Females from the D. pseudoobscura strain were crossed to males of the D. persimilis strain, and the resultant fertile F1 females were backcrossed to males of each parental line. Backcross hybrid males were designated “BCps” if they were offspring of D. pseudoobscura fathers and “BCper” if they were offspring of D. persimilis fathers. Bottles were cleared, and virgin backcross hybrid females and virgin pure species males were harvested 7 h later. The flies were then aged for 7 days in groups of 5–20 individuals.
On day 8, single pure-species males were aspirated into vials containing one backcross hybrid female. Fly pairs were observed for 5 min after the onset of courtship, or 5 min in total if no courtship occurred. Courtship was defined as wing vibration or attempted copulation by the male (27). If no courtship occurred, the flies were discarded. If courtship did occur, we recorded whether the fly was successful at copulating with the female for at least 30 sec during the observation period. Males that failed to achieve 30 sec of copulation were considered to be discriminated against by the female, and the females were scored with a “0.” Females that allowed the males to copulate within their first two attempts were scored as “2.” Other females that allowed a copulation within 5 min of courtship initiation but after two attempts were scored as “1.” All observations were performed between 0700 and 1100 hours, and each male and female were used only once.
Because F1 females mate readily with males of either species, we only paired BCps females with D. persimilis males and BCper females with D. pseudoobscura males. Preliminary experiments of BCps females with D. pseudoobscura males documented that virtually all pairings resulted in an immediate copulation (data not shown).
After mating experiments, backcross females were genotyped for 16 molecular genetic markers (see Fig. 1): 14 microsatellites and 2 restriction fragment length polymorphisms. These markers, their recombinational distances, and details of amplification procedures have been described elsewhere (19, 21, 26, 28). The names of individual markers are available by request from the authors. Females were scored as either homozygous or heterozygous for alleles at each locus, and over 500 females were assayed for each backcross. The data were analyzed by the composite interval mapping method (29) in QTL Cartographer (ref. 30, http://statgen.ncsu.edu) by using a conditioning window of 10 centiMorgans. The threshold for significance in this interval analysis was estimated by permuting traits relative to genotypes 1,000 times. Composite interval mapping analyses were conditioned on markers shown to have strong associations with the phenotypes in separate single marker regression analyses. We also used the conditional empirical threshold protocol of Doerge and Churchill (31) to identify potentially weak quantitative trait loci. When a strong effect was associated with a particular marker, the backcrosses were stratified into the separate classes for that marker, and a reanalysis was performed with 1,000 permutations again. No additional effects were noted following this protocol.
Genetics of Hybrid Male Sterility Following Introgressions.
D. pseudoobscura females were crossed to D. persimilis males to produce F1 females. These females were backcrossed to D. pseudoobscura males. The male offspring of this backcross were paired individually with D. pseudoobscura females. Approximately 20% of these pairings produced offspring. Offspring then were mated with their siblings in single pairs, hence producing homozygous D. persimilis segments in a predominantly D. pseudoobscura genetic background in the progeny. Male progeny from this cross then were scored for fertility based on the presence of motile sperm (see refs. 21 and 32) 8 days after eclosion.
All these progeny were genotyped for molecular markers as described above. At least 100 such progeny for each introgression were scored, and all genotypes were observed consistently at or close to expected proportions. The X and second chromosome regions bearing the fixed inversion differences between these species were never able to introgress, because the male offspring of the first backcross that were heterozygous or hemizygous for these regions were almost invariably sterile (21).
Results
Female Species Preferences.
We produced backcross hybrids between D. pseudoobscura and D. persimilis and mapped the genetic basis of discrimination exercised by females of the two species. The results are presented in Table 1. In short, the only regions of the genome that had any detectable association with the species discrimination exercised by females were the left arm of the X chromosome (XL) and second chromosome inverted regions (see Fig. 1). In the case of the second chromosome, the effect mapped unambiguously to microsatellite marker DPS2002, which is located within the inversion that distinguishes these species. BCps females bearing one D. persimilis XL or one second chromosome inversion had 20% greater mating success with D. persimilis males than BCps females homozygous for D. pseudoobscura arrangements. BCper females bearing one D. pseudoobscura XL inversion had 20% greater mating success with D. pseudoobscura males than those homozygous for the D. persimilis arrangement, but BCper females bearing one D. pseudoobscura second chromosome inversion had 40% greater mating success with D. pseudoobscura males than BCper females homozygous for the D. persimilis arrangement.
Table 1.
Mating success of female D. pseudoobscura, D. persimilis, F1 hybrids, and backcross offspring within two attempted copulations
| Females | To male D. pseudoobscura | To male D. persimilis |
|---|---|---|
| D. pseudoobscura | 88% (112) | 30% (110) |
| D. persimilis | 30% (106) | 78% (120) |
| F1 hybrids | 85% (236) | 75% (175) |
| BCper* | 64% (654) | — |
| BCps† | — | 64% (573) |
Significant effects detected by composite interval mapping:
Mating of BCper females to D. pseudoobscura males:
2 quantitative trait loci completely linked to XL and 2nd chromosome (DPS2002) inversions.
Mating of BCps females to D. persimilis males:
2 quantitative trait loci completely linked to XL and 2nd chromosome (DPS2002) inversions.
Sample sizes are presented parenthetically.
Offspring of mating between D. persimilis males and F1 hybrid females.
Offspring of mating between D. pseudoobscura males and F1 hybrid females.
No effect was detected with any uninverted region or with the large regions of the third chromosome and right arm of X inverted between these two strains. This finding suggests that the preference is not highly polygenic or that the genes contributing to it are nonrandomly distributed across the genome and clustered in regions that bear fixed inversion differences between the species. This finding is unlikely to result from a bias in mapping caused by linkage between markers and traits created by the inversions. The XL and second chromosome inversion differences are smaller than the inversion differences between these strains on the right arm of the X chromosome (XR) and third chromosome, but no effect was associated with the latter inversions (see Discussion).
Hybrid Male Sterility in Introgressed Segments.
Second, we introgressed 10 autosomal segments from D. persimilis into D. pseudoobscura, subsequently making them homozygous in a D. pseudoobscura genetic background. A similar protocol was followed in genetic studies of hybrid sterility in D. simulans/D. mauritiana and D. simulans/D. sechellia (5, 6), all of which are homosequential and allopatric to each other. In the D. simulans clade species, half of the autosomal introgressions were associated with hybrid male sterility (5, 6). In contrast to the results from these hybridizations, we failed to observe any hybrid sterility whatsoever associated with our introgressions (see Fig. 1). This finding is particularly noteworthy given that the introgressions of True et al. (5) were reportedly much smaller. Even the cointrogression of the three third chromosome markers, spanning ≈15% of the physical genome based on cytological locations, produced no fertility consequences. Although unlikely, double recombinants may have been produced within this introgression, but the third chromosome inversion itself still constitutes over 7% of the genome. This inversion was introgressed from D. persimilis into D. pseudoobscura and made homozygous with no fertility consequence.
Discussion
Genetic studies of two barriers to gene exchange, hybrid male sterility and female species preferences, typically have found these characters to be highly polygenic (for reviews see refs. 1–4). For example, the two races of D. melanogaster may have over 15 genes contributing to their sexual isolation (33). Although both sterility and female species preferences may sometimes have simple genetic bases (e.g., refs. 34 and 35), such examples are confined to taxa that likely diverged much more recently than D. pseudoobscura and D. persimilis.
Here, we have mapped the genetic basis of hybrid male sterility as associated with homozygous introgressions and female species preferences in D. pseudoobscura and D. persimilis. These species diverged ≈500,000 ago (15, 36), slightly greater in estimated divergence time than the species of the D. simulans clade. In contrast to results from genetic studies of the D. simulans clade, none of our introgressions from D. persimilis into D. pseudoobscura were associated with hybrid male sterility, and female species preferences exercised by both species mapped exclusively to two regions of the genome that are inverted between all strains of the two species.
This association with inversions may result from a linkage between markers and traits caused by the effective suppression of recombination between differing arrangements. However, we consider this conclusion unlikely. The strains used in our current and previous genetic studies differed by two inversions in addition to the two that separate all individuals of these two species. These latter two inversions are larger than the two that are fixed differently, yet no (or very little) effect mapped to these inversions in our studies. Combining our results from the current study with our previous analyses by using the offspring of backcrosses (21), we observed that the strongest effects were associated with the two fixed inversion differences in four of five traits: hybrid male sterility, male mating success, female species preferences, and hybrid inviability were most strongly associated with both of the fixed inversion differences, whereas the hybrid courtship dysfunction was associated most strongly with one of the fixed inversion differences and one of the other inversions (21). The probability of the two strongest effects being associated most strongly with both of the two fixed inversion differences in four of five traits, conservatively assuming that all four inversions are of similar size, would be ≈0.3% (P = 0.003) following a binomial distribution.
Recent genetic studies of D. pseudoobscura and D. persimilis have documented extensive recent introgression between these species in uninverted regions but little or no introgression in those regions bearing fixed inversion differences (37, 38). Coupled with our genetic data, we conclude that hybridization may have homogenized uninverted regions of the genome of these species, but inverted regions were protected from such homogenization because of linkage to barriers to gene exchange.
From all these observations, we propose a preliminary model whereby paracentric inversions that differentiate hybridizing taxa may contribute to the persistence of these taxa in the face of ongoing gene flow. This model is similar to one recently suggested by Rieseberg (39). Our suggestion is based on three assumptions. First, recombination within inverted regions is suppressed effectively between chromosomes of differing arrangements. Second, incompatibilities resulting in hybrid sterility are typically asymmetric in their effects. For example, the OdysseusH allele from D. mauritiana confers hybrid sterility in a genetic background of D. simulans, but the D. simulans allele of OdysseusH does not confer sterility in D. mauritiana (40). Similarly, the frequent observation of sterility in the offspring of one hybridization but not the reciprocal hybridization suggests that incompatibilities are often asymmetric (41). Finally, we assume that many loci possess alleles that confer hybrid sterility between very closely related species. This assumption is also consistent with genetic data from D. simulans/D. mauritiana and other species (3, 42).
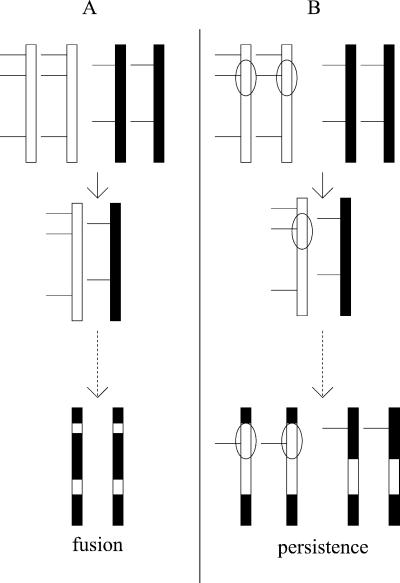
If two taxa that do not bear paracentric inversion differences were to hybridize, such as D. simulans and D. mauritiana, we predict that alleles that confer hybrid sterility will be selected against and removed from these taxa (see Fig. 2A). For example, we predict that the D. mauritiana OdysseusH allele would be selected against, and the D. simulans allele of OdysseusH would spread into D. mauritiana. If there are many such alleles at loci across the genome, the alleles that do not confer sterility in a heterospecific genetic background will be selected at all such loci over time. The taxa then will be fully compatible and will fuse completely.
Figure 2.
Model for the long term effect of inversions on introgression and species persistence with hybridization. Two species are designated by diploid chromosomes of a single color (black or white) at the top of the figure. Horizontal lines indicate the locations of alleles that confer hybrid sterility in a heterospecific genetic background. The oval indicates an inverted gene arrangement relative to the other species. (A) No inversion difference between species. (B) One inversion differentiates the species.
In contrast, if one or more paracentric inversions differentiate hybridizing taxa, genes possessing alleles that confer hybrid sterility in one genetic background are linked to genes possessing alleles that confer hybrid sterility in the other genetic background (see Fig. 2B). Selection cannot eliminate either allele (or either arrangement) from both taxa, because the remaining allele will be associated with hybrid sterility in one genetic background. The resulting inefficiency of selection will cause the species to persist without fusing longer than if no inversion was present. This additional time could provide the opportunity for natural selection to decrease the frequency of hybridization by strengthening mate discrimination (reinforcement). Our model would thereby predict that hybridizing taxa differing by paracentric inversions would be less prone to fusion than taxa that are homosequential (uninverted) in their gene arrangements. Hence, many recently diverged species that co-occur in a region should differ by one or more paracentric inversions, because hybridization will act as a sieve that favors persistence of species separated by such inversions.
The genetic expectations from such a model would be that gene flow and homogenization would occur in uninverted regions of the genome or regions in which both species share one or more arrangements: alleles conferring hybrid sterility will be eliminated from both species. Conversely, gene flow will not occur in the regions of the genome bearing fixed inversion differences. Greater genetic differentiation between the species would be expected in inverted regions than uninverted regions. Correspondingly, all remaining differences between the species including differences causing hybrid sterility would map primarily or exclusively to the inverted regions and probably to those that in which the species are fixed for differing arrangements. All these observations are consistent with the data from D. pseudoobscura and D. persimilis.
We have also evaluated our model by examining the extensive literature survey of reproductive isolation between Drosophila species pairs provided by Coyne and Orr (41, 43) to determine the possible association of inversion differences with postzygotic isolation (hybrid sterility and inviability in particular; see Table 2). For 84 of the pairs that they surveyed, we identified whether the taxa differed by one or more inversions. We excluded the semispecies from the Drosophila paulistorum complex, because their sterility is associated with an endosymbiont rather than being entirely genic (44). Of the remaining pairs, 44 are known to be separated by some sterility or inviability. Of these 44 pairs, 8 pairs were homosequential in their gene arrangement. 7 of these 8 were allopatric taxa (e.g., Drosophila plantibia/Drosophila silvestris and D. mauritiana/D. sechellia), and the 8th (Drosophila aldrichi/Drosophila mulleri) were separated by sterility of all F1 hybrids, precluding any possibility for recombination to allow for fusion. In contrast, species pairs of similar genetic divergence that differed by one of more inversions were frequently sympatric (e.g., Drosophila borealis/Drosophila montana and Drosophila arizonae/Drosophila mojavensis). These findings are consistent with our model, although the small number of phylogenetically independent taxa that are homosequential in their gene arrangement precludes a definitive statistical test.
Table 2.
Relative abundance of homosequential Drosophila taxa vs. those bearing inversion differences compared by sympatry (sym)/allopatry (allo) and genetic distance
| Patry* | D† | Sterility‡ | ||
|---|---|---|---|---|
| Drosophila species/subspecies not differing by one or more inversions | ||||
| heteroneura | planitibia | allo | 0.134 | 0.500 |
| differens | planitibia | allo | 0.138 | 0.500 |
| planitibia | silvestris | allo | 0.191 | 0.500 |
| pseudoobscura Bogota | pseudoobscura USA | allo | 0.194 | 0.250 |
| sechellia | simulans | allo | 0.280 | 0.500 |
| simulans | sechellia | allo | 0.280 | 0.500 |
| mauritiana | simulans | allo | 0.300 | 0.500 |
| mauritiana | sechellia | allo | 0.320 | 0.500 |
| “aldrichi”§ | mulleri | sym | 1.051 | 1.000 |
| Drosophila species differing by one or more inversions | ||||
| flavomontana | lacicola | allo | 0.180 | 0.500 |
| borealis | montana | sym | 0.210 | 0.500 |
| arizonae | mojavensis baja | sym | 0.212 | 0.250 |
| flavomontana | montana | sym | 0.290 | 0.500 |
| borealis | flavomontana | sym | 0.380 | 1.000 |
| persimilis | pseudoobscura | sym | 0.410 | 0.500 |
Inversions that differentiate hybridizing species may facilitate divergence in an additional manner as well. Several theoretical studies have suggested that the reinforcement of barriers to gene flow may be more likely if recombination is prevented or reduced between loci conferring hybrid sterility and those conferring mate discrimination (45–47). In our model, hybridizing species are more likely to persist longer if inversions are present, and the linkage between loci conferring sterility and those conferring mate discrimination that will occur can also facilitate subsequent selection for greater discrimination. Hence, the presence of inversions not only allows species to persist despite hybridization, but it also may aid the completion of speciation by preventing further hybridization.
The subject of chromosomal speciation has been debated hotly in the literature (7, 8, 10, 12, 48, 49, †). We present a genic explanation for why species may differ frequently in gene arrangement (50), but unlike other models of chromosomal speciation, it does not suffer from the problem of underdominance. In contrast, standard models of chromosomal speciation predict that novel chromosomal arrangements must be at a selective disadvantage when they first appear in a population. We have produced additional data, both genetic and from the literature, that are consistent with our model but contrast genetic data collected from other species not separated by inversions. We do acknowledge that our data do not conclusively support the model, but we suggest that it merits further investigation. Clearly, other forces are also involved in species formation such as ecological differentiation or strong sexual selection, and these forces sometimes may be stronger than what we propose here. This is evident by the existence of hybridizing species not separated by any known hybrid sterility or inviability such as Drosophila heteroneura/D. silvestris or Allonemobius fasciatus/Allonemobius socius.
Rieseberg (39) has also suggested that rearrangements can reduce gene flow between hybridizing species by summing the effects of individual sterility (or other isolating) factors and that this effect can contribute to the persistence of species. Like Rieseberg, we also suggest that rearrangements may reduce gene flow more by suppressing recombination than by directly reducing fitness. However, we suggest that this effect may result from the asymmetry of gene incompatibilities that cause hybrid dysfunctions rather than the summation of minor gene effects. These hypotheses are not mutually exclusive; the summation of various isolating effects, possibly including ecological differences, in the inversions of D. pseudoobscura and D. persimilis may have contributed to their divergence. Further research using artificially induced inversions and population cage experiments may help to demonstrate empirically this chromosome rearrangement effect.
Acknowledgments
This research was supported by National Institutes of Health Grant GM58060 and National Science Foundation Grants DEB-9980797 and DEB-0100816.
Abbreviation
- XL
left arm of the X chromosome
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Butlin, R. K. (1993) Nature (London) 366, 27 (abstr.).
References
- 1.Orr H A, Presgraves D C. BioEssays. 2000;22:1085–1094. doi: 10.1002/1521-1878(200012)22:12<1085::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Hollocher H. Curr Opin Genet Dev. 1998;8:709–714. doi: 10.1016/s0959-437x(98)80041-5. [DOI] [PubMed] [Google Scholar]
- 3.Wu C-I, Hollocher H. In: Endless Forms: Species and Speciation. Howard D J, Berlocher S H, editors. New York: Oxford Univ. Press; 1998. pp. 39–351. [Google Scholar]
- 4.Coyne J A, Orr H A. Philos Trans Roy Soc London B. 1998;353:287–305. doi: 10.1098/rstb.1998.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.True J R, Weir B S, Laurie C C. Genetics. 1996;142:819–837. doi: 10.1093/genetics/142.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollocher H, Wu C-I. Genetics. 1996;143:1243–1255. doi: 10.1093/genetics/143.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King M. Species Evolution: The Role of Chromosome Change. Cambridge: Cambridge Univ. Press; 1993. [Google Scholar]
- 8.Spirito F. In: Endless Forms: Species and Speciation. Howard D J, Berlocher S H, editors. New York: Oxford Univ. Press; 1998. pp. 320–329. [Google Scholar]
- 9.Searle J B. Genome Res. 1998;8:1–3. doi: 10.1101/gr.8.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Coyne J A, Meyers W, Crittenden A P, Sniegowski P. Genetics. 1993;134:487–496. doi: 10.1093/genetics/134.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyne J A, Aulard S, Berry A. Genetics. 1991;129:791–802. doi: 10.1093/genetics/129.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro A, Ruiz A. Genetics. 1997;147:931–933. doi: 10.1093/genetics/147.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobzhansky T. Genetics of the Evolutionary Process. New York: Columbia Univ. Press; 1970. [Google Scholar]
- 14.Anderson W W, Watanabe T K. Proc Natl Acad Sci USA. 1997;94:7742–7747. doi: 10.1073/pnas.94.15.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R L, Wakeley J, Hey J. Genetics. 1997;147:1091–1106. doi: 10.1093/genetics/147.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell J R. Proc Natl Acad Sci USA. 1983;80:492–495. doi: 10.1073/pnas.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noor M A F. Nature (London) 1995;375:674–675. doi: 10.1038/375674a0. [DOI] [PubMed] [Google Scholar]
- 18.Noor M A F, Coyne J A. Genet Res. 1996;68:117–123. doi: 10.1017/s0016672300034005. [DOI] [PubMed] [Google Scholar]
- 19.Williams M A, Blouin A G, Noor M A F. Heredity. 2001;86:68–77. doi: 10.1046/j.1365-2540.2001.00811.x. [DOI] [PubMed] [Google Scholar]
- 20.Orr H A. Genetics. 1987;116:555–563. doi: 10.1093/genetics/116.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noor M A F, Grams K L, Bertucci L A, Almendarez Y, Reiland J, Smith K R. Evolution (Lawrence, Kans) 2001;55:512–521. doi: 10.1554/0014-3820(2001)055[0512:tgoria]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Tan C C. Genetics. 1946;31:558–573. doi: 10.1093/genetics/31.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan C C. Genetics. 1935;20:392–402. doi: 10.1093/genetics/20.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturtevant A H, Dobzhansky T. Genetics. 1936;21:473–490. doi: 10.1093/genetics/21.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobzhansky T, Tan C C. Z Indukt Abstamm Vererbungsl. 1936;72:88–114. [Google Scholar]
- 26.Noor M A F, Smith K R. J Hered. 2000;91:99–103. doi: 10.1093/jhered/91.2.99. [DOI] [PubMed] [Google Scholar]
- 27.Noor M A F. Evolution (Lawrence, Kans) 1997;51:809–815. doi: 10.1111/j.1558-5646.1997.tb03663.x. [DOI] [PubMed] [Google Scholar]
- 28.Noor M A F, Schug M D, Aquadro C F. Genet Res. 2000;75:25–35. doi: 10.1017/s0016672399004024. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Z B. Genetics. 1994;136:1457–1468. doi: 10.1093/genetics/136.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basten C J, Weir B S, Zeng Z-B. QTL Cartographer: A Reference Manual and Tutorial for QTL Mapping. Raleigh, North Carolina: North Carolina State University; 1999. [Google Scholar]
- 31.Doerge R W, Churchill G A. Genetics. 1996;142:285–294. doi: 10.1093/genetics/142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coyne J A. Proc Natl Acad Sci USA. 1984;81:4444–4447. doi: 10.1073/pnas.81.14.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ting C-T, Takahashi A, Wu C-I. Proc Natl Acad Sci USA. 2001;98:6709–6713. doi: 10.1073/pnas.121418898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orr H A, Irving S. Genetics. 2001;158:1089–1100. doi: 10.1093/genetics/158.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doi M, Matsuda M, Tomaru M, Matsubayashi H, Oguma Y. Proc Natl Acad Sci USA. 2001;98:6714–6719. doi: 10.1073/pnas.091421598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aquadro C F, Weaver A L, Schaeffer S W, Anderson W W. Proc Natl Acad Sci USA. 1991;88:305–309. doi: 10.1073/pnas.88.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R-L, Hey J. Genetics. 1996;144:1113–1126. doi: 10.1093/genetics/144.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machado, C. A., Kliman, R. M., Markert, J. A. & Hey, J. (2001) Mol. Biol. Evol. in press. [DOI] [PubMed]
- 39.Rieseberg L H. Trends Ecol Evol. 2001;16:351–358. doi: 10.1016/s0169-5347(01)02187-5. [DOI] [PubMed] [Google Scholar]
- 40.Palopoli M F, Wu C-I. Genetics. 1994;138:329–341. doi: 10.1093/genetics/138.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coyne J A, Orr H A. Evolution (Lawrence, Kans) 1989;43:362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. [DOI] [PubMed] [Google Scholar]
- 42.Barton N H, Hewitt G M. Annu Rev Ecol Syst. 1985;16:113–148. [Google Scholar]
- 43.Coyne J A, Orr H A. Evolution (Lawrence, Kans) 1997;51:295–303. doi: 10.1111/j.1558-5646.1997.tb02412.x. [DOI] [PubMed] [Google Scholar]
- 44.Somerson N L, Ehrman L, Kocka J P, Gottlieb F J. Proc Natl Acad Sci USA. 1984;81:282–285. doi: 10.1073/pnas.81.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trickett A J, Butlin R K. Heredity. 1994;73:339–345. doi: 10.1038/hdy.1994.180. [DOI] [PubMed] [Google Scholar]
- 46.Servedio M R. Evolution (Lawrence, Kans) 2000;54:21–29. doi: 10.1111/j.0014-3820.2000.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 47.Felsenstein J. Evolution (Lawrence, Kans) 1981;35:124–138. doi: 10.1111/j.1558-5646.1981.tb04864.x. [DOI] [PubMed] [Google Scholar]
- 48.Rieseberg L H, Fossen C V, Desrochers A M. Nature (London) 1995;375:313–316. [Google Scholar]
- 49.Reed K M, Greenbaum I F, Sites J W. Evolution (Lawrence, Kans) 1995;49:37–47. doi: 10.1111/j.1558-5646.1995.tb05956.x. [DOI] [PubMed] [Google Scholar]
- 50.Kliman R M, Rogers B T, Noor M A F. J Theor Biol. 2001;209:131–140. doi: 10.1006/jtbi.2000.2242. [DOI] [PubMed] [Google Scholar]
- 51.Krebs R A, Barker J S F. Drosophila Inf Serv. 1994;75:133–134. [Google Scholar]