Abstract
The Epstein-Barr virus (EBV), which is a ubiquitous γ-herpesvirus, establishes a latent infection in more than 90% of the global adult population. EBV-associated malignancies have increased by 14.6% over the last 20 years, and account for approximately 1.5% of all cancers worldwide and 1.8% of all cancer deaths. However, the potential involvement/contribution of lytic proteins to the pathophysiology of EBV-associated cancers is not well understood. We have previously demonstrated that the EBV-deoxyuridine triphosphate nucleotidohydrolase (dUTPase) modulates innate and adaptive immune responses by engaging the Toll-Like Receptor 2 (TLR2), which leads to the modulation of downstream genes involved in oncogenesis, chronic inflammation, and in effector T-cell function. Furthermore, examination of serum samples from diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia patients revealed the presence of increased levels of anti-dUTPase antibodies in both cohorts compared to controls with the highest levels (3.67-fold increase) observed in DLBCL female cases and the lowest (2.12-fold increase) in DLBCL males. Using computer-generated algorithms, dUTPase amino acid sequence alignments, and functional studies of BLLF3 mutants, we identified a putative amino acid motif involved with TLR2 interaction. These findings suggest that the EBV-dUTPase: TLR2 interaction is a potential molecular target that could be used for developing novel therapeutics (small molecules/vaccines).
Keywords: Epstein-Barr virus (EBV), deoxyuridine triphosphate nucleotidohydrolase (dUTPase), Toll-like receptor 2 (TLR2), diffuse large B cell lymphoma (DLBCL), chronic lymphocytic leukemia (CLL)
1. Introduction
Epstein-Barr virus, which is a γ herpesvirus, is a ubiquitous virus that establishes a latent infection in over 90% of the global adult population. In addition to being the etiological agent of infectious mononucleosis (IM), it is implicated in several human malignancies including Burkitt’s lymphoma (BL), nasopharyngeal carcinoma (NPC), classical Hodgkin lymphoma (cHL), gastric cancer, and diffuse large B cell lymphoma (DLBCL) [1]. DLBCL is a heterogeneous disease that is classified based on micro-array-based gene expression profiling as germinal center B-cell like (GCB) DLBCL or activated B-cell-like (ABC) DLBCL [2]. A small percentage (10–20%) of DLBCLs are EBV-genome positive and such tumors are usually classified in the ABC DLBCL group [3]. While EBV-genome positive tumors were originally discovered in older (>50 years of age) immunocompetent individuals [4,5,6,7,8], they have recently been reclassified to EBV+ DLBCL-NOS due to the increased occurrence of EBV+ DLBCL in younger immunocompetent patients [9]. EBV is an independent factor that adversely affects risk and/or survival among patients with DLBCL [5,7]. Conversely, while EBV is not generally associated with developing chronic lymphocytic leukemia (CLL), which is the most common leukemia in adults in western countries [10], it is associated with Richter Syndrome (RS). RS, which occurs in 10% to 15% of patients with CLL, is a histological transformation to DLBCL resulting in a more aggressive lymphoma with a poorer prognosis [11,12,13,14]. While several studies have implicated EBV in RS [15,16], a mechanistic relationship has not been determined. However, a recent study demonstrated that therapy related to immunosuppression in patients with CLL resulted in EBV reactivation, which drove RS and the formation of the ABC subtype DLBCL in some patients [17]. This study as well as a recent study of DLBCL [18] has suggested that products of lytic EBV may contribute to the development of these malignancies, but additional studies need to be performed.
Studies to examine the roles of EBV-encoded proteins in cellular transformation have focused primarily on those proteins and RNAs expressed during latency. These studies have demonstrated unequivocally the roles of the latent membrane proteins LMP1 and LMP2A in the transformation process and the immunological response of the host to these proteins [19,20]. Until recently, there have been very few studies directed toward determining the role(s) of proteins expressed during the lytic replication of EBV in immune modulation or in transformation despite the fact that low levels of EBV reactivation and expression of genes associated with lytic replication are typically observed in a small number of cells in many tumors [21,22,23,24,25,26,27,28]. However, recent studies have demonstrated the expression of a large number of lytic genes in cell lines and, more importantly, in biopsy tissue [29,30,31]. It has been suggested that proteins encoded by these genes may contribute to EBV oncogenesis by modulating the tumor microenvironment through the release of growth factors and/or immunosuppressive cytokines [32] or more directly by inducing genomic instability [28]. Additional data obtained from studies using SCID and humanized mouse models support this premise [33,34,35,36].
The EBV gene BLLF3 encodes for a deoxyuridine triphosphate nucleotidohydrolase (dUTPase), which is expressed during lytic/abortive lytic replication of the virus. While it has been difficult to quantify the amount of EBV-dUTPase present in tissue or serum because of the lack of sensitive assays, Ersing et al. [37] recently examined virus-host interactions during lytic replication using systemic proteomic quantitative analysis with tandem mass tags and mass spectrometry and estimated that the concentration of the EBV-dUTPase was 6000 nM and 7500 nM, respectively, in Akata and P3HR1 cells. There is indirect evidence to support the premise that EBV-encoded dUTPase is expressed and released from cells in vivo by following lytic and/or abortive replication. We have demonstrated, using quantitative real-time PCR, the expression of BLLF3 in tumors (9/10) obtained from SCID mice injected with C666-1 cells, which is an EBV-genome positive NPC cell line [38]. Zhang et al. [39], using microarray technology, demonstrated the expression of BLLF3 in PBMCs from a patient with acute phase IM and in EBV genome positive tumor cell lines established from patients with nasal NK/T-cell lymphoma. In addition, the EBV-encoded dUTPase protein has been detected using immuno-histochemical techniques in the upper epithelial layers of oral hairy leukoplakia (HL) lesions and the expression pattern was the same for BZLF-1 [40]. Similar results were obtained with lymphoid cells in tonsils from patients with IM and in NPC tissue [40,41]. Furthermore, we recently demonstrated by using immunohistochemistry the presence of the EBV- dUTPase in kidney biopsies from class III/IV Lupus nephritis (LN) patients. The EBV-dUTPase localized in infiltrating plasma-cell aggregates near glomeruli where neighboring cells expressing increased toll-like receptor 2 (TLR2) and IL-17 protein levels were observed, which suggests that EBV-dUTPase may exacerbate the immunopathologies in some LN patients [42]. We, as well as others, have demonstrated the presence of specific anti-EBV-encoded dUTPase antibodies in the sera of patients with IM, in reactivated and chronic EBV infections, in immunocompromised patients with HIV infections, and in immunocompetent patients with EBV genome positive diffuse large B-cell lymphoma, chronic lymphocytic leukemia and NPC [43,44,45], and unpublished data.
We have demonstrated that the dUTPases encoded by the human herpesviruses represent a new class of pathogen-associated molecular pattern (PAMP) proteins that have novel immuno-regulatory and neuro-regulatory functions, which may contribute to the pathophysiology of diseases caused by these viruses. Using the EBV-dUTPase as the prototype, our studies have demonstrated that it possesses novel functions independent of its enzymatic activity. Among them, the EBV-dUTPase acts as a trigger for TLR2, which leads to the activation of NF-κB and subsequent modulation of downstream genes involved in chronic inflammation and oncogenesis [46]. We have also demonstrated that these viral dUTPases are capable of differentially inducing the secretion of the pro-inflammatory TH1/TH17 cytokines IL-1β, IL-6, IL-8, IL-12p70, TNF-α, CCL20, and IFN-γ as well as the anti-inflammatory cytokine IL-10 in human primary immune cells [47,48,49,50,51]. Not only is CCL20 reported to promote cellular proliferation and differentiation of numerous cell types including malignant cells but IL-6, which is a positive regulator of CCL20, also functions as an autocrine growth factor for EBV-immortalized B-cells [52,53,54].
Since the interaction of EBV-dUTPase with TLR2 is the critical step for initiating the signaling cascade that leads to the establishment of a microenvironment that may support the survival and proliferation of EBV-transformed cells, the purpose of the present study was to elucidate the amino acid residues in the EBV-dUTPase important for interacting with TLR2.
2. Results
2.1. Identification of a Putative TLR2 Binding Motif within the EBV-dUTPase
The EBV-encoded dUTPase is composed of 278 amino acids and, while it is the smallest of the human herpesviruses’ dUTPases, it contains all five motifs characteristic of dUTPases [55] as well as a unique motif (motif 6) found in herpesviruses’ dUTPases (see Figure 1) [56].
Figure 1.
Epstein-Barr Virus deoxyuridine triphosphate nucleotidohydrolase (EBV-dUTPase) amino acid sequence. Typical dUTPase motifs 1–5 and the unique motif 6 characteristic of the Herpesviruses dUTPase family are depicted.
Using computer-generated algorithms (hydrophilicity, flexibility, mobility, solvent exposure, amphiphilicity, reverse turns, α-helical properties, and protrusion) to predict amino acid sequences that have the potential to interact with other proteins, we identified five sequences, which were then computer-ranked based upon their respective algorithms [51,57]. The amino acid sequences were 83–103 (rank 2), 109–140 (rank 1 contains the entire conserved motif 1), 174–194 (rank 4 contains most of motif 6, which is restricted to herpesviruses’ dUTPases), 210–237 (rank 5 contains conserved motif 4), and 253–276 (rank 3 contains conserved motif 5).
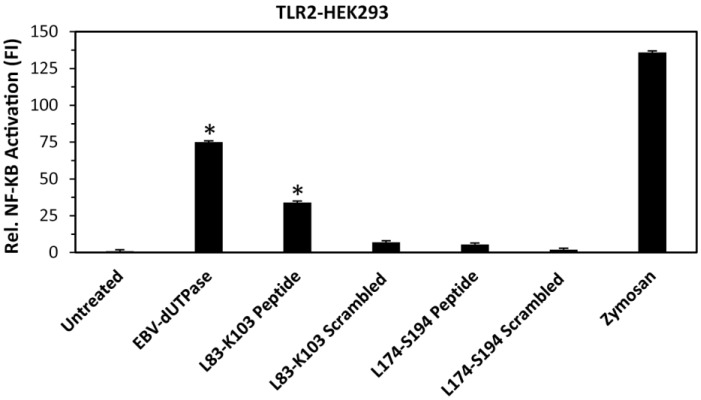
Since amino acid residues 83 to 103 were identified by computer-generated algorithms as the only non-conserved motif containing sequences with a high likelihood for interacting with other proteins and amino acid sequence 174 to 194 contains most of the unique motif 6 of unknown function, we next constructed synthetic peptides corresponding to amino acid residues leucine 83 through lysine 103 (L83-K103) as well as leucine 174 through serine 194 (L174-S194) and tested whether or not these peptides could induce NF-κB activation by engaging TLR2. As shown in Figure 2, only the L83-K103 peptide induced the transcriptional activation of the NF-κB reporter gene by 34-fold, which is approximately 50% of that exhibited by the full-length EBV-dUTPase protein (74-fold) while L174-S194 and corresponding scrambled control peptides did not cause a significant activation of NF-κB in human embryonic kidney 293 cells (HEK293)—stably expressing TLR2.
Figure 2.
Activation of NF-κB by L83-K103 peptide in HEK293 cells stably expressing TLR2. Cells were transiently transfected with NF-κB luciferase reporter plasmid as we have described [40,43,44]. After 24–36 h, cells were treated with wild-type EBV-dUTPase, EBV-dUTPase peptide L83-K103, scrambled peptide L83-K103, EBV-dUTPase peptide L174-S194, scrambled peptide L174-S194 (10 μg/mL), zymosan (10 μg/mL), or left untreated for 8 h and luciferase reporter gene activity was measured. Values represent the mean fold induction (FI) ± SD relative to control (n = 3). Values represent the mean fold induction (FI) ± SD relative to control (n = 3). * p < 0.05 (Groups compared: wild-type dUTPase or synthetic peptide treated vs. untreated).
Further cytokine analysis of synthetic peptides in human PBMCs revealed that stimulation of cells with the EBV-dUTPase synthetic peptide L83-K103 but not scrambled peptide resulted in an increased production of IL-6 (9-fold increase over scrambled control peptide), IL-8 (2.5-fold increase), TNF-α (5-fold increase), IL-10 (4.8-fold increase), and IL-1β (3.3-fold increase) cytokines compared to untreated control cells. However, the cytokine response induced by L83-K103 peptide was not as strong as that observed in cells stimulated with the full-length EBV-dUTPase protein especially for IL-1β, TNFα, and IL-10 (see Table 1).
Table 1.
Cytokine profile induced by EBV-dUTPase peptide L83-K103 in human a PBMCs at 48 h.
| Treatments (10 µg/mL) | IL-6 (pg/mL) |
IL-1β (pg/mL) |
TNFα (pg/mL) |
IL-8 (pg/mL) |
IL-10 (pg/mL) |
|---|---|---|---|---|---|
| Untreated | 5 ± 0.8 | 31 ± 22.8 | 5 ± 4.9 | 407 ± 5.7 | 8 ± 3.8 |
| EBV-dUTPase | 9570 ± 5.7 | 978 ± 15 | 379 ± 123 | 35,039 ± 219 | 311 ± 35 |
| Scrambled peptide L83-K103 | 369 ± 312 | 34 ± 24.4 | 9 ± 6.2 | 8738 ± 267.6 | 9 ± 5.2 |
| EBV-dUTPase peptide L83-K103 | 3272 ± 6 | 111 ± 21.5 | 45 ± 6.6 | 21,934 ± 14 | 43 ± 6.2 |
a PBMCS from healthy donors were treated with EBV-dUTPase full-length protein, EBV-dUTPase peptide L83-K103, scrambled control peptide (10 µg/mL), or left untreated for 48 h. Culture supernatants were collected for cytokine analysis by ELISA. Cytokine levels are expressed as pg/mL. Values represent mean ± SD of an n = 3.
While the size and sequence homologies of the dUTPases encoded by members of the Herpesviridae Family vary considerably, a common feature in the dUTPases encoded by EBV, HSV-2, HHV-6, HHV-8, and VZV is their ability to trigger the activation of TLR2 [40,43,44]. Blast analyses of the amino acid sequences of the herpesviruses dUTPases as well as the human nuclear dUTPase demonstrated that the herpesviruses contained a sequence that was somewhat divergent especially with the β-herpesviruses that may represent a conserved sequence (see Table 2).
Table 2.
TLR2 putative binding motif.
| dUTPases | Amino Acid Sequence |
|---|---|
| EBV | 81GELRLILQNQ90 |
| HHV-8 | 109GEIQVILLNK118 |
| HSV-1/2 | 102GTVMAVVAP110 |
| VZV | 130GVISALLYYR139 |
| HHV-6A | 207TDISVFLMNL116 |
| HHV-7 | 215NVISISLINL224 |
| HCMV | 173LQVPQLDVVNL183 |
| Human | 84GNVGVVLFNF93 |
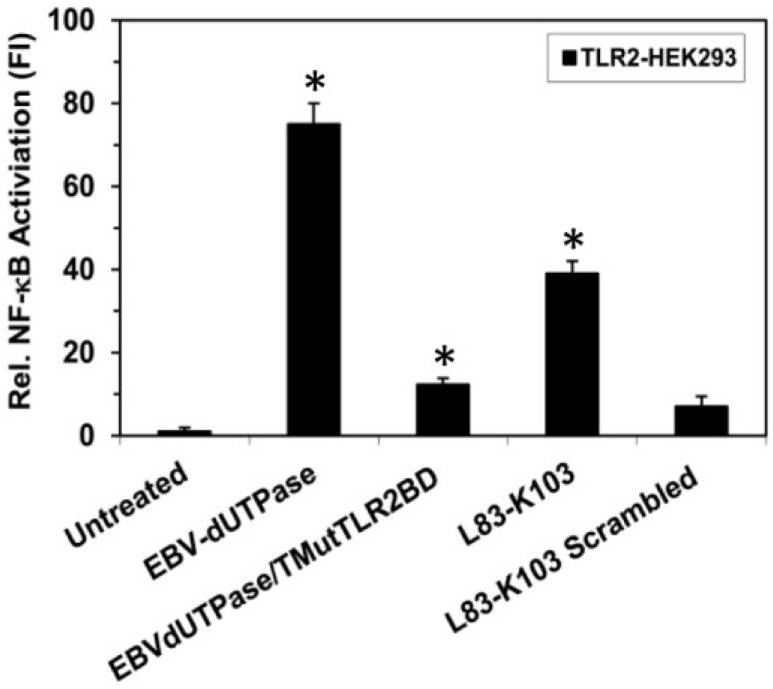
This TLR2 putative interactive motif is located in the β6 strand structure adjacent to motif 3, which is part of the catalytic site. This motif contains eight amino acid residues, which are included in the computer-generated algorithm sequence 83-103. The possibility of this motif being part of the TLR2 interactive domain is further supported by the studies described above, which demonstrate that a synthetic peptide corresponding to amino acids L83-K103 of the EBV-dUTPase induced NF-κB activation (see Figure 1) in TLR2-HEK293 cells and stimulated the secretion of pro-inflammatory cytokines in human PBMCs (see Table 2). To further confirm the importance of this motif for triggering TLR2 signaling, we generated a recombinant EBV-dUTPase protein containing a triple mutation (82ELR84 to 82GGG84; EBVdUTPase/TMutTLR2BD) and demonstrated that it significantly reduced NF-κB activation by 83% to 12.31-fold compared to the wild-type EBV-dUTPase protein (75-fold increase) (see Figure 3). Decreased cytokine secretion by stimulated PBMCs was also observed (data not shown), which highlights the importance of amino acid residues glutamate (E) 82, leucine (L) 83, and arginine (R) 84 in the activation of TLR2 signaling and further supporting this motif as a TLR2 putative interactive motif.
Figure 3.
Site-directed mutagenesis of the putative TLR2 binding motif in the EBV-dUTPase inhibits NF-κB activation. TLR2-HEK293 cells were transiently transfected with NF-κB luciferase reporter plasmid as we have described [40,43,44]. After 24–36 h, cells were treated with wild-type EBV-dUTPase, a triple mutant (82ELR84 to 82GGG84) of the EBV-dUTPase TLR2 putative binding motif (EBVdUTPase/TMutTLR2BD), EBV-dUTPase peptide L83-K103, scrambled peptide L83-K103 (10 μg/mL) or left untreated for 8 h and luciferase reporter gene activity was measured. Values represent the mean fold induction (FI) ± SD relative to control (n = 3). * p < 0.05 (Groups compared: dUTPase treated vs. untreated and dUTPase triple mutant vs. wild-type dUTPase).
2.2. Anti-EBV-Encoded dUTPase Antibody in Patients with DLBCL and Chronic Lymphocytic Leukemia (CLL)
A better understanding of the diversity in the humoral response to EBV-dUTPase in health and disease states may enable us to identify EBV-dUTPase antibody patterns that could be used as markers for early diagnosis and/or to monitor treatment. Using our standard neutralization assay [44], we next conducted a pilot study to examine the humoral response to the EBV-dUTPase in healthy EBV carriers (n = 89) and in the B cell malignancies DLBCL (n = 36) and CLL (n = 66) sera samples from the European EPILYMPH case-control published study [58] exhibiting either a normal or abnormal/reactive antibody pattern to EBV, which was determined by de Sanjose et al. [58]. Sera from a control cohort of 431 individuals with no known health problems (268 females and 163 males) ranging in age from 18–92 years with a median age 63.38 ± 12.52 for females and 62.48 ± 13.46 for males, a DLBCL cohort of 36 cases (21 females and 15 males) ranging in age from 23 to 80 years old with a median age of 59.22 ± 18.39 and 49.40 ± 15.95 years, respectively, and a CLL cohort of 66 patients (33 females and 34 males) ranging in age from 30 to 87 years old with a median age of 69.52 ± 9.61 and 68.99 ± 11.75, respectively, were tested for the presence of anti-EBV dUTPase neutralizing antibodies. This study revealed an overall increase in neutralizing antibodies specific to the EBV-dUTPase in the case cohorts (36.11% and 39.39% for DLBCL and CLL, respectively) compared to the controls (12.76%) (see Table 3). Data analysis by gender shows a difference in the prevalence of dUTPase neutralizing antibodies between females and males within each cohort and across disease type relative to the controls. Within the DLBCL sera samples, there was a higher prevalence of dUTPase neutralizing antibodies in females than males (42.86% versus 26.66% in males). Additionally, the opposite was observed in the CLL cohort with males exhibiting a higher prevalence of dUTPase neutralizing antibodies than females (34.37% versus 44.12% in males). No differences were found within the control cohort between females and males, which suggests that the prevalence of dUTPase neutralizing antibodies in this group is independent of gender. More importantly, when comparing dUTPase antibody prevalence across disease type, it was found that the highest increase was observed in CLL male (44.12% versus 13.98% in controls) and DLBCL female cases (42.86% versus 11.67% in controls) (a 3.16-fold and 3.67-fold increase respectively), which were followed by the CLL female (34.37%, 2.95-fold increase) and DLBCL male cases (29.67%, 2.12-fold).
Table 3.
Detection of anti-EBV-dUTPase antibodies (Ab) in patients with DLBCL or CLL.
| Clinical Status | Gender | % Positive EBV dUTPase Ab a | % Abnormal Reactive Ab Pattern to EBV (ARP_EBV) b | % ARP_EBV & dUTPase Seropositive c |
|---|---|---|---|---|
| Controls | Females | 11.67 (32/268) | 22.22 (10/45) | 8.88 (4/45) |
| Males | 13.98 (23/163) | 11.36 (5/44) | 4.54 (2/44) | |
| DLBCL | Females | 42.86 (9/21) | 33.33 (7/21) | 14.28 (3/21) |
| Males | 26.66 (4/15) | 20.00 (3/15) | 0.00 (0/15) | |
| CLL | Females | 34.37 (11/32) | 53.12 (17/32) | 18.75 (6/32) |
| Males | 44.12 (15/34) | 20.59 (7/34) | 14.70 (5/34) |
a dUTPase neutralizing assays were performed as described previously [44]. Values in parentheses represent the number of positive sera in either cases or controls/total sera. The total number of control sera (n = 431) include 89 samples from the EPILYMPH case-control study [58] as well as 352 samples from other published studies [44,45,48]. b Individuals’ sera exhibiting an abnormal reactive Ab pattern to EBV (ARP_EBV) was determined by de Sanjose et al., as part of the EPILYMPH case-control previously published study [58]. Values represent the percentage of sera samples exhibiting EBV-IgG reactivity to combined immuno-dominant epitopes of EBNA1 and VCA-p18-based ELISA assays and abnormal reactivity/intensity score on immunoblots to EBV antigens (ex: EAd-p47/54, EAd-p138) other than/besides EBNA1, VCA-p40, VAC-p18, and ZEBRA predominantly recognized by healthy EBV immunocompetent individuals [58]. Values in parentheses represent the number of individuals exhibiting increased/abnormal Ab responses to EBV proteins in either cases or controls sera/total sera. c Values represent the percentage of sera samples that were positive for anti-EBV-dUTPase antibodies and also had increased/abnormal EBV reactivity. Values in parentheses represent the number of positive sera in either case or controls/total sera.
3. Discussion
EBV-associated malignancies are reported to account for approximately 1.5% of all cancers worldwide and represent 1.8% of all cancer deaths [59]. While most studies have focused on the contribution of EBV latency proteins such as LMP-1 and LMP-2A in oncogenesis, few studies have addressed the role, if any, that EBV proteins produced during lytic/abortive lytic replication may have in this process.
In the current study, we demonstrate the presence of increased neutralizing antibodies against the EBV-dUTPase in the sera of DLBCL and CCL patients from the EPYLYMPH study, which examined abnormal humoral responses to EBV [58]. In the EPYLYMPH study, the investigators reported that patients with aberrant EBV activity were identified by a broad immuno-reactive profile including antibodies to several peptides from proteins composing the Early Antigen diffuse (EA-D) complex (BMRF1, BALF2, BGLF5, and BXLF1) as well as EBNA1, VCA, and BZLF1 while uncomplicated carriers and sera from some patients with lymphomas exhibited a more restricted antibody pattern (EBNA1, VCA, and BZLF1). The dUTPase, which is encoded by the BLLF3 gene, is an early protein that forms part of the EA-D complex. An important finding of our study is the observation that CLL male cases exhibited the highest prevalence of dUTPase neutralizing antibodies (44.12% versus 13.98% in controls) of all cases examined and had the lowest prevalence of an abnormal reactive antibody pattern to EBV. By contrast, CLL females had the highest increase in abnormal reactive antibody patterns to EBV (53.12% versus 22.22% in controls as determined by de Sanjose et al. [58]) but had the second lowest prevalence in dUTPase antibodies. Furthermore, within the DLBCL cohort, 67% (6/9) of female and all male sera samples (4) that tested positive for the presence of dUTPase neutralizing antibodies also expressed a normal/non-reactive antibody pattern to EBV. Overall, an increased prevalence of dUTPase neutralizing antibodies was consistently observed in sera of DLBCL and CLL patients who exhibited a normal/non-reactive antibody pattern to EBV. A recent study demonstrated that immediate early and early EBV proteins expressed during lytic replication of EBV are expressed in some tumor cells in patients with EBV+ DLBCL and that antibodies against these proteins are detected in patients’ sera. This led the investigators to propose that products from lytic/abortive lytic replication may contribute to tumor growth and survival [18]. Our previous studies have demonstrated that the EBV-dUTPase protein induces IL-6 in primary dendritic cells and PBMCs [46,47,48]. IL-6 is a growth factor for EBV-immortalized B cells and IL-6 over-expression has been shown to enhance growth of EBV-transformed lymphoblastoid cell lines (LCL’s) in SCID mice [60,61,62,63]. In addition, we have shown that EBV-dUTPase up-regulates the expression of CCL20 (335-fold) [49], which, in turn, may increase migration and trafficking of regulatory T cells (Tregs) into the tumor environment. Therefore, this dampens the immune response to EBV [54]. EBV-dUTPase also up-regulates the expression of BIC/miR155 [38], which is associated with aberrant inflammatory responses and oncogenesis, enhanced B-cell transformation, and the development of Tregs [64]. In all, these data support the premise that the dUTPase could modify the tumor microenvironment [32,38,65] and that the presence of antibodies directed against the dUTPase may be a useful marker for detecting aberrant virus replication in a subset of patients with DLBCL.
While several studies have demonstrated increases in EBV viral load, EBV miRNA and anti-EBV-antibodies in the sera of patients with CLL [66,67,68,69,70], the potential role of EBV in the development of CLL still remains poorly understood. Another study by de Sanjose et al. [58] reported that CLL samples exhibited the highest prevalence of abnormal anti-EBV antibody reactivity (40%) of any lymphomas examined. This finding was also observed in our analyses of anti-EBV dUTPase antibodies in the same samples. Two independent studies have recently demonstrated that a subgroup (53–59%) of patients presenting with CLL had significantly higher EBV-DNA copy numbers [69,70]. These patients required early treatment [69] and exhibited shorter survival rates [69,70]. It is also well documented that a small percentage (10–15%) of patients with CLL will undergo histological transformation into an aggressive form of DLBCL [11,12,13,14] referred to as Ritchter syndrome (RS). Recently, it was reported that RS might occur following aggressive therapy during CLL that results in the reactivation of EBV [17]. Therefore, it is possible that the CLL patients tested in this study and the study by de Sanjose et al. [58] represent two subgroups one in which EBV is a negative prognostic factor for patients with CLL and a second subgroup in which EBV contributes to RS transformation. While additional studies are necessary to delineate additional markers to distinguish these subgroups, the results of this study as well as that of de Sanjose et al. [58] suggest that antibodies against EBV-dUTPase and EA-D, which the dUTPase is a component of, could be useful markers for initially identifying such patients.
There is a growing body of evidence demonstrating that the reactivation of latent herpesviruses, as indicated by higher antibody titers to proteins expressed during lytic or abortive-lytic replication, occurs when the immune system is compromised [45,48,71,72,73,74]. Several studies on EBV have established that reactivation of the virus usually results in abortive-lytic replication in which only immediate early and early genes are expressed and, therefore, no new virus is produced [75,76,77]. Since the dUTPase is expressed as an early protein, this would suggest that abortive and/or lytic replication occurs in a subset of patients with DLBCL and CLL.
We have previously demonstrated that the EBV-dUTPase triggers NF-κB activation by engaging TLR2 homodimers [46] while the dUTPases encoded by HSV-2, HHV-6, HHV-8, and VZV require ligation of the TLR2/1 heterodimer complex to activate NF-κB [50]. Follow-up studies demonstrated that these viral dUTPases are capable of differentially inducing the secretion of the pro-inflammatory TH1/TH17 cytokines IL-1β, IL-6, IL-8, IL-12p70, TNF-α, and IFN-γ as well as the anti-inflammatory cytokine IL-10 in human primary immune cells [46,47,48,49,50,51]. This suggests that they can modulate the cellular microenvironment [32,38,65]. While sequence analyses have demonstrated that the α and γ herpesvirus members contain the five highly conserved motifs characteristic of the homotrimeric and monomeric dUTPases, members of the β herpesvirus group do not. However, all the human herpesviruses contain an additional conserved motif (domain 6) that is absent in the homotrimeric dUTPases [56]. It has been suggested that this novel herpesvirus-specific domain may contribute to some unknown novel function. With the exception of the EBV-dUTPase [55], no crystal structure data is available for the other human herpesviruses’ dUTPases. The results shown in this study demonstrate that amino acid residues between 81G to 103K of the EBV-dUTPase are important for binding to and activating TLR2 signaling. This TLR2 putative interactive motif is located in the β6 strand structure adjacent to motif 3, which is part of the catalytic site.
The lack of control of EBV abortive/lytic replication may reflect a variety of physiological processes including stress and aging, which affect T-cell function and, therefore, the role of EBV in oncogenesis may or may not be a direct effect. However, our results suggest that lytic/abortive lytic replication occurs in patients with DLBCL as well as CLL. The EBV-dUTPase may alter the tumor microenvironment [32,38,65] by providing a selective advantage (growth/survival) to the malignant cell. Future studies using a larger cohort of patients will be necessary to determine whether there is a possible relationship between EBV-dUTPase expression and malignant progression as well as whether or not the presence of anti-EBV-dUTPase antibodies could be useful for diagnostic purposes. While additional experiments involving crystal structures of TLR2: EBV-dUTPase complexes are needed to confirm the specific amino acid residues of the EBV-dUTPase that interacts with TLR2, the results from the current study support the premise that the EBV-dUTPase-TLR2 interaction could be used as a target for developing novel therapeutics specifically small molecules and/or vaccines.
4. Materials and Methods
4.1. Construction of EBV-dUTPase Triple Mutant
EBV-dUTPase containing a triple point mutation E82G, L83G, and R84G (82ELR84 to 82GGG84) was generated by site-directed mutagenesis using the QuikChange Lightning Mutagenesis system (Stratagene, Santa Clara, CA, USA), which was previously described [78] and the primer set: Forward: 5′-CCGGTCACGTCTCATGTTGGCATCATCGATCCCGGCTACACG-3′; Reverse: 5′-CGTGTAGCCGGGATCGATGATGCCAACATGAGACGTGACCGG-3′. The PCR conditions used include one cycle at 95 °C for 2 min, which was followed by 18 cycles of 95 °C for 20 s, 60 °C for 10 s, and 68 °C for 2.5 min, and one cycle at 68 °C for 5 min. Amplified products were DpnI digested and screened for the β-galactosidase (β-gal+) phenotype. DNA was then purified and the amino acid changes E82G, L83G, and R84G were verified by using sequence analysis.
4.2. Peptide Synthesis
EBV-dUTPase peptides 83LRLILQNQRRYNSTLRPSELK103, 174LAMQGILVKPCRWRRGGVDVS194 as well as the respective scrambled controls peptides 83ELQPKRTLQSRLYRINLSNRL103 and 174KRLGVCIQWVGLPRDVMRSAG194 were synthesized in house in the Peptide Protein Engineering Laboratory at the Tzagrournis Medical Research Facility at The Ohio State University. Peptide synthesis was performed on a Milligen/Biosearch 9600 solid-phase peptide synthesizer (Bedford, MA, USA) using Fmoc/t-But chemistry. The C-terminal amino acid loaded on CLEAR ACID resin (0.32 mmol/gm) (e.g., in case of peptide WILL 83-103, Fmoc-Ile-CLEAR ACID Resin (Peptides International, Louisville, KY, USA) was used for the synthesis. All peptides were cleaved from the resin using cleavage reagent B (Trifluoroacteic acid:Phenol:Water:Triisopropyl silane 90:4:4:2) and crude peptides were purified on preparative Reverse Phase-High Pressure Liquid Chromatography (RP-HPLC) using Vydac C-4 column and the acetonitrile-water (0.1% TFA) gradient system. All fractions were analyzed on analytical RP-HPLC and characterized by using Matrix Assisted Laser Desorption Ionization mass spectroscopy (MALDI) at The Ohio State University Campus Chemical Instrumentation Center. RP-HPLC fractions showing the same mass spectrum peak were pooled together and lyophilized. The respective scrambled peptides were synthesized using the number of amino acids present in natural sequences and were scrambled manually. All pure peptides were further characterized using MALDI mass spectroscopy analysis to confirm the calculated and observed molecular weight, which include L83-K103 (M+H+) Cal/Obs 2598.49/2598.47, SCRL83-K103 (M+H+) Cal/Obs 2598.49/2598.23; L174-S194 (M+H+) Cal/Obs 2341.82/2341.13, SCRL174-S194 (M+H+) Cal/Obs 2341.84/2341.42.
4.3. Purification of Recombinant EBV-dUTPase Protein
Sub-cloning and purification of recombinant EBV-dUTPase mutant and wild-type proteins were performed as previously described [40,41,44]. All recombinant dUTPase protein preparations were tested for the presence of contaminants, which was described previously [40,41,44], and were free of detectable levels of LPS, peptidoglycan (SLP-HS), DNA, or RNA. Protein concentration was determined using the Qubit fluorimeter (Invitrogen, Carlsbad, CA, USA). The purified recombinant dUTPase proteins used in these studies were stored at −80 °C until further use.
4.4. EBV-dUTPase Neutralization Assays
Neutralization assays for the EBV dUTPase were performed as previously described [44]. In brief, 5 µL of human serum were mixed with 5 µL of purified EBV-dUTPase (3–5 units of enzyme) for 30 min at room temperature prior to assaying for enzymatic activity. EBV-dUTPase activity was determined as described previously [44]. For positive controls, assays were performed in the presence of human serum that lacked detectable antibodies to the EBV. Negative controls were also performed in the absence of the enzyme preparation. A unit of EBV-dUTPase activity was defined as the amount of enzyme required to convert 1 nmole of dUTPase to dUMP and pyrophosphate/min/mL of enzyme at 37 °C. Units of enzymatic activity neutralized per mL of serum were obtained as follows: (Ucontrol–Userum). Serum with neutralizing units greater than or equal to two standard deviations from the control were considered “positive” for dUTPase neutralizing antibodies.
4.5. Patients
The patient samples in this study were collected from 1988–2003 as part of the EPILYMPH case-control study carried out in six European countries by de Sanjose et al. [58]. Cases were defined as all consecutive patients having their initial diagnosis of lymphoid malignancy during the study period. The diagnosis of lymphoma was verified by histology and 99% of cases were supplemented by immunohistochemistry tests and flow cytometry. The cases were categorized according to the World Health Organization (WHO) Classification for Neoplastic Diseases of the Lymphoid Tissues and included all B-cell, T-cell, and NK-cell neoplasms as well as Hodgkin’s lymphoma [79]. Additionally, 20% of all diagnosed cases in each country were externally reviewed by a panel of international pathologists. The panel diagnosis is the one used in this analysis in the rare circumstance of disagreement between the local and the panel pathologists. Subjects with a diagnosis of uncertain malignant potential such as post-transplant lymphoproliferative disorder or monoclonal gammopathies of undetermined significance were excluded. Immunosuppressed patients were excluded from the analysis.
4.6. Cell Culture
Human embryonic kidney (HEK293) cells stably expressing human TLR2 (TLR2-HEK293; Invivogen, San Diego, CA, USA) were maintained in DMEM-supplemented medium, as recommended by the manufacturer [46,49,50,78]. Human peripheral blood mononuclear cells (PBMCs) from healthy subjects were obtained from Astarte Biologics (Bothell, WA, USA).
4.7. Luciferase Reporter Gene Assays
HEK293 cells (2.5 × 105) were seeded into 12-well plates and 24 h later transiently transfected with pNFκB-Luc, pRL-TK reporter vectors (Promega, Madison, WI, USA), or with empty vectors as described [46,49,50,78]. About 24 h to 36 h after transfection, cells were treated with recombinant wild-type or truncated EBV dUTPase proteins (10 μg/mL), zymosan (10 μg/mL; positive control for TLR2 activation) for 8 h or left untreated. After treatment, cell lysates were prepared and reporter gene activities were measured using the dual-luciferase reporter assay system (Promega). Data were normalized for transfection efficiency by measuring Renilla luciferase activity and expressed as mean relative stimulation ± SD.
4.8. Cytokine Profile Induced by Herpesviruses-Encoded dUTPases
PBMCs were seeded at a density of 2.5 × 105 in 24-well plates and cultured in AIM-V serum-free medium supplemented with l-glutamine (2 mM), streptomycin (50 μg/mL), and gentamycin (10 μg/mL). The next day, cells were stimulated with wild-type or truncated dUTPases (10 μg/mL), EBV-dUTPase peptide L83-K103, scrambled peptide L83-K103 or left untreated for 24 h. Following treatment, cell culture supernatants were collected and cytokine levels were measured by using ELISA (MSD Multi-array and Multi-spot human cytokine kit), which we described previously [46,49,50,78]. Concentrations are expressed as pg/mL and represent the mean ± SD of an n of 3.
4.9. Statistical Analysis
Statistical analyses were performed using a paired two-sample t-test for the means and p values were reported when displaying a significant value (p < 0.05). Values represent the mean ± SD of at least three independent experiments.
5. Conclusions
Examination of serum samples from diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia (CLL) patients revealed the presence of increased anti-dUTPase neutralizing antibodies in both cohorts compared to controls with the highest levels (3.67-fold increase) observed in DLBCL female cases and the lowest (2.12-fold increase) in the DLBCL males. Furthermore, using computer-generated algorithms, dUTPase amino acid sequence alignments, and functional studies of BLLF3 mutants, we identified a putative amino acid motif involved with TLR2 interaction and demonstrated that amino acid residues between 81G to 103K of the EBV-dUTPase are important for binding to and activating TLR2 signaling. These findings suggest that the EBV-dUTPase: TLR2 interaction is a potential molecular target that could be used for developing novel therapeutics (small molecules/vaccines).
Acknowledgments
We would like to thank the European sample collectors in the EPYLYMPH study as well as Silvia de Sanjose and Yolanda Benavente for enabling us to obtain the case and control sera samples used in this study via the NIH cancer repository.
Abbreviations
The following abbreviations are used in this manuscript:
| TLR2 | Toll-like receptor 2 |
| DLBCL | Diffuse large B cell lymphoma |
| CLL | Chronic lymphocytic leukemia |
| EBV | Epstein-Barr virus |
| dUTPase | Deoxyuridine triphosphate nucleotidohydrolase |
| HSV-2 | Herpes simplex virus type 2 |
| HHV-6 | Human herpesvirus 6 |
| HHV-8 | Human herpesvirus 8 |
| VZV | Varicella-zoster virus |
| HEK293 | Human embryonic kidney cells |
| PBMCs | Peripheral blood mononuclear cells |
Author Contributions
Maria Eugenia Ariza and Marshall Williams conceived, designed, and performed the experiments, analyzed the data, and wrote the paper.
Funding
This research was supported by the National Institutes of Health grant R01 A1084898 to M.W. and M.E.A., and by funds from the University of South Carolina Centenary plan initiative to M.E.A.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Kutok J.L., Wan F. Spectrum of Epstein-Barr virus-associated diseases. Annu. Rev. Pathol. Mech. Dis. 2006;1:375–404. doi: 10.1146/annurev.pathol.1.110304.100209. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh A.A., Eisen M.B., Davis E.R., Ma C., Lossos I.S., Rosenwald A., Boldrick J.C., Sabet H., Tran T., Yu X., et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 3.Shannon-Lowe C., Rickinson A.B., Bell A.I. Epstein-Barr virus-associated lymphomas. Philos. Trans. R. Soc. Lond. B Boil. Sci. 2017;372 doi: 10.1098/rstb.2016.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oyama T., Yamamoto K., Asano N., Oshiro A., Suzuki R., Kagami Y., Morishima Y., Takeuchi K., Izumo T., Mori S., et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: A study of 96 patients. Clin. Cancer Res. 2007;13:5124–5132. doi: 10.1158/1078-0432.CCR-06-2823. [DOI] [PubMed] [Google Scholar]
- 5.Park S., Lee J., Ko Y.H., Han A., Jun H.J., Lee S.C., Hwang I.G., Park Y.H., Ahn J.S., Jung C.J., et al. The impact of Epstein-Barr virus status on clinical outcome in diffuse large B-cell lymphoma. Blood. 2007;110:972–978. doi: 10.1182/blood-2007-01-067769. [DOI] [PubMed] [Google Scholar]
- 6.Shimoyama Y., Yamamoto K., Asano N., Oyama T., Kinoshita T., Nakamura S. Age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorders: Special references to lymphomas surrounding this newly recognized clinicopatholoogic disease. Cancer Sci. 2008;99:1085–1091. doi: 10.1111/j.1349-7006.2008.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asano N., Yamamoto K., Tamaru J.I., Oyama T., Ishida F., Ohshima K., Yoshino T., Nakamura N., Mori S., Yoshie O., et al. Age-related Epstein-Barrvirus (EBV)-associated B-cell lymphoproliferative disorders: Comparison with EBV-positive classic Hodgkin lymphoma in elderly patients. Blood. 2009;113:2629–2636. doi: 10.1182/blood-2008-06-164806. [DOI] [PubMed] [Google Scholar]
- 8.Gibson S.E., His E.D. Epstein-Barr virus-positive B-cell lymphoma of the elderly at a United States tertiary medical center: An uncommon aggressive lymphoma with a nongerminal center B-cell phenotype. Hum. Pathol. 2009;40:653–661. doi: 10.1016/j.humpath.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow S.H., Campo E., Pileri S.A., Harris N.L., Stein H., Siebert R., Advanti R., Gheelmini M., Salles G.A., Zelenets A.D., et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghia P., Ferreri A.J.M., Caligaris-Cappio F. Chronic lymphocytic leukemia. Crit. Rev. Oncol./Hematol. 2007;64:234–246. doi: 10.1016/j.critrevonc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Campo E., Swerdlow S., Harris N.L., Pileri S., Stein H., Jaffe E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain P., O’Brien S. Richter’s transformation in chronic lymphocytic leukemia. Oncology. 2012;26:1146–1152. [PubMed] [Google Scholar]
- 13.Parikh S.A., Shanafelt T.D. Risk factors for Richter Syndrome in chronic lymphocytic leukemia. Curr. Hematol. Malig. Rep. 2014;9:294–299. doi: 10.1007/s11899-014-0223-4. [DOI] [PubMed] [Google Scholar]
- 14.Vitale C., Ferrajoli A. Richter syndrome in chronic lymphocytic leukemia. Curr. Hematol. Malig. Rep. 2016;11:43–51. doi: 10.1007/s11899-016-0300-y. [DOI] [PubMed] [Google Scholar]
- 15.Ansell S.M., Li C.Y., Lloyd R.V., Phyliky R.L. Epstein-Barr virus infection in Richter’s transformation. Am. J. Hematol. 1999;60:99–104. doi: 10.1002/(SICI)1096-8652(199902)60:2<99::AID-AJH3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Dolcetti R., Carbone A. Epstein-Barr virus infection and chronic lymphocytic leukemis: A possible progression factor? Infect. Agents Cancer. 2010;5:22. doi: 10.1186/1750-9378-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Barchino M.J., Sarasquete M.E., Panizo C., Morscio J., Martinez A., Alcoceba M., Fresquet V., Gonzalez-Farre B., Paiva B., Young K.H., et al. Richter transformation driven by Epstein-Barr virus reactivation during therapy-related immunosuppression in chronic lymphocytic leukemia. J. Pathol. 2018 doi: 10.1002/path.5060. [DOI] [PubMed] [Google Scholar]
- 18.Cohen M., Vistraop A.G., Huaman F., Narbaitz M., Metrebian F., De Matteo E., Preciado M.V., Chabay P.A. Epstein-Barr virus lytic cycle involvement in diffuse large B cell lymphoma. Hematol. Oncol. 2018;36:98–103. doi: 10.1002/hon.2465. [DOI] [PubMed] [Google Scholar]
- 19.Khanna R., Burrows S.R. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu. Rev. Microbiol. 2000;54:19–48. doi: 10.1146/annurev.micro.54.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Hislop A.D., Taylor G.S., Sauce D., Rickinson A.B. Cellular responses to viral infection in humans: Lessons from Epstein-Barr virus. Annu. Rev. Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Farre B., Rovira J., Martinez D., Valera A., Garcia-Herrera A., Marcos M.A., Sole C., Rouge G., Colomer D., Gonzalvo E., et al. In vivo intratumoral Epstein–Barr virus replication is associated with XBP1 activation and early-onset post-transplant lymphoproliferative disorders with prognostic implications. Mod. Pathol. 2014:1599–1611. doi: 10.1038/modpathol.2014.68. [DOI] [PubMed] [Google Scholar]
- 22.Cochet C., Martel-Renoir D., Greunewald V., Bosq J., Cochet G., Schwaab G., Bernaudin J.F., Joab I. Expression of the Epstein-Barr virus immediate early gene, BZLF1, in nasopharyngeal carcinoma tumor cells. Virology. 1993;197:358–365. doi: 10.1006/viro.1993.1597. [DOI] [PubMed] [Google Scholar]
- 23.Martel-Renoir A., Grunewald V., Touitou R., Schwaab G., Joab I. Qualitative analysis of the expression of Epstein-Barr virus lytic genes in nasopharyngeal biopsies. J. Gen. Virol. 1995;76:1401–1408. doi: 10.1099/0022-1317-76-6-1401. [DOI] [PubMed] [Google Scholar]
- 24.Montone K.T., Hodinka R.L., Salhany K.E., Lavi E., Rostami A., Tomaszewski J.E. Identification of Epstein-Barr virus lytic activity in post-transplantion lymphoproliferative disease. Mod. Pathol. 1996;9:621–630. [PubMed] [Google Scholar]
- 25.Xue S.A., Labrecque L.G., Lu Q.L., Ong S.K., Lampert I.A., Kazembe P., Molyneux E., Broadhead R.L., Borgstein E., Griffin B.E. Promiscuous expression of Epstein-Barr virus in Burkitt’s lymphoma from the central Africian country Malawi. Int. J. Cancer. 2002;99:635–643. doi: 10.1002/ijc.10372. [DOI] [PubMed] [Google Scholar]
- 26.Kroll J., Li S., Levi M., Weinberg A. Lytic and latent EBV gene expression in transplant recipients with and without post-transplant lymphoproliferative disorder. J. Clin. Virol. 2011;52:231–235. doi: 10.1016/j.jcv.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Strong M.J., Xu G., Coco J., Baribault C., Vinay D.S., Lacey M.R., Strong A.L., Lehman T.A., Seddon M.B., Lin L., et al. Differences in gastric carcinoma microenvironment stratify according to EBV infection intensity: Implications for possible immune adjuvant therapy. PLoS Pathog. 2013;9:e1003341. doi: 10.1371/journal.ppat.1003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai M.H., Raykova A., Klinke O., Bernhardt K., Gartner K., Leung C.S., Geletneky K., Sertel S., Munz C., Feederie R., et al. Spontaneous lytic replication and epitheliotropism define an Epstein-Barr Virus strain found in carcinomas. Cell Rep. 2013;5:458–470. doi: 10.1016/j.celrep.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Cao S., Strong M.J., Wang X., Moss W.N., Concha M., Lin Z., O’Grady T., Baddoo M., Fewell C., Renne R., et al. High-throughput RNA sequencing-based virome analysis of 50 lymphoma cell lines from the cancer cell line encyclopedia project. J. Virol. 2015;89:713–729. doi: 10.1128/JVI.02570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tierney R.J., Shannon-Lowe C.D., Fitzsimmons L., Bell A.I., Rowe M. Unexpected patterns of Epstein-Barr virus transcription revealed by a high throughput PCR array for absolute quantification of viral mRNA. Virology. 2015;474:117–130. doi: 10.1016/j.virol.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramayanti O., Juwana H., Verkuijlen S.A.M.W., Adham M., Pegtel M.D., Greijer A.E., Middeldorp J. Epstein-Barr virus mRNA profiles and viral DNA methylation status in nasopharyngeal brushings from nasopharyngeal carcinoma patients reflect tumor origin. Int. J. Cancer. 2017;140:149–162. doi: 10.1002/ijc.30418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolcetti R., Dal Col J., Martorelli D., Carbone A., Klein E. Interplay among viral antigens, cellular pathways and tumor microenvironment in the pathogenesis of EBV-driven lymphomas. Semin. Cancer Biol. 2013;23:441–456. doi: 10.1016/j.semcancer.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Mui U.N., Haley C.T., Tyring S.K. Viral oncology, molecular biology and pathogenesis. J. Clin. Med. 2017;6:111. doi: 10.3390/jcm6120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong G.K., Gulley M.L., Feng W.H., Delecluse H.J., Holley-Guthrie E., Kenney S.C. Epstein-Barr Virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J. Virol. 2005;79:13993–14003. doi: 10.1128/JVI.79.22.13993-14003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma S.D., Hedge S., Young K.H., Sullivan R., Rajesh D., Zhou Y., Jankowska-Gan E., Burlingham W.J., Sun X., Gulley M.L., et al. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J. Virol. 2011;85:165–177. doi: 10.1128/JVI.01512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma S.D., Yu X., Mertz J.E., Gumperz J.E., Reinheim E., Zhou Y., Tang W., Burlingham W.J., Gulley M.L., Kenney S.C. An Epstein-Barr virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J. Virol. 2012;86:7976–7987. doi: 10.1128/JVI.00770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ersing I., Nobre L., Wang L.W., Soday L., Ma Y., Paulo J.A., Narita Y., Ashbaugh C.W., Jiang C., Grayson N.E., et al. A temporal proteomic map of Epstein-Barr virus lytic replication in B cells. Cell Rep. 2017;19:1479–1493. doi: 10.1016/j.celrep.2017.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ariza M.E., Williams M.V. EBV-dUTPase modulates host immune responses potentially altering the tumor microenvironment in EBV-associated malignancies. J. Curr. Res. HIV/AIDS. 2016;2016:1–9. [Google Scholar]
- 39.Zhang Y., Ohyashiki J.H., Takaku T., Shinizu N., Okhyashiki K. Transcriptional profiling of Epstein-Barr virus (EBV) genes and host cellular genes in nasal NK/T-cell lymphoma and chronic EBV infection. Br. J. Cancer. 2006;94:599–608. doi: 10.1038/sj.bjc.6602968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sommer P., Kremmer E., Bier S., Konig S., Zalud P., Zeppezauer M., Jones J.F., Mueller-Lantzsch N., Grasser F.A. Cloning and expression of the Epstein-Barr virus encoded dUTPase: Patients with acute, reactivated or chronic virus infection develop antibodies against the enzyme. J. Gen. Virol. 1996;77:2795–2805. doi: 10.1099/0022-1317-77-11-2795. [DOI] [PubMed] [Google Scholar]
- 41.Nicholls J.M., Sommer P., Kremmer E., Ong K.S., Fung K., Lee J.M.F., Ng M.H., Grasser F.A. A new lytic antibody, 7D6, detects Epstein-Barr virus dUTPase in nonkeratinizing undifferentiated nasopharyngeal carcinomas. Lab. Investig. 1998;78:1031–1032. [PubMed] [Google Scholar]
- 42.Young N.A., Williams M., Jarjour W.N., Bruss M.S., Bolton B., Parikh S., Satoskar A., Ariza M.E. Epstein-Barr virus (EBV) encoded dUTPase exacerbates the immune pathology of lupus nephritis in vivo. Int. J. Immunol. Immunother. 2016;3:023. doi: 10.23937/2378-3672/1410023. [DOI] [Google Scholar]
- 43.Fleischmann J., Kremmer E., Greenspan J.S., Grasser F.A., Niedobitek G. Expression of viral and human dUTPase in Epstein-Barr virus-associated diseases. J. Med. Virol. 2002;68:568–573. doi: 10.1002/jmv.10234. [DOI] [PubMed] [Google Scholar]
- 44.Lerner A.M., Ariza M.E., Williams M.V., Jason L., Beqaj S., Fitzgerald J.T., Lemeshow S., Glaser R. Antibody to Epstein-Barr virus deoxyuridine triphosphate nucleotidohydrolase and deoxyribonucleotide polymerase in a Chronic Fatigue Syndrome subset. PLoS ONE. 2012;7:e47891. doi: 10.1371/journal.pone.0047891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halpin P., Williams M.V., Klimas N.G., Fletcher M.A., Barnes Z., Ariza M.E. Myalgic encephalomyelitis/chronic fatigue syndrome and gulf war illness patients exhibit increased humoral responses to the herpesviruses-encoded dUTPase: Implications in disease pathophysiology. J. Med. Virol. 2017;89:1636–1645. doi: 10.1002/jmv.24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ariza M.E., Glaser R., Kaumaya P.T.P., Jones C., Williams M. The Epstein-Barr Virus (EBV)-encoded dUTPase activates NF-κB through the TLR2 and MyD88-dependent signaling Pathway. J. Immunol. 2009;182:851–859. doi: 10.4049/jimmunol.182.2.851. [DOI] [PubMed] [Google Scholar]
- 47.Glaser R., Litsky M.L., Padget D.A., Baiocchi R.A., Yang E.V., Chen M., Yeh P.E., Green-Church K.B., Caligiuri M.A., Williams M.V. The EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology. 2006;346:205–218. doi: 10.1016/j.virol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 48.Waldman W.J., Williams M.V., Lemeshow S.A., Binkley P., Guttridge D., Kiecolt-Glaser J.K., Knight D.A., Ladner K.J., Glaser R. Epstein-Barr virus encoded dUTPase enhances proinflammatory cytokine production by macrophages in contact with endothelial cells: Evidence for depression-induced atherosclerotic risk. Brain Behav. Immun. 2008;22:215–223. doi: 10.1016/j.bbi.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ariza M.E., Rivailler P., Glaser R., Chen M., Williams M.V. Epstein-Barr virus encoded dUTPase containing exosomes modulate innate and adaptive immune responses in human dendritic cells and peripheral blood mononuclear cells. PLoS ONE. 2013;8:e69827. doi: 10.1371/journal.pone.0069827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ariza M.E., Glaser R., Williams M.V. Human herpesviruses encoded dUTPases: A family of proteins that modulate dendritic cells function and innate immunity. Front. Microbiol. 2014;5:504. doi: 10.3389/fmicb.2014.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams M.V., Cox B., Ariza M.E. Herpesviruses dUTPases: A new family of Pathogen-Associated Molecular Pattern (PAMP) proteins with implications for human disease. Pathogens. 2017;6:2. doi: 10.3390/pathogens6010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang K.P., Hao S.P., Chang J.H., Wu C.C., Tsang N.M., Lee Y., Hsu C.L., Ueng S.H., Liu S.C., Wei P.C., et al. Macrophage inflammatory protein-3α is a novel serum marker for nasopharyngeal carcinoma detection and prediction of treatment outcomes. Clin. Cancer Res. 2008;14:6979–6987. doi: 10.1158/1078-0432.CCR-08-0090. [DOI] [PubMed] [Google Scholar]
- 53.Okudaira T., Yamamoto K., Kawakami H., Uchihara J.N., Tomita M., Masuda M., Matsuda T., Sairenji T., Iha H., Jeang K.T., et al. Transactivation of CCL20 gene by Epstein-Barr virus latent membrane protein 1. Br. J. Haematol. 2006;132:293–302. doi: 10.1111/j.1365-2141.2005.05877.x. [DOI] [PubMed] [Google Scholar]
- 54.Baumforth K.R., Birgersodder A., Reynolds G.M., Wei W., Kapatai G., Flavell J.R., Kalk E., Piper K., Lee S., Machado L., et al. Expression of the Epstein Barr virus-encoded Epstein-Barr nuclear antigen 1 in Hodgkin’s lymphoma cells mediates up-regulation of CCL20 and the migration of regulatory T cells. Am. J. Pathol. 2008;173:195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarbouriech N., Buisson M., Seigneurin J.M., Cusack S., Burmeiste W.P. The monomeric dUTPase from Epstein-Barr virus mimic trimeric dUTPases. Structure. 2005;13:1299–1310. doi: 10.1016/j.str.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Davidson A.J., Stow N.D. New genes from old: Redeployment of dUTPase by herpesviruses. J. Virol. 2005;79:12880–12892. doi: 10.1128/JVI.79.20.12880-12892.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobs-Conrad S., Lee H., DiGeorge A.M., Kaumaya P.T.P. Engineered topographic Determinants with βα, βαβ and βαβα topologies show high affinity binding to native protein antigen (Lactate Dehydrogenase-C4) J. Biol. Chem. 1993;268:25285–25295. [PubMed] [Google Scholar]
- 58.De Sanjose S., Bosch R., Schouten T., Verkuijlen S., Nieters A., Foretova L., Maynadie M., Cocco P.L., Staines A., Becker N., et al. Epstein-Barr virus infection and risk of lymphoma: Immunoblot analysis of antibody responses against EBV-related proteins in a large series of lymphoma subjects and matched controls. Int. J. Cancer. 2007;121:1806–1812. doi: 10.1002/ijc.22857. [DOI] [PubMed] [Google Scholar]
- 59.Cao Y. EBV based cancer prevention and therapy in nasopharyngeal carcinoma. NPJ Precis. Oncol. 2017:1–10. doi: 10.1038/s41698-017-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tosato G., Tanner J., Jones K.D., Revel M., Pike S.E. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J. Virol. 1990;64:3033–3041. doi: 10.1128/jvi.64.6.3033-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scala G., Quinto I., Ruocco M.R., Arcucci A., Mallardo M., Caretto P., Forni G., Venuta S. Expression of an endogenous interleukin 6 gene in human Epstein Barr virus B cell confers growth advantage and in vivo tumorigenicity. J. Exp. Med. 1990;172:61–68. doi: 10.1084/jem.172.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durandy A., Emilie D., Peuchmaur M., Forveille M., Clement C., Wijdenes J., Fischer A. Role of IL-6 in promoting growth of human EBV-induced B-cell tumors in severe combined immunodeficient mice. J. Immunol. 1994;152:3561–3567. [PubMed] [Google Scholar]
- 63.Jones R.J., Seaman W.T., Feng W.H., Barlow E., Dickerson S., Delecluse H.J., Kenney S.C. Roles of lytic viral infection and IL-6 in early versus late passage lymphoblastoid cell lines and EBV-associated lymphoproliferative disease. Int. J. Cancer. 2007;121:1274–1281. doi: 10.1002/ijc.22839. [DOI] [PubMed] [Google Scholar]
- 64.Mashima R. Physiological roles of MIR-155. Immunology. 2015;145:323–333. doi: 10.1111/imm.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan G.W., Visser L., Tan L.P., vanden Berg A., Diepstra A. The microenvironment of Epstein-Barr virus-associated malignancies. Pathogens. 2018;7:40. doi: 10.3390/pathogens7020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrella T., Yaziji N., Colin F., Rifle G., Morlevat F., Arnould L., Fargeot P., Depret O. Implication of the Epstein-Barr virus in the progression of chronic lymphocytic leukaemia/small lymphocytic lymphoma to Hodgkin-like lymphomas. Anticancer Res. 1997;17:3907–3913. [PubMed] [Google Scholar]
- 67.Tsimberidou A.M., Keating M.J., Bueso-Ramos C.E., Kurzrock R. Epstein-Barr virus in patients with chronic lymphocytic leukemia. Leuk. Lymphoma. 2006;47:827–836. doi: 10.1080/10428190500398856. [DOI] [PubMed] [Google Scholar]
- 68.Ferrajoli A., Ivan C., Ciccone M., Shimizu M., Kita Y., Ohtsuka M., D’Abundo L., Qiang J., Lerner S., Nouraee N., et al. Epstein-Barr virus microRNAs are expressed in patients with chronic lymphocytic leukemia and correlate with overall survival. EBioMedicine. 2015;2:572–582. doi: 10.1016/j.ebiom.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grywalska E., Rolinski J., Pasiarski M., Korona-Glowniak I., Maj M., Surdacka A., Grafka A., Stelmach-Goldys A., Zgurski M., Gozdz S., et al. High viral loads of Epstein-Barr virus DNA in peripheral blood of patients with chronic lymphocytic leukemia associated with unfavorable prognosis. PLoS ONE. 2015;10:e0140178. doi: 10.1371/journal.pone.0140178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Visco C., Falisi E., Young K.H., Pascarella M., Perbellini O., Carli G., Novella E., Rossi D., Giaretta I., Cavallini C., et al. Epstein-Barr virus DNA load in chronic lymphocytic leukemia is an independent predictor of clinical course and survival. Oncotarget. 2015;6:18653–18663. doi: 10.18632/oncotarget.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glaser R., Pearson G.R., Bonneau R.H., Esterling B.A., Atkinson C., Kiecolt-Glaser J.K. Stress and the memory T-cell response to the Epstein-Barr virus in healthy medical students. Health Psychol. 1993;12:435–442. doi: 10.1037/0278-6133.12.6.435. [DOI] [PubMed] [Google Scholar]
- 72.Aiello A.E., Simanek A.M., Galea S. Population levels of psychological stress, herpesvirus reactivation and HIV. AIDS Behav. 2010;14:308–317. doi: 10.1007/s10461-008-9358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bennett J.M., Glaser R., Malarkey W.B., Beversdorf D.Q., Peng J., Kiecolt-Glaser J.K. Inflammation and reactivation of latent herpesviruses in older adults. Brain Behav. Immun. 2012;26:739–746. doi: 10.1016/j.bbi.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Christian L.M., Iams J.D., Porter K., Glaser R. Epstein-Barr virus reactivation during pregnancy and postpartum: Effects of race and racial discrimination. Brain Behav. Immun. 2012;26:1280–1287. doi: 10.1016/j.bbi.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laichalk L., Thorley-Lawson D.A. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 2005;79:1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al Tabaa Y., Tuaillon E., Bollore K., Foulongne V., Petitjean G., Seigneurin J.M., Duperray C., Desgranges C., Vendrell J.P., et al. Functional Epstein-Barr virus reservoir in plasma cells derived from infected peripheral blood memory B cells. Blood. 2009;113:604–611. doi: 10.1182/blood-2008-02-136903. [DOI] [PubMed] [Google Scholar]
- 77.Al Tabaa Y., Tuaillon E., Jeziorski E., Ouedraogo D.E., Bolloré K., Rubbo P.A., Foulongne V., Rodiere M., Vendrell J.P. B-cell polyclonal activation and Epstein-Barr viral abortive lytic cycle are two key features in acute infectious mononucleosis. J. Clin. Virol. 2011;52:33–37. doi: 10.1016/j.jcv.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 78.Ariza M.E., Williams M.V. A Human Endogenous Retrovirus K dUTPase Triggers a TH1, TH17 Cytokine Response: Does it play a role in psoriasis? J. Investig. Dermatol. 2011;131:2419–2428. doi: 10.1038/jid.2011.217. [DOI] [PubMed] [Google Scholar]
- 79.Jaffe E.S., Harris N.L., Stein H., Vardiman J. Pathology and Genetics of Human Tumors of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2001. [Google Scholar]