Abstract
The translation of mRNAs plays a critical role in the regulation of gene expression and therefore, in the regulation of cell proliferation, differentiation and apoptosis. Unrestricted initiation of translation causes malignant transformation and plays a key role in the maintenance and progression of cancers. Translation initiation is regulated by the ternary complex and the eukaryotic initiation factor 4F (eIF4F) complex. The p53 tumor suppressor protein is the most well studied mammalian transcription factor that mediates a variety of anti-proliferative processes. Post-transcriptional mechanisms of gene expression in general and those of translation in particular play a major role in shaping the protein composition of the cell. The p53 protein regulates transcription and controls eIF4F, the ternary complex and the synthesis of ribosomal components, including the down-regulation of rRNA genes. In summary, the induction of p53 regulates protein synthesis and translational control to inhibit cell growth.
Keywords: p53, eukaryotic initiation factor 4F complex, ternary complex, translation regulation, the mammalian target of rapamycin, Casein Kinase 2
1. Introduction
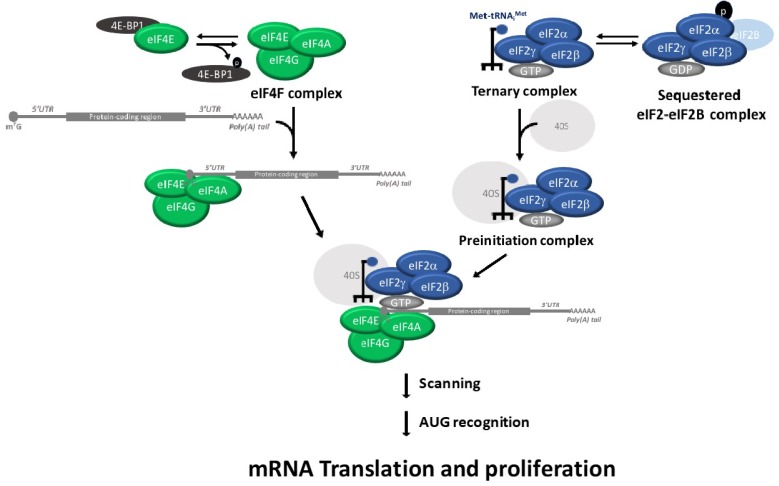
Translation initiation plays a critical role in the regulation of cell proliferation, differentiation and apoptosis (reviewed in [1]). Translation initiation is regulated by the assembly of ternary complex and eIF4F complexes [2]. The eukaryotic Initiation factor 2 (eIF2) and the initiating methionyl-tRNA (Met-tRNAi) form the ternary complex. The ternary complex recruits the 40S ribosomal subunit to form the 43S pre-initiation complex. The 43S pre-initiation complex binds to the mRNA cap with the participation of other translation initiation factors, such as the eIF4F complex. The pre-initiation complex scans the 5′ untranslated region (5′ UTR) of the mRNA for the initiator AUG codon, which is the location where the 60S ribosomal subunit joins to form the 80S ribosome. An important point of translation initiation regulation is the guanosine diphosphate (GDP)/guanosine triphosphate (GTP) exchange of eIF2, which is catalyzed by eIF2B in order to initiation a new round of translation. This GDP–GTP exchange of eIF2 is inhibited when the alpha subunit of eIF2 (eIF2α) is phosphorylated on S51 [3]. The eIF2α phosphorylation results in a reduction in the overall rate of translation initiation [4]. Phosphorylated eIF2α binds with high affinity to the guanine nucleotide exchange factor eIF2B and thereby inhibits the exchange of the GDP for GTP in eIF2 (Figure 1) [5,6,7]. The inhibitory effects of the ternary complex reduce the expression of oncogenic proteins and increases the expression of tumor-suppressor and pro-apoptotic proteins [8,9].
Figure 1.
Scheme showing the eukaryotic translation initiation pathway.
Although most mRNAs are recruited to the ribosome via the recognition of their 5′ end cap structure (m7GpppN, where N can be any nucleotide), a subset of mRNAs can be translated using an internal ribosome entry site (IRES) [2]. As mentioned, the translation initiation is regulated by the ternary complex and eIF4F assembly [10]. The eIF4F is a complex comprised of eIF4A (an RNA helicase), eIF4E (the cap-binding subunit) and eIF4G (a large scaffolding protein for eIF4A, which is called eIF4E). The eIF4F complex binds to the cap structure, unwinds the complex secondary structures of the mRNA 5′ UTRs template and recruits the 43S pre-initiation complex. The eIF4F complex stimulates the recruitment of ribosomes to the mRNA template. eIF4A stimulates translation of both capped and uncapped mRNAs in vitro [10].
A key regulatory step of translation is the initiation stage, which involves the recruitment of ribosomes to the 5′ UTR of the mRNA. The eIF4E-binding proteins (consists of three members: 4E-BP1, 4E-BP2 and 4E-BP3) are repressors of eIF4F. Hypo-phosphorylated 4E-BPs binds to eIF4E and prevents recruitment of the translation machinery to mRNA. In contrast, 4E-BP hyper-phosphorylation causes disruption of the 4E-BP:eIF4E complex and renders eIF4E available for eIF4F formation, which results in an abrogation of eIF4E-binding activity (Figure 1) [11]. Similarly, the tumor suppressor protein programmed cell death protein 4 regulates the protein translation by preventing eIF4A from interacting with eIF4G, leading to translational suppression [12].
The p53 tumor suppressor protein is the most well studied mammalian transcription factor that mediates a variety of anti-proliferative processes [13]. Elucidating the effect of p53 on translation initiation in the context of cell cycle regulation is essential in understanding the role that mutations or deregulation of p53 play in cancer biology. The post-transcriptional mechanisms of gene expression in general, especially those of translation, play a major role in shaping the protein composition of the cell [14]. The p53 protein regulates transcription and also controls ribosome biogenesis and eukaryotic initiation factors. In this review article, we examine the role of p53 as a regulator of the ternary complex, the eIF4F complex and translation ribosome biogenesis.
2. p53 Restricts the Ribosome Biogenesis
Ribosomes are responsible for transferring the information contained in mRNAs to proteins. Ribosome biogenesis takes place in the nucleolus [15,16]. The ribosomal DNA (rDNA) genes are organized at the nucleolar organizer regions [17,18]. The human 60S ribosome subunit is a complex molecule composed of three ribosomal RNA (5S, 5.8S and 28S rRNA) and 47 distinct ribosomal proteins (RPs) [19]. This complex is responsible for peptide bond formation and quality control of nascent peptides [20]. The human 40S ribosome subunit, which is responsible for unwinding and scanning mRNAs, is also a complex molecule composed of one strand of 18S ribosomal RNA (rRNA) and 33 distinct RPs. Both experimental models and clinical studies indicate that disturbances in the ribosomal biosynthesis and/or protein synthesis have been shown to play a major role in tumorigenesis [21,22,23].
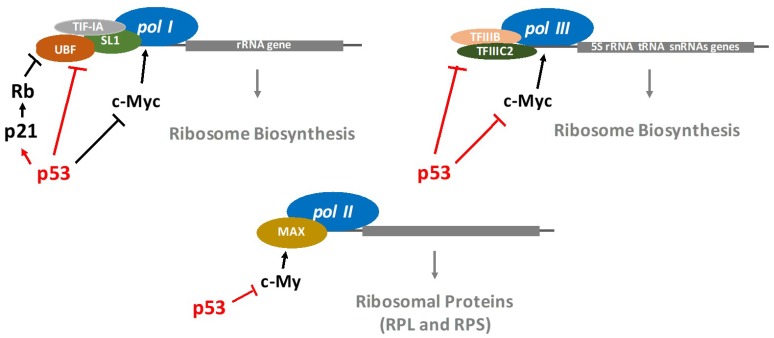
It is well established that p53 restricts the ribosome biogenesis. Indeed, the regulation of RNA polymerases (pol) by p53 has been extensively studied. RNA pol I synthesizes ribosomal RNAs (except for 5S rRNA) [24]. RNA pol II synthesizes mRNA precursors as well as most small nuclear RNA and microRNAs, while RNA pol III manufactures transfer RNA (tRNAs), the 5S rRNA as well as other small RNAs involved in RNA processing and transport. The p53-mediated RNA pol I transcriptional repression involves p53 interfering with a set of proteins required for the assembly and initiation of transcriptional machinery on the rRNA promoter [25]. Interestingly, Zhai et al. demonstrated that p53-mediated growth suppression occurred by repressing rRNA gene transcription [26]. Specifically, p53 directly binds to the TATA (the Hogness box)-binding protein (TBP) and TBP-associated factors, disrupting their interaction with upstream binding factors and thereby repressing RNA pol I transcription. It has also been reported that p53 is able to activate the transcription of RNA pol I and II through repression of c-Myc expression [27] (Figure 2). A novel, selective RNA pol I transcription inhibitor, CX-5461 (Cylene Pharmaceuticals/Senhwa Biosciences), promotes cancer-specific activation of p53 and apoptosis in malignant B cells [28], which is an interesting development in the search for new anti-cancer therapies.
Figure 2.
Key mechanism by which p53 inhibits RNA polymerases.
Furthermore, p53 inhibits RNA pol III activity, which generates tRNAs, the 5S rRNA as well as other small RNAs involved in RNA processing and transport. Indeed, the wild-type p53 directly interacts with the TBP-containing general factor (TFIIIB), which prevents the attachment of RNA pol III onto DNA [29,30,31] (Figure 3).
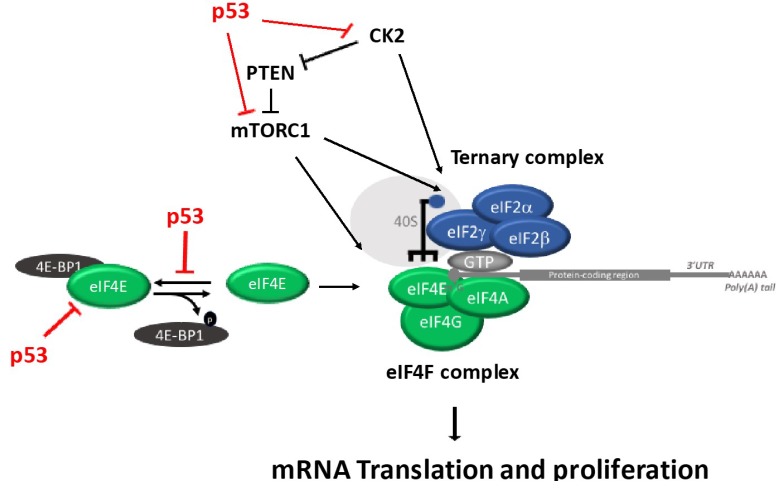
Figure 3.
Schematic diagram outlining the regulation of eIF4F and Ternary Complex and the different steps known to be modulated by p53.
3. p53 Regulates the Transcription of RP Genes
Newly synthesized ribosomal proteins (RPs) are imported into the nucleolus from the cytosol. In response to nucleolar stress, several RPs (RPL5, RPL11, RPL23, RPS3, RPS7) as well as 5.8S and 5S rRNA translocate from the nucleolus to the nucleoplasm. This subset of nucleolar elements binds to and inhibits the activity of murine double minute 2 (MDM2), resulting in p53-mediated cell cycle arrest and apoptosis [32]. Cancer cells that harbor mutant p53 (R248W) leads to an up-regulation of L37, P1 and S2 expression [33]. Interestingly, in response to DNA damage, the ribosomal protein RPL26 binds to both 5′-UTR and 3′-UTR of p53 mRNA, enhancing p53 translation and leading to p53-dependent and MDM2-independent cell cycle arrest [34,35]. Upon genotoxic stress, p53 directly induces the expression of the ribosomal protein S27-like (RPS27L), thus positively regulating p21 protein expression. This ultimately leads to p21-mediated cell cycle arrest [36,37].
p53 not only regulates rRNA transcription, but also controls the processing of pre-rRNAs. Fibrillarin (FBL) is a nucleolar protein vital for methylation and processing of pre-rRNAs [38]. Marcel et al. demonstrated that p53 inhibits FBL expression levels. The p53-dependent FBL inhibition leads to a high translational fidelity (i.e., nonsense suppression or amino acid misincorporation) and increases the initiation of internal ribosome entry site (IRES)-dependent translation [39]. Understanding the role of p53 in pre-RNAs processing has the potential to identify future therapeutic targets on the ribosome population with altered rRNA methylation patterns.
4. p53 Regulates Ternary Complex and eIF4F Assembly Regulation
It remains largely unknown how the two major regulatory branches that regulate translation, ternary complex and eIF4F complex are coordinated. Interestingly, Gandin et al. demonstrated a coordinated regulation of eIF2α and eIF4E via CK2 and mTORC1 [40]. The mTOR (a phosphatidylinositol 3-kinase) forms functionally distinct complexes, including: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). The mTORC1 regulates mRNA translation, protein synthesis and cell growth, while mTORC2 regulates cellular metabolism, survival and the cytoskeletal organization. Protein kinase casein kinase 2 (CK2) belongs to the serine/threonine protein kinase family and consists of two catalytic (alpha and/or alpha’) subunits and two regulatory (beta) subunits [41,42]. CK2 regulates key cell growth and survival pathways, including translation regulation and DNA damage response pathways [42].
A 50% reduction of global protein synthesis was determined in vitro utilizing thermosensitive murine erythroleukemic cells carrying wild-type p53 [43]. Specifically, it was demonstrated that the inhibition of the ribosomal protein S6 kinase (p70S6k), which is the upstream kinase of 4E-BP1, by p53 strongly attenuated the global protein synthesis [43].
Recent data showed that TRIM22, a p53 target gene, inhibits the binding of eIF4E to eIF4G [44]. Furthermore, p53 activation causes dephosphorylation and cleavage of the initiation factor eIF4GI and the eIF4E-binding protein 4E-BP1 [45,46]. Interestingly, Petroulakis et al. demonstrated that the p53 controls the 4E-BP-dependent senescence and transformation. Specifically, p53-deficient mice have an increased risk of tumorigenesis in the absence of both 4E-BP1 and 4E-BP2. Conversely, primary fibroblasts expressing p53, but missing 4E-BPs, undergo premature senescence and are resistant to transformation. These findings indicate that the combined effect of absence of 4E-BPs and p53 loss synergistically enhanced cell proliferation and tumorigenesis, which provides new insights into anticancer drug therapy [47]. p53 inhibits protein synthesis through directly inhibiting the expression of eIF4E [48] and inhibiting the eIF4F complex assembly by enhancing the de-phosphorylation of 4E-BP1 [45]. Although p53 does not directly interact with components of the ternary complex, it controls translation by interacting with several components of the eIF4F complex. Specifically, Rahman and colleagues showed that p53 is not able to control the ternary complex pathway through PKR-eIF2α activation [49]. Instead, the p53 inhibits mTOR signaling [50] and CK2 protein kinase activity (Figure 3).
The eukaryotic translation factor 5A (eIF5A) promotes the elongation of translation [51]. Posttranslational hypusine modification of eIF5A by deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase (DOHH) regulates eIF5A activity [51]. Preukschas et al. demonstrated that targeting eIF5A with a specific DHS-inhibitor has an antiproliferative effect in glioblastoma cell lines, while sparing the normal human astrocytes. Furthermore, targeting eIF5A results in p53-mediated premature senescence in glioblastoma cell lines [52].
As mentioned, CK2 and mTORC1 coordinate the activation of the ternary complex and elF4F complex. The phosphorylation of elF2B by CK2 increases the ternary complex assembly, which also up-regulates 4E-BP phosphorylation through mTORC1. It has been shown that there is phosphorylation of p53 in a highly-conserved residue (S392) by CK2 [53,54]. Catrogiovanni and colleagues showed that the phosphorylation at residue S392 regulates p53 mitochondrial translocation and transcription-independent apoptosis after cell exposure to genotoxic agents. Furthermore, the regulatory beta subunit of CK2 can interact with p53 and this interaction reduces the DNA binding activity and the transactivation function of p53 [55,56]. Conversely, the wild-type p53 interacts with and inhibits CK2 protein kinase activity [57]. As described above, the role of CK2 and mTORC1 are major regulators of translation [40]. The exact mechanism by which the interaction between CK2, mTOR and p53 regulates translation awaits further studies.
5. Genome-Wide Analysis
To investigate the effects of p53 on the genome-wide translational regulation, Zaccara et al. used the polysome profiling technique [58]. The authors identified about 340 genes whose translation is regulated by p53 [59]. Loayza-Puch et al. combined RNA sequencing and ribosomal profiling analyses in order to methodically discover transcriptional and translational control in cells under the following conditions: quiescence, senescence, normal proliferation and neoplastic transformation. The authors demonstrated that the global repression of protein synthesis is mediated by p53 activation and consequent mTOR inhibition. The authors conclude that transcriptional regulation mediates cell-cycle arrest, while the global translation inhibition impacts cell growth [60].
6. Conclusions
p53 is associated with numerous signaling pathways, which are involved in the regulation of cellular responses to stress. Its effect on growth arrest or programmed cell death is context-dependent: both in terms of cellular type and of physiological state. It is therefore not surprising that translation regulation is critically affected by p53. A better understanding of these intricate pathways and the complex interplay between p53 and various signaling pathways that regulate translation and ribosome synthesis could open up novel strategies for cancer diagnosis, prevention and p53-based therapies.
Acknowledgments
This study was supported by the NCI of the NIH under award numbers SC1 CA2005 (M.S.). We apologize to those whose work we were not able to include due to size constraints.
Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- 1.Chu J., Pelletier J. Therapeutic Opportunities in Eukaryotic Translation. Cold Spring Harb. Perspect. Biol. 2018 doi: 10.1101/cshperspect.a032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinnebusch A.G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 2014;83:779–812. doi: 10.1146/annurev-biochem-060713-035802. [DOI] [PubMed] [Google Scholar]
- 3.Pain V.M. Initiation of protein synthesis in eukaryotic cells. Eur. J. Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 4.Brostrom C.O., Chin K.V., Wong W.L., Cade C., Brostrom M.A. Inhibition of translational initiation in eukaryotic cells by calcium ionophore. J. Biol. Chem. 1989;264:1644–1649. [PubMed] [Google Scholar]
- 5.Aktas H., Fluckiger R., Acosta J.A., Savage J.M., Palakurthi S.S., Halperin J.A. Depletion of intracellular Ca2+ stores, phosphorylation of eIF2alpha, and sustained inhibition of translation initiation mediate the anticancer effects of clotrimazole. Proc. Natl. Acad. Sci. USA. 1998;95:8280–8285. doi: 10.1073/pnas.95.14.8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benzaquen L.R., Brugnara C., Byers H.R., Gatton-Celli S., Halperin J.A. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat. Med. 1995;1:534–540. doi: 10.1038/nm0695-534. [DOI] [PubMed] [Google Scholar]
- 7.Voigts-Hoffmann F., Klinge S., Ban N. Structural insights into eukaryotic ribosomes and the initiation of translation. Curr. Opin. Struct. Biol. 2012;22:768–777. doi: 10.1016/j.sbi.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Bjornsti M.A., Houghton P.J. Lost in translation: Dysregulation of cap-dependent translation and cancer. Cancer Cell. 2004;5:519–523. doi: 10.1016/j.ccr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J. Biol. Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 10.Malka-Mahieu H., Newman M., Desaubry L., Robert C., Vagner S. Molecular Pathways: The eIF4F Translation Initiation Complex-New Opportunities for Cancer Treatment. Clin. Cancer Res. 2017;23:21–25. doi: 10.1158/1078-0432.CCR-14-2362. [DOI] [PubMed] [Google Scholar]
- 11.Gingras A.C., Raught B., Gygi S.P., Niedzwiecka A., Miron M., Burley S.K., Polakiewicz R.D., Wyslouch-Cieszynska A., Aebersold R., Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H.S., Cho M.H., Zakowicz H., Hegamyer G., Sonenberg N., Colburn N.H. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol. Cell. Biol. 2004;24:3894–3906. doi: 10.1128/MCB.24.9.3894-3906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kastenhuber E.R., Lowe S.W. Putting p53 in Context. Cell. 2017;170:1062–1078. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux P.P., Topisirovic I. Signaling pathways involved in the regulation of mRNA translation. Mol. Cell. Biol. 2018 doi: 10.1128/MCB.00070-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenner L., Melnikov S., de Loubresse N.G., Ben-Shem A., Iskakova M., Urzhumtsev A., Meskauskas A., Dinman J., Yusupova G., Yusupov M. Crystal structure of the 80S yeast ribosome. Curr. Opin. Struct. Biol. 2012;22:759–767. doi: 10.1016/j.sbi.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Anger A.M., Armache J.P., Berninghausen O., Habeck M., Subklewe M., Wilson D.N., Beckmann R. Structures of the human and Drosophila 80S ribosome. Nature. 2013;497:80–85. doi: 10.1038/nature12104. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons J.G., Branco A.T., Godinho S.A., Yu S., Lemos B. Concerted copy number variation balances ribosomal DNA dosage in human and mouse genomes. Proc. Natl. Acad. Sci. USA. 2015;112:2485–2490. doi: 10.1073/pnas.1416878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stults D.M., Killen M.W., Pierce H.H., Pierce A.J. Genomic architecture and inheritance of human ribosomal RNA gene clusters. Genome Res. 2008;18:13–18. doi: 10.1101/gr.6858507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentilella A., Kozma S.C., Thomas G. A liaison between mTOR signaling, ribosome biogenesis and cancer. Biochim. Biophys. Acta. 2015;1849:812–820. doi: 10.1016/j.bbagrm.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen P.S., Park J., Qin Y., Li X., Parsawar K., Larson M.H., Cox J., Cheng Y., Lambowitz A.M., Weissman J.S., et al. Protein synthesis. Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science. 2015;347:75–78. doi: 10.1126/science.1259724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robichaud N., Sonenberg N. Translational control and the cancer cell response to stress. Curr. Opin. Cell Biol. 2017;45:102–109. doi: 10.1016/j.ceb.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Quin J.E., Devlin J.R., Cameron D., Hannan K.M., Pearson R.B., Hannan R.D. Targeting the nucleolus for cancer intervention. Biochim. Biophys. Acta. 2014;1842:802–816. doi: 10.1016/j.bbadis.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Sulima S.O., Hofman I.J.F., De Keersmaecker K., Dinman J.D. How Ribosomes Translate Cancer. Cancer Discov. 2017;7:1069–1087. doi: 10.1158/2159-8290.CD-17-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cramer P., Armache K.J., Baumli S., Benkert S., Brueckner F., Buchen C., Damsma G.E., Dengl S., Geiger S.R., Jasiak A.J., et al. Structure of eukaryotic RNA polymerases. Annu. Rev. Biophys. 2008;37:337–352. doi: 10.1146/annurev.biophys.37.032807.130008. [DOI] [PubMed] [Google Scholar]
- 25.Budde A., Grummt I. p53 represses ribosomal gene transcription. Oncogene. 1999;18:1119–1124. doi: 10.1038/sj.onc.1202402. [DOI] [PubMed] [Google Scholar]
- 26.Zhai W., Comai L. Repression of RNA polymerase I transcription by the tumor suppressor p53. Mol. Cell. Biol. 2000;20:5930–5938. doi: 10.1128/MCB.20.16.5930-5938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho J.S., Ma W., Mao D.Y., Benchimol S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol. Cell. Biol. 2005;25:7423–7431. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bywater M.J., Poortinga G., Sanij E., Hein N., Peck A., Cullinane C., Wall M., Cluse L., Drygin D., Anderes K., et al. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22:51–65. doi: 10.1016/j.ccr.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cairns C.A., White R.J. p53 is a general repressor of RNA polymerase III transcription. EMBO J. 1998;17:3112–3123. doi: 10.1093/emboj/17.11.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chesnokov I., Chu W.M., Botchan M.R., Schmid C.W. p53 inhibits RNA polymerase III-directed transcription in a promoter-dependent manner. Mol. Cell. Biol. 1996;16:7084–7088. doi: 10.1128/MCB.16.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein T., Crighton D., Boyle J.M., Varley J.M., White R.J. RNA polymerase III transcription can be derepressed by oncogenes or mutations that compromise p53 function in tumours and Li-Fraumeni syndrome. Oncogene. 2002;21:2961–2970. doi: 10.1038/sj.onc.1205372. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., Wang W., Wang H., Wang M.H., Xu W., Zhang R. Identification of ribosomal protein S25 (RPS25)-MDM2-p53 regulatory feedback loop. Oncogene. 2013;32:2782–2791. doi: 10.1038/onc.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loging W.T., Reisman D. Elevated expression of ribosomal protein genes L37, RPP-1, and S2 in the presence of mutant p53. Cancer Epidemiol. Biomark. Prev. 1999;8:1011–1016. [PubMed] [Google Scholar]
- 34.Takagi M., Absalon M.J., McLure K.G., Kastan M.B. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 35.Chen J., Guo K., Kastan M.B. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J. Biol. Chem. 2012;287:16467–16476. doi: 10.1074/jbc.M112.349274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J., Tan J., Zhuang L., Banerjee B., Yang X., Chau J.F.L., Lee P.L., Hande M.P., Li B., Yu Q. Ribosomal protein S27-like, a p53-inducible modulator of cell fate in response to genotoxic stress. Cancer Res. 2007;67:11317–11326. doi: 10.1158/0008-5472.CAN-07-1088. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y., Tan M., Liu X., Xiong X., Sun Y. Inactivation of ribosomal protein S27-like confers radiosensitivity via the Mdm2-p53 and Mdm2-MRN-ATM axes. Cell Death Dis. 2018;9:145. doi: 10.1038/s41419-017-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shishova K.V., Khodarovich Y.M., Lavrentyeva E.A., Zatsepina O.V. High-resolution microscopy of active ribosomal genes and key members of the rRNA processing machinery inside nucleolus-like bodies of fully-grown mouse oocytes. Exp. Cell Res. 2015;337:208–218. doi: 10.1016/j.yexcr.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Marcel V., Ghayad S.E., Belin S., Therizols G., Morel A.P., Solano-Gonzàlez E., Vendrell J.A., Hacot S., Mertani H.C., Albaret M.A., et al. p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell. 2013;24:318–330. doi: 10.1016/j.ccr.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandin V., Masvidal L., Cargnello M., Gyenis L., McLaughlan S., Cai Y., Tenkerian C., Morita M., Balanathan P., Jean-Jean O. mTORC1 and CK2 coordinate ternary and eIF4F complex assembly. Nat. Commun. 2016;7:11127. doi: 10.1038/ncomms11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Litchfield D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003;369:1–15. doi: 10.1042/bj20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruzzene M., Pinna L.A. Addiction to protein kinase CK2: A common denominator of diverse cancer cells? Biochim. Biophys. Acta. 2010;1804:499–504. doi: 10.1016/j.bbapap.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 43.Horton L.E., Bushell M., Barth-Baus D., Tilleray V.J., Clemens M.J., Hensold J.O. p53 activation results in rapid dephosphorylation of the eIF4E-binding protein 4E-BP1, inhibition of ribosomal protein S6 kinase and inhibition of translation initiation. Oncogene. 2002;21:5325–5334. doi: 10.1038/sj.onc.1205662. [DOI] [PubMed] [Google Scholar]
- 44.Petersson J., Ageberg M., Sanden C., Olofsson T., Gullberg U., Drott K. The p53 target gene TRIM22 directly or indirectly interacts with the translation initiation factor eIF4E and inhibits the binding of eIF4E to eIF4G. Biol. Cell. 2012;104:462–475. doi: 10.1111/boc.201100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Constantinou C., Clemens M.J. Regulation of the phosphorylation and integrity of protein synthesis initiation factor eIF4GI and the translational repressor 4E-BP1 by p53. Oncogene. 2005;24:4839–4850. doi: 10.1038/sj.onc.1208648. [DOI] [PubMed] [Google Scholar]
- 46.Constantinou C., Clemens M.J. Regulation of translation factors eIF4GI and 4E-BP1 during recovery of protein synthesis from inhibition by p53. Cell Death Differ. 2007;14:576–585. doi: 10.1038/sj.cdd.4402045. [DOI] [PubMed] [Google Scholar]
- 47.Petroulakis E., Parsyan A., Dowling R.J., LeBacquer O., Martineau Y., Bidinosti M., Larsson O., Alain T., Rong L., Mamane Y., et al. p53-dependent translational control of senescence and transformation via 4E-BPs. Cancer Cell. 2009;16:439–446. doi: 10.1016/j.ccr.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 48.Nathan C.O., Sanders K., Abreo F.W., Nassar R., Glass J. Correlation of p53 and the proto-oncogene eIF4E in larynx cancers: Prognostic implications. Cancer Res. 2000;60:3599–3604. [PubMed] [Google Scholar]
- 49.Rahman M., Lem C., Muaddi H., Koromilas A.E. PKR is not a universal target of tumor suppressor p53 in response to genotoxic stress. Cell Cycle. 2009;8:3606–3607. doi: 10.4161/cc.8.21.9848. [DOI] [PubMed] [Google Scholar]
- 50.Budanov A.V., Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dever T.E., Dinman J.D., Green R. Translation Elongation and Recoding in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2018 doi: 10.1101/cshperspect.a032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preukschas M., Hagel C., Schulte A., Weber K., Lamszus K., Sievert H., Pällmann N., Bokemeyer C., Hauber J., Braig M., et al. Expression of eukaryotic initiation factor 5A and hypusine forming enzymes in glioblastoma patient samples: Implications for new targeted therapies. PLoS ONE. 2012;7:e43468. doi: 10.1371/journal.pone.0043468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herrmann C.P., Kraiss S., Montenarh M. Association of casein kinase II with immunopurified p53. Oncogene. 1991;6:877–884. [PubMed] [Google Scholar]
- 54.Meek D.W., Simon S., Kikkawa U., Eckhart W. The p53 tumour suppressor protein is phosphorylated at serine 389 by casein kinase, II. EMBO J. 1990;9:3253–3260. doi: 10.1002/j.1460-2075.1990.tb07524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prowald A., Schuster N., Montenarh M. Regulation of the DNA binding of p53 by its interaction with protein kinase CK2. FEBS Lett. 1997;408:99–104. doi: 10.1016/S0014-5793(97)00399-2. [DOI] [PubMed] [Google Scholar]
- 56.Schuster N., Prowald A., Schneider E., Scheidtmann K.H., Montenarh M. Regulation of p53 mediated transactivation by the beta-subunit of protein kinase CK2. FEBS Lett. 1999;447:160–166. doi: 10.1016/S0014-5793(99)00273-2. [DOI] [PubMed] [Google Scholar]
- 57.Schuster N., GoÈtz C., Faust M., Schneider E., Prowald A., Jungbluth A., Montenarh M. Wild-type p53 inhibits protein kinase CK2 activity. J. Cell. Biochem. 2001;81:172–183. doi: 10.1002/1097-4644(20010401)81:1<172::AID-JCB1033>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 58.Ingolia N.T., Ghaemmaghami S., Newman J.R., Weissman J.S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaccara S., Tebaldi T., Pederiva C., Ciribilli Y., Bisio A., Inga A. p53-directed translational control can shape and expand the universe of p53 target genes. Cell Death Differ. 2014;21:1522–1534. doi: 10.1038/cdd.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loayza-Puch F., Drost J., Rooijers K., Lopes R., Elkon R., Agami R. p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol. 2013;14:R32. doi: 10.1186/gb-2013-14-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]