Abstract
DICER1 syndrome is a rare genetic disorder that predisposes individuals to multiple cancer types. Through mutations of the gene encoding the endoribonuclease, Dicer, DICER1 syndrome disrupts the biogenesis and processing of miRNAs with subsequent disruption in control of gene expression. Since the first description of DICER1 syndrome, case reports have documented novel germline mutations of the DICER1 gene in patients with cancers as well as second site mutations that alter the function of the Dicer protein expressed. Here, we present a review of mutations in the DICER1 gene, the respective protein sequence changes, and clinical manifestations of DICER1 syndrome. Directions for future research are discussed.
Keywords: DICER1 syndrome, DICER1 germline mutations, miRNA, rare genetic disorder, cancer
1. Introduction
DICER1 syndrome, or pleuropulmonary blastoma familial tumor susceptibility syndrome (ORPHA: 284343; OMIM: 601200), is a rare genetic disorder predisposing individual to the development of tumors, both benign and malignant [1,2,3]. Recently, mutations documented in endocrine tumors (thyroid, parathyroid, pituitary, pineal gland, endocrine pancreas, paragangliomas, medullary, adrenocortical, ovarian, and testicular tumors) have been reviewed [4]. One copy of the altered gene is sufficient to cause an increased risk of developing tumors; however, many individuals who carry a mutation in the DICER1 gene do not develop abnormal growths. Patients may acquire a second mutation during tumorigenesis that has the potential to affect the catalytic activity of the enzyme. The prevalence of DICER1 syndrome is currently unknown and the full spectrum of clinical manifestation may not yet be fully defined [2,5,6]. However, documented cases of DICER1 syndrome have been linked to pleuropulmonary blastomas, cystic nephroma, rhabdomyosarcoma, multinodular goiter, thyroid cancer, ovarian Sertoli–Leydig cell tumors, and other neoplasias [7,8,9]. DICER1 syndrome may also include neuroblastoma [10].
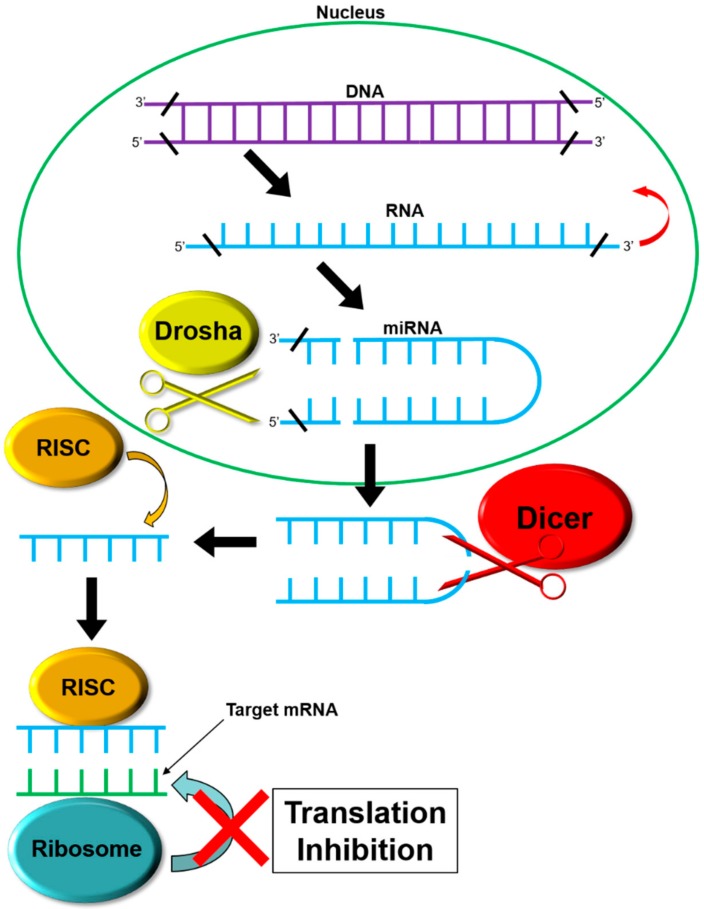
The DICER1 gene, located on chromosome 14, position q32.13, encodes the endoribonuclease Dicer protein of the ribonuclease III family. Discovered in 2001 by Bernstein, the Dicer endoribonuclease plays a role in protein translational control [11]. MicroRNAs (miRNAs) are created by the Dicer endoribonuclease protein [12,13,14]. Dicer is a component of the RNA-induced silencing complex (RISC) loading complex (RLC), which is composed of Dicer, Argonaute-2 (AGO2), and trans-activation-responsive RNA binding protein 2 (TARBP2). Dicer carries out its function downstream of DROSHA, a nuclear homolog of Dicer. miRNAs are produced from hairpin-folded pre-miRNAs that are approximately 60 nucleotides long. The pre-miRNAs are exported to the cytoplasm by exportin 5, where they are then processed by Dicer into the ~22 nt double stranded RNAs (dsRNAs). dsRNAs are loaded into the Argonaut family member protein by the RLC. The resulting RISC asymmetrically processes the ~22 nt RNA at specific 3′-overhang and 5′-phosphate cleavage [15]. Each miRNA binds to specific mRNAs, inhibiting ribosomal access and subsequent translation to control gene expression [16] (see Figure 1). miRNA-mediated effects that lead to DICER1 syndrome may be due to loss-of-function in genes that normally contribute to the prevention of cancer (tumor suppressors), or alternatively, changes that lead to gain of function in genes that contribute to the onset of cancer in an active manner (oncogenes) [17]. Dysregulation of miRNA production is related to several tumor types [18]. Mutations in the DICER1 gene are found in approximately 50–70% of pleuropulmonary blastoma patients [1].
Figure 1.
miRNA Production Pathway. DNA sequences are transcribed into RNA sequences that form a ‘hairpin’ structure of precursor miRNA. Drosha, a nucleic endoribonuclease, cleaves the hairpin from the primary RNA strand. Transported out of the nucleus by exportin 5, they are further processed by Dicer. After processing by Dicer and its accessory proteins, the hairpin structure of the precursor miRNA is degraded, leaving a single, linear piece of miRNA (the opposing piece is degraded by intracellular processes). This single piece is then bound by the RNA-induced silencing complex RISC. The RISC-miRNA complex binds to target mRNA strands, inhibiting translation by the ribosome.
Dicer plays additional diverse roles other than miRNA regulation [19]. Dicer plays a role in the processing of rRNA and, indirectly, ribosome biogenesis prior to export from the nucleus. In addition, Dicer plays a role in DNA processing and apoptosis [20]. Upon initiation of apoptosis, Dicer initiates the breakdown of chromosomal DNA, a key step in controlled cell death.
2. DICER1 Germline Mutations
Research into the causal factors and mechanisms of DICER1 syndrome has been represented in the scientific literature since 2009. Generation and dissemination of new knowledge about DICER1 syndrome is increasing as demonstrated by the increasing number of publications each year over the past decade. Mutations that lead to DICER1 syndrome are reviewed here.
Pathogenic germline mutations of the DICER1 gene linked to DICER1 syndrome are included in this review. Case studies provided detailed information regarding the germline mutation, patient(s), and physical manifestation. Mosaic DICER1 mutations have also been associated with DICER1 syndrome [21], in addition to the inherited germline mutations. Cases represented in the PubMed database [22] were identified using keywords “DICER1 syndrome”, “DICER1 mutations”, and “Pleuropulmonary Blastoma Familial Tumor Susceptibility Syndrome”. A total of 244 articles were collected since the discovery of DICER1 syndrome, and 36 were selected that represented DICER1 pathogenic germline mutations. Articles were excluded from this review if they did not address germline mutations in the DICER1 gene, if they did not identify novel mutations, or if detailed documentation was absent from the report.
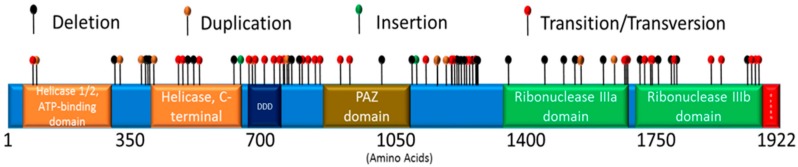
Eighty-eight DICER1 mutations are included in this review. While the majority of identified mutations are located within regions that encode one of Dicer’s seven defined domains (helicase domains, the Dicer dimerization domain (DDD), the Piwi/Argonaute, Zwille (PAZ) domain, the RNase III domains, and the double-stranded RNA-binding domain), some mutations were found to lie outside these domains (see Figure 2). Mutations, resulting protein changes, patient information, and background, clinical manifestation, and references are presented in Tables 1–3.
Figure 2.
DICER1 Pathogenic Germline Mutations. Mutations reported in DICER1 include deletions, duplications, insertions, transitions, or transversions. The DICER1 gene encodes 1922 amino acids, arranged into specific domains including the helicase 1/2, ATP-binding domain, the helicase, C-terminal domain, the Dicer dimerization domain (DDD), the PAZ domain (PAZ), the ribonuclease IIIa domain, and the ribonuclease IIIb domain. (see Tables 1–3).
3. Manifestations of DICER1 Gene Mutations
While the range of clinical symptoms associated with DICER1 syndrome is varied, some symptoms are prevalent among patients. These include multinodular goiter, pleuropulmonary blastoma, cystic nephroma, and ovarian Sertoli–Leydig Cell Tumor.
3.1. Multinodular Goiter (MNG)
Multinodular goiters, abnormal, cancerous growths of the thyroid gland, are associated with DICER1 syndrome and are a subsection of thyroid growths, which have been reported as a prevalent manifestation of DICER1 syndrome. Seventy-five percent of women and 17% of men with DICER1 syndrome were shown to harbor abnormal thyroid growths, multinodular goiters included, compared to the control population: 8% and 0% for women and men, respectively [23]. Specifically, a recent study indicated a correlation between truncating germline DICER1 mutations and familial multinodular goiter, among other cancers [24]. Women are more likely to develop thyroid cancer than men, regardless of DICER1 variant status [25]. Frequent malignant neoplasms of the endocrine system [26] such as thyroid cancers are linked to both environmental and genetic factors, with studies indicating similar links between multinodular goiters and both environmental and genetic factors [27]. DICER1 mutations and subsequent global downregulation of miRNAs were found in multinodular goiter [28].
3.2. Pleuropulmonary Blastoma
Pleuropulmonary blastoma is a manifestation of DICER1 syndrome [29]. Primarily observed in children, it is a rare cancer that originates in the pleural cavity or the lungs [30]. Although rare, many cases of pleuropulmonary blastoma have been identified to be associated with DICER1 syndrome. First described in 1988 [31], cases are now documented in an international registry.
Four types of pleuropulmonary blastomas have been characterized. Type I is defined by cystic growths, has malignant potential, and may undergo malignant transformation in childhood. Type Ir is very similar to Type I, being defined by cystic growths, but contains no cancerous cells and is therefore not malignant. Type II consists of a hybrid of cystic and cancerous tumors, and type III solely consists of solid cancerous tumors. Types II and III have been associated with increased metastasis, primarily to the brain [32], and were found to be more aggressive than type I pleuropulmonary blastomas [33] (See Table 1).
Table 1.
Pathogenic germline mutations in the DICER1 gene related to Pleuropulmonary Blastoma.
| Mutation Type | Chromosomal Mutation | Protein Change | Clinical Manifestation | Reference |
|---|---|---|---|---|
| dup | c.1196_1197dupAG | p.Trp400Serfs*59 | 4-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| tran | c.1376+1G>A | p.splice | 13-year old female, peritoneal cysts of right & left round ligaments, nasal polyps, Sertoli–Leydig cell tumor. History: 5 years, type II pleuropulmonary blastoma, 8 years, thyroid nodules. | Schultz, 2016 [34] |
| tran | c.1507G>T | p.Glu503* | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.1684_1685delAT | p.Met562Valfs*11 | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.1716delT | p.Phe572Leufs*15 | 0.8-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| dup | c.1910dupA | p.Tyr637* | 5-month old female, pleuropulmonary blastoma, and cervical embryonal rhabdomyosarcoma; pleuropulmonary blastoma & embryonal rhabdomyosarcoma. | Hill, 2009 [1]; Doros, 2012 [35] |
| tran | c.1966C>T | p.Arg656* | pleuropulmonary blastoma; 7-year old, pleuropulmonary blastoma. | Slade, 2011 [7] Hill, 2009 [1] |
| tran | c.2040+1G>T | p.splice | 10-year female, nasal chondromesenchymal hamartoma. History: Pleuropulmonary blastoma. | Stewart, 2014 [36] |
| dup | c.2245_2248dupTACC | p.Pro750Leufs*12 | pleuropulmonary blastoma. | Hill, 2009 [1] |
| tran | c.2247C>A | p.Tyr749* | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.2268_2271delTTTG | p.Cys756* | 0.9-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| tran | c.2379T>G | p.Tyr793* | 11.5-years male, bilateral papillary thyroid carcinoma in follicular adenoma. History: 32 months, type II pleuropulmonary blastoma and cystic nephroma. | de Kock, 2014 [37] |
| dup | c.2392dupA | p.Thr798Asnfs*33 | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.2399delG | p.Arg800fs*5 | 3.5-year old and 13-year old, Wilms’ tumor. | Palculict, 2016 [38] |
| tran | c.2830C>T | p.Arg944* | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.2863delA | p.Thr955fs | 7-year old male, nasal chondromesenchymal hamartoma. History: pleuropulmonary blastoma. | Stewart, 2014 [36] |
| tran | c.3019C>T | p.Gln1007* | 27-year old woman, nasal chondromesenchymal hamartoma and pleuropulmonary blastoma. History: multinodular goiter. | Stewart, 2014 [36] |
| del | c.3505delT | p.Ser1169Glnfs*23 | 3-year-old, Pleuropulmonary blastoma. | Slade, 2011 [7] |
| dup | c.3505dupT | p.Ser1169Phefs*8 | 7-year old female, thyroid goiter, multiple nodules on both lobes. History: 4.3 years, pleuropulmonary blastoma in left back musculature. 23 months, type II pleuropulmonary blastoma. | de Kock, 2014 [37] |
| tran | c.3540C>A | p.Tyr1180* | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.3583_3584delGA | N/A | 6-year-old, intraocular medulloepithelioma. History: pleuropulmonary blastoma. | Slade, 2011 [7] |
| del | c.3665delT | p.Leu1222Tyrfs*17 | 4.2-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| tran | c.3726C>A | p.Tyr1242* | 4-year old, pleuropulmonary blastoma; 27-year old female, pleuropulmonary blastoma. History: 13 years, Sertoli–Leydig cell tumor and multinodular goiter, 21 years, nasal chondromesenchymal hamartoma. | Slade, 2011 [7]; Stewart, 2014 [36] |
| del | c.4309_4312delGACT | p.Asp1437Metfs*16 | 8-year old female, embryonal rhabdomyosarcoma. History: 4 years, pleuropulmonary blastoma; Median Age 34 months, female, cystic nephroma, pleuropulmonary blastoma. | Doros, 2012 [35]; Bahubeshi, 2010 [39] |
| del | c.4403_4406delCTCT | p.Ser1468Phefs*21 | 1.5-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| del | c.4407_4410delTTCT | p.Leu1469fs | 11-year old male, nasal chondromesenchymal hamartoma. History: pleuropulmonary blastoma. | Stewart, 2014 [36] |
| del | c.4555delG | p.Glu1519Lysfs*41 | 3-year old female, Polish, type II pleuropulmonary blastoma. | de Kock, 2013 [40] |
| tran | c.4616C>T | p.Thr1539Met | 11-year-old male, Hodgkin lymphoma, pleuropulmonary blastoma Type I. History: thyroid cysts, syringomyelia. | Kuhlen, 2016 [41] |
| tran | c.4748T>G | p.Leu1583Arg | pleuropulmonary blastoma. | Hill, 2009 [1] |
| tran | c.5104C>T | p.Gln1702* | 9-year old female, pleuropulmonary blastoma & ERMS. | Doros, 2012 [35] |
| del | c.5221_5232delAACAACACCATC | p.Asn1741_1744del | 9-year old male, multinodular goiter, pleuropulmonary blastoma. History: 20 months, cystic nephroma. | Rath, 2014 [42] |
| del | c.5299delC | premature stop in exon 24 | 11-year-old male, Hodgkin lymphoma, pleuropulmonary blastoma Type I. History: thyroid cysts, syringomyelia. | Kuhlen, 2016 [41] |
| tran | c.5387C>T | p.Gln1783* | 14-month old female, type I pleuropulmonary blastoma. History: cystic nephroma. | Fernandez-Martinez, 2017 [43] |
| tran | c.5465A>T | p.Asp1822Val | 1.8-year-old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| tran | c.5477C>A | p.Ser1826* | Median Age 34 months, female, cystic nephroma, pleuropulmonary blastoma. | Bahubeshi, 2010 [39] |
del—deletion, dup—duplication, tran—transversion/transition.
3.3. Cystic Nephroma
Cystic nephromas have been reported in approximately 12% of children with pleuropulmonary blastomas or those with a family member with cystic nephroma [44,45]. The frequency of DICER1 germline mutations in cystic nephroma patients was found to be 73.2% [46,47].
Cystic nephromas, multilocular cystic nephroma, and cystic renal hamartoma [48] are benign lesions in the kidney [49]. Common symptoms include hematuria, flank pain, and increased abdominal mass [50]. Enucleation of characteristic cysts can be associated with recurrence [51]. Treatment includes radical nephrectomy to prevent renal cell carcinoma [48].
A recent study of a patient with a cystic nephroma diagnosed as a multicystic left renal tumor was diagnosed with germline and somatic mutations in the DICER1 gene [52]. Additionally, a nascent anaplastic sarcoma of the kidney (ASK) was reported within a cystic nephroma, associated with the presence of a germline DICER1 mutation, or alternatively due to a somatic mutation [44,53].
3.4. Sertoli–Leydig Cell Tumor
Ovarian sex cord-stromal tumors have been associated with DICER1 mutations [54,55], specifically, ovarian Sertoli–Leydig cell tumors. While rare among all ovarian neoplasms (<0.5%) [56], a recent study indicated that 57% of individuals with ovarian Sertoli–Leydig cell tumors also harbored DICER1 germline mutations [46]. Another study confirmed this, indicating that more than 60% of ovarian Sertoli–Leydig cell tumors diagnosed harbored DICER1 mutations within the RNase III domains [57]. A more recent study examined 34 Sertoli–Leydig cell tumors, with 88% containing one-or-more DICER1 mutations [58].
Ovarian Sertoli–Leydig cell tumors are composed of several cell types, including Sertoli cells and Leydig cells [59]. They are responsible for an increase in testosterone production [60], and can lead to masculinization, voice deepening, and acne [61]. Interestingly, a recent study noted that the simultaneous occurrence of Sertoli–Leydig cell tumor and thyroid carcinoma is a reliable indicator of DICER1 syndrome [62] (See Table 2).
Table 2.
Pathogenic germline mutations in the DICER1 gene related to Sertoli–Leydig Cell Tumor.
| Mutation Type | Chromosomal Mutation | Protein Change | Clinical Manifestation | Reference |
|---|---|---|---|---|
| tran | c.325C>T | p.Gln109* | 11-year old female, multinodular goiter. History: Sertoli–Leydig cell tumor. | Canfarotta M, 2016 [5] |
| del | c.876_879delAAAG | p.Arg293Ilefs*4 | 18-year old female, Sertoli–Leydig cell tumor. History: 16 years, multinodular goiter. | Rio Frio, 2011 [8] |
| tran | c.1376+1G>A | p.splice | 13-year old female, peritoneal cysts of right & left round ligaments, nasal polyps, Sertoli–Leydig cell tumor. History: 5 years, type II pleuropulmonary blastoma, 8 years, thyroid nodules. | Schultz, 2016 [34] |
| del | c.1532_1533delAT | N/A | 28-year old female, Sertoli–Leydig cell tumor. History: None | 16-year-old, Sertoli–Leydig cell tumor. | Oost, 2015 [63] |
| tran | c.2457C>G | p.Ile813_Tyr819del | 32-year old female, Sertoli–Leydig cell tumor. History: 18 years, multinodular goiter. | Rio Frio, 2011 [8] |
| del/ins | c.3270-6_4051—1280delinsG | p.Tyr1091Ser*28 | 14-year old female, multinodular goiter. History: Sertoli–Leydig cell tumor, primitive neuroectodermal tumor. | Sabbaghian, 2013 [64] |
| tran | c.3540C>A | p.Tyr1180* | 16-year old female, Sertoli–Leydig cell tumor. History: 14 years, bilateral multinodular goiter. 16-year old female, ovarian Sertoli–Leydig cell tumor, and lung lesion. History: 14 years, multinodular goiter. |
de Kock, 2016 [65] Wu, 2014 [66] |
| tran | c.3647C>A | p.Ser1216* | 13-year old female, Danish, multinodular goiter and Sertoli–Leydig cell tumor. | Rossing, 2014 [67] |
| tran | c.3649T>A | p.Tyr1217Asn | 13-year old female, Danish, multinodular goiter and Sertoli–Leydig cell tumor. | Rossing, 2014 [67] |
| tran | c.3726C>A | p.Tyr1242* | 27-year old female, pleuropulmonary blastoma. History: 13 years, Sertoli–Leydig cell tumor and multinodular goiter, 21 years, nasal chondromesenchymal hamartoma. | Stewart, 2014 [36] |
| del | c.4050+1delG | p.Val351Valfs*11 | 20-year old female, primitive neuroectodermal tumor & multinodular goiter. History: 9 years, Sertoli–Leydig cell tumor. | Foulkes, 2011 [68] |
| del | c.5018_5021delTCAA | p.Ile1673Thrfs*31 | 32-year old female, Sertoli–Leydig cell tumor. History: 18 years, multinodular goiter. | Rio Frio, 2011 [8] |
| del | c.5122_5128delGGAGATG | p.Gly1708Argfs*7 | 21-year old, Sertoli–Leydig cell tumor. History: 17 years, Sertoli–Leydig cell tumor. | Slade, 2011 [7] |
del—deletion, dup—duplication, tran—transversion/transition.
4. Additional Symptoms and Presentations Related to DICER1 Syndrome
4.1. Hodgkin Lymphoma
DICER1 syndrome includes novel symptoms which may facilitate early diagnosis. A rare form of Hodgkin lymphoma was diagnosed in an 11-year old boy with DICER1 syndrome in 2016 [41]. The patient had two DICER1 mutations (c.5299delC and c.4616C>T), and several of his family members shared these mutations. Prior to this diagnosis, Hodgkin lymphoma had not been linked to DICER1 syndrome; additionally, this form of Hodgkin lymphoma is considered rare. Most Hodgkin and Reed–Sternberg cells arise from mature B cells, but a rare subset of cells arise from T cells. In this patient, the cells were found to be of the T-cell lineage, indicating a unique symptom. All affected family members developed at least one type of tumor with differing origins [5].
4.2. Pineoblastoma
Pineoblastoma may be associated with a DICER1 mutation. Individuals with pineoblastomas were tested by de Kock and colleagues for the presence of DICER1 mutations. They suggested that germline DICER1 mutations make a clinically significant contribution to pineoblastoma; however, additional studies may confirm a causal relationship [69]. Additionally, the study of a single patient implicated a DICER1 germline mutation in a pineoblastoma. The mutation was found to be heterozygous for germline but hemizygous in the tumor itself [70]. Further studies may determine the relationship between DICER1 mutations and pineoblastomas (See Table 3).
Table 3.
Pathogenic germline mutations in the DICER1 gene related to cystic nephroma, pineoblastomas, Wilms’ tumor, multinodular goiter, medulloblastoma, rhabdomyosarcoma, pituitary blastoma, endometrial cancer, and seminoma.
| Mutation Type | Chromosomal Mutation | Protein Change | Clinical Manifestation | Reference |
|---|---|---|---|---|
| dup | c.328_338dupGTGTCAGCTGT | p.Arg114Cysfs*18 | 3-year old, cystic nephroma. | Slade, 2011 [7] |
| dup | c.912_919dupAGACTGTC | p.Arg307Glnfs*8 | 4-year old male, Wilms’ tumor. | Foulkes, 2011 [68] |
| del | c.1128_1132delAGTAA | p.Lys376Asnfs*11 | Pineoblastomas. | Sabbaghian, 2012 [70] |
| del | c.1153delC | p.Arg385Alafs*73 | 13-year old, Medulloblastoma/infratentorial primitive neuroectodermal tumor. | Slade, 2011 [7] |
| dup | c.1196_1197dupAG | p.Trp400Serfs*59 | 16-year old female, Ashkenazi Jewish/Anglo-Saxon, fibroadenoma of the breast. History: 6 years, ovarian embryonal rhabdomyosarcoma, 11 years, radiologic focal nodular liver hyperplasia, 12 years, cystic nephroma, 13 years, multinodular goiter. | de Kock, 2015 [71] |
| del | c.1284delGA | N/A | 23-month old female, pituitary blastoma | de Kock, 2014 [72] |
| dup | c.1306dupT | p.Ser436Phefs*41 | 2-year old male, Wlims’ tumor. | Foulkes, 2011 [68] |
| tran | c.1525C>T | p.Arg509* | 12-year old female, multinodular goiter. History: 6 years, dermoid cyst. | Darrat, 2013 [28] |
| tran | c.1966C>T | p.Arg656* | 15-month old female, Pulmonary sequestration & cystic nephroma. 14-year old female, Belarusian-Serbian, hepatic focal nodular hyperplasia. History: Right Brain ventricle tumor (part teratoma, party embryonic carcinoma) at 8 months old. pilomatrixoma at 3 years, Renal cysts at 4 years, thyroid nodules at 10 years, basal cell carcinoma at 13 years. |
Foulkes, 2011 [68] Mehraein, 2016 [73] |
| tran | c.2026C>T | N/A | 17-year old female, pituitary blastoma. | de Kock, 2014 [72] |
| tran | c.2062C>T | p.Arg688* | 8 year, a 9-month-old girl, anaplastic sarcoma of the kidney. History: pneumothorax, left upper lung cyst and left renal cyst at 10 months. cysts multiplied and increased in size over next few years. | Wu, 2016 [74] |
| tran | c.2117-1G>A | p.Gly706Aspsfs*8 | 10-year old female, multinodular goiter. History: 5 years, Wilms’ tumor. | Foulkes, 2011 [68] |
| tran | c.2247C>A | p.Tyr749* | 6-week old male, embryonal rhabdomyosarcoma. | Doros, 2012 [35] |
| del | c.2399delG | p.Arg800fs*5 | 3.5-year old and 13-year old, Wilms’ tumor. | Palculict, 2016 [38] |
| tran | c.2407G>A | p.Gly803Arg | The average age of 44 months, Wilms’ tumor. | Palculict, 2016 [38] |
| del | c.2450delC | p.Pro817Leufs*15 | 7-month old female, Polish, multiseptated cystic mass in abdomen (early anaplastic sarcoma). | Wu, 2016 [52] |
| tran | c.2455T>C | p.Tyr819His | 34 & 32-year old male family members, hepatocellular tumors. | Caruso, 2016 [75] |
| tran | c.2457C>G | p.Ile813_Tyr819del | 53-year-old female, cERMS. History: multinodular goiter. | de Kock, 2015 [76] |
| tran | c.2516C>T | p.Ser839Phe | 15-year old female, multinodular goiter. | Rio Frio, 2011 [8] |
| tran | c.2805-1G>T | p.Tyr936_Arg996del | The patient died at 20 years from alveolar rhabdomyosarcoma. History: multinodular goiter. | Rio Frio, 2011 [8] |
| del | c.3046delA | p.Ser1016Valfs*1065 | 12-month old female, pituitary blastoma. | Sahakitrungruang, 2014 [77] |
| tran | c.2379T>G | N/A | 3-year old male, pituitary blastoma. | de Kock, 2014 [72] |
| del | c.3277_3280delAACT | N/A | 7-year old female, pituitary blastoma. | de Kock, 2014 [72] |
| ins | c.3288_3289insTTTC | p.Gly1097Phefs*8 | 1.5-year old, cystic nephroma. | Slade, 2011 [7] |
| tran | c.3334A>G | p.Asn1112Asp | 55-year old female, endometrial cancer. | Yang, 2015 [78] |
| dup | c.3405dupA | p.Gly1136Arg | 12-year old female, renal cysts & focal nodular hyperplasia of the liver. History: 6 months, eRMS of the bladder and a cystic lesion in the lung at. 3 & 4.5 years, Ciliary body medulloepithelioma. | Fremerey, 2016 [79] |
| del | c.3535_3538delTCTT | p.Ser1179Thrfs*12 | 13-year old female, cervical sarcoma botryoides. | Tomiak, 2014 [80] |
| tran | c.3540C>G | p.Tyr1180 | 2-year old female, a multilocular cyst in left kidney, 2 cystic lesions in the lung, multicystic nephroma extended from left kidney. | Bardon-Cancho, 2016 [81] |
| del | c.3611_3616delACTACAinsT | p.Tyr1204Leufs*29 | 14-year old female, cERMS and thyroid goiter. | Foulkes, 2011 [68] |
| dup | c.3665dupT | p.Leu1222fs*13 | 30–39 year old female, soft tissue sarcoma | de Kock, 2017 [9] |
| del | c.3793delA | p.Thr1265Glnfs*37 | 6-year-old, ovarian sex cord stromal tumour. | Slade, 2011 [7] |
| del | c.3907_3908delCT | p.Leu1303Valfs*4 | 13-year old female, cervical embryonal rhabdomyosarcoma & two small lung cysts. History: 11 years, multinodular goiter. | Foulkes, 2011 [68] |
| del | c.4309_4312delGACT | N/A | Male, deceased 8 months post-surgery, pituitary blastoma. | de Kock, 2014 [72] |
| dup | c.4566_4579dupCTTTG | p.Val1524fs*38 | 14-month old female, neuroblastoma & cystic nephroma, multinodular goiter at age 7. | Saskin, 2017 [10] |
| tran | c.4740G>T | p.Gln1580His | 32-year old, seminoma. | Slade, 2011 [7] |
| tran | c.5096-12G>A | N/A | 10-year old female, undifferentiated sarcoma at ovary. | de Kock, 2017 [9] |
| tran | c.5125G>C(de novo) | N/A | 21-month old male, pituitary blastoma. | de Kock, 2014 [72] |
| del | c.5221_5232delAACAACACCATC | p.Asn1741_1744del | 9-year old male, multinodular goiter, pleuropulmonary blastoma. History: 20 months, cystic nephroma. | Rath, 2014 [42] |
| del/ins | c.5426_5442 del GGGATATTTTTGAGTCGinsCA | p.Gly1809_Ser1814delinsAla | 15-year old female, thyroid follicular carcinoma. History: ASK for 12 years & multiple cystic-appearing thyroid nodules, no malignancy. | Yoshida, 2017 [82] |
| tran | c.5441C>T | p.Ser1814Leu | 12.5-year-old female, ovarian tumor. History: 12 years, multinodular goiter. | Wu, 2016 [83] |
del—deletion, dup—duplication, tran—transversion/transition.
4.3. Global Developmental Delay, Lung Cysts, Overgrowth, and Wilms Tumor (GLOW)
Documented by Klein and colleagues, symptoms include Global developmental delay, Lung cysts, Overgrowth, and Wilms tumor (GLOW). These symptoms were identified in patients with DICER1 mutations in the RNase IIIb domain of Dicer [84]. Mutations were associated with Lung cysts and Wilms tumors, but also developmental delays and overgrowth, marked by large body size and mass. While both mutations reported were de novo missense mutations, the symptoms were similar to that of other patients diagnosed with germline mutations in similar loci within the DICER1 gene. The current understanding of GLOW syndrome is limited, and more inquiry is required to determine how widespread GLOW syndrome is in relation to DICER1 syndrome.
4.4. Macrocephaly
A recently conducted study indicated that macrocephaly is associated with DICER1 syndrome [85]. Further studies are needed to confirm the link between macrocephaly and DICER1 syndrome, as this may help to identify individuals with DICER1 syndrome at an early stage.
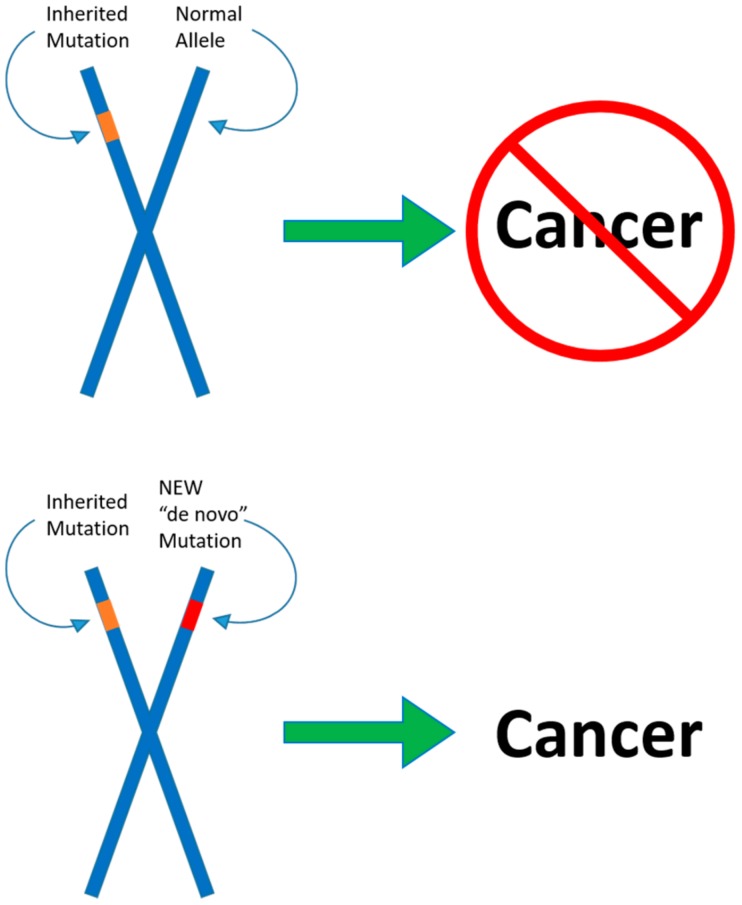
5. Molecular Mechanisms of DICER1 Mutations—The Two-Hit Hypothesis
The two-hit hypothesis, also known as the Knudson hypothesis, was originally suggested in 1953, and formally postulated by Knudson in 1971 [86,87]. The hypothesis suggests that, for abnormalities to arise, an individual requires two separate mutations in each allele, and that only one mutation in a single allele is not sufficient to induce the formation of tumors. Generally, a single mutation is inherited, which by itself is relatively harmless. However, a second mutation may act in tandem with the first to give rise to cancer. In a number of cases, the two-hit hypothesis describes the mechanism by which tumor suppressor gene deactivation occurs [88,89].
DICER1 syndrome has been recognized as an autosomal-dominant disease, inherited and expressed in a haploinsufficient manner [1,5]. This proposed mechanism has been substantiated by a number of cases of individuals with DICER1 syndrome. Specifically, these cases involved individuals with only one apparent germline mutation, and symptoms characteristic of DICER1 syndrome. However, recent studies have indicated that patients with DICER1 syndrome have not only inherited mutations in one allele of the DICER1 gene but also acquired a somatic mutation in the second allele of the DICER1 gene. The two-hit hypothesis applies to DICER1 mutations and the role of Dicer as a tumor suppressor gene (see Figure 3).
Figure 3.
The two-hit hypothesis. One germline mutation in a DICER1 allele predisposes the individual to an increased risk for benign and malignant tumors. A second somatic mutation in the other allele arising during tumorigenesis may lead to malignant rare cancers. While the first mutation by itself is overtly harmless, it only acts in tandem with the second to induce cancerous formation, according to the hypothesis.
Second-hit, somatic mutations have been found in the RNase IIIb domain of the DICER1 gene. A study involving three children with Wilms’ tumor suggested that the two-hit hypothesis applied to DICER1 syndrome, in the formation of Wilms’ tumor [90]. The patients were found to harbor germline DICER1 mutations, and upon screening for somatic DICER1 mutations, somatic mutations in the RNase IIIb domain on the second allele were found. Additionally, this finding highlights DICER1 somatic gene mutations occurring in Wilms Tumor patients in the RNase IIIb domain. The regions of the gene encoding the RNase III domains are genetic hotspots for somatic mutations within the DICER1 gene [91,92].
Biallelic DICER1 mutations are common in pleuropulmonary blastomas, with the second mutation occurring within the RNase IIIb domain [93]. A study on 11 pleuropulmonary blastoma patients revealed that, out of 11 patients with DICER1 gene mutations with sporadic pleuropulmonary blastomas, eight harbored biallelic DICER1 gene mutations in which one of the mutations was within the RNase IIIb domain.
A recent report of biallelic DICER1 mutations in an ovarian fibrosarcoma from a 9-year-old patient demonstrated a germline single base insertion in the DICER1 gene, causing a frameshift and premature stop codon as well as a second point mutation within the tumor that resulted in a substitution at amino acid position 1813 within the RNase IIIb domain (p.E1813G) [94].
Another case study involved a 14-month-old female patient diagnosed with pleuropulmonary blastoma and a previously removed cystic nephroma at 11 months [43]. Mutation analysis was conducted on available tissue and peripheral blood, revealing both alleles of the DICER1 gene to be compromised. A missense heterozygous somatic mutation (c.5425G>A; p.G1809R) was detected in the DNA obtained from the cystic nephroma in addition to the germline truncating mutation detected from peripheral blood (c.5347C>T; p.Q1783*) within exon 24, which encodes the RNase IIIb domain. This germline mutation was confirmed in the patient’s mother and grandmother. In addition, the patient’s 21-year-old female cousin was found to also harbor the germline mutation and had previously been treated for embryonal rhabdomyosarcoma at age 14 and multimodal goiter at age 20. A unique missense heterozygous somatic mutation was detected in the embryonal rhabdomyosarcoma (c.5428G>C; p.D1810H) from a cousin of the patient. This case demonstrates that biallelic mutations in DICER1 alleles, rather than haploinsufficiency, contribute to the mechanism of DICER1 syndrome.
Brenneman and colleagues [95] discussed the concept of biallelic mutations of the DICER1 gene, with a focus on second, somatic, hot-spot mutations in the RNase IIIb domain. A cohort of individuals diagnosed with pleuropulmonary blastoma underwent analysis to determine the mutation status of DICER1 alleles. Mutations within the RNase IIIb domain may represent hotspot mutations [96], and may be the rate-limiting step in the pathogenesis of DICER1 syndrome. Loss of function germline mutations in one allele of DICER1 was found to be common among patients, and the RNase IIIb hotspot mutations were less common, but more frequently found within the tumor. RNase IIIb mutations may also predispose patients to additional mutations due to the role of Dicer in DNA replication and repair.
6. Future Directions
The breadth of knowledge regarding DICER1 syndrome continues to grow since its recent discovery, but promising treatments and management options need further investigation. As Dicer deficiency stems from DICER1 mutations, experiments on Dicer in model animals have attempted to determine viable methods of upregulation of Dicer and related proteins to combat the effects of DICER1 syndrome. One such study conducted by Blandino and colleagues treated diabetic mice with metformin, reducing the incidence of cancer [97]. Mice treated with metformin showed reduced tumor growth and an upregulation of miRNAs and an increase in DICER1 gene expression. A similar, recent experiment confirmed these results, as metformin was found to induce higher Dicer levels in mouse models and human patients by altering the localization of AUF1, a DICER1 mRNA binding protein that down-regulates DICER1, leading to increased DICER1 mRNA stability [98,99]. While the treatments described in these experiments may not be beneficial to patients with biallelic DICER1 mutations, those with a single, functional DICER1 allele might benefit from metformin and compounds similar to it, as this single allele could hypothetically be targeted and upregulated to achieve increased Dicer protein production.
DICER1 germline mutations have been identified as nonsense mutations, leading to stop codons within the coding sequence and truncated proteins or nonsense-mediated RNA degradation. While these mutations lead to cancerous and non-cancerous tumors, certain antibiotic treatments have been shown to promote the read-through of similar premature stop-codons, leading to restored transcription and translation of otherwise unreadable sequences [100]. One study demonstrated this novel approach by treating human cells containing known nonsense mutations with Ataluren, a pharmaceutical drug utilized in treating genetic disorders. Ataluren successfully promoted the read-through of all three nonsense codons within the mutated alleles [101]. A more recent study built upon these findings by modifying the fluorine number and position of Ataluren, which led to the increased read-through ability of stop codons in nonsense mutations [102]. While no research has been conducted on DICER1 nonsense mutations utilizing drugs such as Ataluren, this drug and synonymous compounds may prove beneficial in treating patients with DICER1 syndrome stemming from nonsense mutations similar to those already tested. While treatments for pleuropulmonary blastoma and cystic nephroma have received wide attention from the scientific community, the root of these abnormal formations, DICER1 germline mutations and acquired somatic mutations, require more study.
7. Conclusions
The mechanisms of DICER1 syndrome and its presentation in patients have become more fully understood over the past several years as more cases are diagnosed with novel mutations and presentations discovered and documented, extending the phenotypic range of this disorder. Successful treatments for DICER1 syndrome will require a combination of basic, translational, and clinical research. The outcome of the inaugural International DICER1 Symposium was the development of consensus testing and surveillance as well as treatment recommendations. Recommendations for genetic testing—including intronic sequencing [103]; whole exome sequencing [104]; or screening products such as the ThyroSeq, which is a DNA- and RNA-based next-generation sequencing assay that analyzes 112 genes for genetic alterations, including point mutations, insertions/deletions, gene fusions, and copy number alterations [105], prenatal management—and surveillance for DICER1-associated symptoms have been developed and described in recent publications [106,107,108].
Acknowledgments
Authors would like to dedicate this manuscript to Kennedy C., her family, and other pediatric patients diagnosed with DICER1 syndrome. Authors acknowledge support from the Department of Biological Sciences at Boise State University.
Abbreviations
| AGO2 | argonaute-2 |
| AUF1 | heterogeneous nuclear ribonucleoprotein D |
| DDD | Dicer dimerization domain |
| GLOW | Global developmental delay, Lung cysts, Overgrowth, and Wilms tumor |
| miRNAs | MicroRNA |
| PAZ | Piwi/Argonaute, Zwille domain |
| RISC | RNA-induced silencing complex |
| RLC | RNA-induced silencing complex (RISC) loading complex |
| RNase IIIb | ribonuclease IIIb |
| rRNA | ribosomal RNA |
| TARBP2 | trans-activation responsive RNA binding protein 2 |
Funding
This research was funded by Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health grant numbers P20GM103408 and P20GM109095.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hill D.A., Ivanovich J., Priest J.R., Gurnett C.A., Dehner L.P., Desruisseau D., Jarzembowski J.A., Wikenheiser-Brokamp K.A., Suarez B.K., Whelan A.J., et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orphanet: Pleuropulmonary Blastoma Familial Tumor Susceptibility Syndrome. [(accessed on 19 April 2017)]; Available online: http://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=64742.
- 3.DICER1 Syndrome|Children’s Hospital of Philadelphia. [(accessed on 19 April 2017)]; Available online: http://www.chop.edu/conditions-diseases/dicer1-syndrome.
- 4.Solarski M., Rotondo F., Foulkes W.D., Priest J.R., Syro L.V., Butz H., Cusimano M.D., Kovacs K. DICER1 gene mutations in endocrine tumors. Endocr. Relat. Cancer. 2018;25:R197–R208. doi: 10.1530/ERC-17-0509. [DOI] [PubMed] [Google Scholar]
- 5.Canfarotta M., Riba-Wolman R., Orsey A.D., Balarezo F., Finck C. DICER1 syndrome and thyroid disease. J. Pediatr. Surg. Case Rep. 2016;11:31–34. doi: 10.1016/j.epsc.2016.05.014. [DOI] [Google Scholar]
- 6.DICER1-Related Pleuropulmonary Blastoma Cancer Predisposition Syndrome|Genetic and Rare Diseases Information Center (GARD)—An NCATS Program. [(accessed on 19 April 2017)]; Available online: https://rarediseases.info.nih.gov/diseases/10734/dicer1-related-pleuropulmonary-blastoma-cancer-predisposition-syndrome.
- 7.Slade I., Bacchelli C., Davies H., Murray A., Abbaszadeh F., Hanks S., Barfoot R., Burke A., Chisholm J., Hewitt M., et al. DICER1 syndrome: Clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J. Med. Genet. 2011;48:273–278. doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- 8.Rio Frio T., Bahubeshi A., Kanellopoulou C., Hamel N., Niedziela M., Sabbaghian N., Pouchet C., Gilbert L., O’Brien P.K., Serfas K., et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA. 2011;305:68–77. doi: 10.1001/jama.2010.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Kock L., Rivera B., Revil T., Thorner P., Goudie C., Bouron-Dal Soglio D., Choong C.S., Priest J.R., van Diest P.J., Tanboon J., et al. Sequencing of DICER1 in sarcomas identifies biallelic somatic DICER1 mutations in an adult-onset embryonal rhabdomyosarcoma. Br. J. Cancer. 2017;116:1621–1626. doi: 10.1038/bjc.2017.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saskin A., de Kock L., Sabbaghian N., Apellaniz-Ruiz M., Bozkurt C., Bouron-Dal Soglio D., Foulkes W.D. A case of neuroblastoma in DICER1 syndrome: Chance finding or noncanonical causation? Pediatr. Blood Cancer. 2018;65:e26715. doi: 10.1002/pbc.26715. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein E., Caudy A.A., Hammond S.M., Hannon G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 12.Murray M.J., Bailey S., Raby K.L., Saini H.K., de Kock L., Burke G.A.A., Foulkes W.D., Enright A.J., Coleman N., Tischkowitz M. Serum levels of mature microRNAs in DICER1-mutated pleuropulmonary blastoma. Oncogenesis. 2014;3:e87. doi: 10.1038/oncsis.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 14.Wightman B., Ha I., Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 15.Hammond S.M. Dicing and slicing. FEBS Lett. 2005;579:5822–5829. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 16.Catalanotto C., Cogoni C., Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016;17:1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansson M.D., Lund A.H. MicroRNA and cancer. Mol. Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kian R., Moradi S., Ghorbian S. Role of components of microRNA machinery in carcinogenesis. Exp. Oncol. 2018;40:2–9. [PubMed] [Google Scholar]
- 19.Song M., Rossi J.J. Molecular mechanisms of DICER: Endonuclease and enzymatic activity. Biochem. J. 2017;474:1603–1618. doi: 10.1042/BCJ20160759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matskevich A.A., Moelling K. Stimuli-dependent cleavage of DICER during apoptosis. Biochem. J. 2008;412:527–534. doi: 10.1042/BJ20071461. [DOI] [PubMed] [Google Scholar]
- 21.De Kock L., Wang Y.C., Revil T., Badescu D., Rivera B., Sabbaghian N., Wu M., Weber E., Sandoval C., Hopman S.M.J., et al. High-sensitivity sequencing reveals multi-organ somatic mosaicism causing DICER1 syndrome. J. Med. Genet. 2016;53:43–52. doi: 10.1136/jmedgenet-2015-103428. [DOI] [PubMed] [Google Scholar]
- 22.Home-PubMed-NCBI. [(accessed on 19 April 2017)]; Available online: https://www.ncbi.nlm.nih.gov/pubmed.
- 23.Khan N.E., Bauer A.J., Schultz K.A.P., Doros L., Decastro R.M., Ling A., Lodish M.B., Harney L.A., Kase R.G., Carr A.G., et al. Quantification of Thyroid Cancer and Multinodular Goiter Risk in the DICER1 Syndrome: A Family-Based Cohort Study. J. Clin. Endocrinol. Metab. 2017;102:1614–1622. doi: 10.1210/jc.2016-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apellaniz-Ruiz M., de Kock L., Sabbaghian N., Guaraldi F., Ghizzoni L., Beccuti G., Foulkes W.D. Familial multinodular goiter and Sertoli-Leydig cell tumors associated with a large intragenic in-frame DICER1 deletion. Eur. J. Endocrinol. 2018;178:K11–K19. doi: 10.1530/EJE-17-0904. [DOI] [PubMed] [Google Scholar]
- 25.Cancer of the Thyroid—Cancer Stat Facts. [(accessed on 19 April 2017)]; Available online: https://seer.cancer.gov/statfacts/html/thyro.html.
- 26.Zarkesh M., Zadeh-Vakili A., Azizi F., Foroughi F., Akhavan M.M., Hedayati M. Altered Epigenetic Mechanisms in Thyroid Cancer Subtypes. Mol. Diagn. Ther. 2017;22:41–46. doi: 10.1007/s40291-017-0303-y. [DOI] [PubMed] [Google Scholar]
- 27.Knudsen N., Laurberg P., Perrild H., Bülow I., Ovesen L., Jørgensen T. Risk Factors for Goiter and Thyroid Nodules. Thyroid. 2002;12:879–888. doi: 10.1089/105072502761016502. [DOI] [PubMed] [Google Scholar]
- 28.Darrat I., Bedoyan J.K., Chen M., Schuette J.L., Lesperance M.M. Novel DICER1 mutation as cause of multinodular goiter in children. Head Neck. 2013 doi: 10.1002/hed.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Kock L., Bah I., Brunet J., Druker H., Astigarraga I., Bosch-Barrera J., Soglio D.B.-D., Nguyen V.-H., Malkin D., Priest J.R., et al. Somatic DICER1 mutations in adult-onset pulmonary blastoma. Eur. Respir. J. 2016;47:1879–1882. doi: 10.1183/13993003.00172-2016. [DOI] [PubMed] [Google Scholar]
- 30.Dishop M.K., Kuruvilla S. Primary and metastatic lung tumors in the pediatric population: A review and 25-year experience at a large children’s hospital. Arch. Pathol. Lab. Med. 2008;132:1079–1103. doi: 10.5858/2008-132-1079-PAMLTI. [DOI] [PubMed] [Google Scholar]
- 31.Manivel J.C., Priest J.R., Watterson J., Steiner M., Woods W.G., Wick M.R., Dehner L.P. Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer. 1988;62:1516–1526. doi: 10.1002/1097-0142(19881015)62:8<1516::AID-CNCR2820620812>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Fosdal M.B. Pleuropulmonary Blastoma. J. Pediatr. Oncol. Nurs. 2008;25:295–302. doi: 10.1177/1043454208323292. [DOI] [PubMed] [Google Scholar]
- 33.Priest J.R., Magnuson J., Williams G.M., Abromowitch M., Byrd R., Sprinz P., Finkelstein M., Moertel C.L., Hill D.A. Cerebral metastasis and other central nervous system complications of pleuropulmonary blastoma. Pediatr. Blood Cancer. 2007;49:266–273. doi: 10.1002/pbc.20937. [DOI] [PubMed] [Google Scholar]
- 34.Schultz K.A.P., Harris A., Messinger Y., Sencer S., Baldinger S., Dehner L.P., Hill D.A. Ovarian tumors related to intronic mutations in DICER1: A report from the international ovarian and testicular stromal tumor registry. Fam. Cancer. 2016;15:105–110. doi: 10.1007/s10689-015-9831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doros L., Yang J., Dehner L., Rossi C.T., Skiver K., Jarzembowski J.A., Messinger Y., Schultz K.A., Williams G., André N., Hill D.A. DICER1 Mutations in embryonal rhabdomyosarcomas from children with and without familial PPB-tumor predisposition syndrome. Pediatr. Blood Cancer. 2012;59:558–560. doi: 10.1002/pbc.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart D.R., Messinger Y., Williams G.M., Yang J., Field A., Schultz K.A.P., Harney L.A., Doros L.A., Dehner L.P., Hill D.A. Nasal chondromesenchymal hamartomas arise secondary to germline and somatic mutations of DICER1 in the pleuropulmonary blastoma tumor predisposition disorder. Hum. Genet. 2014;133:1443–1450. doi: 10.1007/s00439-014-1474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Kock L., Sabbaghian N., Soglio D.B.D., Guillerman R.P., Park B.K., Chami R., Deal C.L., Priest J.R., Foulkes W.D. Exploring the association between DICER1 mutations and differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2014;99:E1072-7. doi: 10.1210/jc.2013-4206. [DOI] [PubMed] [Google Scholar]
- 38.Palculict T.B., Ruteshouser E.C., Fan Y., Wang W., Strong L., Huff V. Identification of germline DICER1 mutations and loss of heterozygosity in familial Wilms tumour. J. Med. Genet. 2015;53:1–4. doi: 10.1136/jmedgenet-2015-103311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahubeshi A., Bal N., Rio Frio T., Hamel N., Pouchet C., Yilmaz A., Bouron-Dal Soglio D., Williams G.M., Tischkowitz M., Priest J.R., et al. Germline DICER1 mutations and familial cystic nephroma. J. Med. Genet. 2010;47:863–866. doi: 10.1136/jmg.2010.081216. [DOI] [PubMed] [Google Scholar]
- 40.De Kock L., Plourde F., Carter M.T., Hamel N., Srivastava A., Meyn M.S., Arseneau J., Soglio D.B.-D., Foulkes W.D. Germ-line and somatic DICER1 mutations in a pleuropulmonary blastoma. Pediatr. Blood Cancer. 2013;60:2091–2092. doi: 10.1002/pbc.24692. [DOI] [PubMed] [Google Scholar]
- 41.Kuhlen M., Hönscheid A., Schemme J., Merz H., Mauz-Körholz C., Borkhardt A., Troeger A. Hodgkin lymphoma as a novel presentation of familial DICER1 syndrome. Eur. J. Pediatr. 2016;175:593–597. doi: 10.1007/s00431-015-2660-z. [DOI] [PubMed] [Google Scholar]
- 42.Rath S.R., Bartley A., Charles A., Powers N., Baynam G., Jones T., Priest J.R., Foulkes W.D., Choong C.S.Y. Multinodular Goiter in children: An important pointer to a germline DICER1 mutation. J. Clin. Endocrinol. Metab. 2014;99:1947–1948. doi: 10.1210/jc.2013-3932. [DOI] [PubMed] [Google Scholar]
- 43.Fernández-Martínez L., Villegas J.A., Santamaría Í., Pitiot A.S., Alvarado M.G., Fernández S., Torres H., Paredes Á., Blay P., Balbín M. Identification of somatic and germ-line DICER1 mutations in pleuropulmonary blastoma, cystic nephroma and rhabdomyosarcoma tumors within a DICER1 syndrome pedigree. BMC Cancer. 2017;17:146. doi: 10.1186/s12885-017-3136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doros L.A., Rossi C.T., Yang J., Field A., Williams G.M., Messinger Y., Cajaiba M.M., Perlman E.J., Schultz K.A., Cathro H.P., et al. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod. Pathol. 2014;27:1267–1280. doi: 10.1038/modpathol.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boman F., Hill D.A., Williams G.M., Chauvenet A., Fournet J.-C., Soglio D.B.-D., Messinger Y., Priest J.R. Familial association of pleuropulmonary blastoma with cystic nephroma and other renal tumors: A report from the International Pleuropulmonary Blastoma Registry. J. Pediatr. 2006;149:850–854. doi: 10.1016/j.jpeds.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 46.Cai S., Zhao W., Nie X., Abbas A., Fu L., Bihi S., Feng G., Liu T., Lv Y., Ma X., et al. Multimorbidity and Genetic Characteristics of DICER1 Syndrome Based on Systematic Review. J. Pediatr. Hematol. Oncol. 2016;39:355–361. doi: 10.1097/MPH.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 47.Turbiner J., Amin M.B., Humphrey P.A., Srigley J.R., De Leval L., Radhakrishnan A., Oliva E. Cystic nephroma and mixed epithelial and stromal tumor of kidney: A detailed clinicopathologic analysis of 34 cases and proposal for renal epithelial and stromal tumor (REST) as a unifying term. Am. J. Surg. Pathol. 2007;31:489–500. doi: 10.1097/PAS.0b013e31802bdd56. [DOI] [PubMed] [Google Scholar]
- 48.Bal N., Kayaselçuk F., Polat A., Bolat F., Yilmaz Z., Tuncer I. Familial cystic nephroma in two siblings with pleuropulmonary blastoma. Pathol. Oncol. Res. 2005;11:53–56. doi: 10.1007/BF03032407. [DOI] [PubMed] [Google Scholar]
- 49.Kuzgunbay B., Turunc T., Bolat F., Kilinc F. Adult cystic nephroma: A case report and a review of the literature. Urol. Oncol. 2009;27:407–409. doi: 10.1016/j.urolonc.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Paal E., Thompson L.D., Heffess C.S. A clinicopathologic and immunohistochemical study of ten pancreatic lymphangiomas and a review of the literature. Cancer. 1998;82:2150–2158. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2150::AID-CNCR9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 51.Bastian P.J., Kuhlmann R., Vogel J., Bastian H.-P. Local recurrence of a unilateral cystic nephroma. Int. J. Urol. 2004;11:329–331. doi: 10.1111/j.1442-2042.2004.00787.x. [DOI] [PubMed] [Google Scholar]
- 52.Wu M.K., Cotter M.B., Pears J., McDermott M.B., Fabian M.R., Foulkes W.D., O’Sullivan M.J. Tumor progression in DICER1-mutated cystic nephroma-witnessing the genesis of anaplastic sarcoma of the kidney. Hum. Pathol. 2016;53:114–120. doi: 10.1016/j.humpath.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Wu M.K., Vujanic G.M., Fahiminiya S., Watanabe N., Thorner P.S., O’Sullivan M.J., Fabian M.R., Foulkes W.D. Anaplastic sarcomas of the kidney are characterized by DICER1 mutations. Mod. Pathol. 2018;31:169–178. doi: 10.1038/modpathol.2017.100. [DOI] [PubMed] [Google Scholar]
- 54.Croce S., de Kock L., Boshari T., Hostein I., Velasco V., Foulkes W.D., McCluggage W.G. Uterine Tumor Resembling Ovarian Sex Cord Tumor (UTROSCT) Commonly Exhibits Positivity With Sex Cord Markers FOXL2 and SF-1 but Lacks FOXL2 and DICER1 Mutations. Int. J. Gynecol. Pathol. 2016;35:301–308. doi: 10.1097/PGP.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 55.Lim D., Oliva E. Ovarian sex cord-stromal tumours: An update in recent molecular advances. Pathology. 2018;50:178–189. doi: 10.1016/j.pathol.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Nwogu L.C., Showalter J.A., Roy S., Deavers M.T., Zhao B. Retiform Sertoli-Leydig Cell Tumor in a 38-Year-Old Woman: A Case Report, Retrospective Review, and Review of Current Literature. Case Rep. Pathol. 2017;2017:3421832. doi: 10.1155/2017/3421832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conlon N., Schultheis A.M., Piscuoglio S., Silva A., Guerra E., Tornos C., Reuter V.E., Soslow R.A., Young R.H., Oliva E., et al. A survey of DICER1 hotspot mutations in ovarian and testicular sex cord-stromal tumors. Mod. Pathol. 2015;28:1603–1612. doi: 10.1038/modpathol.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Kock L., Terzic T., McCluggage W.G., Stewart C.J.R., Shaw P., Foulkes W.D., Clarke B.A. DICER1 Mutations Are Consistently Present in Moderately and Poorly Differentiated Sertoli-Leydig Cell Tumors. Am. J. Surg. Pathol. 2017;41:1178–1187. doi: 10.1097/PAS.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 59.Sigismondi C., Gadducci A., Lorusso D., Candiani M., Breda E., Raspagliesi F., Cormio G., Marinaccio M., Mangili G. Ovarian Sertoli-Leydig cell tumors. A retrospective MITO study. Gynecol. Oncol. 2012;125:673–676. doi: 10.1016/j.ygyno.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 60.Mooney E.E., Nogales F.F., Bergeron C., Tavassoli F.A. Retiform Sertoli-Leydig cell tumours: Clinical, morphological and immunohistochemical findings. Histopathology. 2002;41:110–117. doi: 10.1046/j.1365-2559.2002.01426.x. [DOI] [PubMed] [Google Scholar]
- 61.Bellfield E.J., Alemzadeh R. Recurrent ovarian Sertoli-Leydig cell tumor in a child with Peutz-Jeghers syndrome. Oxford Med. 2016;2016 doi: 10.1093/omcr/omw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durieux E., Descotes F., Mauduit C., Decaussin M., Guyetant S., Devouassoux-Shisheboran M. The co-occurrence of an ovarian Sertoli-Leydig cell tumor with a thyroid carcinoma is highly suggestive of a DICER1 syndrome. Virchows Arch. 2016;468:631–636. doi: 10.1007/s00428-016-1922-0. [DOI] [PubMed] [Google Scholar]
- 63.Oost E.E., Charles A., Choong C.S., Leung Y.C., Salfinger S., Sonnendecker H., Tan J., Townshend S., Witkowski L., Foulkes W.D., et al. Ovarian sex cord-stromal tumors in patients with probable or confirmed germline DICER1 mutations. Int. J. Gynecol. Pathol. 2015;34:266–274. doi: 10.1097/PGP.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 64.Sabbaghian N., Srivastava A., Hamel N., Plourde F., Gajtko-Metera M., Niedziela M., Foulkes W.D. Germ-line deletion in DICER1 revealed by a novel MLPA assay using synthetic oligonucleotides. Eur. J. Hum. Genet. 2014;22:564–567. doi: 10.1038/ejhg.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Kock L., Bah I., Wu Y., Xie M., Priest J.R., Foulkes W.D. Germline and somatic DICER1 mutations in a well-differentiated fetal adenocarcinoma of the lung. J. Thorac. Oncol. 2016;11:e31–e33. doi: 10.1016/j.jtho.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Wu Y., Chen D., Li Y., Bian L., Ma T., Xie M. DICER1 mutations in a patient with an ovarian Sertoli-Leydig tumor, well-differentiated fetal adenocarcinoma of the lung, and familial multinodular goiter. Eur. J. Med. Genet. 2014;57:621–625. doi: 10.1016/j.ejmg.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 67.Rossing M., Gerdes A.-M., Juul A., Rechnitzer C., Rudnicki M., Nielsen F.C., Vo Hansen T. A novel DICER1 mutation identified in a female with ovarian Sertoli-Leydig cell tumor and multinodular goiter: A case report. J. Med. Case Rep. 2014;8:112. doi: 10.1186/1752-1947-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foulkes W.D., Bahubeshi A., Hamel N., Pasini B., Asioli S., Baynam G., Choong C.S., Charles A., Frieder R.P., Dishop M.K., et al. Extending the phenotypes associated with DICER1 mutations. Hum. Mutat. 2011;32:1381–1384. doi: 10.1002/humu.21600. [DOI] [PubMed] [Google Scholar]
- 69.De Kock L., Sabbaghian N., Druker H., Weber E., Hamel N., Miller S., Choong C.S., Gottardo N.G., Kees U.R., Rednam S.P., et al. Germ-line and somatic DICER1 mutations in pineoblastoma. Acta Neuropathol. 2014;128:583–595. doi: 10.1007/s00401-014-1318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabbaghian N., Hamel N., Srivastava A., Albrecht S., Priest J.R., Foulkes W.D. Germline DICER1 mutation and associated loss of heterozygosity in a pineoblastoma. J. Med. Genet. 2012;49:417–419. doi: 10.1136/jmedgenet-2012-100898. [DOI] [PubMed] [Google Scholar]
- 71.De Kock L., Druker H., Weber E., Hamel N., Traubici J., Malkin D., Arseneau J., Stewart C.J.R., Bouron-Dal Soglio D., Priest J.R., et al. Ovarian embryonal rhabdomyosarcoma is a rare manifestation of the DICER1 syndrome. Hum. Pathol. 2015;46:917–922. doi: 10.1016/j.humpath.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 72.De Kock L., Sabbaghian N., Plourde F., Srivastava A., Weber E., Bouron-Dal Soglio D., Hamel N., Choi J.H., Park S.-H., Deal C.L., et al. Pituitary blastoma: A pathognomonic feature of germ-line DICER1 mutations. Acta Neuropathol. 2014;128:111–122. doi: 10.1007/s00401-014-1285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mehraein Y., Schmid I., Eggert M., Kohlhase J., Steinlein O.K. DICER1 syndrome can mimic different genetic tumor predispositions. Cancer Lett. 2016;370:275–278. doi: 10.1016/j.canlet.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Wu M.K., Goudie C., Druker H., Thorner P., Traubici J., Grant R., Albrecht S., Weber E., Charles A., Priest J.R., et al. Evolution of Renal Cysts to Anaplastic Sarcoma of Kidney in a Child With DICER1 Syndrome. Pediatr. Blood Cancer. 2016;63:1272–1275. doi: 10.1002/pbc.25959. [DOI] [PubMed] [Google Scholar]
- 75.Caruso S., Calderaro J., Letouzé E., Nault J.-C., Couchy G., Boulais A., Luciani A., Zafrani E.-S., Bioulac-Sage P., Seror O., et al. Germline and somatic DICER1 mutations in familial and sporadic liver tumors. J. Hepatol. 2016;66:734–742. doi: 10.1016/j.jhep.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 76.De Kock L., Boshari T., Martinelli F., Wojcik E., Niedziela M., Foulkes W.D. Adult-Onset Cervical Embryonal Rhabdomyosarcoma and DICER1 Mutations. J. Low. Genit. Tract Dis. 2016;20:e8–e10. doi: 10.1097/LGT.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 77.Sahakitrungruang T., Srichomthong C., Pornkunwilai S., Amornfa J., Shuangshoti S., Kulawonganunchai S., Suphapeetiporn K., Shotelersuk V. Germline and somatic DICER1 mutations in a pituitary blastoma causing infantile-onset Cushing’s disease. J. Clin. Endocrinol. Metab. 2014;99:E1487-92. doi: 10.1210/jc.2014-1016. [DOI] [PubMed] [Google Scholar]
- 78.Yang L., Wang G., Zhao X., Ye S., Shen P., Wang W., Zheng S. A novel WRN frameshift mutation identified by multiplex genetic testing in a family with multiple cases of cancer. PLoS ONE. 2015;10:e0133020. doi: 10.1371/journal.pone.0133020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fremerey J., Balzer S., Brozou T., Schaper J., Borkhardt A., Kuhlen M. Embryonal rhabdomyosarcoma in a patient with a heterozygous frameshift variant in the DICER1 gene and additional manifestations of the DICER1 syndrome. Fam. Cancer. 2017;16:401–405. doi: 10.1007/s10689-016-9958-5. [DOI] [PubMed] [Google Scholar]
- 80.Tomiak E., de Kock L., Grynspan D., Ramphal R., Foulkes W.D. DICER1 mutations in an adolescent with cervical embryonal rhabdomyosarcoma (cERMS) Pediatr. Blood Cancer. 2014;61:568–569. doi: 10.1002/pbc.24826. [DOI] [PubMed] [Google Scholar]
- 81.Bardón-Cancho E.J., Haro-Díaz A., Alonso-García-de la Rosa F.J., Huerta-Aragonés J., García-Morín M., González-Martínez F., Garrido-Colino C. DICER1 mutation and tumors associated with a familial tumor predisposition syndrome: Practical considerations. Fam. Cancer. 2016;16:291–294. doi: 10.1007/s10689-016-9949-6. [DOI] [PubMed] [Google Scholar]
- 82.Yoshida M. Metachronous anaplastic sarcoma of the kidney and thyroid follicular carcinoma as manifestations of DICER1 abnormalities. Endocr. Relat. Cancer. 2016;23:L1–L5. doi: 10.1016/j.humpath.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 83.Wu M.K., de Kock L., Conwell L.S., Stewart C.J.R., King B.R., Choong C.S., Hussain K., Sabbaghian N., MacRae I.J., Fabian M.R., et al. Functional characterization of multiple DICER1 mutations in an adolescent. Endocr. Relat. Cancer. 2016;23:L1–L5. doi: 10.1530/ERC-15-0460. [DOI] [PubMed] [Google Scholar]
- 84.Klein S., Lee H., Ghahremani S., Kempert P., Ischander M., Teitell M.A., Nelson S.F., Martinez-Agosto J.A. Expanding the phenotype of mutations in DICER1: Mosaic missense mutations in the RNase IIIb domain of DICER1 cause GLOW syndrome. J. Med. Genet. 2014;51:294–302. doi: 10.1136/jmedgenet-2013-101943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khan N.E., Bauer A.J., Doros L., Schultz K.A.P., Decastro R.M., Harney L.A., Kase R.G., Carr A.G., Harris A.K., Williams G.M., et al. Macrocephaly associated with the DICER1 syndrome. Genet. Med. 2017;19:244–248. doi: 10.1038/gim.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nordling C.O. A new theory on cancer-inducing mechanism. Br. J. Cancer. 1953;7:68–72. doi: 10.1038/bjc.1953.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knudson A.G. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis J., Eyre H., Jacka F.N., Dodd S., Dean O., McEwen S., Debnath M., McGrath J., Maes M., Amminger P., et al. A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis. Proc. Natl. Acad. Sci. USA. 2016;65:185–194. doi: 10.1016/j.neubiorev.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berthon A., Faucz F., Bertherat J., Stratakis C.A. Analysis of ARMC5 expression in human tissues. Mol. Cell. Endocrinol. 2017;441:140–145. doi: 10.1016/j.mce.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu M.K., Sabbaghian N., Xu B., Addidou-Kalucki S., Bernard C., Zou D., Reeve A.E., Eccles M.R., Cole C., Choong C.S., et al. Biallelic DICER1 mutations occur in Wilms tumours. J. Pathol. 2013;230:154–164. doi: 10.1002/path.4196. [DOI] [PubMed] [Google Scholar]
- 91.Anglesio M., Wang Y., Yang W., Senz J., Wan A., Heravi-Moussavi A., Salamanca C., Maines-Bandiera S., Huntsman D., Morin G. Cancer-associated somatic DICER1 hotspot mutations cause defective miRNA processing and reverse-strand expression bias to predominantly mature 3p strands through loss of 5p strand cleavage. J. Pathol. 2013;229:400–409. doi: 10.1002/path.4135. [DOI] [PubMed] [Google Scholar]
- 92.De Kock L., Bah I., Revil T., Bérubé P., Wu M.K., Sabbaghian N., Priest J.R., Ragoussis J., Foulkes W.D. Deep Sequencing Reveals Spatially Distributed Distinct Hot Spot Mutations in DICER1-Related Multinodular Goiter. J. Clin. Endocrinol. Metab. 2016;101:3637–3645. doi: 10.1210/jc.2016-1328. [DOI] [PubMed] [Google Scholar]
- 93.Seki M., Yoshida K., Shiraishi Y., Shimamura T., Sato Y., Nishimura R., Okuno Y., Chiba K., Tanaka H., Kato K., et al. Biallelic DICER1 mutations in sporadic pleuropulmonary blastoma. Cancer Res. 2014;74:2742–2749. doi: 10.1158/0008-5472.CAN-13-2470. [DOI] [PubMed] [Google Scholar]
- 94.Melendez-Zajgla J., Mercado-Celis G.E., Gaytan-Cervantes J., Torres A., Gabiño N.B., Zapata-Tarres M., Juarez-Villegas L.E., Lezama P., Maldonado V., Ruiz-Monroy K., et al. Genomics of a pediatric ovarian fibrosarcoma. Association with the DICER1 syndrome. Sci. Rep. 2018;8:3252. doi: 10.1038/s41598-018-21663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brenneman M., Field A., Yang J., Williams G., Doros L., Rossi C., Schultz K.A., Rosenberg A., Ivanovich J., Turner J., et al. Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in DICER1 syndrome: A unique variant of the two-hit tumor suppression model. F1000Research. 2015;4:214. doi: 10.12688/f1000research.6746.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J., Wang Y., McMonechy M.K., Anglesio M.S., Yang W., Senz J., Maines-Bandiera S., Rosner J., Trigo-Gonzalez G., Grace Cheng S.W., et al. Recurrent DICER1 hotspot mutations in endometrial tumours and their impact on microRNA biogenesis. J. Pathol. 2015;237:215–225. doi: 10.1002/path.4569. [DOI] [PubMed] [Google Scholar]
- 97.Blandino G., Valerio M., Cioce M., Mori F., Casadei L., Pulito C., Sacconi A., Biagioni F., Cortese G., Galanti S., et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat. Commun. 2012;3:865. doi: 10.1038/ncomms1859. [DOI] [PubMed] [Google Scholar]
- 98.Noren Hooten N., Martin-Montalvo A., Dluzen D.F., Zhang Y., Bernier M., Zonderman A.B., Becker K.G., Gorospe M., de Cabo R., Evans M.K. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell. 2016;15:572–581. doi: 10.1111/acel.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu X., Yang Y., Huang Y., Chen Y., Wang T., Wu S., Tong L., Wang Y., Lin L., Hao M., et al. RNA-binding protein AUF1 suppresses miR-122 biogenesis by down-regulating DICER1 in hepatocellular carcinoma. Oncotarget. 2018;9:14815–14827. doi: 10.18632/oncotarget.24079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oxford A.E., Jorcyk C.L., Oxford J.T. Neuropathies of Stüve-Wiedemann Syndrome due to mutations in leukemia inhibitory factor receptor (LIFR) gene. J. Neurol. Neuromedicine. 2016;1:37–44. doi: 10.29245/2572.942X/2016/7.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Welch E.M., Barton E.R., Zhuo J., Tomizawa Y., Friesen W.J., Trifillis P., Paushkin S., Patel M., Trotta C.R., Hwang S., et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 102.Pibiri I., Lentini L., Melfi R., Gallucci G., Pace A., Spinello A., Barone G., Di Leonardo A. Enhancement of premature stop codon readthrough in the CFTR gene by Ataluren (PTC124) derivatives. Eur. J. Med. Chem. 2015;101:236–244. doi: 10.1016/j.ejmech.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 103.Verrier F., Dubois d’Enghien C., Gauthier-Villars M., Bonadona V., Faure-Conter C., Dijoud F., Stoppa-Lyonnet D., Houdayer C., Golmard L. Mutiple DICER1-related lesions associated with a germline deep intronic mutation. Pediatr. Blood Cancer. 2018:e27005. doi: 10.1002/pbc.27005. [DOI] [PubMed] [Google Scholar]
- 104.Diets I.J., Waanders E., Ligtenberg M.J., van Bladel D.A.G., Kamping E.J., Hoogerbrugge P.M., Hopman S., Olderode-Berends M.J., Gerkes E.H., Koolen D.A., et al. High Yield of Pathogenic Germline Mutations Causative or Likely Causative of the Cancer Phenotype in Selected Children with Cancer. Clin. Cancer Res. 2018;24:1594–1603. doi: 10.1158/1078-0432.CCR-17-1725. [DOI] [PubMed] [Google Scholar]
- 105.Nikiforova M.N., Mercurio S., Wald A.I., Barbi de Moura M., Callenberg K., Santana-Santos L., Gooding W.E., Yip L., Ferris R.L., Nikiforov Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018 doi: 10.1002/cncr.31245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schultz K.A.P., Williams G.M., Kamihara J., Stewart D.R., Harris A.K., Bauer A.J., Turner J., Shah R., Schneider K., Schneider K.W., et al. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin. Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-17-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wasserman J.D., Sabbaghian N., Fahiminiya S., Chami R., Mete O., Acker M., Wu M.K., Shlien A., de Kock L., Foulkes W.D. DICER1 mutations are frequent in adolescent-onset papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2018;103:2009–2015. doi: 10.1210/jc.2017-02698. [DOI] [PubMed] [Google Scholar]
- 108.Van Engelen K., Villani A., Wasserman J.D., Aronoff L., Greer M.-L.C., Tijerin Bueno M., Gallinger B., Kim R.H., Grant R., Meyn M.S., et al. DICER1 syndrome: Approach to testing and management at a large pediatric tertiary care center. Pediatr. Blood Cancer. 2018;65:e26720. doi: 10.1002/pbc.26720. [DOI] [PubMed] [Google Scholar]