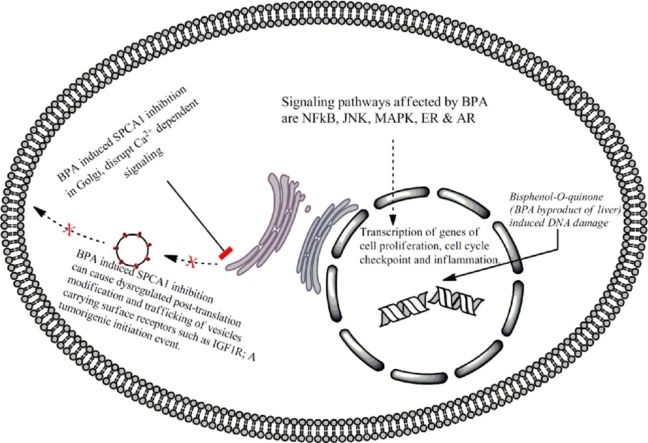
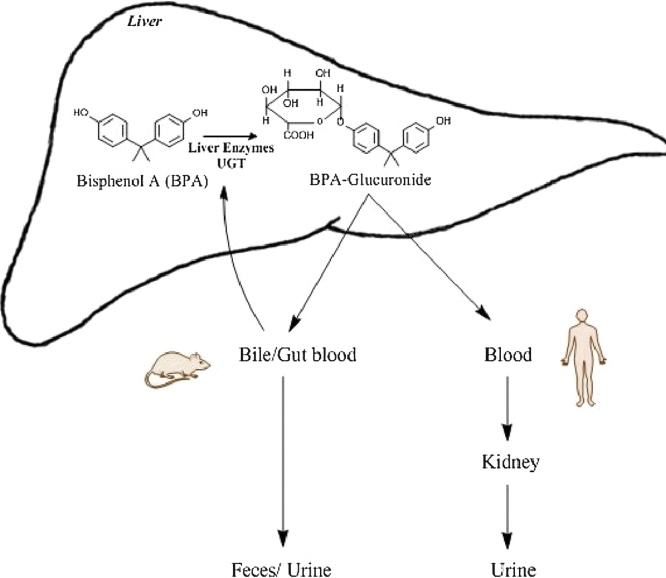
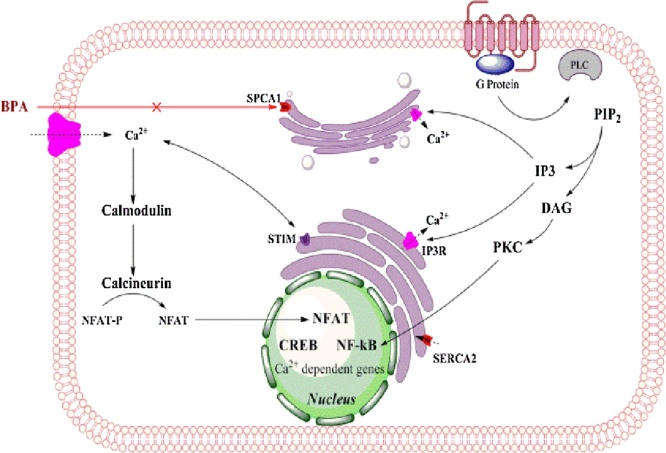
Graphical abstract
Abbreviations: GC–MS, gas chromatography–mass spectrometry; HPLC, high-performance liquid chromatography; ELISA, enzyme linked immunosorbent assay; MS, mass spectrometry; LLE, liquid/liquid extraction; SPE, solid phase extraction; DES, diethyl stilbesterol; SPCA1, secretory pathway calcium ATPase1; IGF1R, insulin-like growth factor 1 receptor; FDA, Food and Drugs Administration; EFSA, European Food Safety Authority; FAO/WHO, Food and Agricultural Organization/World Health Organization
Chemical compound studided in this article: BISPHENOL A (BPA) CCID: 6623
Keywords: Bisphenol A (BPA), DNA damage, Cancer, Mutations, Ca2+ homeostasis, SPCA1 inhibition, IGF1R
Highlights
-
•
Measurement of BPA in human tissues and organs is critically analyzed.
-
•
The tumorigenic effects of low and high dose BPA in-vitro and in-vivo experiments discussed.
-
•
BPA induced DNA damage and activation of signaling pathways that initiate tumorigenic changes in target cell have been discussed.
-
•
New experimental approaches to evaluate the carcinogenic potential of BPA proposed.
Abstract
Bisphenol A (BPA) is one of the most widely used synthetic compounds on the planet. Upon entering the diet, its highest concentration (1–104 ng/g of tissue) has been recorded in the placenta and fetus. This accumulation of BPA can have many health hazards ranging from the easy to repair single strand DNA breaks (SSBs) to error prone double strand DNA breaks (DSBs). Although the Human liver can efficiently metabolize BPA via glucuronidation and sulfation pathways, however the by-product Bisphenol-o-quinone has been shown to act as a DNA adduct. Low doses of BPA have also been shown to interact with various signaling pathways to disrupt normal downstream signaling. Analysis has been made on how BPA could interact with several signaling pathways such as NFκB, JNK, MAPK, ER and AR that eventually lead to disease morphology and even tumorigenesis. The role of low dose BPA is also discussed in dysregulating Ca2+ homeostasis of the cell by inhibiting calcium channels such as SPCA1/2 to suggest a new direction for future research in the realms of BPA induced disease morphology and mutagenicity.
1. Introduction
BPA is a synthetic phenol extensively used in the manufacture of polycarbonate plastics and epoxy resins. It was first reported by a Russian chemist Aleksandr Dianin in 1891 [1] (Fig. 1) and synthesized via the condensation of acetone with two equivalents of phenol by Zincke in 1905 [2].
Fig. 1.
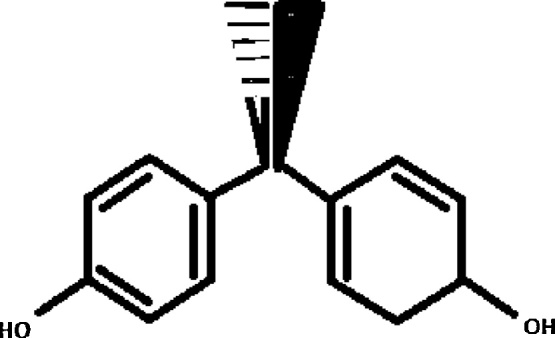
Bisphenol A, molecular formula: C15H16O2, molar mass is 228.29 g/mol.
Although BPA is one of the most widely used synthetic compounds on the planet with an annual production of about 5 million tonnes in the United States, but the biggest growth is being observed in Asia with 13% annual average growth and 19% growth in the demand of polycarbonates in India [3]. A worrisome effect of BPA is that it leaches out from the food and beverage containers that are manufactured by using BPA and leaches into the contents [[4], [5], [6], [7]]. Human BPA exposure through consumption of canned food has been estimated to be 6.6 μg/person/day [8] which then enters the blood stream [9,11,12]. Analysis of urine samples of co-habitating male and female partners were correlated indicating a common exposure source of BPA [13]. With a minimum detection limit of 0.4 μg/L urine, BPA was detected in 92.6% of the samples examined. The mean value reported was 2.6 μg/L (2.6 μg/g creatinine) and the 95th percentile concentration of 15.9 μg/L (11.2 μg/g creatinine). Of the 2517 participants, females had statistically higher BPA concentrations than males (p = 0.043) and children had higher concentrations than adolescents (p < 0.001) whereas adolescents had higher concentrations than adults (p = 0.003). Urine concentrations were linked to race/ethnicity, age, gender and even the household income [14]. Quantification of BPA in sweat via solid phase extraction (SPE) followed by analysis with HPLC has shown that BPA could be recorded in 16 of the 20 samples. Moreover, the team was careful in their sample collection methodology for directly transferring sweat from skin to the glass containers using stainless steel spatulae [15]. In a similar study conducted in 2015 the amount of BPA excreted in sweat of 50 subjects was attempted but used polyurethane sweat patches to later extract BPA by placing the patches in polypropylene plastic tubes. The team reported five-fold higher BPA in sweat than the background [16]. Further, this high concentration of BPA showed up only in 3 out of 50 samples. However, the experimental design and sample sweat collection methodology both need modification as the use of any kind of plastics in BPA biomonitoring could be severely undermined due to external contamination. Isocyanate based polyurethanes raise severe toxicity concerns, however non-isocyanate polyurethanes have also been developed [17]. Biomonitoring of BPA in 69 hair samples collected from urban and rural Greek population was evaluated using LCMS analysis. The results showed that 41.2% of urban samples had a mean BPA burden of 64.1 pg mg−1 while only 14.8% of the rural samples showed BPA at a mean concentration of 40.3 pg mg−1 [18]. These observations are a clear evidence of the link between lifestyle exposure of BPA versus detection in human blood, urine or sweat.
Reported occurrence of BPA in baby bottles raised an alarm in 2003 [9] and was also reported from the animal cages that were manufactured using BPA containing polycarbonates [10]. Factors such as heat, acidic or basic pH levels were also indicted in enhanced leaching of BPA from the containers [12]. Occurrence of BPA in free and conjugated forms was validated in blood of pregnant women and the highest concentration was measured in placenta and the developing fetus [18]. This accumulation can lead to birth defects as shown in animal studies [22]. Human epidemiological studies also reveal the relationship between BPA exposure and repeated miscarriage [23]. Concentrations of 0.3–18.9 ng/mL were measured in maternal plasma and 1–104.4 ng/g in the placental tissue and male fetuses accumulated significantly more BPA than female fetuses. Passage of BPA across the placenta has also been reported and found to in the range of 1–18 ng/mL in the maternal serum, 1–10 ng/mL in amniotic fluid and cord serum at birth and up to 100 ng/g in placenta [19,20]. However the concentration of BPA in human serum is about 0.1–1000 nM [21]. Besides serum, blood and placenta; BPA has also been detected in the milk of healthy women at a concentration of 0.28–0.97 ng/mL [25]. The objective of this study was to explore the possible molecular mechanisms that drive toxicity build-up in humans that affect DNA and signaling pathways in different ways that can initiate tumorigenic events.
2. Quantification of BPA in body fluids
Different analytical techniques have been used since 1999 to measure unconjugated BPA concentrations in human serum at levels ranging from 0.2–20 ng/mL and exceeding 100 ng/g in a study that focused on toxicity in placental tissue [18]. Some of the techniques employed for this purpose are gas chromatography–mass spectrometry (GC–MS), high-performance liquid chromatography (HPLC) and enzyme linked immunosorbent assay (ELISA). Several analytical techniques including the highly sensitive mass spectrometry (MS), specifically isotope dilution-MS, is considered a relatively reliable method for measuring trace levels of BPA and other environmental chemicals in biological samples [24]. The validity of methods used by Schönfelder et al. for BPA quantification in human fluids and use of standard BPA free controls has been debated widely but arguments provided by Völkel et al. relate high BPA urine concentrations to external contamination, sampling error or insensitive analytical methods. But an alternative explanation to the occurrence of free/unconjugated, conjugated or substituted BPA reported in several studies conducted on human blood, serum and the placental tissue was not provided [26]. Furthermore, oral pharmacokinetic studies in rodents and rhesus monkeys using isotopically labeled BPA indicate that intakes of 75 to over 1000 μg/kg BW/day would be required for the high levels of unconjugated BPA reported in the human literature assuming similar kinetic parameters [27]. Thus more sensitive methods were required to determine free/unconjugated BPA in human fluids.
As BPA enters the blood through diet it can be effectively detoxified through glucuronidation and sulfation [45] (please see BPA metabolism in mammals) but until the detoxification process begins the free/unconjugated form of BPA can exist in blood and serum for almost 1–2 h after one oral dose of 100 μg/kg BW of deuterated BPA (d6-BPA) by oral administration. Unconjugated d6-BPA was detected in serum within 5–20 min of oral dosing with a mean Cmax of 6.5 nM (1.5 ng/mL) observed at Tmax of 1.3 ± 0.52 h. Detectable blood levels of unconjugated BPA (d6-BPA) could be measured for up to 48 h [28]. BPA can be conjugated to glucuronide (BPAG) and eliminated through urine [28,29] but can be deconjugated via β-glucuronidase which is present at high concentrations in liver, kidney, intestine and placenta [30]. The deconjugation of BPAG to BPA increases the potential reactivation of BPA induced effects. A new method for the determination of free BPA, BPAG, BPA disulfates (BPADS) and three BPA chlorides, namely BPA mono-(BPAMC), di-(BPADC), trichloride (BPATrC), in human urine and serum samples, using solid-phase extraction (SPE) and LC–MS/MS detection was described recently and attempted to eliminate possible exogenous BPA contamination through the use of glass apparatus, instead of plastic tubes, for sample preparation and washed their apparatus with formic acid. Internal standard for establishing baseline for any possible BPA contamination was used besides verifying instrumental calibration by injecting 10 μL of 0.01–100 ng/mL standards of the target compounds, that demonstrated excellent linearity (regression coefficient = r > 0.99). Upon comparison of two methods (liquid/liquid extraction LLE versus solid phase extraction SPE) the values for conjugated and unconjugated forms of BPA were measured and reported to be 5 times higher in urine using SPE method than measured via LLE method. However, the concentrations of BPA in serum were not significantly different than reported in earlier investigations or LLE method [31]. Other studies also confirm the unconjugated occurrence of BPA in blood and serum even when all possible precautions were taken to avoid external contamination [32].
BPA has been recently reclassified as a class 1B reproductive toxin by the European chemical classification and labeling (CLP) [34] and a similar essential component of epoxy resins called bisphenol A diglycidyl ether, to which humans are widely exposed, was previously classified as a suspected class 2 B carcinogen by the International agency for research on cancers (IARC) [33]. It is noted that toxic exposure data for humans is missing but it was shown to be carcinogenic in animal models. It is also claimed by IARC that glycidaldehyde, a metabolite of bis-phenol A diglycidyl ether, is carcinogenic to experimental animals and classified as possibly carcinogenic to humans (group 2B) in their 1989 report [33].
North America and Europe have taken steps to either ban the use of BPA or shown concern vis-à-vis its use in food containers. Canada became the first Country to ban BPA in October 2008 through its chemicals management plan [35]. Other Countries have issued exposure warnings but not banned the compound due to insufficient direct link between dose and incidence. Asia in general and South Asia in particular has only recently realized the health hazards posed by the biotoxic nature of BPA.
Earlier studies of Food and Drugs Administration (FDA) in 2008 [36,37], based on available research data, but no new research, suggested that BPA is not toxic for humans, even for unborn babies. The European Food safety Authority (EFSA) issued a new tolerable daily intake (TDI) of 4 μg/kg of bw/day of BPA in 2011, which significantly reduced the amount of BPA exposure that the EFSA previously considered “safe” a TDI of 50 μg/kg of body weight (bw)/day (equivalent to 0.05 mg/kg of bw/day) [38]. In the fall of 2014, FDA experts, specializing in toxicology, analytical chemistry, endocrinology, epidemiology, and other related fields, completed a four-year review of more than 300 scientific studies. The FDA review has not found any information in the evaluated studies to prompt a revision of FDA’s safety assessment of BPA in food packaging at this time [41]. On similar guidelines the EFSA concluded on January 21, 2015 that BPA poses no risk to human health at current exposure levels, including for pregnant women, infants, children and the elderly [38]. However the probable toxic nature of BPA used in plastic bottles and containers of canned food was initially realized when higher than normal fetal mortality rates were associated with it [39].
However as a precautionary measure the use of BPA in the manufacture of plastic feeding bottles for infants has been strictly restricted in Europe (EU regulation N0. 321/2011) [40]. The latest report was published after the regulatory agency reduced the TDI to a mere 4 μg/kg BW/day. Following suit by EFSA the new safe TDI of 1.5–4.2 μg BPA/kg BW per day was issued by the Food and Agricultural Organization/World Health Organization (FAO/WHO) in 2010 [42].
Besides all the environmental exposure to BPA from food containers, plastic bottles and plastic appliances (cell phones to laptops) the highest dose that humans can get in one day is from the thermal receipt at a checkout counter. According to the research, when taking hold of a receipt consisting of thermal printing paper for 5 s, roughly 1 μg BPA (0.2–6 μg) was transferred to the forefinger and the middle finger (of the dominant hand) if the skin was dry but it could be ten times more if these fingers were wet or very greasy. The check-out clerk who is exposed to thermal receipts for up to 10 h/day could get an average dose of 71 μg/day [43]. Free BPA (∼20 mg BPA/g paper) is applied to the outer layers of thermal receipt paper. When such receipts are handled along with the use of common hand sanitizers (or other skin care products that contain mixtures of dermal penetration enhancing chemicals) they can increase the transdermal penetration of lipophilic compounds such as BPA by up to 100 fold [44].
Since the food and drug administration authorities have not been able to reach a definite conclusion on BPA induced DNA damage that could potentially lead to several tumorigenic phenotypes, and create various health hazards, this review collects some of the vital and most recent data on the mutagenic power of BPA and how conjugated or unconjugated BPA might be involved in degenerative disease incidence and progression. The aim of this review is to highlight the direct and indirect effects of BPA that could lead to disease or modify major signaling pathways and to suggest new direction for in-vitro and in-vivo research related to low dose exposures.
2.1. BPA metabolism in mammals
Based on the studies of Yokota et al. BPA can be metabolized by mammals via two pathways i.e., glucuronidation and sulfation [45]. They reported that glucuronidation could be catalyzed by UGT2B1, an isoform of UGT (UDP-glucuronosyltransferase), in the rat liver (Fig. 2). The rate of hepatic glucuronidation is slightly lower during pregnancy, since multidrug resistance-associated protein II and UGT both have reduced expression during pregnancy [46]. Levels of UGT in human fetal liver are also reported to be lower as compared to the adult human liver. Once metabolized by sulfation; BPA glucuronide is excreted via the urinary tract (Fig. 3) [47]. This is another contra-indication of the neo-natal exposure to BPA, and also explains why the concentration of BPA in placenta is much higher than the plasma of the mother [18,21]. Atkinson and Roy explored the metabolism via cytochrome p450 s (CYP450s) and demonstrated that this enzyme system could metabolize BPA into bisphenol-o-quinone via 5-hydroxy BPA and a bisphenol semiquinone intermediate [48,49]. Whereas Yoshihara et al. [50] were able to inhibit this metabolic pathway using an inhibitor called SKF 525-A thus proving the involvement of CYP450 enzymes. Sulfation of BPA by sulfotransferases is also sometimes believed to be a part of the detoxification metabolic pathways [51,52]. Among human sulfotransferases, the simple phenol (P)-form phenol sulfotransferase (SULT1A1) and thermostable phenol sulfotransferase (ST1A3) were shown to be involved in the sulfation of BPA [53].
Fig. 2.
Outlines the UDP glucuronosyltransferases (UGT) based metabolism of unconjugated bisphenol A (BPA) in the liver and excretion of metabolite through the urine in humans and urine/feces in rodents. (Single-column fitting image).
Fig. 3.
The metabolism of Bisphenol A (BPA) in mammalian liver via the cytochrome p-450 (Cyp-450) enzyme system, through hydroxylation. (Single-column fitting image).
2.2. BPA In janus kinase (JNK) pathway and neurological disorders
In-vivo experiments with rats exposed to low dose (10–1000 nM) of BPA (0.5 μg/kg body weight/day for 3 days) induced hormonal response. To investigate the influence of BPA on steroidogenesis, researchers investigated the activity of steroidogenic gene Cyp11a1 and its regulatory pathways in mouse Y1 adrenal cortex cells. They reported that Cyp11a1 gene activity was upregulated via the JNK pathway and this association could also have neurological consequences [54] . In a different study, the JNK inhibitor (SP600125) displayed anti-inflammatory properties on BPA mediated IL-6 cytokine response in BV2 cells [55].
2.3. Pro-inflammatory pathway triggered by BPA
The inflammatory role of BPA on target murine microglial BV2 cells was also recently studied and mitogen activated protein (MAPK) pathway was found to be a target of interest [55]. Expression analyses, western blot and immunofluorescence studies indicated that BPA is indeed involved in activating inflammatory responses in murine microglial BV2 cells via upregulated TNF-α and IL-6 which could be reversed using the estrogen receptor antagonist ICI182780. Additionally, the c-Jun N-terminal protein kinase (JNK) inhibitor (SP600125), rather than ERK1/2 blocker (PD98059), displayed anti-inflammatory properties on BPA-elicited cytokine responses, indicating that JNK pathway plays a role in downstream inflammation pathway signaling. The inflammatory transcription factor NF-κB was specifically activated by BPA as well in this study [55]. This data clearly indicates that BPA has a pro-inflammatory role in microglial cells and in the realms of the nervous system might initiate a neurodegenerative response progressing to Alzheimer’s disease (AD) or Parkinson’s disease (PD) in later life.
2.4. BPA as an inhibitor of secretory pathway calcium dependent ATPase-1 (SPCA1)
Accumulation of Ca2+ in the Golgi apparatus is mediated by sarcoendoplasmic reticulum Ca2+ ATPases (SERCAs) or by secretory pathway Ca2+ ATPases (SPCAs). These Golgi localized pumps have a high affinity for Ca2+ but human SPCA2 isoform (hSPCA2) has the ability to transport Ca2+ besides having a Mn2+ transport activity. The SPCA1 isoform is mainly located on the trans-Golgi compartment of mammalian cells (Fig. 4) [56]. Mutations in the human SPCA1 gene (ATP2C1) causes Hailey–Hailey disease which is an autosomal dominant skin disease leading to the detachment of keratinocytes in the suprabasal layer of the epidermis. BPA has been shown to inhibit SPCA1 in the cisternal membrane of Golgi apparatus [57]. Calcium is required in the post-translational modification of proteins being trafficked through the Golgi. Exogenous expression of SPCA1 isoforms (SERCA2b and SPCA1d) in COS 7 cells were used to test inhibitory properties of a variety of compounds and calcium dependent ATPase activity measured in the microsomal membrane. Bis (2-hydroxy-3-tert-butyl-5-methyl-phenyl) methane (bis-phenol) inhibited SPCA1d with high specificity with an IC50 value of 0.13 μM which was a 62-fold greater potency for inhibiting SERCA2b with the same compound. Other compounds similar to BPA, such as, tetrabromobisphenol-A and trifluoperazine inhibited both Ca2+ ATPases in a similar manner. This research pointed out the fact that BPA has the potential to act in sub-toxic doses to inhibit post-translational modification in the Golgi [57].
Fig. 4.
Bisphenol A (BPA) inhibits an important microsomal Golgi protein secretory pathway of calcium ATPase (SPCA1). Same inhibitory effect of BPA has been reported for the smooth endoplasmic reticulum calcium ATPase 2 (SERCA2) protein. (Single-column fitting image).
2.5. BPA in calcium signaling and cancer targeting
The role of intracellular Ca2+ has been highlighted in a Nature Review, which reasons that Ca2+ is a ubiquitous signal and that altered expression of specific Ca2+ channels and pumps is a characteristic feature of many cancers [58]. The ability of Ca2+ to regulate both cell proliferation and death makes it a new potential anti-cancer therapeutic target. In many cases Ca2+ channels, pumps and exporters control the complex calcium homeostasis and chemical compound that alters the normal functioning of these channels renders the channel druggable. It has also been argued that calcium channels undergo up or down-regulation in various cancers, for instance TRPM8 is upregulated in prostate cancer and SERCA3 is downregulated in colon cancer. Resting cytosolic free Ca2+ concentrations are maintained at (∼100 nM) compared to the extracellular free Ca2+ of (∼1.2 mM) [59]. Within the sub-cellular organelles there is also a Ca2+ gradient that needs to be maintained, for example cytosol and endoplasmic reticulum or sarcoplasmic reticulum (ER/SR). These organelles act as Ca2+ stores and the process is facilitated by enrichment with calcium binding proteins such as calsequestrin and calreticulin [60]. Free resting Ca2+ within the ER/SR is reported to be in the range of (100–500 μM) [61].
With Ca2+ channels also located in the Golgi apparatus and used to export post-translationally modified proteins to their destination, it is logical to hypothesize that BPA (an inhibitor of SPCA1) can be a potential inhibitory drug that not only blocks the post-translational modification process but may also have a direct role in breast cancers via this very inhibition. The signaling pathways that are Ca2+ dependent are assumed to also undergo modifications in the downstream targeting of respective signal proteins.
2.6. Insulin-like growth factor 1 receptor (IGF1-R), insulin receptor (IR) and BPA
Recently the role of IGF1R has been highlighted in the progenitor/stem cells from solid and hematopoietic malignancies and progression of certain cancers. It is now known that IGF1R and IR as well as their corresponding ligands are overexpressed in human thyroid progenitor cells cultured as thyrospheres. Isoforms of insulin receptor (IR-A) and insulin like growth factor 2 (IGF2) were found to be overexpressed in thyrospheres from cancers while only IGF2 was required for self-renewal [62]. Inhibition of SPCA1 was shown to produce a significant change in the processing of insulin-like growth factor receptor (IGF1R), with lower levels of functional IGF1Rβ and accumulation of the inactive trans-Golgi network pro-IGF1R form. Calcium regulation via the secretory pathway calcium ATPase1 (SPCA1) has therefore been associated with the IGF1R [63]. Since IGF1R is also involved in cell proliferation and apoptosis as well as overexpressed in solid tumors, the inhibition of SPCA1 by BPA can be hypothesized to affect this cascade rendering it dysfunctional as shown in Fig. 5.
Fig. 5.
Demonstrates the importance of IGF1R in normal cellular metabolism, while BPA induced secretory pathway calcium ATPase (SPCA1) inhibition can cause reduced surface expression of IGF1R while it causes accumulation of the inactive trans-Golgi pro-IGF1R form. (Single-column fitting image).
2.7. BPA: an endocrine disruptor
BPA has been in commercial use since the 1930s, being used as a synthetic estradiol [64]. It lost its medical significance when BPA was soon replaced by another synthetic estradiol called diethyl stilbesterol (DES). DES was prescribed to women between 1940 and 1971 to avoid miscarriages, premature labor and other pregnancy related complications [65]. In 1978, DES was banned by FDA based on the undeniable link between DES and cervical cancer [66]. Now a range of such compounds that mimic endocrine hormones have been shown to basically disrupt the normal hormonal signaling hence described as Endocrine disrupting chemicals or EDCs. In terms of its mechanism of action, BPA has only 10−4 to 10−3 times the estrogen receptor (ER) binding affinity as estradiol. Thus, only high dose exposure to BPA can affect the functions of natural ER ligands, such as estrogens [67].
2.8. Estrogen receptor pathway
The synthetic estrogen BPA can induce cell proliferation, leading to ovarian carcinoma, through estrogen receptor [68,69]. Based on the chemical construction similarity of BPA to beta-estradiol (E2) it has the ability to bind with ERα and ERβ receptors [70] although the affinity is much less than that for estrogen [71]. BPA’s interaction with ER-α and ER-β has been observed to have agonist as well as antagonist activities on ER-α in-vitro [71] and at very low doses can induce effects similar to estrogen besides disrupting the beta cell function in pancreas in-vivo [72]. BPA promotes the transcriptional activity of estrogen response element in BG-1 ovarian carcinoma cell in a dose-dependent manner whereas high dose expedites cell proliferation through ERα and ERβ receptors [69]. In addition, BPA has high affinity for the estrogen receptor-related receptor γ (ERRγ), but not to the estrogen receptor (ER) [73]. It protects the basal constitutive activity of ERRγ, and selectively defends ER modulator 4-hydroxytamoxifen from deactivation of ERRγ [74]. BPA also binds with the membrane estrogen receptor (mER), activating guanylyl cyclase and protein kinase G, which can turn off ATP-dependent K+ channel, induce depolarized Ca2+ flow into L-calcium channel, enhance calcium signal path resulting in cAMP response element-binding protein (CREB) phosphorylation and it can also regulate the transcription of cAMP/Ca2+ response element [75].
2.9. Androgen receptor pathway
Almost 70%-90% primary breast cancer tissues express androgen receptor (AR); this is related to a positive prognosis for small tumor volume, low pathological grading and long patient survival time [76]. Animal model studies have helped to determine that AR agonist 5α-dihydroxytestosterone (DHT) suppress mouse mammary epithelial cell proliferation. Whereas antagonist flutamide enhance mouse mammary gland cell proliferation [77]. Research shows that BPA can be an AR antagonist, since BPA dose-dependently suppresses transcription activity induced by DHT [78]. The IC50 inhibiting concentration is 1 × 10−6M to 7 × 10−7 M supported by human AR reporter gene assay [79]. In addition, BPA may influence AR’s activity and function by changing AR nuclear translocation and transcriptional activation, since BPA and Nonylphenol (NP) cause dispersed distribution of AR between the nuclear and the cytoplasmic compartments in the presence of testosterone [80].
2.10. DNA adducts of BPA
Microsomal p450 enzymes in rat liver take part in the metabolism of BPA and convert it into Bisphenol o-quinone that acts as a DNA adduct [49]. The reaction of BPA with calf thymus DNA, in the presence of S9 mix, resulted in the formation of bulky DNA adducts. The formation of DNA adducts was concentration-dependent in the range of 6.2–100 μM, corresponding to doses of 14.25–228 ng. The detection limit for BPA, corresponding to a detection limit of 0.1 DNA adducts/108 nucleotides, was 13.2 ng (5.8 μM). Upon administration of BPA (dissolved in water) to mice, at a dose of 200 mg/kg body weight for eight days, resulted in the formation of bulky DNA adducts in the liver. Similar results were reported for BPA with mammary gland. The level of DNA adducts in the pooled mammary cells of BPA-treated mice was 4.97 ± 0.61/108 nucleotides versus 1.05 ± 0.13 in controls was significantly higher [81].
2.11. BPA induced DNA damage
A recent study investigated the role of low dose BPA induced DNA damage in breast cancer cells and three dimensional geometric cancer tissue [82]. Immunostaining and single cell gel electrophoresis demonstrated an upregulated c-Myc along with other crucial proteins and induced an increased cell proliferation in estrogen receptor α (ER- α) negative mammary cells. Silencing the c-MYC gene via siRNA inhibition demonstrated that the DNA damaging effects of low dose BPA could be reduced, hence c-Myc plays a pivotal role in controlling the downstream signaling of low dose BPA induced DNA damage [82]. Among all the breaks that a DNA molecule receives, the most dangerous and hard to repair are the double strand breaks (DSBs) [83]. This happens because megabase sized deletion is an inevitable consequence of DSB repair [84]. If the megabase deletions occur in an exon, or worse, in the exon of a tumor suppressor gene, the consequences could be far and wide.
In a similar study rat insulinoma INS-1 cells were tested against doses of BPA (0, 25, 50, 100 μM) for DNA damaging capacity and the expression of proteins associated with DNA damage and apoptosis or proliferation. The team concluded that p-53 and p-Chk2 were significantly increased [85]. This study highlighted that BPA does not only target the breast cancer cells but may have a role also in the pancreatic tissue. In recent years attempts have been made to use analogues of BPA but data now shows that analogues of BPA also exert the same DNA damaging effect on target cells. The evaluated analogues include BPF, BPAF, BPZ, BPS, DMBPA, DMBPS, BP-1, and BP-2. None of these analogues demonstrated a DNA damaging activity in Salmonella typhimurium strains TA98 and TA100. However when tested on HepG2 cells the results showed that even non-cytotoxic doses of these analogues 0.1 μmol L−1 to 10 μmol L−1 could cause significant DNA damage, as tested by comet assay and Ames test [86].
In a recent elaborate study, female dam pups were used to investigate the DNA damaging effects of BPA. Post-BPA exposure histopathological changes in liver tissue and oxidative stress was measured, based on the activity of enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), malondialdehyde (MDA), nitric oxide (NO) and total antioxidant capacity (TAC). The results indicated a reduced antioxidant enzyme activity for SOD, CAT and GPX that leads to hepatotoxicity. The team observed severe DNA damage in BPA (0.5 and 50 mg/kg BW) treated dam female mice, compared to the control group. The study also found out that early life exposure to BPA induced leucocytic cell infiltration, appearance of necrotic areas and histopathological changes in the liver tissue [87].
In a distinct study that measured not just DNA damage but also DNA methylation in MCF-10F normal breast epithelial cells a BPA dose of 10−5 and 10−6 M caused the formation of 73% to 80% less tubules in the collagen. At these concentrations the expression analysis demonstrated an upregulation of DNA repair genes BRCA1/2, BARD1, CtIP, RAD51 and BRCC3 while a downregulation of apoptotic genes PDCD5 and BCL2L11. This showed that increased DNA damage due to BPA exposure must be repaired and delayed apoptosis could lead to extended survival of these cells. Methylation analysis of the BPA exposed cells showed hypermethylation of BCL2L11, PARD6G, FOXP1 and SFRS11 while hypomethylated NUP98 and CtIP genes [88].
2.12. BPA can impair double strand break repair machinery
When Caenorhabditis elegans was used as a research model to investigate the effects of BPA (at concentrations of 100uM, 500uM and 1 M) it was observed that such concentrations caused increased sterility and embryonic lethality. BPA exposure also caused impaired chromosome synapsis and dysregulated meiotic double strand break repair progression. Exposure led to an anti-estrogenic activity in the germline and initiated a germ-line dependent down-regulation of DSBR genes, hence dysregulating the maintenance of genomic integrity at meiosis [89].
2.13. BPA in disease morphology of bone
Bone formation and resorption is affected by estrogens. Since estrogen plays a crucial role in bone homeostasis, it is important to know the effect of BPA in bone biology. Bone contains bone forming osteoblasts and bone resorbing osteoclasts. BPA has been shown to not only inhibit differentiation and activity of osteoblasts and osteoclasts but also stimulate apoptosis of both cells in-vitro [90,91]. Osteoblasts are formed by osteogenic differentiation of mesenchymal stem cells (MSCs). MSCs can also differentiate to adipocytes or chondrocytes. Chamorro-Garcia et al. reported in 2012 that low dose of BPA induced MSCs to differentiate towards adipocytes rather than osteoblasts in-vitro, which can impair bone formation [92]. In addition, Watanabe et al. showed that BPA inhibits the expression of cytochrome P450 aromatase (CYP19) in human fetal osteoblastic cell lines, indicating possible inhibitory effect of BPA on CYP19 mediated bone formation [93]. BPA has catabolic effect on bone forming osteoblasts as well as on bone resorbing osteoclasts in- vitro; therefore, it is difficult to predict the exact effect of BPA on bone homeostasis. Clinical studies have been conducted to analyze the effect of BPA on bone mineral densities (BMDs) in premenopausal and post-menopausal women [94,95]. The results showed that BPA has no effect on BMDs in premenopausal women [94]. Moreover, BPA showed no effect on clinical variables related to bone metabolism and BMDs in post menopausal women [95]. Available literature provides limited information to draw any conclusion that BPA has anabolic or catabolic effect on bone. To elucidate the effect of BPA on bone metabolism, further in-vitro and in-vivo studies, and large scale clinical studies in high-risk population sample of osteoporosis are suggested.
3. Discussion
The available data overwhelmingly points towards the multifarious effects of BPA ranging from cytotoxicity at higher doses to DNA damage at relatively low doses of BPA besides its role in bone development and breast cancer development. A variety of cell lines tested and several analogues of BPA all indicate the same end-result. However our knowledge of DNA damage suggests that most kinds of damage can be efficiently repaired except the double strand DNA damage. Previous research has shown that SPCA1 inhibitors can effectively inhibit Ca2+ loading into the Golgi, and that BPA is also a potent inhibitor of SPCA1 similar to already tested thapsigargin [96]. High concentration of Ca2+ in the lumen of intracellular organelles is a source of activator Ca2+ for a range of processes including transcription, translation, translocation, folding and processes of protein folding [97]. Within the Golgi apparatus luminal Ca2+ is important for intra and inter Golgi transportations and for endosome vesicle fusion [98,99]. Therefore the dynamics of BPA may be more widespread than previously thought. The formation of neurotransmitter vesicles in the neuronal cells could experience an inhibited release from the pre-synaptic membrane, thereby affecting the electrochemical passage of nerve impulses in the brain.
The area of research pertaining to BPA that still remains untouched is the post-translational modification inhibition. This review suggests a new direction of BPA acting as a potent calcium channel inhibitor of Golgi and the Sarcoplasmic reticulum. Since Trans- Golgi is a widely accepted sub-cellular compartment that processes proteins in the post-translational event for further distribution to various parts of the cell, therefore this should be the next logical direction for researchers.
4. Conclusion
Human exposure to the environmental toxin BPA is being debated globally. With the banning of use of BPA in food containers, baby bottles and in some cases complete import ban into a Country drives the inquisition to investigate not only the high dose effects but also the low dose effects of BPA. While food safety and health organizations consider BPA a class 2 B reproductive toxin, there is a lot that needs to be done for reconsideration of the carcinogenicity status. The available data suggests as yet an unclear link between BPA and carcinogenicity, however with the many research directions pointed in this text, this paradigm can be clarified within the next few years.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The contributing authors of this article have no known conflict of interest.
Acknowldgments
We would like to acknowledge all investigators who tirelessly work to investigate the effects of environmental carcinogens that pose a substantial risk to human health.
Contributor Information
Nasir Jalal, Email: nasirjalal@tju.edu.cn.
Austin R. Surendranath, Email: austinrichards@cids.edu.
Janak L. Pathak, Email: j.pathak@tju.edu.cn.
Shi Yu, Email: yushi@tju.edu.cn.
Chang Y. Chung, Email: cychung@tju.edu.cn.
References
- 1.Dianin A.P. Condensation of ketones with phenols. Zhurnal Russkogo Fiziko-Khimicheskogo Obshchestva. J. Russ. Phys. Chem. Soc. St. Petersburg. 1891;23(488–517):601–611. (523–546) [Google Scholar]
- 2.Zincke T. Ueber die Einwirkung von Brom und von Chlor auf Phenole: Substitutionsprodukte, Pseudobromide und Pseudochloride. Justus Liebigs Annalen der Chemie (in German) 1905;343:75–99. [Google Scholar]
- 3.Bisphenol A. 2016. (BPA) Chemical Profile: Asia Phenol. https://www.icis.com/resources/news/2016/06/30/10012759/chemical-profile-asia-phenol/(Last accessed on November 30, 2017) [Google Scholar]
- 4.Vandenberg L.N., Maffini M.V., Sonnenschein C., Rubin B.S., Soto A.M. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo H.W., Ding W.H. Trace determination of bisphenol A and phytoestrogens in infant formula powders by gas chromatography–mass spectrometry. J. Chromatogr. A. 2004;1027:67–74. doi: 10.1016/j.chroma.2003.08.084. [DOI] [PubMed] [Google Scholar]
- 6.Munguía-López E.M., Gerardo-Lugo S., Peralta E., Bolumen S., Soto-Valdez H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit. Contam. 2005;22:892–898. doi: 10.1080/02652030500163674. [DOI] [PubMed] [Google Scholar]
- 7.Le H.H., Carlson E.M., Chua J.P., Belcher S.M. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol. Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe S.R., Borodinsky L., Lyon R.S. Potential exposure to bisphenol A from food-contact use of epoxy coated cans. J. Coat. Technol. 1998;70:69–74. doi: 10.1080/02652039809374653. [DOI] [PubMed] [Google Scholar]
- 9.Brede C., Fjeldal P., Skjevrak I., Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 2003;20:684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 10.Howdeshell K.L., Peterman P.H., Judy B.M., Taylor J.A., Orazio C.E., Ruhlen R.L., vom Saal F.S., Welshons W.V. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ. Health Perspect. 2003;111:1180–1187. doi: 10.1289/ehp.5993. (PMID: 12842771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joskow R., Boyd B.D., Barr J.R., Calafat A.M., Needham L.L., Rubin C. Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J. Am. Dent. Assoc. 2006;137:353–362. doi: 10.14219/jada.archive.2006.0185. (PMID: 16570469) [DOI] [PubMed] [Google Scholar]
- 12.Kang J.H., Kito K., Kondo F. Factors influencing the migration of bisphenol A from cans. J. Food Prot. 2003;66(8):1444–1447. doi: 10.4315/0362-028x-66.8.1444. (PMID: 12929833) [DOI] [PubMed] [Google Scholar]
- 13.Mahalingaiah S., Meeker J.D., Pearson K.R., Calafat A.M., Ye X., Petrozza J., Hauser R. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ. Health Perspect. 2008;116:173–178. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calafat A.M., Ye X., Wong L.-Y., Reidy J.A., Needham L.L. Exposure of the U.S. population to bisphenol a and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzatzarakis M.N., Vakonaki E., Kavvalakis M.P., Barmpas M., Kokkinakis E.N., Xenos K., Tsatsakis A.M. Biomonitoring of bisphenol A in hair of Greek population. Chemosphere. 2015;118:336–341. doi: 10.1016/j.chemosphere.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 16.Porucznik C.A., Cox K.J., Wilkins D.G., Anderson D.J., Bailey N.M., Szczotka K.M., Stanford J.B. A preliminary study of biomonitoring for bisphenol-A in human sweat. J. Anal. Toxicol. 2015;39(7):562–566. doi: 10.1093/jat/bkv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K., Nelson A.M., Talley S.J., Chen M., Margaretta E., Hudson A.G., Moore R.B., Long T.E. Non-isocyanate poly(amide-hydroxyurethane)s from sustainable resources. Green Chem. 2016;18(17):4667–4681. [Google Scholar]
- 18.Schönfelder G., Wittfoht W., Hopp H., Talsness C.E., Paul M., Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110:703–707. doi: 10.1289/ehp.110-1241091. (PMID: 12417499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada H., Furuta I., Kato E.H., Kataoka S., Usuki Y., Kobashi G., Sata F., Kishi R., Fujimoto S. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod. Toxicol. 2002;16:735–739. doi: 10.1016/s0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 20.Padmanabhan V., Siefert K., Ransom S., Johnson T., Pinkerton J., Anderson L., Tao L., Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J. Perinatol. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sajiki J., Takahashi K., Yonekubo J. Sensitive method for the determination of bisphenol-A in serum using two systems of high-performance liquid chromatography. J. Chromatogr. B. 1999;736:255–261. doi: 10.1016/s0378-4347(99)00471-5. (PMCID: PMC1241312) [DOI] [PubMed] [Google Scholar]
- 22.Lee C.K., Kim S.H., Moon D.H., Kim J.H., Son B.C., Kim D.-H., Lee C.-H., Kim H.-D., Kim J.-W., Kim J.-E., Lee C.-U. Effects of bisphenol A on the placental function and reproduction in rats. J. Prev. Med. Pub. Health. 2005;38:330–336. (PMID: 16323634) [PubMed] [Google Scholar]
- 23.Sugiura-Ogasawara M., Ozaki Y., Sonta S., Makino T., Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 2005;20:2325–2329. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 24.Vandenberg L.N., Hauser R., Marcus M., Olea N., Welshons W.V. Human exposure to bisphenol a (BPA) Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y., Irie M., Kishikawa N., Wada N., Kuroda N., Nakashima K. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed. Chromatogr. 2004;18:501–507. doi: 10.1002/bmc.345. [DOI] [PubMed] [Google Scholar]
- 26.Völkel W. Lessons of 15-year exposure to Bisphenol A: a critical discussion of biomonitoring studies. Arch. Toxicol. 2017;91(November (11)):3693–3696. doi: 10.1007/s00204-017-1963-4. https://link.springer.com/article/10.1007%2Fs00204-017-1963-4 [DOI] [PubMed] [Google Scholar]
- 27.vom Saal F.S., Vandevoort C.A., Taylor J.A., Welshons W.V., Toutain P.L., Hunt P.A. Bisphenol A (BPA) pharmacokinetics with daily oral bolus or continuous exposure via silastic capsules in pregnant rhesus monkeys: relevance for human exposures. Reprod. Toxicol. 2014;45:105–116. doi: 10.1016/j.reprotox.2014.01.007. pii: S0890-6238(14)00020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thayer K.A., Doerge D.R., Hunt D., Schurman S.H., Twaddle N.C., Churchwell M.I., Garantziotis S., Kissling G.E., Easterling M.R. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015;83:107–115. doi: 10.1016/j.envint.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Völkel W., Colnot T., Csanády G.A., Filser J.G., Dekant W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem. Res. Toxicol. 2002;15(10):1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg G., Rice D.C. Does rapid metabolism ensure negligible risk from bisphenol A? Environ. Health Perspect. 2009;117(11):1639–1643. doi: 10.1289/ehp.0901010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao C., Kannan K. Determination of free and conjugated forms of bisphenol a in human urine and serum by liquid chromatography–tandem mass spectrometry. Environ. Sci. Technol. 2012;46(9):5003–5009. doi: 10.1021/es300115a. [DOI] [PubMed] [Google Scholar]
- 32.vom Saal F.S., Welshons W.V. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Mol. Cell. Endocrinol. 2014;398:101–113. doi: 10.1016/j.mce.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyon; 2017. BPA Monograph; pp. 1285–1289. http://monographs.iarc.fr/ENG/Monographs/vol71/mono71-71.pdf (Last accessed on November 30, 2017) [Google Scholar]
- 34.European legislation on chemicals . 2008. The CLP Regulation (EC) No 1272/2008 European Regulation on Classification, Labelling and Packaging of Chemical Substances and Mixtures. (Website accessed 08/16/2017. http://www.bisphenol-a-europe.org/regulatory-framework/european-legislation-on-chemicals/) [Google Scholar]
- 35.2008. Chemicals Management Plan of Canada. https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/packaging-materials/bisphenol.html (Last accessed on November 30, 2017) [Google Scholar]
- 36.FDA report . 2008. Draft Assessment of Bisphenol A for Use in Food Contact Applications. [Posted 2008 Aug 14] Available: www.fda.gov/ohrms/dockets/AC/08/briefing/2008-0038b1_01_02_FDA%20BPA%20Draft%20Assessment.pdf (Last accessed on November 30, 2017) [Google Scholar]
- 37.FDA Science Board Subcommittee on Bisphenol A . 2018. Scientific Peer-Review of the Draft Assessment of Bisphenol A for Use in Food Contact Applications [Posted 2008 Oct 31] Available from: www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4386b1-05.pdf (Last accessed on November 30, 2017) [Google Scholar]
- 38.EFSA CEF Panel, EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: executive summary. EFSA J. 2015;13(1):3978. [Google Scholar]
- 39.Ranjit N., Siefert K., Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J. Perinatol. 2010;30(1):2–9. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eorpoean Union Law . 2011. Commission Implementing Regulation (EU) No 321/2011 of 1 April 2011 Amending Regulation (EU) No 10/2011 as Regards the Restriction of Use of Bisphenol A in Plastic Infant Feeding Bottles Text with EEA Relevance. http://data.europa.eu/eli/reg_impl/2011/321/oj (Last accessed on November 30, 2017) [Google Scholar]
- 41.FDA Food Additives, Bisphenol A, Availability . 2014. Document 1 – Memorandum Dated August 31, 2009, Bisphenol A, Review of Low Dose Studies [FDA-2010-N-0100-0001] Supporting & Related Material. Posted: 04/05/2010 ID: FDA-2010-N-0100-0006. Available: http://www.regulations.gov/#!docketBrowser;rpp=25;po=0;dct=SR;D=FDA-2010-N-0100 (Last accessed on November 30, 2017) [Google Scholar]
- 42.FAO/WHO Food and Agricultural Organization/World Health Organization . 2011. Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A. Available from: http://www.who.int/foodsafety/publications/bisphenol-a/en/(Last accessed on November 30, 2017) [Google Scholar]
- 43.Biedermann S., Tschudin P., Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal. Bioanal. Chem. 2010;398:571–576. doi: 10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- 44.Hormann A.M., vom Saal F.S., Nagel S.C., Stahlhut R.W., Moyer C.L., Ellersieck M.R., Welshons W.V., Toutain P.L., Taylor J.A. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA) PLoS One. 2014;9(10):e110509. doi: 10.1371/journal.pone.0110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokota H., Iwano H., Endo M., Kobayashi T., Inoue H., Ikushiro S., Yuasa A. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem. J. 1999;340:405–409. [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue H., Tsuruta A., Kudo S., Ishii T., Fukushima Y., Iwano H., Yokota H., Kato S. Bisphenol A glucuronidation and excretion in liver of pregnant and nonpregnant female rats. Drug Metab. Dispos. 2004;33:55–59. doi: 10.1124/dmd.104.001537. [DOI] [PubMed] [Google Scholar]
- 47.Strassburg C.P., Strassburg A., Kneip S., Barut A., Tukey R.H., Rodeck B., Manns M.P. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50:259–265. doi: 10.1136/gut.50.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atkinson A., Roy D. In-vitro conversion of environmental estrogenic chemical bisphenol A to DNA binding metabolite(s) Biochem. Biophys. Res. Commun. 2002;210:424–433. doi: 10.1006/bbrc.1995.1678. [DOI] [PubMed] [Google Scholar]
- 49.Atkinson A., Roy D. In-vivo DNA adduct formation by bisphenol A. Environ. Mol. Mutagen. 1995;26:60–66. doi: 10.1002/em.2850260109. (PMID: 7641708) [DOI] [PubMed] [Google Scholar]
- 50.Yoshihara S., Makishima M., Suzuki N., Ohta S. Metabolic activation of bisphenol A by rat liver S9 fraction. Toxicol. Sci. 2001;62:221–227. doi: 10.1093/toxsci/62.2.221. [DOI] [PubMed] [Google Scholar]
- 51.Suiko M., Sakakibara Y., Liu M.C. Sulfation of environmental estrogen-like chemicals by human cytosolic sulfotransferases. Biochem. Biophys. Res. Commun. 2000;267:80–84. doi: 10.1006/bbrc.1999.1935. [DOI] [PubMed] [Google Scholar]
- 52.Nishiyama T., Ogura K., Nakano H., Kaku T., Takahashi E., Ohkubo Y., Sekine K., Hiratsuka A., Kadota S. Sulfation of environmental estrogens by cytosolic human sulfotransferase. Drug Metab. Pharmcokinet. 2002;17:221–228. doi: 10.2133/dmpk.17.221. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu M., Ohta K., Matsumoto Y., Fukuoka M., Ohno Y., Ozawa S. Sulfation of bisphenol A abolished its estrogenicity based on proliferation and gene expression in human breast cancer MCF-7 cells. Toxicol. In Vitro. 2002;16:549–556. doi: 10.1016/s0887-2333(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 54.Lan H.C., Lin I.W., Yang A.J., Lin J.H. Low-Dose bisphenol A activates an activates cyp11a1 gene expression and corticosterone secretion in adrenal gland via the JNK signaling pathway. Toxicol. Sci. 2015;148:26–34. doi: 10.1093/toxsci/kfv162. [DOI] [PubMed] [Google Scholar]
- 55.Zhu J., Jiang L., Liu Y., Qian W., Liu J., Zhou J., Gao R., Xiao H., Wang J. MAPK and NF-κB pathways are involved in bisphenol A-induced TNF-α and IL-6 production in BV2 microglial cells. Inflammation. 2015;38:637–648. doi: 10.1007/s10753-014-9971-5. [DOI] [PubMed] [Google Scholar]
- 56.Missiaen L., Dode L., Vanoevelen D., Raeymaekers L., Wuytack F. Calcium in the Golgi apparatus. Cell Calcium. 2007;41:405–416. doi: 10.1016/j.ceca.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Lai P., Michelangeli F. Bis(2-hydroxy-3-tert-butyl-5-methyl-phenyl)-methane (bis-phenol) is a potent and selective inhibitor of the secretory pathway Ca2+ ATPase (SPCA1) Biochem. Biophys. Res. Commun. 2012;424:616–619. doi: 10.1016/j.bbrc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Monteith G.R., McAndrew D., Faddy H.M., Roberts-Thomson S.J. Calcium and cancer: targeting Ca2+ transport. Nat. Rev. Cancer. 2007;7:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 59.Carafoli E. Intracellular calcium homeostasis. Annu. Rev. Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 60.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 61.Palmer A.E., Jin C., Reed J.C., Tsien R.Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malaguarnera R., Frasca F., Garozzo A., Gianì F., Pandini G., Vella V., Vigneri R., Belfiore A. Insulin receptor isoforms and insulin-like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J. Clin. Endocrinol. Metab. 2011;96:766–774. doi: 10.1210/jc.2010-1255. [DOI] [PubMed] [Google Scholar]
- 63.Grice D.M., Vetter I., Faddy H.M., Kenny P.A., Roberts-Thomson S.J., Monteith G.R. Golgi calcium pump secretory pathway calcium ATPase 1 (SPCA1) is a key regulator of insulin-like growth factor receptor (IGF1R) processing in the basal-like breast cancer cell line MDA-MB-231. J. Biol. Chem. 2010;285:37458–37466. doi: 10.1074/jbc.M110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dodds E.C., Lawson W. Synthetic estrogenic agents without phenanthrene nucleus. Nature. 1936;137:996–997. [Google Scholar]
- 65.Professional and Public Relations Committee of the DESAD (Diethylstilbestrol and Adenosis) Project of the Division of Cancer Control and Rehabilitation, Exposure in utero to diethylstilbestrol and related synthetic hormones association with vaginal and cervical cancers and other abnormalities. JAMA. 1976;236:1107–1109. doi: 10.1001/jama.1976.03270110011002. (PMID: 988858) [DOI] [PubMed] [Google Scholar]
- 66.FDA Drug Bulletin November 1971, diethylstilbestrol contraindicated in pregnancy. Calif. Med. 1972;116:85–86. (PMCID: PMC1518220) [PMC free article] [PubMed] [Google Scholar]
- 67.Gould J.C., Leonard L.S., Maness S.C., Wagner B.L., Conner K., Zacharewski T., Safe S., McDonnell D.P., Gaido K.W. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol. Cell. Endocrinol. 1998;142:203–214. doi: 10.1016/s0303-7207(98)00084-7. (PMID: 9783916) [DOI] [PubMed] [Google Scholar]
- 68.Hall J.M., Korach K.S. Endocrine disrupting chemicals promote the growth of ovarian cancer cells via the ER-CXCL12-CXCR4 signaling axis. Mol. Carcinog. 2013;52:715–725. doi: 10.1002/mc.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park S.H., Kim K.Y., An B.S., Choi J.-H., Jeung E.-B., Leung P.C.K., Choi K.-C. Cell growth of ovarian cancer cells is stimulated by xenoestrogens through an estrogen-dependent pathway, but their stimulation of cell growth appears not to be involved in the activation of the mitogen-activated protein kinases ERK-1 and p38. J. Reprod. Dev. 2009;55:23–29. doi: 10.1262/jrd.20094. [DOI] [PubMed] [Google Scholar]
- 70.Barkhem T., Carlsson B., Nilsson Y., Enmark E., Gustafsson J.A., Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol. Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 71.Hiroi H., Tsutsumi O., Momoeda M., Takai Y., Osuga Y., Taketani Y. Differential interactions of bisphenol A and 17beta-estradiol with estrogen receptor alpha (ERalpha) and ERbeta. Endocr. J. 1999;46:773–778. doi: 10.1507/endocrj.46.773. [DOI] [PubMed] [Google Scholar]
- 72.Alonso-Magdalena P., Morimoto S., Ripoll C., Fuentes E., Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okada H., Tokunaga T., Liu X.H., Takayanagi S., Matsushima A., Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ. Health Perspect. 2008;116:32–38. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsushima A., Kakuta Y., Teramoto T., Koshiba T., Liu X.H., Okada H., Tokunaga T., Kawabata S.-I., Kimura M., Shimohigashi Y. Structural evidence for endocrine disruptor bisphenol a binding to human nuclear receptor ERR gamma. J. Biochem. 2007;142:517–524. doi: 10.1093/jb/mvm158. [DOI] [PubMed] [Google Scholar]
- 75.Quesada I., Fuentes E., Viso-Leon M.C., Soria B., Ripoll C., Nadal A. Low doses of the endocrine disruptor Bisphenol-A and the native hormone 17 beta-estradiol rapidly activate the transcription factor CREB. FASEB J. 2002;16:1671–1673. doi: 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- 76.Yu Q., Niu Y., Liu N., Zhang J.Z., Liu T.J., Zhang R.J. Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann. Oncol. 2011;22:1288–1294. doi: 10.1093/annonc/mdq586. [DOI] [PubMed] [Google Scholar]
- 77.Peters A.A., Ingman W.V., Tilley W.D., Butler L.M. Differential effects of exogenous androgen and an androgen receptor antagonist in the peri- and postpubertal murine mammary gland. Endocrinology. 2011;152:3728–3737. doi: 10.1210/en.2011-1133. [DOI] [PubMed] [Google Scholar]
- 78.Xu L.C., Sun H., Chen J.F., Bian Q., Qian J., Song L., Wang X.-R. Evaluation of androgen receptor transcriptional activities of bisphenol A octylphenol and nonylphenol in vitro. Toxicology. 2005;216:197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Stroheker T., Picard K., Lhuguenot J.C., Canivenc-Lavier M.C., Chagnon M.C. Steroid activities comparison of natural and food wrap compounds in human breast cancer cell lines. Food Chem. Toxicol. 2004;42:887–897. doi: 10.1016/j.fct.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 80.Lee H.J., Chattopadhyay S., Gong E.Y., Ahn R.S., Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol. Sci. 2003;75:40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- 81.Izzotti A., Kanitz S., D'Agostini F., Camoirano A., De Flora S. Formation of adducts by bisphenol A an endocrine disruptor in DNA in vitro and in liver and mammary tissue of mice. Mutat. Res. 2009;679:28–32. doi: 10.1016/j.mrgentox.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 82.Pfeifer D., Chung Y.M., Hu M.C.-T. Effects of low-dose bisphenol A on DNA damage and proliferation of breast cells: the role of c-Myc. Environ. Health Perspect. 2015;123(December (12)):1271––279. doi: 10.1289/ehp.1409199. [Epub 2015 May 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rothkamm K., Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McMahon S.J., Schuemann J., Paganetti H., Prise K.M. Mechanistic modelling of DNA repair and cellular survival following radiation-induced DNA damage. Sci. Rep. 2016;6:33290. doi: 10.1038/srep33290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xin F., Jiang L., Liu X., Geng C., Wang W., Zhong L., Yang G., Chen M. Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014;769:29–33. doi: 10.1016/j.mrgentox.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 86.Fic A., Žegura B., Dolenc M.S., Filipic M., Mašic L.P. Mutagenicity and DNA damage of bisphenol a and its structural analogues in Hepg2Cells. Arh. Hig. Rada Toksikol. 2013;64:189–200. doi: 10.2478/10004-1254-64-2013-2319. [DOI] [PubMed] [Google Scholar]
- 87.Eid J.I., Eissa S.M., El-Ghor A.A. Bisphenol A induces oxidative stress and DNA damage in hepatic tissue of female rat offspring. J. Basic Appl. Zool. 2014;71:10–19. [Google Scholar]
- 88.Fernandez S.V., Huang Y., Snider K.E., Zhou Y., Pogash T.J. Expression and DNA methylation changes in human breast epithelial cells after bisphenol A (BPA) exposure. Int. J. Oncol. 2012;41:369–377. doi: 10.3892/ijo.2012.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allard P., Colaiácovo M.P. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. PNAS. 2010;107:20405–20410. doi: 10.1073/pnas.1010386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang J.K., Min K.H., Choi K.H., Hwang Y.C., Jeong I.K., Ahn K.J. Bisphenol A reduces differentiation and stimulates apoptosis of osteoclasts and osteoblasts. Life Sci. 2013;93:367–372. doi: 10.1016/j.lfs.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki N., Hattori A. Bisphenol A suppresses osteoclastic and osteoblastic activities in the cultured scales of goldfish. Life Sci. 2003;73:2237–2247. doi: 10.1016/s0024-3205(03)00603-9. [DOI] [PubMed] [Google Scholar]
- 92.Chamorro-García R., Kirchner S., Li X., Janesick A., Casey S.C., Chow C., Blumberg B. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism. Environ. Health Perspect. 2012;120:984–989. doi: 10.1289/ehp.1205063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watanabe M., Ohno S., Nakajin S. Effects of bisphenol A on the expression of cytochrome P450 aromatase (CYP19) in human fetal osteoblastic and granulosa cell-like cell lines. Toxicol. Lett. 2012;210:95–99. doi: 10.1016/j.toxlet.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 94.Zhao H.Y., Bi Y.F., Ma L.Y., Zhao L., Wang T.G., Zhang L.Z. The effects of bisphenol A (BPA) exposure on fat mass and serum leptin concentrations have no impact on bone mineral densities in non-obese premenopausal women. Clin. Biochem. 2012;45:1602–1606. doi: 10.1016/j.clinbiochem.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 95.Kim D.H., Oh C.H., Hwang Y.C., Jeong I.K., Ahn K.J., Chung H.Y., Chang J.S. Serum bisphenol a concentration in postmenopausal women with osteoporosis. J. Bone Metab. 2012;19:87–93. doi: 10.11005/jbm.2012.19.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mascia F., Denning M., Kopan R., Yuspa S.H. The black box illuminated: signals and signaling. J. Invest. Dermatol. 2012;132:811–819. doi: 10.1038/jid.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Corbett E.F., Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem. Sci. 2000;25:307–311. doi: 10.1016/s0968-0004(00)01588-7. [DOI] [PubMed] [Google Scholar]
- 98.Missiaen L., Van Acker K., Parys J.B., De Smedt H., Van Baelen K., Weidema A.F., Vanoevelen J., Raeymaekers L., Renders J., Callewaert G. Baseline cytosolic Ca2+ oscillations derived from a non-endoplasmic reticulum Ca2+ store. J. Biol. Chem. 2001;276:39161–39170. doi: 10.1074/jbc.M104044200. [DOI] [PubMed] [Google Scholar]
- 99.Pinton P., Pozzan T., Rizzuto R. The Golgi apparatus is an inositol 1, 4, 5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998;17:5298–5308. doi: 10.1093/emboj/17.18.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]