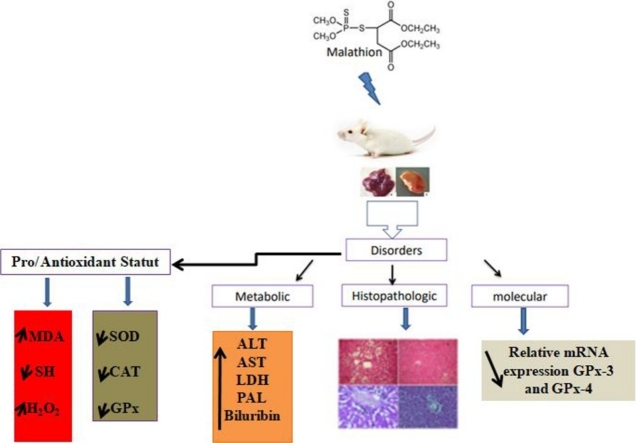
Graphical abstract
Abbreviations: MDA, malondialdehyde; GPx, glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase; OPs, organophosphorus
Keywords: Malathion, Liver, Kidney, GPx-3, GPx-4 expression, Oxidative stress, Mice
Highlights
-
•
Malathion treatment clearly induced kidney and liver dysfunction.
-
•
Malathion induced hepatic and renal changes on mRNA expressions of GPx iso-enzymes.
-
•
This disruption and genotoxicity lead to histopathologic changes in liver and kidney tissues.
-
•
OPs pesticides exposure lead to metabolic, histopathologic and molecular changes witch may be related to oxidative damage.
Abstract
The present study was undertaken to determine the effects of malathion exposure on oxidative stress, functional and metabolic parameters in kidney and liver of prepubertal male mice. For this reason, two separated groups of prepubertal male mice were used in this experiment. Animals were divided into two groups, group 1 served as a control and received the corn oil and group 2 was treated with 200 mg/kg body weight (b.w.) of malathion for 30 days. In result, we found that the malathion administration led to the perturbation of biochemical markers and histopathological as well as molecular damages. These changes were accompanied by an oxidative alternation which was evaluated by lipoperoxidation process and MDA production, a diminution of sulfhydril groups (—SH) content and an antioxidant enzyme activities depletion such as total superoxide dismutase (SOD) and its isoforms, catalase (CAT) and glutathione peroxidase (GPx) in both kidney and liver tissues. These changes were related with many histopathological lesions in the liver and kidney tissues. More importantly, this insecticide clearly caused a decline in the GPx-4 expression in liver as well as GPx-3 in kidney. These data suggest that prepubertal male mice exposure to malathion showed a marked deregulation of liver and kidney functions.
1. Introduction
Extensively using of organophosphorus pesticides in different fields such as agriculture, medicine and industry can cause many disturbances in human and wildlife. These organophosphorus (OP) compounds are immediately degraded in the environment. Their concept was introduced following the ban on organochlorines which can bioaccumulate and biomagnify, which results in ecotoxicological effects [1,2]. Particularly, malathion [O,O-dimethyl-S-(1,2-dicarcethoxyethyl) phosphorodithioate] is an OP pesticide habitually used to eradicate ectoparasites, household insects, to conserve stored grain and to eliminate disease-inducing arthropods [3,4,5]. On the negative side, it is one of OPs agents that exerts diverse toxic effects throught the inactivation of serine esterases [6], mostly acetylcholinesterase (AChE) and butyrylcholinesterase which leads to an overstimulation of the cholinergic pathways [7,8]. The OPs can achieve all the tissues leading eventually to several pathological difficulties, this is due to their lipophilic nature and their simple and rapid intestinal assimilation, including a insufficiency of the immune system [9,10] pancreatitis [11], liver disease [12,13] hematological pathosis disorder [14], kidney injury [15], decrease fertility and reproduction capability [16]. Many studies have reported toxic effects of this OPs in both humans [17,18] and animals [19,20]. Being the main actors of xenobiotic biotransformation, regulation of hepatic gene expression may play a central role in the adaptive response to altered metabolism by changing the capacity of enzymes in relevant metabolic pathways [21]. Hence, liver is the principal metabolizing site for mediating biotransformation of thiono-organophosphates and with kidney contributing to the elimination of toxic products [22]. These tissues are considered among the main targets of malathion toxicity which is mediated through oxidative stress generated by reactive oxygen species (ROS) [23,24,25]. ROS such as superoxide anion, peroxyl radicals, hydroperoxyl radical, hydrogen peroxide are produced from the molecular oxygen as a consequence of normal cellular metabolism [26]. At low or moderate concentrations, ROS are considered as part of normal oxidative metabolism, but at elevated concentrations, they cause tissue injuries, including lipids, proteins oxidation, DNA damage [27], and enzyme inactivation. They are also implicated in many pathological conditions such as cancer, diabetes, cardiovascular, pulmonary and autoimmune diseases, neurological disorders and aging, among others. The main objective of this study was therefore to highlight the role of oxidative stress as a precursor of molecular and histopathological complications following subacute exposure of prepubescent mice to malathion.
2. Materials and methods
2.1. Chemicals
Acetylthiocholine iodide, eosin stain, 5.5′-dithiobis 2-nitrobenzoic acid (DTNB), Malathion (98% purity) (fyfanon 50 EC 500 g/l), RPMI (Roswell Park Memorial Institute) and triton X-100 were purchased from SIGMA and Invitrogen.
2.2. Animals and experimental fields
Female and male mice were purchased from Pasteur Institute of Tunis. All experiments were performed according with the local ethics committee of Tunis University for the use and care of animals in accordance with the NIH recommendations. The animals were provided with food (standard pellet diet- Badr Utique-TN) and water ad libitum and maintained in animal house at controlled conditions: temperature (22 ± 2 °C) and 12 h light-dark cycle. Primiparous females were placed three per cage with one male breeder and vaginal smear was examined daily in the evening. At the weaning age (21days), after the lactational period of their offspring (prepubertal male mice) were separated and then randomly divided into two groups of 16 animals each: Group 1 was served as control and received the corn oil. Group 2 received by intragastrique gavage, the malathion in corn oil at the dose of 200 mg/kg, b.w. during 30 days. The age of animals and the used malathion-dose as well as the treatment duration were chosen based on previous work [16].
On the last day of experiment, animals were anaesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg) and sacrificed by decapitation. The blood was collected in heparinized tubes and the plasma was obtained after centrifugation at 3000g for 15 min. The tissue specimens were removed and placed in a phosphate buffered saline (PBS) solution, homogenized and centrifuged for 15 min at 9000g. Organs supernatants and plasma were stored at −80 °C for biochemical parameters determination.
2.3. Evaluation of body organ weights
The initial and final body weights were recorded. Mice in each group were euthanized and their organs were stripped from fatty tissues and blood vessels. Then, these organs were blotted, and their absolute weights were measured. Clinical signs of body and organs were evaluated for toxicological criteria and organ weights were expressed per 100 g body weight to normalize the data for statistical analysis.
2.4. Functional and metabolic parameters determination
To assess the liver function disorders, plasma alanine aminotransferase (ALT), phosphatase alkaline (PAL), aspartate aminotransferase (AST), total and direct bilirubin were measured using commercially available diagnostic kits (Biomaghreb, Ariana, Tunisia).
Concerning renal function damages, plasma urea, creatinine, uric acid and albumin analyses were also performed using commercially available diagnostic kits (Biomaghreb, Ariana, Tunisia).
2.5. Oxidative deterioration of lipids and protein thiol groups determination
Final products of lipiperoxidation, malondialdehyde (MDA) were determined using the method of Buege and Aust [28] and total thiol groups (—SH) concentration was performed according to Hu and Dillard [29].
2.6. Antioxidant activities determination
The activity of superoxide dismutase (SOD) was determined by using modified epinephrine assays and characterization of SOD isoforms was performed using KCN (2 mM), which inhibits Cu/Zn-SOD or H2O2 (5 mM), affecting both Cu/Zn-SOD and Fe-SOD whereas Mn-SOD was insensitive to both inhibitor [30,31].
The activity of CAT was assessed by measuring the initial rate of H2O2 disappearance at 240 nm [32] and GPx activity was quantified by the procedure of Flohé and Günzler [33].
2.7. Total RNA isolation and RT-PCR analysis
Total RNA was prepared using Trizol reagent according to the manufacturer's instructions. Total RNA (1 μg) reverse was transcribed using MMLV reverse transcriptase (Invitrogen, Tunis, Tunisia) by incubation at 25 °C for 10 min, at 42 °C for 60 min and at 99 °C for 5 min. The synthesized cDNA was amplified using Taq DNA polymerase (Invitrogen, Tunis, Tunisia) and the following specific primers:
GPx-4: F: 5′-AGTACAGGGGTTTCGTGTGC-3′
R: 5′-CGGCAGGTCCTTCTCTATCA-3′
GAPDH: F: 5′-GTGGATATTGTTGCCATCA-3′,
R: 5′-ACTCATACAGCACCTCAG-3′.
PCR conditions were 30 cycles of 94 °C for 30 s, 59 °C for 30 s and 72 °C for 30 s, followed by 5 min incubation at 72 °C. PCR products were run on 1.5% agarose gel and then stained with ethidium bromide.
2.8. Histopathological examination
Immediately after the euthanasia, small pieces of both tissues were harvested and washed with ice cold saline, fixed in a 10% neutral buffered formalin solution, embedded in paraffin and used for histopathological examination. These pieces were cut into 5 μm thick, deparaffinized, hydrated and stained sections with hematoxylin and eosin (HE). The liver and kidney sections were examined in control and malathion treatment.
2.9. Protein determination
Protein concentration was determined according to Bradford method using bovine serum albumin (BSA) as standard [34].
2.10. Statistical analysis
Statistical significance was determined by one-way ANOVA using Statview statistical software. Results were expressed as means ± standard error of the mean (S.E.M.). The data are repre-sentative of 16 independent experiments. All statistical tests were two-tailed, and a p value of 0.05 or less was considered significant.
3. Results
3.1. Body weight, liver and kidney relative weights
As shown in Table 1, the sub-acute exposure of prepubertal male mice to malathion reduced the body weight and mass gain of prepubertal male mice. In contrast, a significant increase in the relative weights of both liver and kidney was observed in malathion-treated mice.
Table 1.
Body weight, mass gain changes in relative and absolute weights of liver and kidney after sub-acute exposure of prepubertal male mice to malathion.
| CTR | Malathion | |
|---|---|---|
| Initial body Weight (g) | 8,71 ± 0,15 | 9,33 ± 0,12 |
| Final body Weight (g) | 29,76 ± 0,99 | 21,41 ± 0.57 ** |
| Mass Gain (g) | 21,05 ± 0,86 | 12,08 ± 4,45 * |
| Absolute Weight of Liver (g) | 2,09 ± 0,12 | 1,67 ± 0.15 * |
| Relative Weight of Liver (g/100 g PC) | 7,03 ± 0,10 | 7,8 ± 0,09 |
| Absolute Weight of Kidney (g) | 0,31 ± 0,01 | 0,29 ± 0,01 |
| Relative Weight of Kidney (g/100 g P.C.) | 1,04 ± 0,01 | 1,35 ± 0.01 * |
Values are means + S.D. of 16 mice in each group.
*and ** represent the statistical difference between control and treated groups, respectively, at p < 0.05 and p < 0.01.
CTR: control group.
3.2. Liver and kidney functions
According to Table 2, the malathion (200 mg/kg) exposure was associated with liver and kidney dysfunctions in prepubertal male mice, while, a significant increase in some liver biochemical parameters including ALT, AST, PAL and LDH was observed in malathion-treated mice. Malathion treatment was also associated with hepatotoxicity in male mice as revealed by an increase in direct and total bilirubin.
Table 2.
Changes in liver function after sub-acute exposure of prepubertal male mice to malathion.
| CTR | Malathion | |
|---|---|---|
| ALT (UI/l) | 42,4 ± 1,5 | 59,2 ± 2,63** |
| AST (UI/l) | 130,6 ± 2,2 | 198,8 ± 14,9** |
| PAL (UI/l) | 75,62 ± 11,3 | 142,6 ± 28,13* |
| LDH (UI/L) | 213 ± 35,6 | 352,1 ± 8,81** |
| Bilirubin (mg/dl) | 5,10 ± 0,21 | 6,89 ± 0,31* |
| total Protein (g/100 g) | 10,2 ± 0,51 | 7,48 ± 0,44** |
Values are means + S.D. of 16 mice in each group.
*and ** represent the statistical difference between control and treated groups, respectively, at p < 0.05 and p < 0.01.
CTR: control group.
In kidney, a significant (p < 0.05) increase in plasmatic creatinine and urea levels as well as a decrease in albumin and uric acid contents in plasma were observed in mices treated with malathion (Table 3).
Table 3.
Changes in liver function after sub-acute exposure of prepubertal male mice to malathion.
| CTR | Malathion | |
|---|---|---|
| Creatinine (μmoles/L) | 102,83 ± 5,36 | 142 ± 6,59** |
| Uric Acide (μmoles/L) | 288,64 ± 14,87 | 143,17 ± 11,42** |
| Urea (mmoles/L) | 7,06 ± 0,4 | 9,72 ± 0,32** |
| Albumin (g/dl) | 5,46 ± 0,29 | 3,62 ± 0,36* |
| total Protein (g/100 g) | 2,98 ± 0,29 | 3,1 ± 0,15 |
Values are means + S.D. of 16 mice in each group.
*and ** represent the statistical difference between control and treated groups, respectively, at p < 0.05 and p < 0.01.
CTR: control group.
3.3. Oxidative stress status
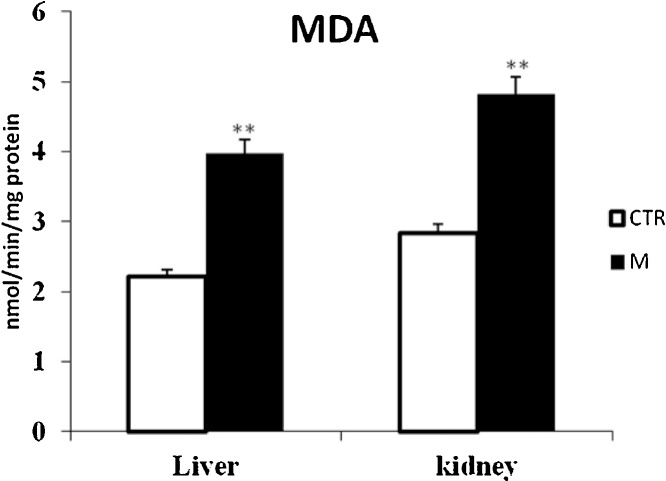
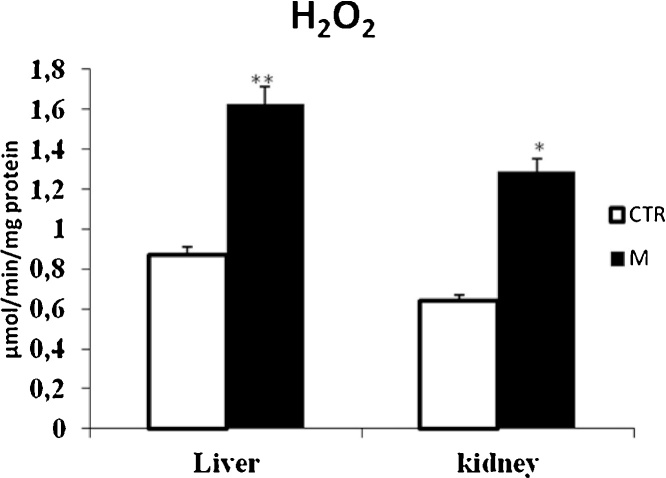
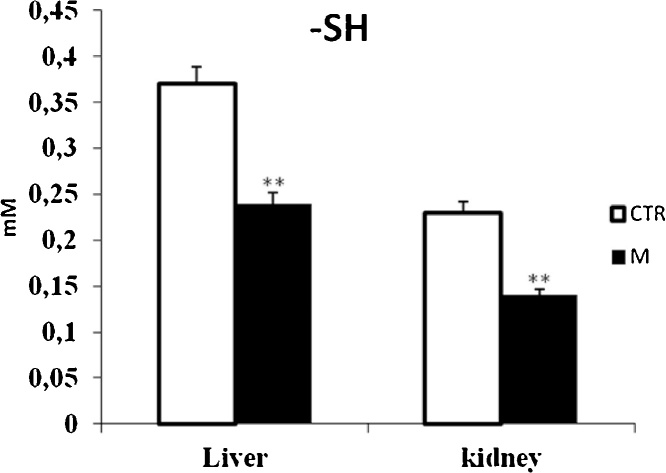
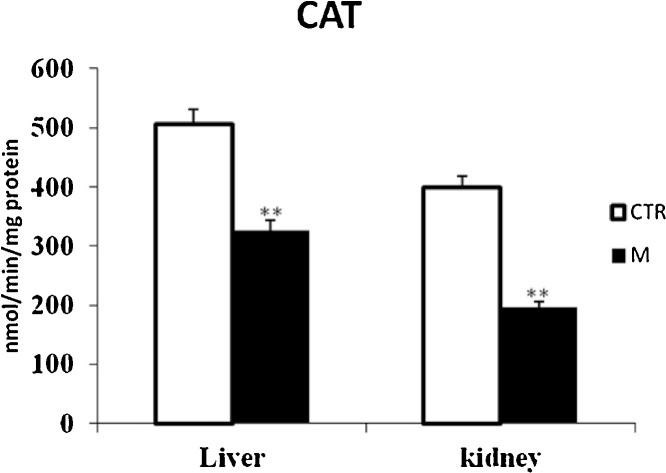
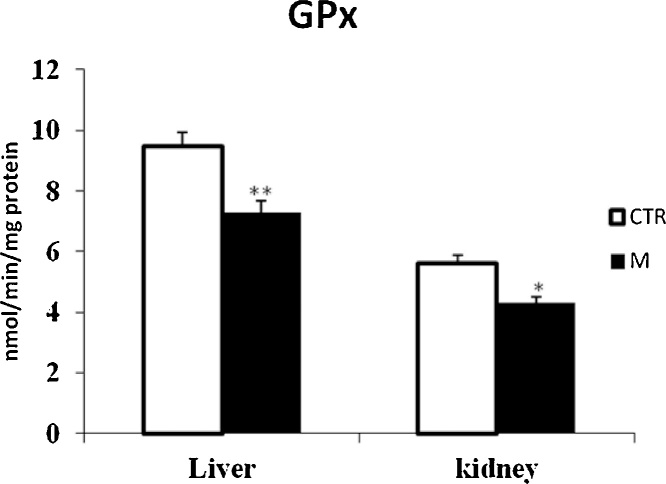
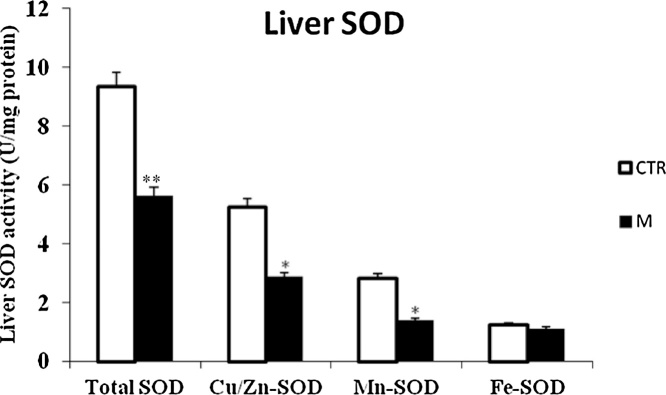
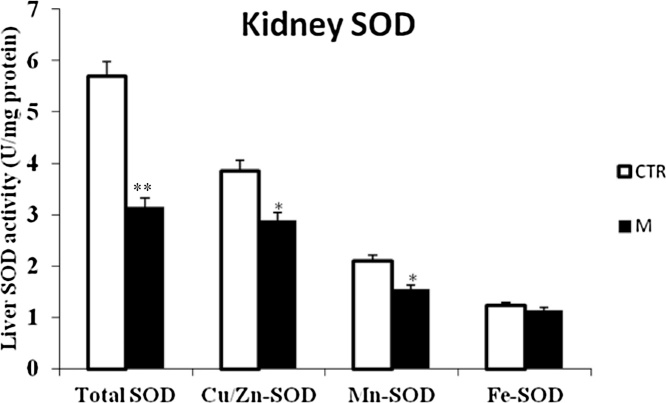
Data from Fig. 1, Fig. 3 clearly revealed that malathion exposure increased significantly (p < 0.05) the MDA and H2O2 contents, respectively. In contrast, it significantly decreased the thiol (—SH) groups (Fig. 2) and reduced the CAT (Fig. 4) and GPx (Fig. 7) activities in both liver and kidney tissues. Total SOD, Cu/Zn–SOD and Mn–SOD activities in liver (Fig. 5) were significantly (p < 0.05) reduced in response to malathion exposure. In kidney tissues, total-SOD and Mn–SOD were reduced in malathion-treated mice (Fig. 6).
Fig. 1.
Effects of malathion exposure (200 mg kg_1 b.w., p.o.) of prepubertal male mice during 30 days on MDA level in liver and kidney tissues (n = 12). *: p < 0.05, **: p < 0.01 versus control group.
Fig. 3.
Effects of malathion exposure (200 mg kg_1 b.w., p.o.) of prepubertal male mice during 30 days on H2O2 level in liver and kidney tissues (n = 12). *: p < 0.05, **: p < 0.01 versus control group.
Fig. 2.
Effects of malathion exposure (200 mg kg_1 b.w., p.o.) of prepubertal male mice during 30 days on thiols group level in liver and kidney tissues (n = 12). *: p < 0.05, **: p < 0.01 versus control group.
Fig. 4.
Effects of malathion exposure (200 mg kg_1 b.w., p.o.) of prepubertal male mice during 30 days on catalase activity in liver and kidney tissues (n = 12). *: p < 0.05, **: p < 0.01 versus control group.
Fig. 7.
Effects of malathion exposure (200 mg kg_1 b.w., p.o.) of prepubertal male mice during 30 days on GPx activity in liver and kidney tissues (n = 12). *: p < 0.05, **: p < 0.01 versus control group.
Fig. 5.
Effects of malathion exposure (200 mg kg_1 b.w., p.o.) of prepubertal male mice during 30 days on SOD and its isoformes activities in liver tissue (n = 12). *: p < 0.05, **: p < 0.01 versus control group.
Fig. 6.
Effects of malathion exposure (200 mg kg_1 b.w., p.o.) of prepubertal male mice during 30 days on SOD and its isoformes activities in kidney tissue (n = 12). *: p < 0.05, **: p < 0.01 versus control group.
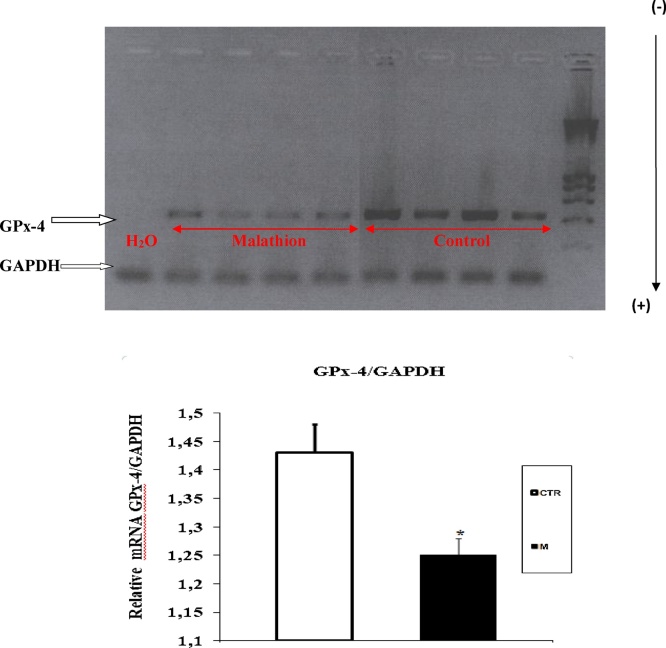
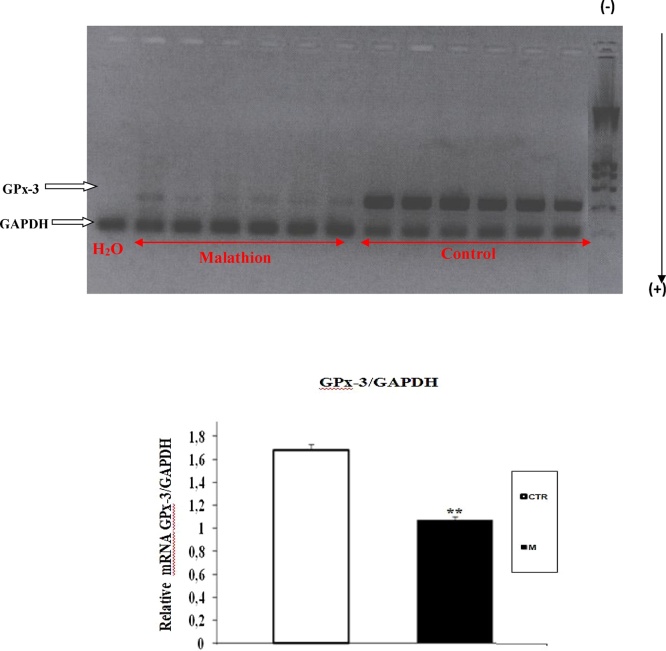
3.4. Expression of GPx-3 and GPx-4 in liver and kidney
Data from Fig. 8, Fig. 9 showed that liver and kidney express both GPx-4 and GPx-3 isozymes, and that their expressions were remarkably depleted by malathion exposure. In contrast, the GADPH expression remained unchanged in both treated and non-treated mice.
Fig. 8.
RT-PCR analysis of malathion effect on GPx-4 expression in liver of male mice. Prepubertal male mice were treated with malathion (200 mg kg−1 b.w., p.o.) during 30 days. The relative expression of GPx isoforms was quantified by densitometry and normalized to GAPDH expression. Data are expressed as mean SEM (n = 4). *: p < 0.05, **: p < 0.01 versus control group.
Fig. 9.
RT-PCR analysis of malathion effect on GPx-3 expression in kidney of prepubertal male mice. Prepubertal male mice were treated with malathion (200 mg kg−1 b.w., p.o.) during 30 days. The relative expression of GPx isoforms was quantified by densitometry and normalized to GAPDH expression. Data are expressed as mean SEM (n = 6). *: p < 0.05, **: p < 0.01 versus control group.
3.5. Histopathological changes in liver and kidney tissues
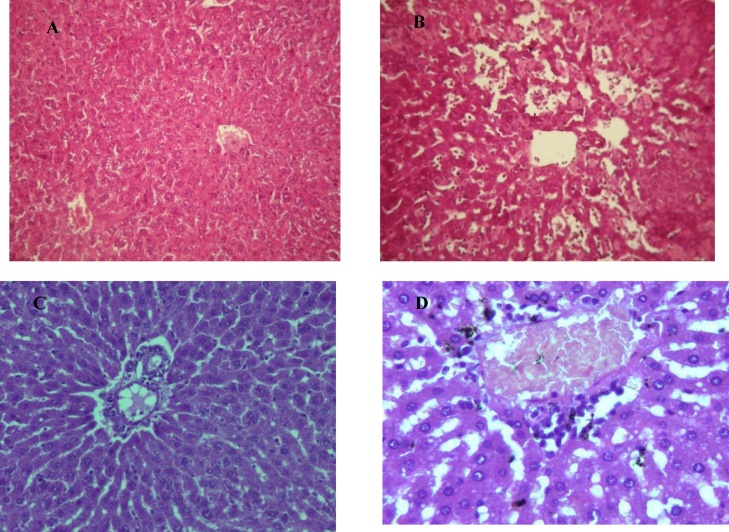
Light microscopic observation of liver of control mice showed regular and compact configuration with well organized hepatic cell and central vain (Fig. 10A and C). The section of malathion treated mice showed different histopathological alteration. The slide from treatment with malathion showed severe damage in hepatic tissue including prominent enlargement of sinusoids, infiltration of mononuclear cell, dilation, hemorrhage and necrosis (Fig. 10B and D).
Fig. 10.
Liver histology showing the effect of malathion exposure (200 mg kg_1 b.w., p.o.) during 30 days of prepubertal male mice. Normal architecture in control group (A) (x100) and (C) (x400). Histological changes in malathion treated group (B) (x100) and (D) (x400).
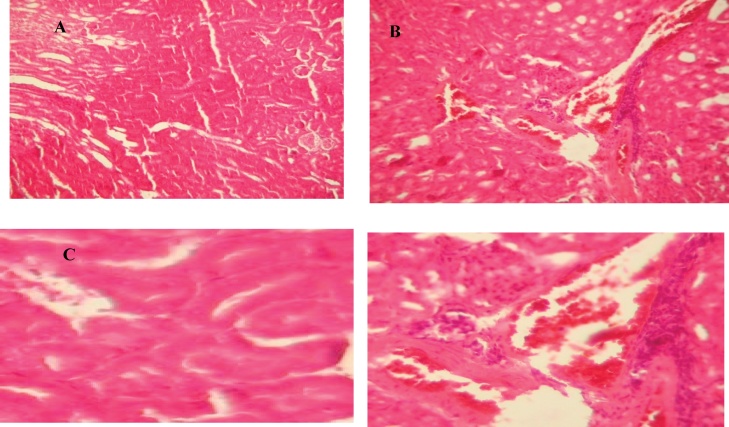
Histopathological study on the kidney of control mice showed regular structure with capillaries, tubules, glomerulus, and Bowman's capsule (Fig. 11A and C). On the other hand, the areas of renal cortex containing renal corpuscles and associated tubules expressed more pronounced changes in malathion-treated animals compared with control. In the case of malathion-treated group, highly degeneration of glomeruli, Bowman’s capsules and associated tubules structure, shrinkage of glomeruli and edema of renal tubules and raising of urinary space was also noticed (Fig. 11B and D).
Fig. 11.
Kidney histology showing the effect of malathion exposure (200 mg kg_1 b.w., p.o.) during 30 days of prepubertal male mice. Normal architecture in control group (A) (x100) and (C) (x400). Histological changes in malathion treated group (B) (x100) and (D) (x400).
4. Discussion
The present research was to evaluate the putative implication of oxidative stress in the sub-acute effects of malathion on the liver and kidney function in male mice. Firstly, we have shown that malathion-induced a significant (p < 0.05) reduction of body weight gain. Conversely, a significant increase of liver and kidney relative weights in mice was observed. These finding are in agreement with previous studies who demonstrated morphologic and symptomatic modifications in morphometric parameters following exposure to malathion. These changes were characteristic of acetylcholinesterase inhibition including accumulation of acetylcholine and subsequent activation of cholinergic, muscarinic and nicotinic receptors as well as the neurological deficits in male mice exposed to malathion [35]. All these disturbances can lead to various toxic effects in male mice, including their feeding ability, and therefore, their metabolisms performances [36]. The sub-acute administration of malathion lead to an increase in weights of liver and kidney which was associated to remarkable injuries. These damages were assessed by numerous perturbations in the metabolism of these organs. Indeed, increased AST, ALT and ALP activities and depletion in direct and total bilirubin demonstrated a hepatotoxicity effect. In addition, an increase in creatinine and urea levels indicated a kidney dysfunction. These disruptions were observed with several cases of OPs poisoning in rats such as chlorfenvinfos, fenthion and dimethoate [37].
To explain these metabolic perturbations, the transaminases such ALT and AST are major cytolysis markers in the liver and their activities increasing in the plasma of male mice resulted from the impairment and necrosis of the function of tissues with subsequent liberation of enzymes into the circulation from the damaged tissues [38,39]. ALP, which is an important critical enzyme in biological processes, is responsible for detoxification, metabolism and biosynthesis of energetic macromolecules for different essential functions. Any interference in this enzyme leads to biochemical alternation and impairment in the tissue and cellular function [40]. In addition, it has been reported that the increase in the activity of ALP in plasma might be due to the increased permeability of plasma membrane or cellular necrosis [41].
To explain renal disturbances, these results corroborated with previous studies in adult rats and their suckling pups intoxicated with dimethoate [42] and in adult rats treated with chlorfenvinfos or with phosphorodithioate [43,44]. Furthermore, plasmatic uric acid levels can be influenced by many drugs witch could affect the net reabsorption of uric acid in the proximal tubule of the nephron [45]. Paradoxically, Bosco et al. [46] showed no changes in glomerular filtration rate of Octodon degus exposed to malathion (200 ppm) as sole drinking fluid for 90 days. This discrepancy might result from the difference in the sensitivity of two species (O. degus and rats). On the another hand, mercuric chloride and dimethoate lead to an increase in urine volume in adult rats exposed to moderate doses of these compounds [42].
In addition to these manifestations, increased lipoperoxidation assessed in term of MDA, and hydrogen peroxide, decreased thiol groups level as well as a depletion of antioxidant enzyme activities such as CAT, total SOD, Cu/Zn-SOD, Mn-SOD and Fe-SOD and GPx was found in malathion-treated prepubertal male mice. Our findings have fully corroborated other work that proves that the administration of organophosphorus compounds to male mice caused an imbalance in antioxidant status in liver and kidney tissues [13]. Being the main actors of xenobiotic biotransformation, regulation of hepatic gene expression may play a central role in the adaptive response to altered metabolism by changing the capacity of enzymes in relevant metabolic pathways [21]. Thus, liver is the principal metabolizing site for mediating biotransformation of thiono-organophosphates and with kidney contributing to the elimination of toxic products [22,23]. Excessive generation of reactive oxygen species (ROS) and oxidative stress are the precursors of many pathologies associated to this organophosphorus exposure [12,25] many findings have reported the enhancement of oxidative stress in human OP poisoning cases [47] and in animals [12,48]. ROS such as superoxide anion, peroxyl radicals, hydroperoxyl radical, hydrogen peroxide produced from the molecular oxygen as a consequence of normal cellular metabolism [26]. At low or moderate concentrations, ROS are considered as part of normal oxidative metabolism, but at elevated concentrations, they cause tissue injuries, including lipids, proteins oxidation, DNA damage [27] and enzyme inactivation. They are also implicated in many pathological conditions such as cancer, diabetes, cardiovascular, pulmonary and autoimmune diseases, neurological disorder and aging, among others [48]. Recently, The clinical importance of this pathologies has led to the development of many pharmaceuticals and researches have already tested several natural compounds to prevent and protecting living organisms from the poisonous effects of pesticides [49,50,51]. On other hand, our findings, oxidative damages in both liver and kidney of treated mice was confirmed by a decrease of GPx-4 and GPx-3 mRNA expression respectively in liver and kidney. This decrease may be due to inhibition of enzyme activity after excessive free radical production. Moreover, free radicals attack not only proteins but also DNA bases; therefore, they have the potential to cause mutagenic lesions. These results were confirmed by RT-PCR analysis of enzyme mRNA levels. Gene expression of GPx-3 and GPx-4 were decreased in the treated group, indicating the disruption of the redox equilibrium in treated mice. The animals were administered malathion orally by gavage and the microscopic slide of selected organs showed major histological changes in their liver and kidney tissues according to previous study with diazinon [10]. Changes observed from light microscopes showed a varied scope of occurrence and different degree of intensity according to the dose of malathion. Results from histological investigation are in accord with diverse earlier studies which elucidate that the introduction to pesticides led to provoke intensive biochemical and physiological turbulence in experimental animals [10]. According to Tos-Luty et al. [52], malathion intoxication led to injurious effects on the organization of the liver and kidney with the persistence of thin subcapsular infiltrations, diffused parenchymatous degeneration of single hepatocytes. Furthermore, they indicated that the histopathological alteration in the kidneys occurred in all animals. These alterations demonstrated parenchymatous deterioration of the cells of renal tubules and hyperemia of the cortical area of the kidney, particularly of renal glomeruli, with infiltrations [10]. A number of experiments indicated that malathion caused testicular toxicity [16], hepatotoxicity [53], hematotoxicity [54,55], genotoxicity [56] and nephrotoxicity [54]. However, diverse works showed that malathion as well as other pesticides provoke histopathological alterations of liver and kidney in different rodent animals [57,58].
5. Conclusion
Taken together, it can be concluded that exposure to the organophosphorus malathion induced production of harmful ROS, causing thereby oxidative stress. Installation of oxidative stress can changed the body, liver and kidney weights, and altered their biochemical markers such as hepatic ALT, AST, PAL, LDH, total and direct bilirubin and renal plasma urea, creatinine and uric acid. In addition to the depletion of antioxidant enzymes system, malathion also induced molecular and histopathological modifications.
Conflict of interest
There is no conflict of interest.
Acknowledgement
Financial support of the Tunisian Ministry of Higher Education and Scientific Research is gratefully acknowledged.
References
- 1.Garcia S.M., Zerbi A., Aliaume C., Do Chi T., Lasserre G. The ecosystem approach to fisheries. Issues, terminology, principles, institutional foundations, implementation and outlook. FAO Fish. Tech. Pap. 2003:443–471. [Google Scholar]
- 2.Kalender A., Selvaraj A., Kim S.Y., Gulati P., BrÛlé S., Viollet B., Kemp B.E., Bardeesy N., Dennis P., Schlager J.J., Marette A., Kozma S.C., Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assini F.L., Zanette K.D., Brocardo P.S., Pandolfo P., Rodrigues A.L.S. Behavioral effects and ChE measures after acute and repeated administration of malathion in rats. Environ. Toxicol. Pharmacol. 2005;20:443–449. doi: 10.1016/j.etap.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Elston D.M. Controversies concerning the treatment of lice and scabies. J. Am. Acad. Dermatol. 2002;46:794–796. doi: 10.1067/mjd.2002.121027. [DOI] [PubMed] [Google Scholar]
- 5.Suresh Babu N., Malik J.K., Rao G.S. Effects of subchronic malathion exposure on the pharmacokinetic disposition of pefloxacin. Environ. Toxicol. Pharmacol. 2006;22:167–171. doi: 10.1016/j.etap.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Taylor P., Radic Z., Hosea N.A. Structural bases for the specificity of cholinesterase catalysis and inhibition. Toxicol. Lett. 1995;83:453–458. doi: 10.1016/0378-4274(95)03575-3. [DOI] [PubMed] [Google Scholar]
- 7.Bartling A., Worek F., Szinicz L., Thiermann H. Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology. 2007;233:166–172. doi: 10.1016/j.tox.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Kwong T.C. Organophosphate pesticides: biochemistry and clinical toxicology. Ther. Drug Monit. 2002;24:144–149. doi: 10.1097/00007691-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Lee A.G., East J.M., Balgauvy P. Interactions of insecticides with biological membranes. Pesticide Sci. 1991;32:317–327. [Google Scholar]
- 10.Handy R.D., Abd-El Samei H.A., Bayomy M.F.F. Chronic diazinon exposure: pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicology. 2002;172:13–34. doi: 10.1016/s0300-483x(01)00575-3. [DOI] [PubMed] [Google Scholar]
- 11.Gokalp O., Buyukvanli B., Cicek E. The effects of diazinon on pancreatic damage and ameliorating role of vitamin E and vitamin C. Pesticide Biochem. Physiol. 2005;81:123–128. [Google Scholar]
- 12.Franco J.L., Posser T., Mattos J.J. Zinc reverses malathion-induced impairment in antioxidant defenses. Toxicol. Lett. 2009;187:137–143. doi: 10.1016/j.toxlet.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Kalender S., Ogutcu A., Uzunhisarcikli M. Diazinon induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. J. Toxicol. 2005:197–206. doi: 10.1016/j.tox.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Kalender Y., Uzunhisarcikli M., Ogutcu A. Effects of diazinon on pseudocholinesterase activity and haematological indices in rats: the protective role of vitamin E. Environ. Toxicol. Pharmacol. 2006;22:46–51. doi: 10.1016/j.etap.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Afshar S., Farshid A.A., Heidari R., Ilkhanipour M. Histopathological changes in the liver and kidney tissues of Wistar albino rat exposed to fenitrothion. Toxicol. Ind. Health. 2008;24:581–586. doi: 10.1177/0748233708100090. [DOI] [PubMed] [Google Scholar]
- 16.Selmi S., Tounsi H., Safra I., Abdellaoui A., Rjeibi M.R., El-Fazaa S., Gharbi N. Histopathological, biochemical and molecular changes of reproductive function after malathion exposure of prepubertal male mice. RSC Adv. 2015;5:13743. [Google Scholar]
- 17.Abdel-Rahman A., Abou-Donia S., El-Masry E. Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. J. Toxicol. Environ. Health. 2004;67:163–192. doi: 10.1080/15287390490264802. [DOI] [PubMed] [Google Scholar]
- 18.Rothlein J., Rohlman D., Lasarev M. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and non-agricultural Hispanic workers. Environ. Health Perspect. 2006;114:691–696. doi: 10.1289/ehp.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brocardo P.S., Assini F., Franco J.L., Pandolfo P., Muller Y.M., Takahashi R.N., Dafre A.L., Rodrigues A.L. Zinc attenuates malathion-induced depressant-like behavior and confers neuroprotection in the rat brain. Toxicol. Sci. 2007;97:140–148. doi: 10.1093/toxsci/kfm024. [DOI] [PubMed] [Google Scholar]
- 20.Da Silva A.P., Meotti F.C., Santos A.R., Farina M. Lactational exposure to malathion inhibits brain acetylcholinesterase in mice. Neurotoxicology. 2006;27:1101–1105. doi: 10.1016/j.neuro.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Lasram M.M., Annabi A.B., Elj N., El-Fazaa S., Gharbi N. Metabolic disorders of acute exposure to malathion in adult Wistar rats. J. Hazard. Mater. 2009;163:1052–1055. doi: 10.1016/j.jhazmat.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 22.Neal R.A. A comparison of the in vitro metabolism of parathion in the lung and liver of the rabbit. Toxicol. Appl. Pharmacol. 1972;23:123–130. doi: 10.1016/0041-008x(72)90211-6. [DOI] [PubMed] [Google Scholar]
- 23.Yang M.C., McLean A.J., Rivory L.P., Le Couteur D.G. Hepatic disposition of neurotoxins and pesticides. Pharmacol. Toxicol. 2000;87:286–291. doi: 10.1034/j.1600-0773.2000.pto870608.x. [DOI] [PubMed] [Google Scholar]
- 24.Delgado E.H., Streck E.L., Quevedo J.L., Dal-Pizzol F. Mitochondrial respiratory dysfunction and oxidative stress after chronic malathion exposure. Neurochem. Res. 2006;31:1021–1025. doi: 10.1007/s11064-006-9111-1. [DOI] [PubMed] [Google Scholar]
- 25.Reus G.Z., Valvassori S.S., Nuernberg H. DNA damage after acute and chronic treatment with malathion in rats. J. Agric. Food Chem. 2008;56:7560–7565. doi: 10.1021/jf800910q. [DOI] [PubMed] [Google Scholar]
- 26.Halliwell B., Gutteridge J.M.C. 3rd ed. Clarendon Press; Oxford: 1999. Free Radicals in Biology and Medicine. [Google Scholar]
- 27.Dal-Pizzol F., Klamt F., Dalmolin R.J.S. Mitogenic signaling mediated by oxidants in retinal treated Sertoli cells. Free Radic. Res. 2001;35:749–755. doi: 10.1080/10715760100301251. [DOI] [PubMed] [Google Scholar]
- 28.Begue J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 29.Hu M.L., Dillard C.J. Plasma SH and GSH measurement. Methods Enzymol. 1994;233:385–387. [Google Scholar]
- 30.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 31.Spitz D., Oberley L. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 32.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 33.Flohé L., Günzler W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 34.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Campana A.D., Sanchez F., Gamboa C., Gomez-Villalobos Mde J., De La Cruz F., Zamudio S., Flores G. Dendritic morphology on neurons from prefrontal cortex, hippocampus, and nucleus accumbens is altered in adult male mice exposed to repeated low dose of malathion. Synapses. 2008;62:283–290. doi: 10.1002/syn.20494. [DOI] [PubMed] [Google Scholar]
- 36.Farag A.T., Ewediah M.H., El-Okazy A.M. Reproductive toxicology of acephate in male mice. Reprod. Toxicol. 2000;14:457–462. doi: 10.1016/s0890-6238(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 37.Kamath V., Rajini P.S. Altered glucose homeostasis and oxidative impairment in pancreas of rats subjected to dimethoate intoxication. Toxicology. 2007;231:137–146. doi: 10.1016/j.tox.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 38.Lasram M.M., Annabi A.B., Rezg R., El-Fazaa S., Gharbi N. Effect of short-time malathion administration on glucose homeostasis in Wistar rat. Pestic. Biochem. Physiol. 2008;92:114–119. [Google Scholar]
- 39.Videira R.A., Antunes-Madeira M.C., Lopes V.I., Madeira V.M. Changes induced by malathion, methyl parathion and parathion on membrane lipid physicochemical properties correlate with their toxicity. Biochim. Biophys. Acta. 2001;1511:360–368. doi: 10.1016/s0005-2736(01)00295-4. [DOI] [PubMed] [Google Scholar]
- 40.Rezg R., Mornagui B., Kamoun A., El-Fazaa S., Gharbi N. Effect of subchronic exposure to malathion on metabolic parameters in the rat. CR Biol. 2007;330:143–147. doi: 10.1016/j.crvi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Rahman A., Tsurumi S., Amakawa T., Soga K., Hoson T., Goto N., Kamisaka S. Involvement of ethylene and gibberellin signalings in chromosaponin I-induced cell division and cell elongation in the roots of Arabidopsis seedlings. Plant Cell Physiol. 2000;41:1–9. doi: 10.1093/pcp/41.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Mahjoubi S.A., Fetoui H., Zeghal N. Nephrotoxicity induced by dimethoate in adult rats and their suckling pups. Pestic. Biochem. Physiol. 2008;91:96–108. [Google Scholar]
- 43.Jablonska J., Brzezinski J. The acute toxic effects of chlorfenvinphos (CHF) on rat kidney. Toxicol. Lett. 1996;88:32–42. [Google Scholar]
- 44.Dheranetra W., Keeadtisuke S., Swatek F.E. Renal effects in the delayed toxicity of O,S,S,-trimethyl-phosphorodithioate. Pestic. Biochem. Physiol. 1988;30:95–100. [Google Scholar]
- 45.Reyes A.J. Cardiovascular drugs and serum uric acid. Cardiovasc. Drugs Ther. 2003;17:397–414. doi: 10.1023/b:card.0000015855.02485.e3. [DOI] [PubMed] [Google Scholar]
- 46.Bosco D., Alma A., Arzone A. Studies on population dynamics and spatial distribution of leafhoppers in vineyards (Homoptera: Cicadellidae) Ann. Appl. Biol. 1997;130:1–11. [Google Scholar]
- 47.Buratti B., Hicks M., Davies A. Spectrophotometry of the small satellites of Saturn and their relationship to Iapetus, Phoebe and Hyperion. Icarus. 2005;175:490–495. [Google Scholar]
- 48.Possamai F.P., Fortunato J.J., Feier G. Oxidative stress after acute and sub-chronic malathion intoxication in Wistar rats. Environ. Toxicol. Pharmacol. 2007;23:198–204. doi: 10.1016/j.etap.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Assayed M.E., Khalaf A.A., Salem H.A. Protective effects of garlic extract and vitamin C against in vivo cypermethrin-induced teratogenic effects in rat offspring. Food Chem. Toxicol. 2010;48:3153–3158. doi: 10.1016/j.fct.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Datta S., Dhar P., Mukherjee A., Ghosh S. Influence of polyphenolic extracts from Enydra fluctuans on oxidative stress induced by acephate in rats. Food Chem. Toxicol. 2010;48:2766–2771. doi: 10.1016/j.fct.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Vijaya Padma V., Sowmya P., Felix Arun T. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem. Toxicol. 2011;49:991–998. doi: 10.1016/j.fct.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Tos-Luty S., Obuchowska-Przebirowska D., Latuszynska J., Tokarska-Rodak M., Haratym-Maj A. Dermal and oral toxicity of malathion in rats. Ann. Agric. Environ. Med. 2003;10:101–106. [PubMed] [Google Scholar]
- 53.Kalender S., Uzun F.G., Durak D., Demir F., Kalender S. Malathion-induced hepatotoxicity in rats: the effects of vitamins C and E. Food Chem. Toxicol. 2010;48:633–663. doi: 10.1016/j.fct.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 54.Al-Attar A.M. Physiological and histopathological investigations on the effects of α-lipoic acid in rats exposed malathion. J. Biomed. Biotechnol. 2010:1–8. doi: 10.1155/2010/203503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durak D., Uzun F.G., Kalender S., Ogutcu A., Uzunhisarcikli M., Kalender A. Malathion induced oxidative stress in human erythrocytes and the protective effect of vitamins C and E in vitro. Environ. Toxicol. 2009;24:235–242. doi: 10.1002/tox.20423. [DOI] [PubMed] [Google Scholar]
- 56.Giri S., Prasad S.B., Giri A., Sharma G.D. Genotoxic effects of malathion an organphosphorous insecticide, using three mammalian bioassays in vivo. Mutat. Res. 2002;514:223–231. doi: 10.1016/s1383-5718(01)00341-2. [DOI] [PubMed] [Google Scholar]
- 57.Farshid A.A., Heidari R., Ilkhanipour M. Histopathological changes in the liver and kidney tissues of Wistar albino rat exposed to fenitrothion. Toxicol. Ind. Health. 2008;24:581–586. doi: 10.1177/0748233708100090. [DOI] [PubMed] [Google Scholar]
- 58.Kerem M., Bedirli N., Gurbuz N., Taylan Ömerfi A.A., Hatice P. Effects of acute fenthion toxicity on liver and kidney function and histology in rats. Turk. J. Med. Sci. 2007;37:281–288. [Google Scholar]