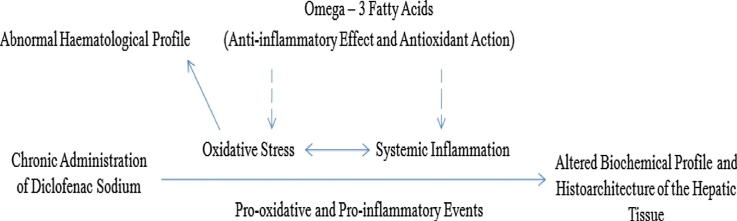
Graphical abstract
A schematic diagram showing the protective effects of omega-3 fatty acids in diclofenac sodium − induced hepatotoxicity.
Chemical compounds studied in this article: Diclofenac sodium, Omega-3 fatty acids, ketamine hydrochloride, Xylazine
Keywords: Omega-3 fatty acids, Hepatoprotective, Diclofenac, Inflammation, Oxidative stress
Highlights
-
•
Diclofenac sodium instigates pro-oxidative and pro-inflammatory responses.
-
•
Dietary supplementation with omega-3 fatty acids (N-3) boost the antioxidant system.
-
•
Low dose of N-3 has more hepatoprotective effects than the high.
Abstract
The global embrace of the Western dietary style has necessitated the need for supplementation with omega-3 fatty acids (N-3) to redress the imbalance in omega-6/omega-3 fatty acids ratio. Therefore, the study investigated the effects of pre-treatment with N-3 in adult male Wistar rats exposed to diclofenac sodium (DF). Twenty adult male Wistar rats were used for this study. They were divided into 4 groups of 5 rats each, which included: Group 1 - Normal control; Group 2 - DF control; Group 3 - Low N-3 + DF; and, Group 4 - High N-3 + DF. The rats in group 2 were administered DF (10 mg/kg b.w./day, im) during the last 7 days of the experiment, while the rats in groups 3 and 4 were pre-treated with N-3 at 100 and 300 mg/kg b.w./day, po respectively for 21 days, afterwards, they received DF at 10 mg/kg b.w./day (im) for 7 days. The result showed that DF significantly increased malondialdehyde, lactate dehydrogenase, and pro-inflammatory markers (total white blood cell count, uric acid, platelet/lymphocyte and neutrophil/lymphocyte ratios). Moreover, DF significantly elevated the activities of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase, but, significant reduced the total antioxidant capacity and the activities of superoxide dismutase, catalase, and glutathione peroxidase. The histological results were parallel to the biochemical and haematological findings. Pre-treatment with N-3 significantly prevented the manifestation of the abnormalities brought about by DF. Although there were indications of the dose-dependent effects of N-3, the low dose was found to be more effective. In conclusion, the pre-administration of N-3, preferably at a low dose, could reduce hepatotoxicity that could result from subsequent exposure to DF.
1. Introduction
Diclofenac belongs to non-steroidal anti-inflammatory drug (NSAID) family. It is a phenylacetic acid derivative which is well-known for its analgesic and anti-inflammatory properties [1]. Also, its antipyretic and anti-bacterial effects have been reported [2,3]. Despite the therapeutic actions of DF, it has notable adverse effects.
The mechanism of DF-induced hepatotoxicity has been partially attributed to; mitochondrial injury [4,5], generation of oxidative stress [6], alteration of the integrity of covalent protein by reactive metabolites [7], and immune-mediated mechanisms [8]. Studies have indicated that the metabolites of DF are capable of causing hepatocytes apoptosis [9]. Therefore, potential therapeutic agent that could arrest any of the pathological pathways activated by DF, could be used to arrest or reverse its cytotoxic action. Omega-3 fatty acids (N-3) have been noted to have NSAIDs sparing effects [10].
Western diets are considered as having omega-6 to omega-3 fatty acids ratio of 15–30:1, whereas diets which we are naturally adapted to, such as, those for palaeolithic and modern hunter-gatherers, contain almost equal amount of omega-6 and omega-3 fatty acids (1:1–2:1) [11]. The high content of omega-6 polyunsaturated fatty acids in Western diets have been linked with a number of inflammatory disorders e.g. rheumatoid arthritis, heart disease, colitis, and osteoarthritis [12]. Hence, dietary supplementation with N-3 has been recommended to redress this imbalance.
Therefore, the present study investigated the effects of pre-treatment with omega-3 fatty acids (eicosapentaenoic acid and docosahexaenoic acid − 3:2) on liver function parameters, antioxidant/pro-oxidant indices, and systemic inflammatory markers in diclofenac-induced hepatotoxicity in male Wistar rats.
2. Materials and methods
2.1. Drugs
Omega-3 fatty acids were procured from Gujarat Liqui Pharmacaps Pvt. Ltd., Vadodara, Gujarat, India, while diclofenac sodium was purchased from Wuhan Grand Pharmaceutical Company, Wuhan, Hubei, China. In addition, ketamine hydrochloride and xylazine were purchased from Nicholas Piramal Ltd., Thane, Maharashtra, India.
2.2. Animal care
Twenty (20) adult male Wistar rats (weight range: 150–200 g) were used for this study. They were acquired from trusted commercial breeders. The rats were kept in wooden cages, which were placed in the animal house of the resident university of the authors, at about 27 °C temperature and photo-periodicity of about 12 h light/12 h dark. The animals were given standard pelletised diet (Ace Feed PLC Ibadan, Nigeria) and water ad libitum daily, and were weighed weekly. After seven (7) days of acclimatisation, but before the administration of different drugs that were used in this study, the rats were randomly divided into separate groups. The animals were well-catered for in accordance with the National Institutes of Health “guide for the care and use of Laboratory animals” (NIH Publications No. 8023, revised 1978).
2.3. Experimental design
The twenty (20) rats that were used for this study were randomly divided into 4 groups, which included: Group 1 − Normal control; Group 2 − Diclofenac (DF) control; Group 3 − Low dose of omega-3 fatty acids (Low N-3) + DF; and, Group 4 − High dose of omega-3 fatty acids (High N-3) + DF. The rats in group 2 were administered DF at 10 mg/kg b.w./day (im) during the last seven (7) days of the experiment i.e. day 21–28. While the rats in groups 3 and 4 were pre-treated with N-3 (eicosapentaenoic acid and docosahexaenoic acid − 3:2) at a low and high dose of 100 and 300 mg/kg body weight (b.w.)/day (po) respectively, during the first 21 days of the experiment, afterwards, they were post-treated with DF for 7 days.
2.4. Biochemical and haematological analyses
Twelve (12) hours after treatments on the 28th day of the experiment, the rats were administered combined doses of ketamine hydrochloride (50 mg/kg/b.w.) and xylazine (5 mg/kg/b.w.) [13] intramuscularly. Afterwards, they were dissected, and blood was collected by cardiac puncture into heparinised tubes which were centrifuged at 4000 revolutions per minute, for 15 min, at − 4 °C, using a cold centrifuge (Bench top centrifuge, Bio-Gene Technology Ltd., Grandtech Centre, Shatin, Hong Kong). The separated plasma samples were collected into separate plain tubes prior to the biochemical analysis, while the blood samples needed for the haematological analysis were collected in ethylenediaminetetraacetic acid (EDTA) tubes.
The diagnostic kits for the determination of superoxide dismutase, lactate dehydrogenase, uric acid, and total antioxidant capacity were purchased from Fortress Diagnostics Limited, Belfast,
Northern Ireland, United Kingdom. Moreover, the analytic kits for the determination of alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, total bilirubin, glutathione peroxidase, malondialdehyde, and catalase were purchased from Elabscience Biotechnology Company Ltd., Wuhan, Hubei, China. The analyses were performed according to the manufacturers’ instruction.
As for the haematological analysis, the total leukocyte count was determined by the haemocytometer method [14], while the differential leukocyte and platelet count were enumerated by the battlement counting method [15].
2.5. Histopathological analysis
Immediately after the rats were sacrificed, the hepatic tissues were fixed in 10% formalin, dehydrated in graded alcohol, cleared in xylene, and then embedded in paraffin wax. Afterwards, the tissues were cut into 2–3 μm thick sections by a microtome, fixed on the slides, and then stained with haematoxylin-eosin (H and E). The slides were examined under a light microscope at x40 magnification (Olympus CH; Olympus, Tokyo, Japan), and photomicrographs were taken with a Sony digital camera (Model number: DSC-W710) [16,17].
2.6. Data analyses
Data were analysed using statistical package for social sciences (SPSS) version 20.0. Statistical evaluations of the differences between the group mean values were tested by one way analysis of variance (ANOVA) following Turkey post-hoc test. The results were expressed as mean ± standard error of mean (SEM) and statistical significance was considered at p < .05.
3. Results
3.1. Effects of omega-3 fatty acids (N-3) on alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (TB), uric acid (UA), and total white blood cell (WBC) count in diclofenac sodium (DF) −induced hepatotoxicity in rats
Compared to normal control group, there were significant (p < 0.05) increases in ALT activity in groups 2–4 (DF control, Low N-3 + DF, and High N-3 + DF) (Table 1). Relative to DF control group, significant decreases in ALT activity were documented in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF). There were significant (p < .05) increases in AST activity in groups 2 (DF control) and 4 (High N-3 + DF), compared to normal control group (Table 1). Relative to DF control group, there were significant reductions in AST activity in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF). Moreover, compared to group 4 (High N-3 + DF), a significant reduction in AST activity was noted in group 3 (Low N-3 + DF). A significant (p < .05) increase in ALP activity was recorded in DF control group, compared to normal control group (Table 1). Relative to the former, there were significant decreases in ALP activity in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF). Compared to normal control group, there were significant (p < .05) increases in TB level in DF control and High N-3 + DF groups (Table 1). A significant (p < .05) elevation in UA level was documented in DF control group, compared to normal control group (Table 1). Relative to the former, significant decreases in UA level were recorded in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF). Moreover, compared to normal control, DF control, and High N-3 + DF groups, there was a significant (p < .05) reduction in total WBC count in Low N-3 + DF group (Table 1).
Table 1.
Effects of omega-3 fatty acids (N-3) on alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (TB), uric acid (UA), and total white blood cell count (WBC) in diclofenac sodium (DF) − induced hepatotoxicity in rats.
| Groups | PLAT (%) | NEUT (%) | LYMPH (%) | PLR | NLR |
|---|---|---|---|---|---|
| 1. Normal Control | 193.20 ± 1.88 | 11.29 ± 0.90 | 91.88 ± 0.85 | 2.21 ± 0.01 | 0.13 ± 0.01 |
| 2. DF Control | 212.62 ± 1.17* | 31.24 ± 2.11* | 73.76 ± 2.11* | 3.04 ± 0.07* | 0.45 ± 0.04* |
| 3. Low N-3 + DF | 150.36 ± 5.55*# | 15.47 ± 0.84 # | 86.45 ± 0.91# | 1.83 ± 0.07*# | 0.19 ± 0.01# |
| 4. High N-3 + DF | 202.86 ± 4.72a | 12.04 ± 0.24 # | 89.18 ± 2.06# | 2.40 ± 0.10#a | 0.14 ± 0.00# |
Values across the column are expressed as mean ± SEM. *p < .05 is significant compared to group 1 (normal control); #p < .05 is significant compared to group 2 (DF control); ap < .05 is significant − Low N–3 + DF vs High N–3 + DF.
3.2. Effects of omega-3 fatty acids (N-3) on platelet count (PLAT), neutrophil count (NEUT), lymphocyte count (LYMPH), platelet-lymphocyte ratio (PLR), and neutrophil-lymphocyte ratio (NLR) in diclofenac sodium (DF) − induced hepatotoxicity in rats
Relative to normal control group, there was a significant (p < .05) increase in PLAT count in DF control group, however, a significant decrease was recorded in group 3 (Low N-3 + DF) (Table 2). Compared to the latter, a significant increase in PLAT count was noted in High N-3 + DF group. Significant (p < .05) elevations in NEUT count and NLR were recorded in DF control group, compared to normal control group (Table 2). Relative to the former, significant decreases in NEUT count and NLR were documented in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF). There was a significant (p < .05) reduction in LYMPH count in DF control group, compared to normal control group (Table 2). Relative to the former, there were significant increases in LYMPH count in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF). Compared to normal control group, there was a significant (p < .05) increase in PLR in DF control group, however, a significant decrease was recorded in group 3 (Low N-3 + DF) (Table 2). Moreover, there were significant decreases in PLR in Low N-3 + DF and High N-3 + DF groups, compared to DF control group. More so, compared to group 4 (High N-3 + DF), a significant reduction in PLR was recorded in group 3 (Low N-3 + DF).
Table 2.
Effects of omega-3 fatty acids (N-3) on platelet count (PLAT), neutrophil count (NEUT), lymphocyte count (LYMPH), platelet-lymphocyte ratio (PLR), and neutrophil-lymphocyte ratio (NLR) in diclofenac sodium (DF) − induced hepatotoxicity in rats.
| Groups | ALT (U/L) | AST (U/L) | ALP (U/L) | TB (mg/dl) | UA (mg/dl) | WBC (x109) |
|---|---|---|---|---|---|---|
| 1. Normal Control | 3.76 ± 0.43 | 1.41 ± 0.16 | 3.72 ± 0.15 | 0.04 ± 0.00 | 1.43 ± 0.05 | 10.62 ± 0.58 |
| 2. DF Control | 11.50 ± 0.82* | 4.44 ± 0.27* | 9.80 ± 1.03* | 0.10 ± 0.00* | 2.17 ± 0.10* | 12.42 ± 0.32 |
| 3. Low N-3 + DF | 7.73 ± 0.73*# | 1.15 ± 0.07# | 1.67 ± 0.36# | 0.05 ± 0.01 | 1.31 ± 0.04# | 8.00 ± 0.63*# |
| 4. High N-3 + DF | 8.42 ± 0.52*# | 2.97 ± 0.09*#a | 3.40 ± 0.57# | 0.10 ± 0.02* | 1.35 ± 0.08# | 11.05 ± 0.67a |
Values across the column are expressed as mean ± SEM. *p < .05 is significant compared to group 1 (normal control); #p < .05 is significant compared to group 2 (DF control); ap < .05 is significant − Low N–3 + DF vs High N–3 + DF.
3.3. Effects of omega-3 fatty acids (N-3) on malondialdehyde (MDA), lactate dehydrogenase (LDH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and total antioxidant capacity (TAC) in diclofenac sodium (DF) − induced hepatotoxicity in rats
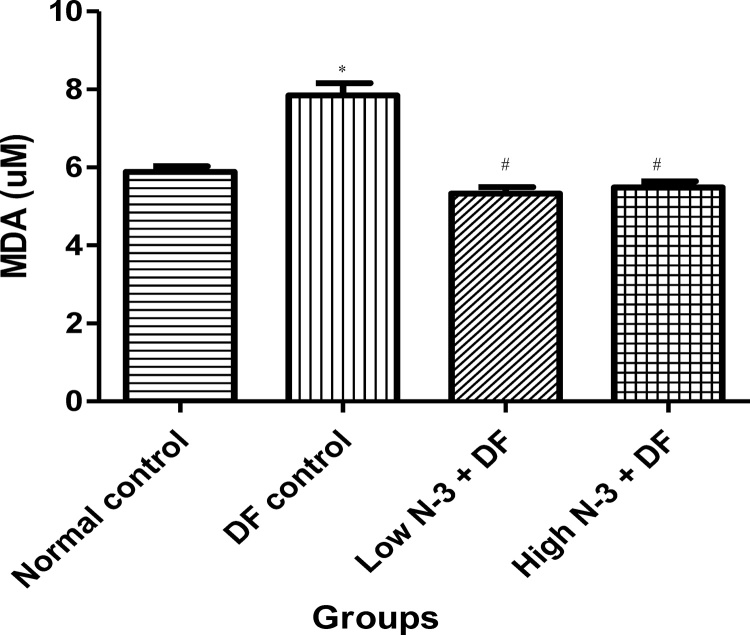
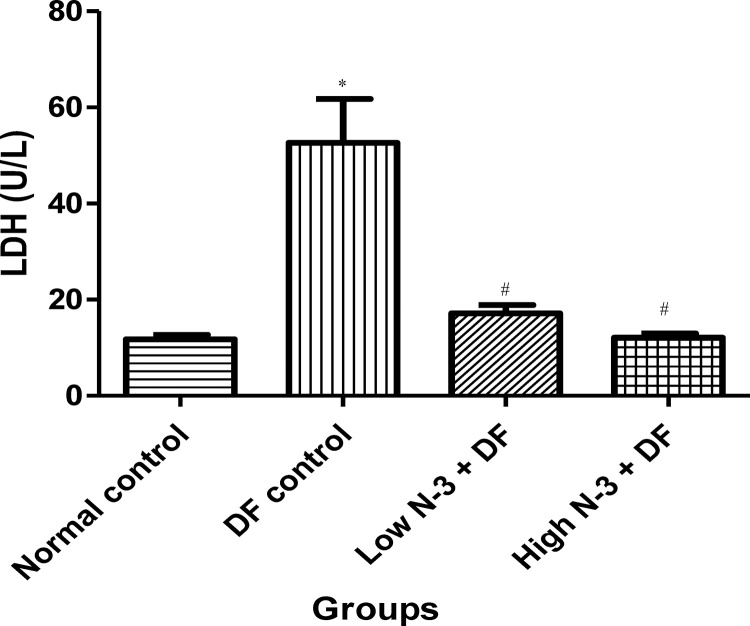
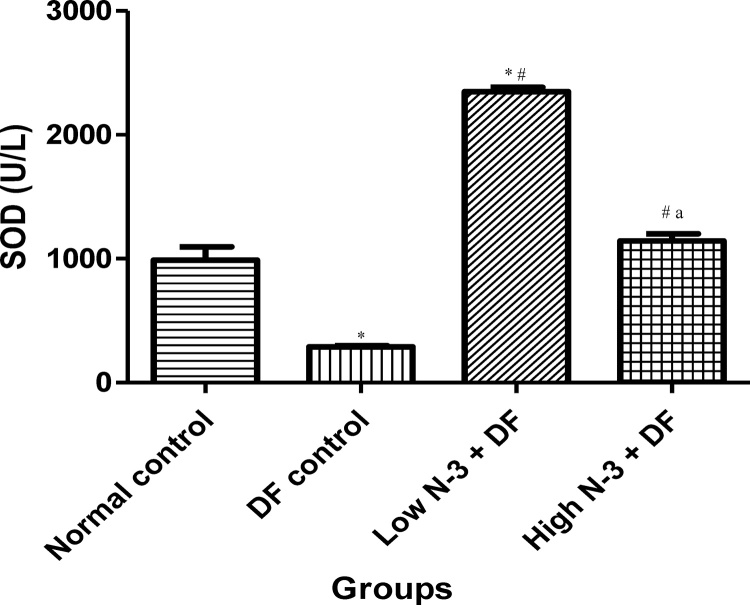
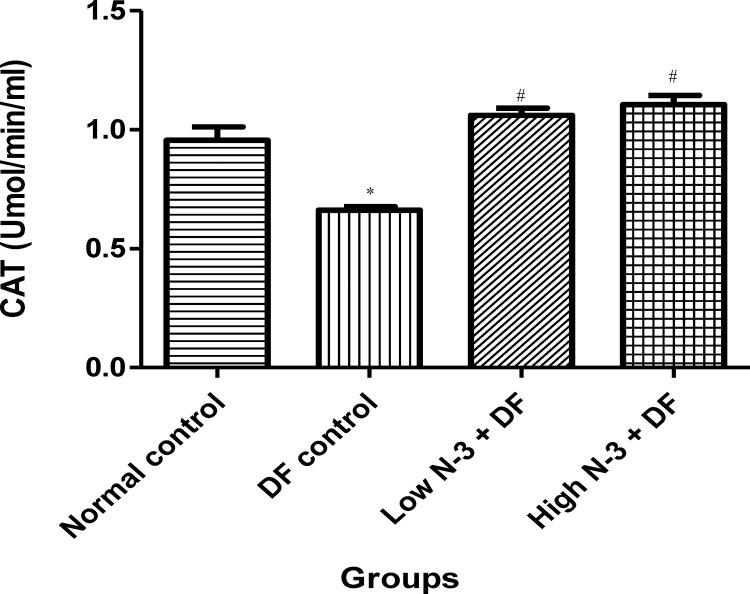
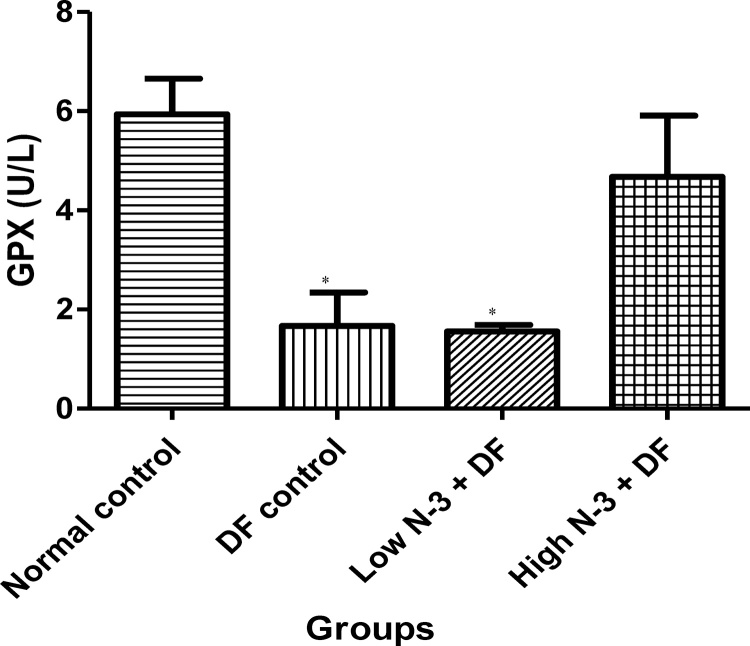
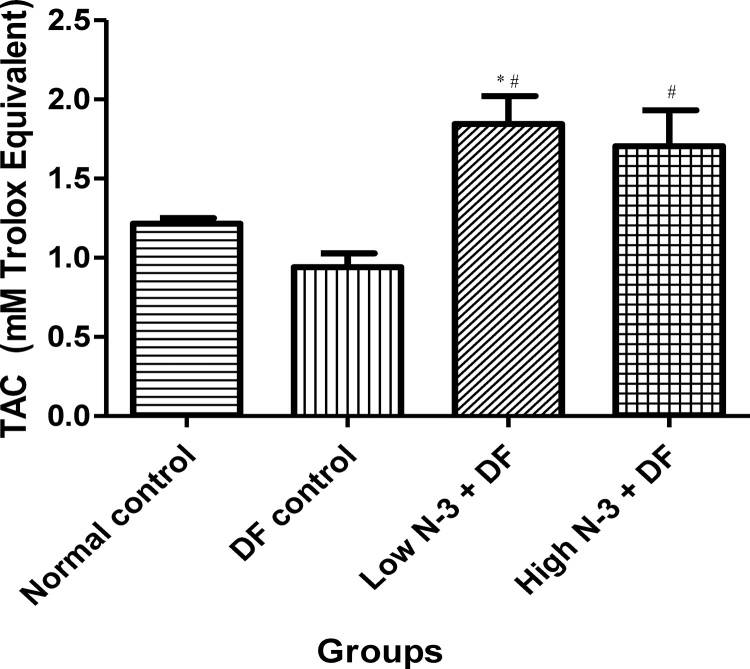
Relative to normal control group, there was a significant (p < .05) elevation in MDA level in the DF control group (Fig. 1). Compared to the latter, there were significant decreases in MDA in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF). There was a significant (p < .05) increase in LDH activity in DF control group, compared to normal control group (Fig. 2). Moreover, a significant decrease in LDH activity was documented in Low N-3 + DF group, relative to DF control group. There was a significant (p < .05) decrease in SOD activity in DF control group, compared to normal control group (Fig. 3). Relative to the former, there were significant increases in SOD activity in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF). Furthermore, relative to group 4, there was a significant increase in SOD activity in group 3. A significant (p < .05) decrease in CAT activity was recorded in DF control group, compared to normal control group (Fig. 4). Compared to the former, significant increases in CAT activity were recorded in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF). Relative to normal control group, significant (p < .05) decreases in GPX activity was documented in groups 2 (DF control) and 3 (Low N-3 + DF) (Fig. 5). There were significant (p < .05) elevations in TAC in groups 3 (Low N-3 + DF) and 4 (High N-3 + DF), compared to DF control group (Fig. 6). In addition, relative to normal control group, there was a significant increase in TAC in group 3 (Low N-3 + DF).
Fig. 1.
Effect of omega-3 fatty acids (N-3) on malondialdehyde (MDA) level (uM) in sodium diclofenac (DF) − induced hepatotoxicity in rats.
Values are expressed as mean ± SEM. *p < .05 is significant compared to normal control; #p < .05 is significant compared to DF control.
Fig. 2.
Effect of omega-3 fatty acids (N-3) on lactate dehydrogenase (LDH) activity (U/L) in sodium diclofenac (DF) − induced hepatotoxicity in rats.
Values are expressed as mean ± SEM. *p < .05 is significant compared to normal control; #p < .05 is significant compared toDF control.
Fig. 3.
Effect of omega-3 fatty acids (N-3) on superoxide dismutase (SOD) (U/L)in sodium diclofenac (DF) − induced hepatotoxicity in rats.
Values are expressed as mean ± SEM. *p < .05 is significant compared to normal control; #p < .05 is significant compared to DF control; ap < .05 is significant − Low N-3 + DF vs High N-3 + DF.
Fig. 4.
Effect of omega-3 fatty acids (N-3) on catalase (CAT) activity (Umol/min/ml) in sodium diclofenac (DF) − induced hepatotoxicity in rats.
Values are expressed as mean ± SEM. *p < .05 is significant compared to normal control; #p < .05 is significant compared to DF control).
Fig. 5.
Effect of omega-3 fatty acids (N-3) on glutathione peroxidase (GPX) activity (U/L) in sodium diclofenac (DF) − induced hepatotoxicity in rats.
Values are expressed as mean ± SEM. *p < .05 is significant compared to normal control.
Fig. 6.
Effect of omega-3 fatty acids (N-3) on total antioxidant capacity (TAC) (mM Trolox Equivalent) in sodium diclofenac (DF) − induced hepatotoxicity in rats.
Values are expressed as mean ± SEM. *p < .05 is significant compared to normal control; #p < .05 is significant compared to DF control.
3.4. Effects of omega-3 fatty acids (N-3) on the histoarchitecture of the hepatic in diclofenac sodium (DF) − induced hepatotoxicity in rats
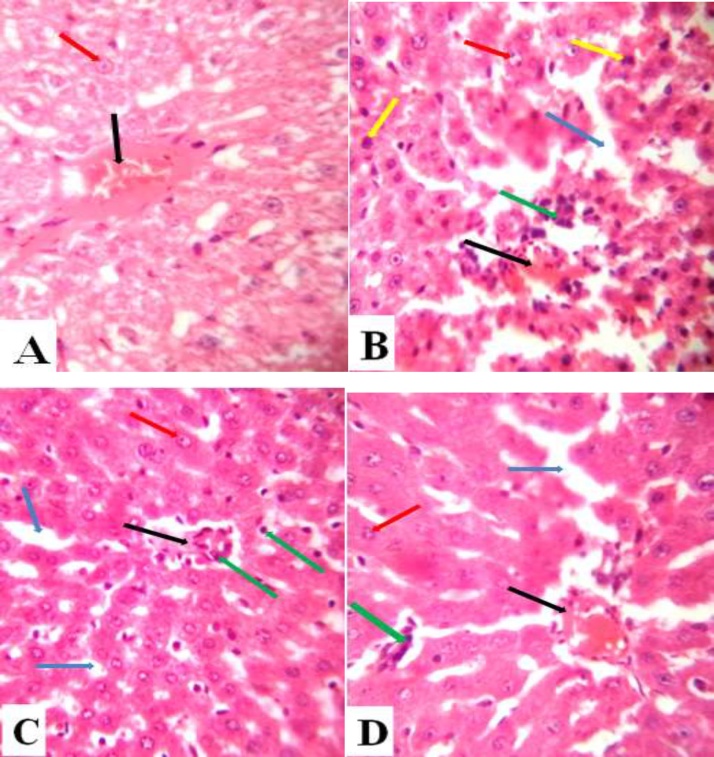
The micrograph of normal control group shows normal central vein and hepatocytes (Fig. 7A). In DF control group, the micrograph shows distorted central vein, significant infiltration with inflammatory cells, normal and dead hepatocytes, and marked degeneration of hepatic tissue (Fig. 7B). In the Low N-3 + DF group, the micrograph shows mild distortion of the central vein, mild infiltration with inflammatory cells, some hepatocytes, and mild degeneration of hepatic tissue (Fig. 7C). Relative to the group 3 (Low N-3 + DF), in the High N-3 + DF group, the micrograph shows more distortion of the central vein, more infiltration with inflammatory cells, few hepatocytes, and notable degeneration of the hepatic tissue (Fig. 7D).
Fig. 7.
(A) Liver section of normal control group showing normal central vein (black arrow) and hepatocytes (red arrow) (H and E X40). (B) Liver section of DF control group showing distorted central vein (black arrow), significant infiltration with inflammatory cells (green arrow), normal and dead hepatocytes (red and yellow arrows respectively), and marked degeneration of the hepatic tissue (blue arrow) (H and E X40). (C) Liver section of Low N-3 + DF group showing mild distortion of the central vein (black arrow), mild infiltration with inflammatory cells (green arrows), some hepatocytes (red arrow), and mild degeneration of the hepatic tissue (blue arrow) (H and E X40). (D) Liver section of High N-3 + DF group. Relative to Fig. 7C, it shows more distortion of the central vein (black arrow), more infiltration with inflammatory cells (green arrow), few hepatocytes, and marked degeneration of the hepatic tissue (H and E X40).
4. Discussion
In the present study, the administration of DF caused an imbalance in the antioxidant system, lipid peroxidation, pro-inflammatory responses, and significant increases in the plasma activities of ALT, AST and ALP. However, pre-treatment with N-3 prior to the administration of DF prevented the overt manifestation of these physiological abnormalities.
The administration of DF has been reported to be accompanied with increased oxidative stress [6] as a result of down-regulation of the antioxidant system. In agreement with previous study, significant decreases in the activities of SOD, CAT, and GPX attended the administration of DF in the present study. Relative to the high dose, pre-treatment with low dose of N-3 before the administration of DF showed preferable pharmacological benefits on TAC and SOD, and not on GPX and CAT. N-3 have been reported to elevate [18,19] or have no significant effect on reactive oxygen species (ROS) level [20]. However, there are also reports on the antioxidant effect of N-3 [21,22]. In the present study, it was probable that pre-treatment with N-3 boosted the antioxidant system, and as such, increased its capacity in handling treats caused by subsequent exposure to DF, which is a pro-oxidative agents. The suggested effect of N-3 possibly account for the significant reduction in lipid peroxidative marker − MDA, and the significant diminution in the incidence of tissue damage or death in the pre-treated groups, indicated by the determination of LDH activity. An inverse association has been established between the efficiency of the antioxidant system and lipid peroxidative event [23]. In the present study, peroxidation of membrane lipid was positively related to the activity of LDH. This proves that an elevation in the activity of LDH is one of the results of oxidative stress. Moreover, elevated activity of LDH has been associated with tissue damage or death [24]. In the pre-treated groups, the low and high doses of N-3 reduced MDA and LDH to a level that was comparable to the normal control group. This is an evidence of the anti-lipid peroxidative effect of N-3, and hence their protective action on cellular integrity.
Inflammation and oxidative stress are closely associated events. Inflammation is considered as one of the consequences of oxidative stress, as the pathways that instigate the production of inflammatory mediators are all triggered by oxidative stress [25]. Some reactive species can initiate intracellular signaling cascade that enhances the expression of pro-inflammatory gene [26,27]. Conversely, inflammatory cells release free radicals at the site of inflammation, resulting to exaggerated pro-oxidative response [28]. Increased WBC count, platelet/lymphocyte and neutrophil/lymphocyte ratios, and UA have been considered as useful index of systemic inflammation [29,30,31,32]. Apart from the fact that UA promotes inflammatory pathways by activating nuclear factor kappa B and p38 mitogen-activated protein kinase [33], it is also considered as one of the primary endogenous danger signals that are released from injured cells [34]. The significant increase in the level of UA in the plasma of DF treated group was positively related to the activity of LDH in this group. This proves that the administered DF was accompanied with compromised cellular integrity and possibly death. In addition to pro-oxidative events, the administration of DF also features pro-inflammatory reactions. This was confirmed by the significant increases in the total WBC count, PLR, NLR, and UA in the DF control. However, in the N-3 pre-treated groups, the systemic levels of these parameters were largely comparable to that which is applicable in a physiological state. Although there was no disparity in the effects of graded doses of N-3 on NLR and UA, however, the determination of total WBC count and PLR proved that the low dose of N-3 have a more desirable effects. It has been documented that supplementation with N-3 at a high dose could be accompanied with some unfavorable events [35]. The noted anti-inflammatory effects of N-3 [36] in this study could be attributed to their metabolites, such as, resolvins [37], neuroprotectin-D1 and docosatrienes [38,39]. Resolvin D1, resolvin E1, and protectin D1 inhibits transendothelial migration of neutrophils into sites of inflammation. While resolvin D1 inhibits IL-1beta production, protectin D1 attenuates the production of TNF-alpha and IL-1beta [40,41,42]. Specifically, protectin D1 seems to have an important role in protecting tissues from extreme damage in a variety of experimental conditions (40) and may be important in preventing neurodegeneration [43].
Although the breakdown of the antioxidant system could precipitate pro-inflammatory responses, it could also be the underlying factor responsible for the recorded significant increases in the activities of AST, ALT, ALP, and the significant elevation in the plasma level of TB. Increased expression of these markers has been associated with the dysfunction of the hepatic tissue [44]. Pre-treatment with N-3 prior to the administration of DF significantly prevented the potential deleterious effects of DF on the hepatic tissue. Even though there was no significant difference in the effect of graded doses of N-3 on ALT and ALP activities, the low dose proved to have more hepatoprotective effect than the high dose. This was evident by the significant reductions in AST activity and TB level in group 3 (low dose of N-3), relative to group 4 (high dose of N-3).
The histological results were parallel to the biochemical and haematological findings. The integrity of the hepatic tissue was significantly distorted by diclofenac sodium [44,45]. The low dose of N-3 showed a more hepatoprotective effect, evident by the less distortion of the histoarchitecture of the liver tissue compared to the diclofenac control and high dose N-3 + DF groups. The study concluded that the pre-administration of N-3, preferably at a low dose, could reduce hepatotoxicity that could result from subsequent exposure to DF.
Acknowledgment
The authors acknowledge Mr. Adebowale Olabanji of Bridge Scientifik Enterprises, Ilorin, Nigeria, for his technical assistance during the biochemical assays.
References
- 1.Todd P.A., Sorkin E.M. Diclofenac sodium, A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1988;35:244–285. doi: 10.2165/00003495-198835030-00004. [DOI] [PubMed] [Google Scholar]
- 2.Armelini P.A. Study comparing the antipyretic potency of diclofenac potassium and dipyrone magnesium in children. Invest. Med. Int. 1984;11:126–129. [Google Scholar]
- 3.Dastidar S.G., Ganguly K., Chaudhuri K., Chakrabarty A.N. The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int. J. Antimicrob. Agents. 2000;14:249–251. doi: 10.1016/s0924-8579(99)00159-4. [DOI] [PubMed] [Google Scholar]
- 4.Masubuchi Y., Nakayama S., Horie T. Role of mitochondrial permeability transition in diclofenac-induced hepatocyte injury in rats. Hepatology. 2002;35:544–551. doi: 10.1053/jhep.2002.31871. [DOI] [PubMed] [Google Scholar]
- 5.Siu W.P., Pun P.B., Latchoumycandane C., Boelsterli U.A. Bax-mediate mitochondrial outer membrane permeabilization (MOMP), distinct from the mitochondrial permeability transition, is a key mechanism in diclofenac-induced hepatocyte injury: multiple protective roles of cyclosporin A. Toxicol. Appl. Pharmacol. 2008;227:451–461. doi: 10.1016/j.taap.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Galati G., Tafazoli S., Sabzevari O., Chan T.S., O'Brien P.J. Idiosyncratic NSAID drug induced oxidative stress. Chem. Biol. Interact. 2002;142:25–41. doi: 10.1016/s0009-2797(02)00052-2. [DOI] [PubMed] [Google Scholar]
- 7.Gill M., Ramirez M.C., Terencio M.C., Castell J.V. Immunochemical detection of protein adducts in cultured human hepatocytes exposed to diclofenac. Bioch. Biophysic. Acta. 1995;1272:140–146. doi: 10.1016/0925-4439(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 8.Lim M.S., Lim P.K., Gupta R., Boelsterli A. Critical role of free cytosolic calcium, but not uncoupling in mitochondrial permeability transition and cell death induced by diclofenac oxidative metabolites in immortalized human hepatocytes. Toxicol. Appl. Pharmacol. 2006;217:322–331. doi: 10.1016/j.taap.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Lechón M.J., Ponsoda X., O'Connor E., Donato T., Castell J.V., Jover V. Diclofenac induces apoptosis in hepatocytes by alteration of mitochondrial function and generation of ROS. Biochem. Pharmacol. 2003;66:2155–2167. doi: 10.1016/j.bcp.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Cleland L.G., James M.J. Omega-3 fatty acids and synovitis in osteoarthritic knees. Nat. Rev. Rheumatol. 2012;8:314–315. doi: 10.1038/nrrheum.2012.60. [DOI] [PubMed] [Google Scholar]
- 11.Simopoulos A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Wluka A.E., Hodge A.M., English D.R., Giles G.G., O’Sullivan R., Cicuttini F.M. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthr. Cartil. 2008;16:579–583. doi: 10.1016/j.joca.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Struck M.B., Andrutis K.A., Ramirez H.E., Battles A.H. Effect of a short-term fast on ketamine-Xylazine anesthesia in rats. J. Am. Assoc. Lab. Anim. Sci. 2011;50:344–348. [PMC free article] [PubMed] [Google Scholar]
- 14.Thrall M.A., Weiser M.G. 4th ed. Mosby Inc.; Missouri: 2002. Laboratory Procedures for Veterinary Technicians; pp. 29–74. [Google Scholar]
- 15.Coles E.H. 4th ed. WB Saunders Company; Philadelphia: 1986. Veterinary Clinical Pathology. [Google Scholar]
- 16.Naiko-Ito A., Asamoto M., Naiki T., Ogawa K., Takahashi S., Sato S., Shirai T. Gap junction dysfunction reduces acetaminophen hepatotoxicity with impact on apoptotic signaling and connexin 43 protein induction in rat. Toxicol. Pathol. 2010;38:280–286. doi: 10.1177/0192623309357951. [DOI] [PubMed] [Google Scholar]
- 17.Içera M., Zengina Y., Gunduza E., Dursuna R., Durguna H.M., Turkcub G., Yuksel H., Üstündağ M., Guloglu C. Is montelukast as effective as N-acetylcysteine in hepaticinjury due to acetaminophen intoxication in rats? Exp. Toxicol. Pathol. 2016;68:55–59. doi: 10.1016/j.etp.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Hatanaka E., Levada-Pires A.C., Pithon-Curi T.C., Curi R. Systematic study on ROS production induced by oleic linoleic, and gamma-linolenic acids in human and rat neutrophils. Free Radic. Biol. Med. 2006;41:1124–1132. doi: 10.1016/j.freeradbiomed.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Obajimi O., Black K.B., Glen I., Ross B.M. Antioxidant modulation of oxidant-stimulated uptake and release of arachidonic acid in eicosapentaenoic acid-supplemented human lymphoma U937 cells. Prostaglandins Leukot. Essent. Fatty Acids. 2007;76:65–71. doi: 10.1016/j.plefa.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Sarkadi-Nagy E., Huang M.C., Diau G.Y., Kirwan R., Chueh C.A., Tschanz C., Brenna J.T. Long chain polyunsaturate supplementation does not induce excess lipid peroxidation of piglet tissues. Eur. J. Nutr. 2003;42:293–296. doi: 10.1007/s00394-003-0422-6. [DOI] [PubMed] [Google Scholar]
- 21.Kesavulu M.M., Kameswararao B., h. Apparao C., Kumar E.G., Harinarayan C.V. Effect of omega-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab. 2002;28:20–26. [PubMed] [Google Scholar]
- 22.Sarsilmaz M., Songur A., Ozyurt H., Kus I., Ozen O.A., Ozyurt B., Söğüt S., Akyol O. Potential role of dietary omega-3 essential fatty acids on some oxidant/antioxidant parameters in rats’ corpus striatum, Prostaglandins Leukot. Essent. Fatty Acids. 2003;69:253–259. doi: 10.1016/s0952-3278(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 23.Surapaneni K.M., Venkataramana G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian J. Med. Sci. 2007;61:9–14. [PubMed] [Google Scholar]
- 24.Najeeb Q., Aziz R. Comparison of alkaline phosphatase lactate dehydrogenase and acid phosphatase levels in serum and synovial fluid between patients with rheumatoid arthritis and osteoarthritis. Int. J. Sci. Res. 2015;4:4. [Google Scholar]
- 25.Haddad J.J. Oxygen-sensitive pro-inflammatory cytokines apoptosis signaling and redox-responsive transcription factors in development and pathophysiology. Cytokines Cell Mol. Ther. 2002;7:1–14. doi: 10.1080/13684730216401. [DOI] [PubMed] [Google Scholar]
- 26.Anderson M.T., Staal F.J.T., Gitler C., Herzenberg L.A., Herzenberg L.A. Separation of oxidant-initiated and redox-regulated steps in the NF-κB signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11527–11531. doi: 10.1073/pnas.91.24.11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flohé L., Brigelius-Flohé R., Saliou C., Traber M.G., Packer L. Redox regulation of NF-Κbactivation. Free Radic. Biol. Med. 1997;22:1115–1126. doi: 10.1016/s0891-5849(96)00501-1. [DOI] [PubMed] [Google Scholar]
- 28.Collins T. 9th ed. W.B. Saunders; Philadelphia: 1999. Robbins Pathologic Basis of Disease. [Google Scholar]
- 29.Bayrakci N., Ozkayar N., Akyel F., Ates I., Akyel S., Dede F. The platelet-to-lymphocyte ratio as an inflammation marker in non-dipper hypertensive patients. Hippokratia. 2015;19:114–118. [PMC free article] [PubMed] [Google Scholar]
- 30.Duffy B.K., Gurm H.S., Rajagopal V., Gupta R., Ellis S.G., Bhatt D.L. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am. J. Cardiol. 2006;97:993–996. doi: 10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Freeman D.J., Norrie J., Sattar N., Neely R.D., Cobbe S.M., Ford I., Isles C., Lorimer A.R., Macfarlane P.W., McKillop J.H., Packard C.J., Shepherd J., Gaw A. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–362. doi: 10.1161/01.cir.103.3.357. [DOI] [PubMed] [Google Scholar]
- 32.Mustafa K.D., Adülkerim B. Evaluation of preoperative neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients undergoing major vascular surgery. Türk. Göğüs. Kalp. Damar. Cerrahisi. Dergisi. 2013;21:930–935. [Google Scholar]
- 33.Kanellis J., Watanabe S., Li J.H., Kang D.H., Li P., Nakagawa T., Wamsley A., Sheikh-Hamad D., Lan H.Y., Feng L., Johnson R.J. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 34.Shi Y., Evans J.E., Rock K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 35.Lewis C.J. United State Food and Drug Administration; 2000. Letter Regarding Dietary Supplement Health Claim for Omega-3 Fatty Acids and Coronary Heart Disease. [Google Scholar]
- 36.Adeyemi W.J., Olayaki L.A. Effects of single or combined administration of salmon calcitonin and omega-3 fatty acids versus diclofenac sodium in sodium monoiodoacetate – induced knee osteoarthritis in male wistar rats. J. Basic Clin. Physiol. Pharmacol. 2017;28(6):573–582. doi: 10.1515/jbcpp-2017-0032. [DOI] [PubMed] [Google Scholar]
- 37.Spite M., Serhan C.N. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ. Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C., Bazan N.G. Lipid-mediated cell signaling protects against injury and neurodegeneration. J. Nutr. 2010;140:858–863. doi: 10.3945/jn.109.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhan C.N., Arita M., Hong S., Gotlinger K. Resolvins, docosatrienes, and neuroprotectins novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 40.Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serhan C.N., Chiang N., van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bazan N.G. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection, Prostaglandins Leukot. Essent. Fatty Acids. 2009;81:205–211. doi: 10.1016/j.plefa.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alabi Q.K., Akomolafe R.O., Olukiran O.S., Adeyemi W.J., Nafiu A.O., Adefisayo M.A., Omole J.G., Kajewole D.I., Odujoko O.O. The Garcinia kola biflavonoid kolaviron attenuates experimental hepatotoxicity induced by diclofenac. Pathophysiology. 2017;24(4):281–290. doi: 10.1016/j.pathophys.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Maity T., Ahmad A., Pahari N., Ganguli S. Hepatoprotective activity of Mikaniascandens (L.) Willd against diclofenac sodium induced liver toxicity in rats. Asian J. Pharm. Clin. Res. 2012;5:185–189. [Google Scholar]