Abstract
Most of the widely used vaginal lubricants in the U.S. and Europe are strongly hyperosmolal, formulated with high concentrations of glycerol, propylene glycol, polyquaternary compounds or other ingredients that make these lubricants 4 to 30 times the osmolality of healthy vaginal fluid. Hyperosmolal formulations have been shown to cause marked toxicity to human colorectal epithelia in vivo, and significantly increase vaginal transmission of genital herpes infections in the mouse/HSV model. They also cause toxicity to explants of vaginal epithelia, to cultured vaginal epithelial cells, and increase susceptibility to HIV in target cells in cell cultures. Here, we report that the osmolality of healthy vaginal fluid is 370 ± 40 mOsm/Kg in women with Nugent scores 0–3, and that a well-characterized three-dimensional human vaginal epithelium tissue model demonstrated that vaginal lubricants with osmolality greater than 4 times that of vaginal fluid (>1500 mOsm/Kg) markedly reduce epithelial barrier properties and showed damage in tissue structure. Four out of four such lubricants caused disruption in the parabasal and basal layers of cells as observed by histological analysis and reduced barrier integrity as measured by trans-epithelial electrical resistance (TEER). No epithelial damage to these layers was observed for hypo- and iso-osmolal lubricants with osmolality of <400 mOsm/Kg. The results confirm extensive reports of safety concerns of hyperosmolal lubricants and suggest the usefulness of reconstructed in vitro vaginal tissue models for assessing safety of lubricants in the absence of direct clinical tests in humans.
Keywords: Vaginal lubricant, Osmolality, EpiVaginal tissue, Epithelial damage
1. Introduction
Hyperosmolal lubricants containing spermicides such as nonoxynol-9 (N-9) induce exfoliation or shedding of the outer layers of the human colorectal epithelium and reduce its barrier properties [[1], [2], [3]]; both these toxic effects are thought likely to increase risk of acquiring and transmitting sexually transmitting infections such as HIV and Herpes Simplex Virus (HSV). Mucosal irritation has been shown to increase with increasing hyperosmolality of several commercially available vaginal lubricants using the slug mucosal irritation assay [4] and perhaps most importantly, to significantly increase vaginal transmission of genital herpes infections in the mouse/HSV model [5]. Lastly, use of some vaginal lubricants is associated with increased incidence of bacterial vaginosis (BV) [[6], [7], [8], [9], [10]]. BV is strongly associated with increased risk of HIV-1 [11] as well as gonorrhea, trichomonas is, upper-tract infections that contribute to preterm deliveries and perinatal complications, pelvic inflammatory disease, and other gynecological and urinary tract infections (see [12]). Here we used a three-dimensional reconstructed model of human vaginal epithelium (EpiVaginal, MatTek Corporation, Ashland, MA), a well-characterized in vitro tissue model for detecting the irritation potential or toxic effects of vaginally applied lubricants. Tests such as these are needed in the continuing absence of clinical safety tests in humans.
The usual toxicity test recommended by the FDA for vaginally formulations/lubricants is the rabbit vaginal irritation model. Unlike in humans, the distal rabbit vagina also acts as a urethra [13] and is directly exposed to the marked variations in the osmolality of urine. In contrast, the human vagina is not normally exposed to hyperosmolal fluids. Moreover, the pH of the rabbit vagina is essentially neutral (pH 7.0), whereas the healthy human vagina is distinctly acidic, with a pH ranging from 3.2 to 4.2 with ∼1% lactic acid [12]. The human vaginal epithelium is unique in that it provides a high concentration of glycogen that can support mono-microbial cultures of lactobacilli that acidify the vagina with lactic acid to levels that likely provide significant first-line of protection against many pathogens, including HIV, HSV, chlamydia, and gonorrhea [12] as well as protection against the polymicrobial communities of BV bacteria [14,15]. Neither the rabbit vagina, nor most cell monocultures are exposed to this level of lactic acid acidity, and therefore may not reliably reflect how the human vagina will respond to vaginal lubricants formulated to match, and support, human vaginal acidity, nor is the rabbit vagina likely to be as susceptible to hyperosmolal lubricants as the human vagina. Thus, three-dimensional vaginal tissue models derived from human vaginal and cervico-vaginal cells represent a useful tool for detecting toxic effects of vaginal lubricants in the continuing absence of human clinical toxicity trials.
The osmolality of at least 44 personal lubricants have been reported [5,[16], [17], [18]]. Thirty-eight of these (86%) are hyperosmolal, and only six are approximately iso-osmolal or hypo-osmolal. The hyperosmolal lubricants range from 4 to more than 30 times the osmolality of vaginal fluids (370 ± 40 mOsm/kg as reported here). Since most of the over-the-counter (OTC) lubricants exceed the osmolality of vaginal fluid, the World Health Organization (WHO) recommended on an interim basis a maximum acceptable osmolality limit of 1200 mOsm/kg [17,19]. In addition 45 of 52 lubricants are formulated with a pH > 4 well above the protective lactic acid acidity of the healthy human vagina, pH < 4 with ∼1% lactic acid [12].
Even though the toxic effects of hyperosmolal lubricants have been examined following rectal application in humans, the toxic effects of commercially available lubricants have not been tested clinically in humans, nor on 3D vaginal tissue models. The overall aim of this investigation was to use one of the best characterized in vitro vaginal tissue models to detect disruption of epithelial barrier functions caused by widely available lubricants following a single topical exposure as a function of their osmolality.
2. Material and methods
2.1. Osmolality of vaginal fluid in humans
Vaginal fluid collection was performed as published previously [20]. Briefly, undiluted vaginal fluid samples, averaging 0.5 g per sample, were obtained from women of reproductive age by using a self-sampling menstrual collection device following protocols approved by the Institutional Review Board of the Johns Hopkins University (protocol # HIRB00000526). Donors inserted the device into the vagina for about 30 s, removed it, and placed it into a 50 ml centrifuge tube. Samples were centrifuged at 200g for 2 min to collect the vaginal fluid. Samples were collected at random times throughout the menstrual cycle; the menstrual cycle date was estimated based on the last menses date reported by the donor and normalized to a 28-day cycle. Samples that were non-uniform in color or consistency were discarded. Donors stated they had not used vaginal products nor participated in unprotected intercourse within 3 days prior to donating. A total of 8 donors were recruited at the Johns Hopkins University Homewood campus, a population with very low incidence of BV and all 8 donors had healthy vaginal microbiota (Nugent scores 0–3). Each donor provided an average of 5 samples. Osmolality was measured using a VAPRO® 5520 vapor pressure osmometer (ELITech Group, Logan, UT) at room temperature and calibrated at 100, 290, and 1000 mOsm/Kg. For vaginal secretions, a Wiretrol (Drummand, Broomall, PA) was used to deposit 10 μl on the test disc of paper. For watery test agents, 10 μl was delivered with the manufacturer’s pipet. For gels too thick for this delivery method, the test disc of paper was submerged in the gel, and squeezed between two layers of Parafilm “M”® (Bemis, Neenah, WI) to leave ∼10 μl of gel saturated in the disc paper (thereby avoiding contaminating the thermocouple with the gel). For hyperosmolal products, the product was diluted on a wt/wt basis with deionized water to bring the osmolality into the ∼300 mOsm/Kg range, and then corrected for this dilution factor.
2.2. Source of vaginal epithelial cells
Human vaginal-ectocervical (VEC) tissue was obtained from otherwise healthy women (age 29–48) undergoing hysterectomies for benign indications via the National Disease Research Interchange (NDRI, Philadelphia, PA) following Internal Review Board (IRB) approval. The ectocervix was used as a source of vaginal epithelial cells; the ectocervix forms a part of the posterior vaginal wall and is covered by stratified squamous epithelium that is histologically indistinguishable from the epithelium lining the lateral vaginal wall. It is also the vaginal site most exposed to pooled vaginal lubricants, and is likely to be susceptible to toxic effects of vaginally administered products. Epithelial cell isolation and expansion was performed using optimized cells expansion medium (MatTek Corporation) as described previously [21].
2.3. In vitro tissue reconstruction
Cryopreserved cells from a single donor were thawed and plated on 150-mm petri dishes. When the cell density reached 60–70% confluence, cells were trypsinized, counted and seeded at a density of 5 × 105 cells/cm2 onto polycarbonate tissue culture treated microporous membrane cell culture inserts (MatTek, Ashland, MA). Inserts were cultured at 37 °C, 5% CO2, 98% rH for 4 days submerged and 7 days at the air liquid interface using a serum free VEC-100-MM (MatTek Corporation, Ashland, MA) differentiation medium to produce the VEC tissue. This culture procedure yields a well-stratified and non-cornified vaginal-ectocervical tissue model (VEC, EpiVaginal) that recapitulates the phenotypic and structural features of in vivo vaginal epithelium.
2.4. Test products and dose volume
In this study we chose several widely available over-the-counter (OTC) vaginal lubricants characteristic of two groups, those with ∼iso-osmolal formulations, and those formulated with hyperosmolal concentrations of glycerol or propylene glycol: The ∼iso-osmolal formulations tested were Good Clean Love (GCL, Eugene, OR), Aloe Cadabra (Seven Oaks Farm, Ventura, CA), Pre-Seed (Lil' Drug Store Products, Inc, Cedar Rapids, IA), and Restore (GCL, Eugene, OR). The hyperosmolal lubricants tested were RepHresh (Lil' Drug Store Products, Inc, Cedar Rapids), K-Y Jelly (Reckitt Benckiser LLC, Parsippany, NJ), ID Glide (Westridge Laboratories, Inc., Newport Beach, CA), Astroglide (Biofilm, Inc., Vista, CA), and K-Y Warming Jelly, (Reckitt Benckiser, LLC., Parsippany, NJ). Culture medium was used as a negative control and deionized water was used to detect toxic effects of hypo-osmolality. Gynol II (with 3% of the detergent nonoxynol-9; Revive Personal Products Company, Madison, NJ), which is known for its significant toxicity to epithelial cells, was used as a positive control.
2.5. Dose volume
To examine toxicity of the vaginal lubricants EpiVaginal 3D tissues were dosed NEAT with 100 μl of each test article by topical application onto the apical surface of the tissue. The 100 μl volume was chosen to match the in vivo dose used in the RVI test, based on the following calculation. The rabbit vagina has an approximate surface area of 10–15 cm2. In standard RVI tests, up to 1.5 ml of test article are applied daily resulting in a dose = 0.1–0.15 ml/cm2. Thus, a 100 μl dose onto the in vitro tissues (surface area = 0.6 cm2) for 24 h approximates the in vivo dose of 0.15 ml/cm2 and a dose regimen in rabbit RVI assays in which daily vaginal administration is recommended. The dosed tissues were incubated for 24 h (37 °C/5% CO2). The in vitro 24 h exposure time was selected to mimic the rabbit vaginal irritation assay for which daily vaginal administration of test articles is recommended. Further, we have also established ET 50 values of commercially available virginally applied products in our previous publications [21]. After 24 h, the dosed 3D EpiVaginal tissues were rinsed with PBS and analyzed using the MTT viability assay, TEER and histology, as described below. In this manuscript, we report the 24 h time point results as specified in the rabbit vaginal irritation model.
2.6. MTT viability assay
Tissue viability was determined using the MTT assay. The extent to which MTT is reduced to a purple formazan dye has been correlated to cell viability [22]. After exposure of tissues to the positive control or test material, the tissues were rinsed with PBS and then loaded with MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide; Sigma). Loading of MTT was accomplished by placing the tissues into a 24-well plate containing 300 μl of MTT dye (1 mg/ml) in culture medium. The plates were then placed into a 37 °C, 5% CO2 incubator for 3 h. After 3 h, the tissues were transferred to a second 24-well plate containing 2.0 ml of isopropyl alcohol in order to stop the reaction and extract the formazan. The 24-well plate was sealed in a plastic bag and the extraction was allowed to proceed overnight at room temperature in the dark. Afterwards, 200 μl l of the formazan extract was quantified by measuring optical density (OD) at 570 nm in an E-MAX 96- well plate reader (Molecular Devices, Menlo Park, CA). The tissue viability was determined by normalizing the OD for treated tissues as a percent of unexposed control tissues, which are loaded with MTT and extracted in an identical manner. Tissue viability (%) was determined using the equation: % viability (treated tissue)/OD (control tissue) X100.
2.7. Transepithelial electrical resistance (TEER)
Changes in barrier function were quantified using TEER. Functional tight junctions are primarily responsible for the barrier function that limits paracellular permeation of water and solutes. Unlike histology sections that only assay limited regions of the epithelium, TEER quantitatively measures the barrier property of the entire tissue surface. Similar methods have been used by others to evaluate epithelial toxicity of candidate microbicides. TEER measurements were made using an EVOM volt-ohmmeter equipped with an EndOhm electrode chamber (World Precision Instruments, Sarasota, FL). Calculations of Ω*cm2 were made by multiplying the readings for each tissue by the surface area of the tissue (0.6 cm2). TEER measurements were normalized as a percentage of the untreated control tissues: % TEER = TEER (Ω *cm2) of treated tissues (TTT) divided by the TEER of untreated tissues (TUT) times 100 (% TEER = (TTT/TUT × 100).
2.8. Histology
To examine the morphology of the in vitro reconstructed VEC tissues, the tissues were fixed by submerging the tissue-containing inserts in 10% formalin overnight at room temperature. Standard histology procedures were used and tissue cross-sections were cut (5–7 μm thick), mounted on microscopic slides, and stained with hematoxylin and eosin (H&E). The stained cross-sections were observed and photographed using a Nikon Diaphot microscope. To evaluate structural changes to the 3D vaginal tissue model following test article exposure, tissues were processed as above and scored visually compared with untreated control tissues. Three observers, blinded as to the test or control articles, scored the disruption of the basal and parabasal cell layers on a scale of 0–4, where 0 was indistinguishable from the control tissue, and 4 was the most extensively disrupted. The average of these scores are reported; the ranges varied from 0 to 1 for the hypo-and iso-osmolar lubricants, and ±∼1 for the others. The ranges are omitted in the figure for clarity.
3. Results
3.1. Osmolality of unmodified vaginal secretion
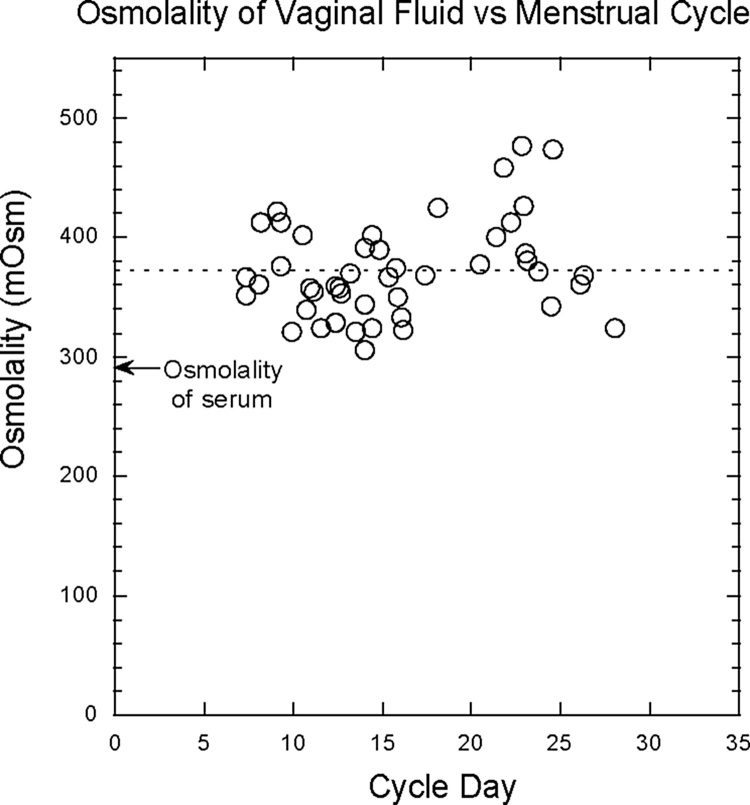
Fig. 1 shows the osmolality of unmodified vaginal secretions collected at random times during the menstrual cycle from eight donors with an average of five samples per donor, and plotted normalized to a 28-day cycle. The dashed line shows the average osmolality: 370 ± 40 mOsm/Kg (range: 300–480 mOsm/Kg). Note that the osmolality is significantly higher than that of serum (290 mOsm/Kg) possibly due to the high concentration of lactic acid, 110 mOsm/Kg, even though lactic acid is only partially osmotically active since the vaginal epithelium is somewhat permeable to it (Fig. 1).
Fig. 1.
Osmolality of unmodified vaginal secretions collected at random times during the menstrual cycles of women.
3.2. Osmolality of control and test lubricants
Table 1 summarizes the osmolality of the controls and products, and the results of the study. Nine lubricants and three controls are ranked in order of increasing osmolality are shown in Table 1. The culture medium served as the negative control, and deionized water was used to detect the effects of maximum hypo-osmolality. Gynol II was used as a positive control since this detergent-based (N9) contraceptive gel has well-established toxicity that increases susceptibility to HIV [23]. Four of the lubricants ranged from 120 to 370 mOsm/Kg and were hypo- or iso-osmolal to vaginal fluid, 370 mOsm/kg. The remaining 5 lubricants were hyperosmolal, with 4–23 times the osmolality of healthy vaginal fluid.
Table 1.
Effect of high osmolality vaginal lubricants on tissue viability, barrier integrity, and tissue morphology. Rank ordering of commercially available vaginal lubricants from low to high osmolality as a function tissue damage (reduced membrane integrity, TEER, and structural damage, histology) are shown N = the number of tissues examined. The histology scores were determined as described in the methods.
| Category | Treatment | Osmolality (mOsm) | # Tissues (N) | % MTT Viability | % TEER (Ω*cm2) | Histology description | Histology Score |
|---|---|---|---|---|---|---|---|
| Controls | H2O control | 0 | 6 | 100 ± 14 | 100 ± 3 | Healthy tissue Minor effects on easily friable apical layers | 0 |
| Culture Medium | 290 | 3 | 103 ± 9 | 100 ± 15 | Healthy tissue Minor effects on easily friable apical layers | 0 | |
| Gynol | 1404 | 10 | 6 ± 0.2 | 2–8 ± 0.5 | Separation of epithelial layers from porous support layer. | 4 | |
| Non-Irritant | Aloe Cadabra | 118 | 3 | 107 ± 15 | 100 ± 10 | Healthy tissue Minor effects on easily friable apical layers | 0.3 |
| Good Clean Love | 194 | 6 | 117 ± 13 | 108 ± 27 | Healthy tissue Minor effectson easily friable apical layers | 0.3 | |
| Preseed | 295 | 3 | 102.4 ± 21 | 103 ± 5 | Healthy tissue Minor effectson easily friable apical layers | 0.3 | |
| Restore | 340 | 2 | 103 ± 9 | 97 ± 11 | Loss of all apical layers. Basal and parabasal layers intact. | 0.5 | |
| Irritant | RepHresh | 1500 | 3 | 103 ± 10 | 155 ± 28 | Loss of apical layers. Basal and parabasal layers mostly intact | 1.3 |
| KY personal lubricant | 2200 | 3 | 108 ± 8 | 71 ± 27 | Modest effects on easily friable apical surface Basal and parabasal layers mostly intact | 1 | |
| ID Glide | 2900 | 3 | 103 ± 9 | 58 ± 13 | Loss of apical layers. Disorganization of parabasal and basal cell layers. | 3 | |
| Astroglide | 4500 | 3 | 110 ± 18 | 66 ± 8 | Loss of apical layers. Disorganization of parabasal layers. | 3 | |
| Ky Warming Jelly | 8600 | 6 | 100 ± 11 | 25 ± 1–29 ± 4 | Separation of epithelial layers from porous support layer. Cells become pyknotic. | 4 |
3.3. Major ingredients in lubricant formulations
Table 2 lists the four ingredients with the highest concentrations as indicate by the product labels. Gel forming polymers have little osmotic activity, but high concentrations of small molecules like glycerin/glycerol and propylene glycol with high osmolality were associated with reduced barrier integrity and histological damage in the 3D vaginal tissue model.
Table 2.
List of four ingredients (indicate by the product labels) with the highest concentrations of each lubricant used in the study.
| Lubricant | Top four Ingredients on product labels | |
|---|---|---|
| Positive Control | Gynol (3% N9) | Nonoxynol 9, lactic acid, methylparaben, povidone |
| Non-Toxic | Aloe Cadabra | Organic aloe barbadensis leaf juice, mixed, tocopheryls, xanthan gum,citric acid |
| Good Clean Love | Organic aloe barbadensis leaf juice, xanthan gum, agar, lactic acid | |
| Preseed | Purified water, hydroxyethylcellulose, pluronic, sodium chloride | |
| Restore | Organic aloe barbadensis leaf juice, xanthan gum, lactic acid, natural flavor | |
| RepHresh | Purified water, glycerin, polycarbophil, carbomer homopolymer type B | |
| Toxic | KY personal Lubricant | Water, glycerin, hydroxyethylcellulose, chlorhexidine |
| ID Glide | Water, glycerin, propylene glycol, cellulose gum | |
| Astroglide | Purified water, glycerin, propyleneglycol, polyquaternium 15 | |
| KY Warming Jelly | Propylene glycol, PEG-8, hydroxypropylcellulose, tocopherol |
3.4. MTT viability assay
The toxicity results for 9 commercially available vaginal lubricants tested on EpiVaginal tissues are shown in Table 1. The MTT viability assay was not able to discriminate test materials of different osmolality values indicating that few surviving cells in the tissue were enough to metabolize and reduce the tetrazolium dye MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to its insoluble formazan, purple color.
3.5. Transepithelial electrical resistance (TEER)
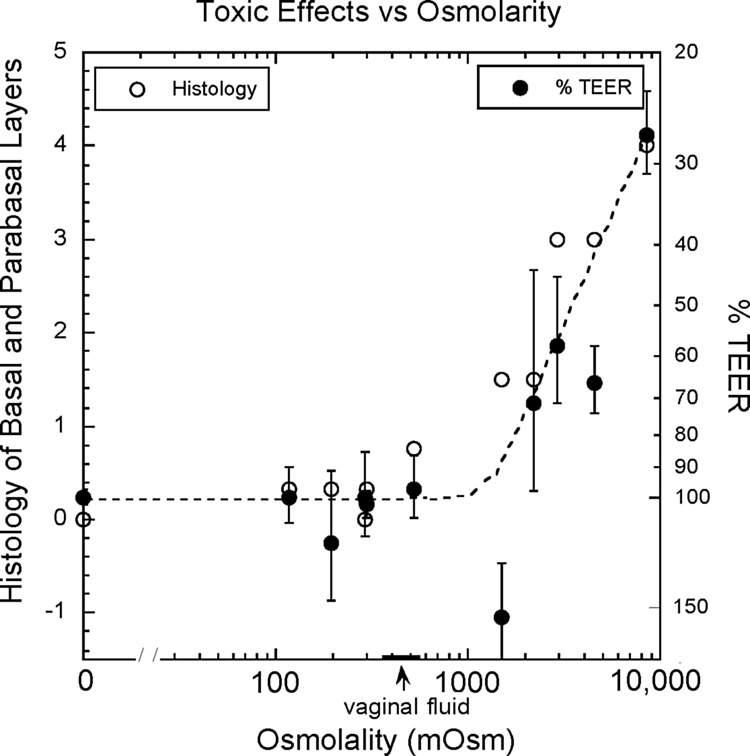
As the osmolality became increasingly hyperosmolal, TEER values fell: The 4 lubricants (KY Personal, ID Glide, Astroglide, and KY Warming jelly) with osmolality values greater than 4 times the osmolality of the vaginal fluid showed reductions in TEER values of 30–70% (Table 1 and Fig. 2). This showed that a decrease in TEER values maybe a sensitive and objective biomarker for detecting changes in epithelial integrity at an early stage of product development. The epithelial barrier disruption, as indicated by decreased TEER values, increased as the osmolality rose above 1500 mOsm/Kg.
Fig. 2.
Shows a trend line of a decrease barrier integrity (quantified by TEER measurement) and compromised tissue morphology (histology scores) as a function of an increase in osmolality of vaginal lubricants. Dark dots indicate% TEER measurement and Open circles indicate histological scores.
3.6. Histology
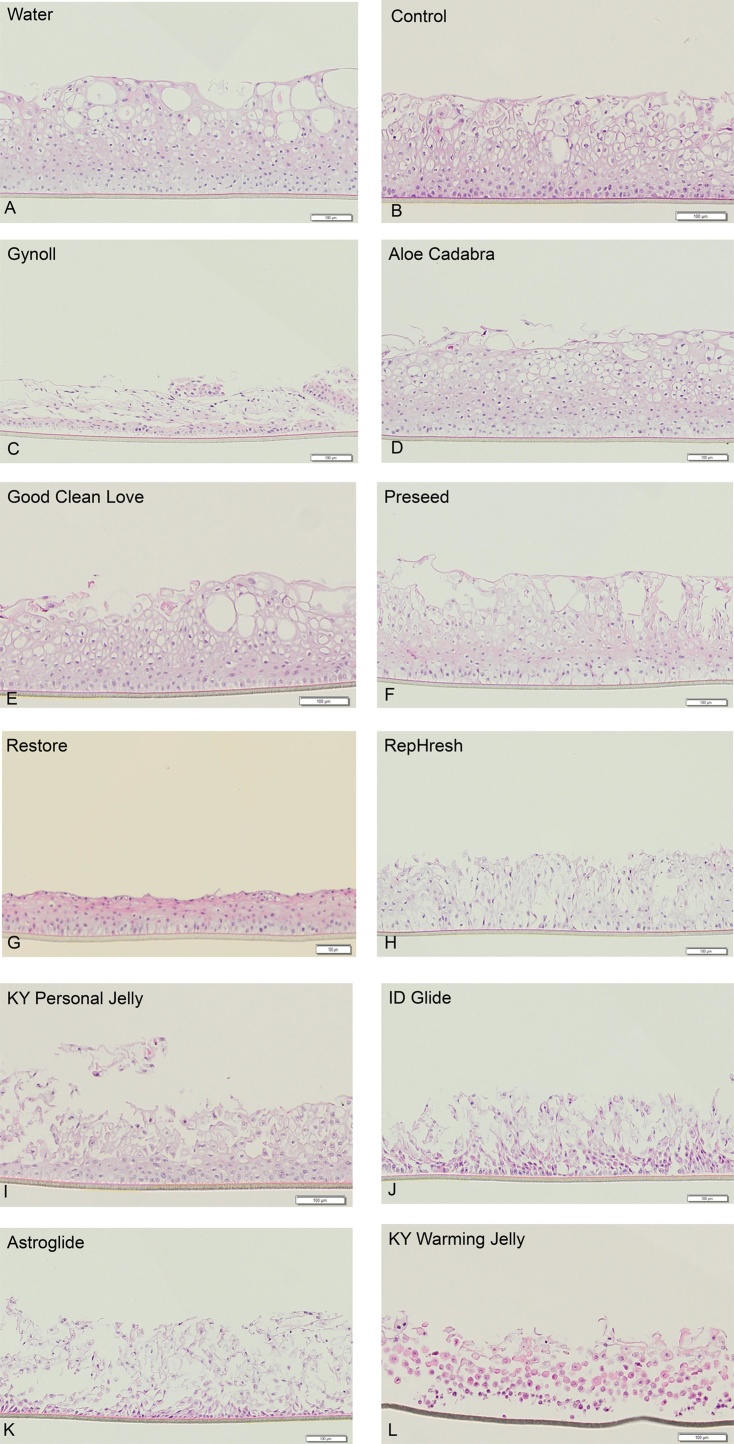
Histological evaluation provides an independent assessment of the effect of lubricants on the tissue. Representative micrographs of EpiVaginal tissue histology 24 h after a single application of the lubricants are presented in Fig. 3A–L. Treatment with four lubricants hypo- to iso-osmolal to that of healthy vaginal fluid, (Aloe Cadabra, Good Clean Love, PreSeed, and Restore) had a range of effects on the apical tissue layers but essentially no detected effect on the basal and parabasal tissue layers. In contrast, the four lubricants with osmolalities greater than 4 times the osmolality of the vaginal fluid (KY Personal, ID Glide, Astroglide, and KY Warming Jelly) showed minor to severe disruption of the basal and parabasal layers, as scored visually by histology.
Fig. 3.
A–L: H&E stained histological cross-sections of in vitro reconstructed Epivaginal epithelial tissue model treated with over the counter vaginal lubricants for 24 h. Water treated tissues were used as controls. Histology sections are arranged in order of increasing osmolality of the controls, and the lubricants.
RepHresh, with an osmolality of 1500 mOsm/Kg, showed minor histological disruption to all these layers, but inexplicably increased TEER.
4. Discussion
In this study, we found that hyperosmolal lubricants induced greater epithelial damage than hypo- and iso-osmolal lubricants. Given the level of breach in the epithelial barrier by hyperosmolal lubricants, the results suggest the potential of an increase in susceptibility to sexually transmitted infections such as HIV and HSV in individuals who are regular users of hyperosmolal lubricants. Such effects could be attributed to: 1) reduction in barrier integrity of the epithelial membrane, which makes the epithelium “leaky” to allow viral and microbial entry and 2) alteration of the microbiota in the vaginal environment. In support of the second possibility [18] showed that hyperosmolal personal lubricants such as KY Jelly, and the surfactant N9 were found to be toxic to lactobacilli that can help protect against infections by acidifying the vagina with lactic acid, a broad antiviral and anti-bacterial agent.
The hypo-osmolal agents, including deionized water, showed no evident toxicity in the reconstructed EpiVaginal tissue model. This surprising result is consistent with earlier reports in the slug mucosal model [4] and in the mouse vaginal model [5] as well as the quite modest effects of tap water in the human colo-rectum [3].
In marked contrast, hyperosmolal concentrations of glycerol and propylene glycol caused obvious toxicity that increased markedly as the osmolality increased. in this study, lubricants containing glycerin/glycol, propylene glycol, and Polyethylene glycol (PEG-8) as one of the top four ingredients were associated with marked reduction in barrier properties and tissue morphological damage. The presence of glycerin as one of the ingredients might have also contributed to an increase in osmolality which resulted in the loss of apical layer in RepHresh exposed EpiVaginal tissues. The reduction in barrier function, as indicated here quantitatively by the reduction in TEER, was reported in the human colorectum [3] and the slug mucosal model [4] and later corroborated in the mouse vaginal and rectal models [5,24,25]. The shedding of the outer layers of the epithelium as a result of the toxic effects of hyperosmolal lubricants was also shown for the human colorectum [3] here we showed a similar toxic effect using human primary cell based organotypic vaginal epithelial tissue model. The findings of this study have significant implications for the development of safe lubricants that are intended to enhance sexual pleasure without increasing risks of infections.
Historically, the significance of a highly toxic agent, the detergent nonoxynol-9, was not adequately realized until after performing large HIV prevention trials of N9 [23]. This detergent was, and still is, used as a vaginal contraceptive. Despite its significant toxic effects, it does not cause obvious pain or discomfort in most users. Similarly, hyperosmolal lubricants cause little or no obvious pain or discomfort to most users. But they cause marked toxicity to the human colorectal epithelium [3]. They increase susceptibility to genital herpes (HSV) infections in the mouse/vaginal HSV model [5] and they cause obvious toxic effects in the slug mucosal model [4].
4.1. Limitations of this study
A much larger array of lubricants have been tested in the literature [16] and osmolality has been directly varied in the slug mucosal model using the same composition but increasing the concentration of a single osmotically active ingredient [4]. Here we tested only a relatively small selection of lubricants to determine whether one of the now available three-dimensional tissue models will be useful for future studies with more breadth and detail. Subsequent studies should include a full array of inflammatory cytokines as well as anti-inflammatory cytokines, since vaginal levels of lactic acid acidity have been reported to have anti-inflammatory effects [26,27]. Finally, the results of this initial study on a 3D reconstructed vaginal tissue model should be validated against other such models.
5. Conclusion
Sexual lubricants have been associated in several studies with increased risk of episodes of BV [[6], [7], [8], [9], [10]] and most sexual lubricants are hyperosmolal with respect to the osmolality of healthy vaginal fluids (370 mOsm/Kg as reported here). Hyperosmolal vaginal lubricants disrupted the barrier functions of the basal and parabasal layers and shedding of the apical layers. These results clearly suggest osmolality-induced disruption of epithelial barrier may be one of the mechanisms by which use of vaginal lubricants is associated with the risk of bacterial vaginosis [9] and may increase susceptibility to sexually transmitted infections.
The broad range of results to date on the toxicity of hyperosmolal lubricants, should encourage manufactures of vaginal lubricants to devise iso- or hypo- osmolal formulations that do not disrupt the barrier properties of the vaginal epithelium. The ingredients that typically cause hypertonicity are small molecules formulated at high concentrations such as glycerol and propylene glycol [5]. Lastly, regulatory agencies should consider on toxicity testing in more appropriate, human-relevant models than the rabbit vagina.
Acknowledgement
The authors would like to thank Wendy Strgar, Founder, and CEO of Good Clean Love, for providing the test articles and a limited financial support for this study.
References
- 1.Phillips D.M., Taylor C.L., Zacharopoulos V.R., Maguire R.A. Nonoxynol-9 causes rapid exfoliation of sheets of rectal epithelium. Contraception. 2000;62(3):149–154. doi: 10.1016/s0010-7824(00)00156-6. [DOI] [PubMed] [Google Scholar]
- 2.Phillips D.M., Sudol K.M., Taylor C.L., Guichard L., Elsen R., Maguire R.A. Lubricants containing N-9 may enhance rectal transmission of HIV and other STIs. Contraception. 2004;70(2):107–110. doi: 10.1016/j.contraception.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs E.J., Lee L.A., Torbenson M.S., Parsons T.L., Bakshi R.P., Guidos A.M., Wahl R.L., Hendrix C.W. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J. Infect. Dis. 2007;195(5):703–710. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 4.Adriaens E., Remon J.P. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex. Transm. Dis. 2008;35(5):512–516. doi: 10.1097/OLQ.0b013e3181644669. [DOI] [PubMed] [Google Scholar]
- 5.Moench T.R., Mumper R.J., Hoen T.E., Sun M., Cone R.A. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect. Dis. 2010;10:331. doi: 10.1186/1471-2334-10-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan W.M., Lavreys L., Chohan V., Richardson B.A., Mandaliya K., Ndinya-Achola J.O., Kiarie J., Jaoko W., Holmes K.K., McClelland R.S. Associations between intravaginal practices and bacterial vaginosis in Kenyan female sex workers without symptoms of vaginal infections. Sex. Transm. Dis. 2007;34(6):384–388. doi: 10.1097/01.olq.0000243624.74573.63. [DOI] [PubMed] [Google Scholar]
- 7.McClelland R.S., Richardson B.A., Graham S.M., Masese L.N., Gitau R., Lavreys L., Mandaliya K., Jaoko W., Baeten J.M., Ndinya-Achola J.O. A prospective study of risk factors for bacterial vaginosis in HIV-1-seronegative African women. Sex. Transm. Dis. 2008;35(6):617–623. doi: 10.1097/OLQ.0b013e31816907fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brotman R.M., Ravel J., Cone R.A., Zenilman J.M. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex. Transm. Infect. 2010;86(4):297–302. doi: 10.1136/sti.2009.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrazzo J.M., Thomas K.K., Agnew K., Ringwood K. Prevalence and risks for bacterial vaginosis in women who have sex with women. Sex. Transm. Dis. 2010;37(5):335–339. [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J.M., Hess K.L., Brown S., Murphy C., Waldman A.L., Hezareh M. Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States. Obstet. Gynecol. 2013;121(4):773–780. doi: 10.1097/AOG.0b013e31828786f8. [DOI] [PubMed] [Google Scholar]
- 11.Gosmann C., Anahtar M.N., Handley S.A., Farcasanu M., Abu-Ali G., Bowman B.A., Padavattan N., Desai C., Droit L., Moodley A., Dong M., Chen Y., Ismail N., Ndung'u T., Ghebremichael M.S., Wesemann D.R., Mitchell C., Dong K.L., Huttenhower C., Walker B.D., Virgin H.W., Kwon D.S. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young south african women. Immunity. 2017;46(1):29–37. doi: 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Hanlon D.E., Moench T.R., Cone R.A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One. 2013;8(11):e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castle P.E., Hoen T.E., Whaley K.J., Cone R.A. Contraceptive testing of vaginal agents in rabbits. Contraception. 1998;58(1):51–60. doi: 10.1016/s0010-7824(98)00059-6. [DOI] [PubMed] [Google Scholar]
- 14.O'Hanlon D.E., Moench T.R., Cone R.A. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011;11:200. doi: 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldunate M., Tyssen D., Johnson A., Zakir T., Sonza S., Moench T., Cone R., Tachedjian G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013;68(9):2015–2025. doi: 10.1093/jac/dkt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Begay O., Jean-Pierre N., Abraham C.J., Chudolij A., Seidor S., Rodriguez A., Ford B.E., Henderson M., Katz D., Zydowsky T., Robbiani M., Fernandez-Romero J.A. Identification of personal lubricants that can cause rectal epithelial cell damage and enhance HIV type 1 replication in vitro. AIDS Res. Hum. Retroviruses. 2011;27(9):1019–1024. doi: 10.1089/aid.2010.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunha A.R., Machado R.M., Palmeira-de-Oliveira A., Martinez-de-Oliveira J., das Neves J., Palmeira-de-Oliveira R. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceutics. 2014;6(3):530–542. doi: 10.3390/pharmaceutics6030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dezzutti C.S., Brown E.R., Moncla B., Russo J., Cost M., Wang L., Uranker K., Kunjara Na Ayudhya R.P., Pryke K., Pickett J., Leblanc M.A., Rohan L.C. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS One. 2012;7(11):e48328. doi: 10.1371/journal.pone.0048328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO/UNFPA/FHI . WHO/UNFPA/FHI; WHO; Geneva, Switzerland: 2012. Use and Procurement of Additional Lubricants for Male and Female Condoms. [Google Scholar]
- 20.Boskey E.R., Moench T.R., Hees P.S., Cone R.A. A self-sampling method to obtain large volumes of undiluted cervicovaginal secretions. Sex. Transm. Dis. 2003;30(2):107–109. doi: 10.1097/00007435-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Ayehunie A., Cannon C., Larosa K., Lamore L., Kabilus J., Anderson D.J., Pudney J., Klausner M. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicology. 2006;20(5):689–698. doi: 10.1016/j.tiv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann T. Rapid Calorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Van Damme L., Ramjee G., Alary M., Vuylsteke B., Chandeying V., Rees H., Sirivongrangson P., Mukenge-Tshibaka L., Ettiegne-Traore V., Uaheowitchai C., Karim S.S., Masse B., Perriens J., Laga M., C. O. L. S. Group Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 24.Ensign L.M., Hoen T.E., Maisel K., Cone R.A., Hanes J.S. Enhanced vaginal drug delivery through the use of hypotonic formulations that induce fluid uptake. Biomaterials. 2013;34(28):6922–6929. doi: 10.1016/j.biomaterials.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maisel K., Chattopadhyay S., Moench T., Hendrix C., Cone R., Ensign L.M., Hanes J. Enema ion compositions for enhancing colorectal drug delivery. J. Control. Release. 2015;209:280–287. doi: 10.1016/j.jconrel.2015.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hearps A.C., Tyssen D., Srbinovski D., Bayigga L., Diaz D.J., Aldunate M., Cone R.A., Gugasyan R., Anderson D.J., Tachedjian G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017;10(6) doi: 10.1038/mi.2017.27. ([Epub ahead of print] PubMed PMID: 28401934, Apr 12) [DOI] [PubMed] [Google Scholar]
- 27.Aldunate M., Srbinovski D., Hearps A.C., Latham C.F., Ramsland P.A., Gugasyan R., Cone R.A., Tachedjian G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015;6(164) doi: 10.3389/fphys.2015.00164. (eCollection 2015. Review. PubMed PMID: 26082720; PubMed Central PMCID: PMC4451362. Jun 2) [DOI] [PMC free article] [PubMed] [Google Scholar]