Abstract
Yersinia enterocolitica is the causative agent of yersiniosis, a zoonotic disease of growing epidemiological importance with significant consequences for public health. This pathogenic species has been intensively studied for many years. Six biotypes (1A, 1B, 2, 3, 4, 5) and more than 70 serotypes of Y. enterocolitica have been identified to date. The biotypes of Y. enterocolitica are divided according to their pathogenic properties: the non-pathogenic biotype 1A, weakly pathogenic biotypes 2–5, and the highly pathogenic biotype 1B. Due to the complex pathogenesis of yersiniosis, further research is needed to expand our knowledge of the molecular mechanisms involved in the infection process and the clinical course of the disease. Many factors, both plasmid and chromosomal, significantly influence these processes. The aim of this study was to present the most important virulence markers of Y. enterocolitica and their role during infection.
Keywords: Yersinia enterocolitica, virulence markers, pYV, mucoid Yersinia factor MyfA, Yersinia adhesin YadA, invasin InvA, attachment-invasion locus protein Ail, Yersinia outer membrane proteins Yops, Yersinia-stable toxin Yst
1. Introduction
The genus Yersinia belonging to the Enterobacteriaceae family consists of 18 species, of which only three, Yersinia pestis, Yersinia enterocolitica, and Yersinia pseudotuberculosis, are pathogenic for humans and animals [1]. According to a recent report of the European Food Safety Authority (EFSA), yersiniosis caused by Y. enterocolitica is one of the most important foodborne zoonotic diseases in Europe [2]. This pathogenic species has been intensively studied for many years. Six biotypes (1A, 1B, 2, 3, 4, 5) and more than 70 serotypes of Y. enterocolitica have been identified to date [3]. The biotypes of Y. enterocolitica are divided regarding their pathogenic properties: the non-pathogenic biotype 1A, weakly pathogenic biotypes 2–5, and the highly pathogenic biotype 1B [4]. Y enterocolitica infections are influenced by several structures, both plasmid and chromosomal, referred to as virulence markers or virulence determinants [5]. The proteins encoded by these genes enable bacteria to invade a susceptible organism, colonize it, evade the immune response and grow under unfavorable conditions.
The plasmid of Yersinia virulence (pYV) with a size of 64–75 kb is the most known and important virulence marker of Y. enterocolitica [1,6]. All biotypes are capable of invading intestinal mucosa, but only strains with a plasmid can migrate from Peyer’s patches to mesenteric lymph nodes and internal organs, where they multiply and lead to the necrotic abscesses formation [7]. Biotype 1B Y. enterocolitica strains harboring pYV also carry the chromosomal high pathogenicity island (HPI) associated with the iron acquisition system (yersiniabactin) which facilitates the uptake and utilization of iron by Y. enterocolitica, and promotes their growth under iron-limiting conditions [8]. Several genes that are directly responsible for the pathogenicity of Y. enterocolitica are located within the pYV, such as yadA encoding the Yersinia adhesin (YadA), or the yop virulon encoding Yersinia outer membrane proteins (Yops) [6]. Unfortunately, the determination of the pathogenicity of Y. enterocolitica strains based on plasmid markers alone can produce false negative results, due to a spontaneous loss of pYV by bacteria caused by, for example, prolonged strain storage, frequent passaging or temperatures higher than 37 °C [9]. The search for chromosomal, genetically-stable virulence markers, such as ail, invA, myfA, and yst genes, which encode the production of Ail (attachment-invasion locus) protein, primary internalization factor invasin InvA, mucoid Yersiniae factor MyfA and Yst (Yersinia-stable toxin) enterotoxin, respectively, is more justified from the diagnostic point of view [9].
Physicochemical parameters, such as temperature, the concentration of calcium and iron ions, pH, and osmolarity also play a role during infection [8,10,11,12]. The expression of many plasmid-encoded virulence genes, selected genes of the flagellar regulon and chromosomal virulence genes, is strictly regulated by temperature [11]. The genes of the flagellar regulon and early virulence genes (invA) are expressed below 30 °C, whereas plasmid-encoded virulence genes (ysc, yop, yadA) are expressed at 37 °C [11]. The expression of virulence markers is also regulated by regulatory genes. The most important regulatory gene is virF, encoding the transcriptional activator of the Yersinia virulence regulon. VirF, a DNA-binding protein of 30 kDa, belonging to the AraC family, is produced only at 37 °C, although other factors are also required to initiate the transcription of VirF target genes [13]. VirF is a key transcriptional activator of the yop and yadA genes [14]. The Yersinia modulator, YmoA, negatively regulates the transcription of virF and invA [15,16]. Invasion is also mediated by the virulence regulator, RovA, a dimeric winged helix transcriptional regulator which stimulates invA expression [17]. Chaperone Hfq modulates the expression of transcriptional regulator rovA; therefore, it acts as a global coordinator of surface virulence markers in Y. enterocolitica, which suggests that it can be an excellent target for new antimicrobial strategies [17]. The mechanisms of virulence marker expression are generally complicated, and the role of selected regulatory genes and temperature in expression processes is described below.
The aim of this study was to present the most important virulence markers of Y. enterocolitica and their role during infection.
2. Pathogenesis of Y. enterocolitica Infection
Bacteria most often enter the body per os with contaminated water or food [1]. In pigs, Y. enterocolitica usually colonize palatine tonsils, where they multiply and reach further segments of the gastrointestinal tract. Before the pathogen can come into contact with enteric epithelial cells, bacteria have to penetrate the layer of gastrointestinal mucus which is secreted by goblet cells. Gastrointestinal mucus contains mucins which are responsible for its gel-like properties [18]. Mantle and Husar [19] demonstrated that 1B and 2–5 Y. enterocolitica biotypes may adhere to human and rabbit mucin to a greater extent than 1A biotype strains which that do not possess pYV. Lipopolysaccharide (LPS) is encoded chromosomally, it is an integral component of the external cell membrane which forms complex structures with proteins and phospholipids, protects bacterial cells against bile, and complements system components [20]. Free-living Y. enterocolitica cells, including cells that enter the intestinal lumen, contain smooth type (S) LPS that varies substantially in the chemical content of the polysaccharide fraction (O-specific chains, O antigen). At 37 °C, LPS is transformed to smooth-rough type (S-R) LPS, which contains less core fraction and O-specific polysaccharide [21,22]. LPS acts as an endotoxin after the breakdown of bacterial cells.
Follicle-associated epithelium (FAE) is the primary site of host–pathogen interactions in Y. enterocolitica infection [23]. Y. enterocolitica penetrates microfold cells (M cells) and, subsequently, induces the destruction of Peyer’s patches. Autenrieth et al. [23] showed that after Y. enterocolitica infection, adjacent villi were dilated from lymphangiectasis, and transmigrating polymorphonuclear leucocytes were found within the epithelium. Interestingly, a pathogenic Y enterocolitica serotype 0:8 colonizes ileal Peyer’s patches about 1000 times more densely than surrounding epithelium of a comparable surface area [24]. The FAE and parts of Peyer’s patches are usually destroyed 5–7 days after infection [23]. The target for pathogenic Y. enterocolitica are abdominal lymph nodes, where the microorganism multiplies and causes inflammatory changes and ulcerations, and from where it can invade internal organs [23].
One of the most intriguing factors, MyfA, plays an important role at the beginning of infection [24]. MyfA closely resembles CS3 fimbriae of enterotoxigenic Escherichia coli, which could suggest that MyfA promotes the adhesion to enterocytes [25]. MyfA is also in 44% identical to PsaA, the major subunit of pH6 antigen of Y. pestis [25]. The myfA gene encoding a fibrillar subunit of MyfA was found in Y. enterocolitica strains of bioserotype 4/O:3 isolated from clinical cases of yersiniosis [26]. It was also detected in some Y. enterocolitica biotype 1A strains isolated from patients with diarrhea [26]. According to Rastawicki et al. [25], Myf fibrillae are immunogenic at the beginning of disease, and immune responses to recombinant MyfA are more frequent in children than in adult patients.
2.1. Adhesion and Invasion
The proteins encoded by three genes, yadA, invA, and ail, are mainly involved in the processes of adhesion and invasion [27]. YadA (previously called YopA) protein is encoded by the structural gene yadA, located extrachromosomally on pYV. YadA is a member of the trimeric autotransporter adhesin (TAA) family, and it forms fibrous, lollipop-like structures on the cell surface that mediate binding to human epithelial cells (HEp-2), as well as microvilli that constitute the intestinal brush border [28]. YadA binds collagen I, II, IV, and laminin, and the interactions between YadA and collagen may contribute to chronic Y. enterocolitica infections, such as reactive arthritis [5]. YadA supports the creation of densely packed microcolonies of Yersinia in three-dimensional collagen gels [29]. Leo et al. [30] examined the binding of YadA to collagen Toolkits, libraries of triple-helical peptides spanning the sequences of type II and III human collagen. YadA was bound to many of these structures, in particular, to hydroxyproline rich peptides [30].
YadA also elicits an inflammatory response in epithelial cells by inducing the production of interleukin-8 (IL-8), which is mediated by mitogen-activated protein kinase (MAPK), and by contributing to the intestinal inflammatory cascade [5]. Additionally, YadA mediates cell adhesion and host cell responses induction, like cytokine production, autoagglutination, and serum resistance [30,31,32,33]. Mühlenkamp et al. [33] demonstrated direct YadA-mediated interactions between selected Y. enterocolitica strains with serum glycoprotein vitronectin (Vn), which acts as an inhibitory regulator of the terminal complement complex. According to these authors, YadA-mediated Vn binding is caused by the “uptake region” of the protein’s N-terminus, and it promotes the adhesion of selected Y. enterocolitica strains. This “uptake region”, similar to the “uptake region” of Y. pseudotuberculosis YadA, was defined as a crucial for the high-affinity binding of Vn [33]. It is worth mentioning that YadA plasmid protein is produced at 37 °C.
The production of YadA is promoted by the RNA chaperone Hfq, a widely conserved protein that stabilizes sRNAs, facilitates sRNA–mRNA pairing, and modulates the degradation of target RNAs [34]. According to Kakoschke et al. [17], Hfq is probably the main regulator in Yersinia. They observed that hfq mutants exhibited decreased translocation of proteins into host cells by the type III secretion system (T3SS), consistent with decreased YadA production [17]. They assumed that Hfq-dependent control exerted at the transcriptional (at 27 °C), post-transcriptional (at 37 °C in stationary phase) and, possibly, post-translational (37 °C in stationary phase) level is involved in fine-tuning the amount of YadA present at the bacterial surface in different environments. Nieckarz et al. [35] demonstrated that another regulatory system, OmpR, can specifically bind to the yadA promoter region, which suggests that expression is inhibited by a direct mechanism. They identified yadA as a new member of the OmpR regulon, and postulated that OmpR negatively regulates yadA, and that the downregulation of YadA could increase the survival of Y. enterocolitica by preventing bacterial binding to host cells, which promotes further spread to deeper tissues. To summarize, YadA seems to be the most important single contributor to the virulence of Y. enterocolitica, in particular, because it acts not only as an adhesin, but also as an invasin to facilitate the penetration of host cells [28]. YadA also plays a central role in promoting serum resistance. Kirjavainen et al. [36] showed that YadA acted as C4-binding protein (C4bp) receptors, and binding of C4bp could help Y. enterocolitica to evade complement-mediated clearance in the human host. Kinetic killing tests, which were conducted by Biedzka-Sarek et al. [37], revealed that the most potent single-serum resistance factor needed for long-term survival was YadA.
Some studies showed that in an early phase of Y. enterocolitica infection, InvA is required for effective bacteria translocation into M cells and Peyer’s patches colonization [38]. The 987 aminoacids polypeptide InvA, a member of the intimin/invasin protein family, consists of a membrane-associated β-barrel and extracellular C-terminal domains, and its production is encoded by the invA chromosomal gene [39]. The β-barrel at the N-terminus and the extracellular domain at the C-terminus invert the arrangement of InvA relative to the classical autotransporter system (type Va secretion system), which could represent a new type of autotransporter system (Ve) [40]. InvA binds to integrins, which leads to the creation of integrin clusters, triggers remodeling of the actin cytoskeleton and leads to the internalization of Y. enterocolitica to epithelial cells. The above is known as the “zipper” invasion mechanism, and internalization allows the delivery of Yops to host cells [41].
The penetration of M cells by Y. enterocolitica begins with a direct interaction between InvA and any of the five types of β1 integrins (α3β1, α4β1, α5β1, α6β1, αvβ1) on the surface of target epithelial cells [42]. InvA binds directly, whereas YadA binds indirectly via extracellular matrix (ECM) proteins to β1 integrins on host cells [43]. According to Thinwa et al. [44], the invasin–integrin interaction provides the first signal for inflammasome activation, and the T3SS translocon provides the second signal for inflammasome activation, which results in the release of IL-18. The binding of InvA to β1 integrins rapidly induces IL-18 mRNA expression, which could suggest that integrins provide the first signal for inflammasome activation [44]. After binding, InvA activates multiple signaling cascades, which leads to the chemotactic cytokines production like IL-8, monocyte-chemoattractant protein-1 (MCP-1) and the granulocyte-macrophage colony-stimulating factor [45]. According to Thinwa et al. [44], the inflammatory reaction triggered by Yersinia InvA leads to the recruitment of phagocytes and tissue disruption. Wiedemann et al. [46] demonstrated that after coming into contact with β1 integrins, InvA induces phagocytosis of nonopsonized Y. enterocolitica by activating actin polymerization. Y. enterocolitica resists macrophage killing, becomes sequestered inside these cells, and the invasin-triggered recruitment of macrophages creates replicative niches for Y. enterocolitica and enables the pathogen to avoid exposure to the host’s immune system [47]. According to Deuretzbacher et al. [48], InvA also mediates the induction of autophagy in macrophages.
InvA synthesis in Y. enterocolitica is dependent on the growth phase and, according to recent research, on serotype. Uliczka et al. [27] observed that in serotypes O:8 and O:9, InvA was expressed efficiently only at environmental temperatures, whereas in serotype O:3, InvA production was constitutive also at higher temperatures. These authors found that the difference in regulation resulted from the insertion of IS1667 into the invA promoter region in Y. enterocolitica serotype O:3. However, Pepe et al. [10] demonstrated that pH was also an important determinant of invA expression. invA is expressed maximally at 26 °C and neutral pH, and is only poorly expressed at 37 °C and neutral pH; however, a decrease in pH increases invA expression at 37 °C. The expression of InvA in response to environmental signals is regulated by several proteins, and genetic studies have revealed the complex nature of this regulatory process [49]. InvA expression is regulated mostly by RovA. Single substitution of amino acid P98S increases the thermostability of RovA, leading to higher expression of InvA in serotype O:3 [27]. Admittedly, Raczkowska et al. [50] demonstrated that RovA may activate invA expression regardless of the presence of histone-like nucleoid structuring (H-NS) protein, however, in general, the thermoregulation of invA gene transcription in Y. enterocolitica is controlled by three regulatory proteins: RovA, H-NS, and YmoA [51]. RovA also upregulates yaxA and yaxB genes [52]. Wagner et al. [52] found that YaxA and YaxB proteins are required for cytotoxic activity, and that they are associated. YaxAB acts as a virulence factor by inducing cell lysis through the creation of pores in the host cell membrane [52].
Ail protein is yet another virulence marker which plays an important role during attachment and invasion processes. Due to its small size (17 kDa), Ail protein is easily masked by other surface structures, like the LPS, decreasing its biological efficiency [37]. Ail has eight membrane-spanning amphipathic β-strands and four extracellular loops. The C-terminal half of loop 2 is involved in interactions with host cell surface components [47]. Miller et al. [53] showed that mutations in loop 2 of Ail lead to the elimination of the invasion phenotype and serum resistance of Y. enterocolitica. During the logarithmic phase of growth, Ail is synthesized at 30 °C; although in the stationary phase, the ail gene is only expressed at 37 °C [41]. Ail mediates the attachment and invasion of host cells and confers serum resistance to Y. enterocolitica [54]. Ail in combination with YadA guarantees a high level of serum resistance by binding factor H, an abundant serum glycoprotein essential for controlling the alternative pathway in blood and on cell surfaces [52,55]. Ail is also the key determinant of serum resistance during exponential growth [52]. It confers serum resistance to Y. enterocolitica by binding C4bp, a complement component, which inactivates C3 convertase and destabilizes the formation of active attack complexes on the bacterial membrane [55]. Bliska and Falkow [56] examined the ail mutant of Y. enterocolitica and the expression of the ail gene in E. coli, and observed that Ail increased serum resistance 100-fold. O-group LPS side chains sugars could mask the Ail receptor-binding regions, thereby weakening its serum resisting properties, but the loss of O-group sugars at 37 °C exposes Ail [47]. Because of these temperature-dependent changes, the cells cultured at 37 °C are more resistant to serum killing than the cells cultured at 30 °C [57]. In contrast to YadA and InvA, chaperone Hfq inhibited the production of Ail post-transcriptionally [17].
The ail gene is an important virulence marker which is widely used as a target in molecular analyses of the pathogenicity of Y. enterocolitica strains [58,59,60]. In contrast to the invA sequence, sequences homologous to the ail gene have been identified only in Y. enterocolitica strains that are epidemiologically associated with clinical yersiniosis in humans, however, they are now more often detected in the 1A biotype [3,61,62,63,64]. According to Sihvonen et al. [63], the nucleotide sequence of the ail gene of a biotype 1A Y. enterocolitica strain (isolated from food in Finland, GenBank FN812733) differed from a biotype 1B Y. enterocolitica strain (isolated from a human with clinical yersiniosis, GenBank AM286415) in only one point mutation—transition G2008088T. This finding suggests that the pathogenicity of Y. enterocolitica cannot be reliably determined based on ail detection in simple PCR. Bancerz-Kisiel et al. [4] recently proposed the high-resolution melting analysis (HRMA) method supporting rapid determination of ail single nucleotide polymorphisms (SNPs) correlated with the biotypes of the tested Y. enterocolitica strains. According to these authors, the HRMA supported reliable discrimination of three genotypes and phylogenetic groups: 1A—non-pathogenic Y. enterocolitica strains, 1B—highly pathogenic Y. enterocolitica strains, and 2/4—weakly pathogenic Y. enterocolitica strains [4]. This method could pose an alternative to standard biotyping, serotyping, and sequencing of ail-positive Y. enterocolitica strains.
2.2. Interaction with the Immune Response
Antigen transcytosis across the FAE of Peyer’s patches by M cells is significant for invasion and induction of effective immune responses to mucosal antigens [65]. Bacteria transported by M cells to Peyer’s patches are surrounded, ingested, and destroyed by phagocytic cells. Antigens are presented to lymphocytes by antigen presenting cells (APC) in Peyer’s patches, which leads to the induction of the immune response and, ultimately, local and systemic mucosal immunity. However, enteropathogenic Y. enterocolitica strains have developed resistance mechanisms, mainly with the involvement of effector Yops encoded by the yop virulon. The yop virulon has four components: a type III secretion system, T3SS, known as Ysc-Yops, translocator Yops (YopB, YopD), control element YopN, and effector Yops (YopE, YopH, YopM, YopO, YopP, YopT) [66]. Selected Yops form pores in the membrane of eukaryotic target cells, whereas other Yops are effector proteins that are transported through the pores to the cytosol of target cells [67]. The transport of Yops also requires close contact between bacterial and host cells, and it is mediated by YadA and InvA that bind to β1 integrins [5].
The T3SS transports Yops to the cytoplasm of host cells through a syringe-like system, formed mainly by YscF and translocator Yops [66,68]. YopB and YopD, two similar hydrophobic proteins referred to as translocators, form a multimeric integral membrane complex in the membrane of eukaryotic cells [69]. YopB acts as a potential suppressor of tumor necrosis factor alpha (TNF-α) mRNA expression in macrophages and Peyer’s patches in vivo, and it has also been found to play a role in the regulation and/or secretion of YopD [70]. Y. enterocolitica strains lacking YopD are unable to induce cytotoxicity in macrophages. These properties were not inherent to YopD, but indirectly related to its role in the secretion of YopE and YopH into host cells [70]. YopN, a secreted protein of 32.3 kDa, is a multi-domain protein with several functions, including the control of Yops secretion [71]. Effector Yops have numerous properties which enable them to block local immune mechanisms. After transport, they inhibit phagocytosis and the production of inflammatory molecules, and they induce host cell apoptosis. YopE, YopH, YopO and YopT have a negative role in cytoskeleton dynamics [67]. YopE has been notified to regulate the translocation of effector Yops into host cells [72]. YopH promotes the inhibition of phagocytosis, inhibits cytokine production by T cells and T-cell proliferation, and prevents the expression of the costimulatory receptor CD86 on B cells [73]. YopO inhibits phagocytosis by disrupting actin filament regulation processes [74], and YopT disrupts the actin cytoskeleton of the host cell [75]. YopM is a crucial immunosuppressive effector of pathogenic Yersinia: it enters the nucleus of host cells, but the mechanisms governing its nucleocytoplasmic shuttling and its intranuclear activities have not been explained to date [76]. YopP has numerous roles: it suppresses the production of TNF-α and IL-8, blocks the activation of MAPK and nuclear factor κB (NF-κB), and induces apoptosis in macrophages [67].
The injection of Yops into host cells by T3SS is an important immune evasion mechanism of Y. enterocolitica. Although leukocytes are the target of Yops injected by Y. enterocolitica, it remains unknown which adhesins and leukocyte receptors are required for Yops injection. Deuschle et al. [43] investigated the role of YadA, InvA, and β1 integrins in Yops injection into leukocytes. Based on the results of a β-lactamase reporter assay, they found that the adhesion of Y. enterocolitica through InvA or YadA is sufficient to promote Yops injection into leukocytes. Serum factors inhibit YadA-mediated, but not InvA-mediated Yops injection into B and T cells, and YadA-mediated Yops injection is shifted towards neutrophils and other myeloid cells [43]. The above authors also found that YadA is essential for Y. enterocolitica virulence and Yops injection into leukocytes, whereas InvA is not required for virulence, and plays only a temporary and minor role in the injection of Yops in the early phase of infection [43]. Deuschle et al. [43] demonstrated that β1 integrins are not required for YadA-mediated Yops injection into leukocytes, but they contribute to InvA-mediated Yops injection. Keller et al. [77] reported that in the absence of β1 integrins, YadA mediates Yops injection by interacting with αV integrins, and unidentified cofactors expressed by epithelial cells, but not fibroblasts. They revealed that by indirectly binding to a broad range of ECM host cell receptors in different cell types, YadA is a versatile tool for Yops injection [77].
2.3. Diarrhea Induction
Enterotoxin Yst is one of the significant factors determining Y. enterocolitica virulence. Yst is a polypeptide chain composed of a 30 amino acid C-terminal domain as the mature component of the toxin and an 18 amino acid N-terminal signal sequence that is cut off during transport through the cytoplasmic membrane [78]. Three types of YstI enterotoxins (A, B, and C) and a recently discovered, but weakly understood YstII enterotoxin, have been identified to date [79]. Y. enterocolitica strains of biotypes 1B and 2–5 produce the YstIA heat-stable enterotoxin [80,81]. Research into YstIA-negative mutants and their transcription demonstrated that YstIA could be essential for virulence in Y. enterocolitica [82,83]. Biotype 1A strains produce mainly YstIB, and they rarely produce YstIC, which is not commonly encountered [84]. YstII, a biologically active enterotoxin with a completely different mechanism of action and an unidentified coding gene, has also been identified [80]. YstIC consists of 53 amino acids, has higher molecular mass, and C-terminal and N-terminal YstIC and YstIA chains share approximately 50% similarity at the amino acid level. The C-terminal 13 amino acid regions of YstIA and YstIB correspond to a strongly conservated sequence that is characteristic of all thermostable toxins produced by enterotoxigenic E. coli [85].
Pathogenic Y. enterocolitica strains isolated from humans with yersiniosis were capable of producing YstI, which implies that YstI plays a significant role in the etiology of diarrhea in yersiniosis. Researchers opposing the theory that YstI is the key determinant of diarrhea have pointed out that YstI is not produced at temperatures higher than 30 °C. However, Singh et al. [81] demonstrated that YstI can be produced both at 37 °C in a slightly alkaline environment (pH 7.5) and at temperatures below 30 °C. The YstIA enterotoxin has highly similar physicochemical and antigenic properties and mechanism of action to the heat-stable enterotoxin type I or type a (STI, STa) produced by enterotoxigenic E. coli, which are manifested by the activation of guanylate cyclase, an increase in cyclic guanosine monophosphate (cGMP) levels in epithelial cells, and the accumulation of fluids in the intestinal lumen, which leads to diarrhea [78,86]. Interestingly, not all yst-positive strains produce enterotoxins, which could imply the presence of “silent genes”. This can probably be attributed to the presence of the ymoA gene, which encodes the production of YmoA protein (8.1 kDa). The YmoA protein belongs to the growing family of Hha proteins, referred to as YmoA in Y. enterocolitica, and it is similar to the N-terminal, dimerized domains of H-NS proteins [87,88]. In intestinal bacteria, H-NS proteins play a host of important roles as structural proteins and gene expression modulators, including those of virulence markers [89,90,91,92,93,94]. There is evidence to suggest that ymoA inhibits the expression of the invA gene, and participates in VirF regulation [16] and temperature-dependent production of Yops and YadA [15].
The possible effect of ymoA gene mutations on YstIA production was first postulated by Cornelis et al. [15]. They identified two Tn5-Tc1 chromosomal mutants responsible for producing YstI with its typical temperature dependence. These mutations were insertions in ymoA gene. Cornelis et al. [15] postulated that ymoA mutations unblock the silencing of the yst gene and stimulate YstI production. Mikulskis et al. [83] also postulated the inherence of a mechanism switching the yst expression to a silent state, and other authors showed that yst gene presence is not always correlated with YstI production [85,95]. In the study of Bancerz-Kisiel et al. [96], no mutations were found in the coding region of the examined ymoA gene fragments. Two point mutations in the non-coding region have been identified in some of the tested Y. enterocolitica strains, regardless of their enterotoxic properties [96]. Hence, the suggestion that ymoA gene mutations influence ystA gene silencing was not confirmed.
2.4. Pathogenicity of Y. enterocolitica Biotype 1A Strains
Y. enterocolitica strains belonging to biotype 1A are commonly regarded as non-pathogenic; they are highly heterogeneous and include many O serogroups [95]. Biotype 1A strains isolated from clinical cases also colonize the gastrointestinal tract, both small and large intestines, and replicate in enterocytes. Despite the fact that Y. enterocolitica strains belonging to biotype 1A are regarded as avirulent and devoid of pYV, various forms of fimbriae have been observed in this biotype [8]. Biotype 1A strains isolated from clinical cases of gastritis and enteritis show higher virulence and capacity to invade enterocytes than environmental strains [95]. They penetrate HEp-2 cells less effectively than the strains that possess the pYV plasmid, but more effectively than strains obtained from other sources [80]. This suggests a different mechanism of epithelial cell invasion than in the biotypes that are regarded as pathogenic [81]. The pathogens escape from macrophages or epithelial cells without causing detectable cytolysis, which implies the presence of an escape mechanism resembling exocytosis [97,98]. The fact that clinical biotype 1A Y. enterocolitica strains are much more resistant to macrophage killing and more likely to escape from host cells than nonclinical strains suggest that these properties may be responsible for virulence [97,98]. Although the 1A biotype rarely produces YstIA enterotoxin, more than 80% of these strains contain the ystB gene that encodes the production of homologous and biologically active enterotoxin YstIB. Rammamurthy et al. [99] showed that 88.9% of clinical Y. enterocolitica strains belonging to biotype 1A caused the accumulation of fluids in the intestines of suckling mice, which points to the toxigenic potential of the tested strains. Additionally, the minimum effective dose (MED) of purified YstIB enterotoxin (0.4 pmol) is much lower than an analogous dose of YstIA enterotoxin (7.6 pmol), which confirms its pathogenic potential [59]. According to McNally et al. [100], biotype 1A strains are emerging as the predominant pathogenic agent of yersiniosis in the Commonwealth countries. A case-control study of diarrheic patients in Finland also revealed that the majority of the isolated Y. enterocolitica strains belonged to biotype 1A [101].
The above suggests that pathogenicity cannot be reliably determined based on bioserotype classification alone.
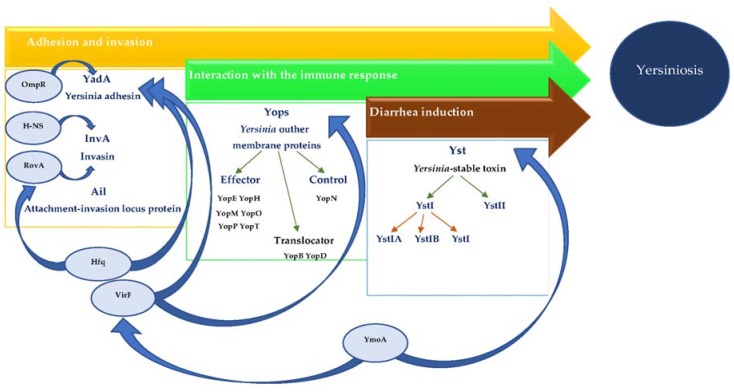
Major virulence markers of Yersinia enterocolitica are summarized in Table 1, and the role of selected regulatory genes in their expression is presented on Figure 1.
Table 1.
Major virulence markers of Y. enterocolitica.
| Gene | Gene Product | Plasmid/Chromosomal |
|---|---|---|
| myfA | mucoid Yersiniae factor (MyfA) | Chromosomal |
| yadA | Yersinia adhesin (YadA) | Plasmid |
| invA | invasin (InvA) | Chromosomal |
| ail | attachment-invasion locus (Ail) protein | Chromosomal |
| yop virulon | Yersinia outer membrane proteins (Yops) | Plasmid |
| ystA | Yersinia-stable toxin type I A (YstIA) | Chromosomal |
| ystB | Yersinia-stable toxin type I B (YstIB) | Chromosomal |
| ystC | Yersinia-stable toxin type I C (YstIC) | Chromosomal |
| virF | transcriptional activator of the Yersinia virulence regulon (VirF) | Plasmid |
| ymoA | Yersinia modulator (YmoA) | Chromosomal |
Figure 1.
The role of selected regulatory genes in Yersinia enterocolitica virulence markers expression.
3. Conclusions
Yersinia enterocolitica is the causative agent of yersiniosis, a zoonotic disease of growing epidemiological importance with significant consequences for public health. Due to its complex pathogenesis, further research is needed to expand our knowledge of the molecular mechanisms involved in the infection process and the clinical course of the disease. Many factors, both plasmid and chromosomal, significantly influence these processes.
Acknowledgments
Funded by KNOW (Krajowy Naukowy Ośrodek Wiodący)—Leading National Research Centre) Scientific Consortium “Healthy Animal—Safe Food”, decision of Ministry of Science and Higher Education No. 05-1/KNOW2/2015.
Author Contributions
A.B.K. researched and wrote this review; M.P. and P.Ł. edited the paper and W.S. reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bottone E.J. Yersinia enterocolitica: Revisitation of an enduring human pathogen. Clin. Microbiol. Newslett. 2015;37:1–8. doi: 10.1016/j.clinmicnews.2014.12.003. [DOI] [Google Scholar]
- 2.EFSA (European Food Safety Authority and European Centre for Disease Prevention and Control) EU summary report on zoonoses, zoonotic agents and food-borne outbreaks 2015. EFSA J. 2016;14:4634–4865. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancerz-Kisiel A., Platt-Samoraj A., Szczerba-Turek A., Syczyło K., Szweda W. The first pathogenic Yersinia enterocolitica bioserotype 4/O:3 strain isolated from a hunted wild boar (Sus scrofa) in Poland. Epidemiol. Infect. 2015;143:2758–2765. doi: 10.1017/S0950268814003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bancerz-Kisiel A., Szczerba-Turek A., Platt-Samoraj A., Michalczyk M., Szweda W. Characterisation of ail-positive Yersinia enterocolitica of different biotypes using HRMA. Int. J. Food Microbiol. 2018;269:46–51. doi: 10.1016/j.ijfoodmicro.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Galindo C.L., Rosenzweig J.A., Kirtley M.L., Chopra A.K. Pathogenesis of Y. enterocolitica and Y. pseudotuberculosis in human yersiniosis. J. Pathog. 2011 doi: 10.4061/2011/182051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gierczyński R. Evaluation of the usefulness of selected virulence markers for identification of virulent Yersinia enterocolitica strains. II. Genotypic markers associated with the pYV plasmid. Med. Dośw. Mikrobiol. 2000;52:35–49. [PubMed] [Google Scholar]
- 7.Hein J., Volkhard A.J., Kempf J.D., Bücheler N., Preger S., Horak I., Sing A., Kramer U., Autenrieth I.B. Interferon consensus sequence binding protein confers resistance against Yersinia enterocolitica. environmental regulation of Yersinia pathophysiology. Infect. Immun. 2000;68:1408–1417. doi: 10.1128/IAI.68.3.1408-1417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabina Y., Rahman A., Ramesh C.R., Montet D. Yersinia enterocolitica: Mode of transmission, molecular insights of virulence, and pathogenesis of infection. J. Pathog. 2011:429069. doi: 10.4061/2011/429069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gierczyński R. Evaluation of the usefulness of selected virulence markers for identification of virulent Yersinia enterocolitica strains. III. Chromosome markers of virulence. Med. Dośw. Mikrobiol. 2000;52:51–65. [PubMed] [Google Scholar]
- 10.Pepe J.C., Badger J.L., Miller V.L. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol. Microbiol. 1994;11:123–135. doi: 10.1111/j.1365-2958.1994.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 11.Horne S.M., Prüss B.M. Global gene regulation in Yersinia enterocolitica: Effect of FliA on the expression levels of flagellar and plasmid-encoded virulence genes. Arch. Microbiol. 2006;185:115–126. doi: 10.1007/s00203-005-0077-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen S., Thompson K., Francis M.S. Environmental regulation of Yersinia Pathophysiology. Front. Cell. Infect. Microbiol. 2016;6:25. doi: 10.3389/fcimb.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wattiau P., Cornelis G.R. Identification of DNA sequences recognized by VirF, the transcriptional activator of the Yersinia yop regulon. J. Bacteriol. 1994;176:3878–3884. doi: 10.1128/jb.176.13.3878-3884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rouvroit C.L., Sluiters C., Cornelis G.R. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol. Microbiol. 1992;6:395–409. doi: 10.1111/j.1365-2958.1992.tb01483.x. [DOI] [PubMed] [Google Scholar]
- 15.Cornelis G.R., Sluiters C., Delor I., Geib D., Kaniga K., Lambert de Rouvroit C., Sory M.P., Vanooteghem J.C., Michiels T. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol. Microbiol. 1991;5:1023–1034. doi: 10.1111/j.1365-2958.1991.tb01875.x. [DOI] [PubMed] [Google Scholar]
- 16.Ellison D.W., Young B., Nelson K., Miller V.L. YmoA negatively regulates expression of Invasin from Yersinia enterocolitica. J. Bacteriol. 2003;185:7153–7159. doi: 10.1128/JB.185.24.7153-7159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakoschke T.K., Kakoschke S.C., Zeuzem C., Bouabe H., Adler K., Heesemann J., Rossier O. The RNA chaperone Hfq is essential for virulence and modulates the expression of four Adhesins in Yersinia enterocolitica. Sci. Rep. 2016;6:29275. doi: 10.1038/srep29275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelaseyed T., Bergström J.H., Gustafsson J.K., Ermund A., Birchenough G.M., Schütte A., van der Post S., Svensson F., Rodríguez-Piñeiro A.M., Nyström E.E., et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantle M., Husar S.D. Adhesion of Yersinia enterocolitica to purified rabbit and human intestinal mucin. Infect. Immun. 1993;61:2340–2346. doi: 10.1128/iai.61.6.2340-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsura M. Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front. Immunol. 2013;4:109. doi: 10.3389/fimmu.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skurnik M., Toivanen P. Yersinia enterocolitica lipopolysaccharide: Genetic and virulence. Trends Microbiol. 1993;1:248–152. doi: 10.1016/0966-842X(93)90130-J. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L., Radziejewska-Lebrecht J., Krajewska-Pietrasik D., Toivanen P., Skurnik M. Molecular and chemical characterization of the lipopolysaccharide O-antigen and its role in the virulence of Yersinia enterocolitica serotype O:8. Mol. Microb. 1997;23:63–76. doi: 10.1046/j.1365-2958.1997.1871558.x. [DOI] [PubMed] [Google Scholar]
- 23.Autenrieth I.B., Firsching R. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: An ultrastructural and histological study. J. Med. Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 24.Grutzkau A., Hanski C., Hahn H., Riecken E. Involvement of M cells in the bacterial invasion of Peyer’s patches: A common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rastawicki W., Gierczyński R. Expression, purification, and characterization of the humoral immune response to recombinant MyfA protein of Yersinia enterocolitica. Eur. J. Clin. Microbiol. Infect. Dis. 2009;28:1491–1494. doi: 10.1007/s10096-009-0812-7. [DOI] [PubMed] [Google Scholar]
- 26.Bhagat N., Virdi J.S. Distribution of virulence-associated genes in Yersinia enterocolitica biovar1A correlates with clonal groups and not the source of isolation. FEMS Microbiol. Lett. 2007;266:177–183. doi: 10.1111/j.1574-6968.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- 27.Uliczka F., Pisano F., Schaake J., Stolz T., Rohde M., Fruth A., Strauch E., Skurnik M., Batzilla J., Rakin A., et al. Unique cell adhesion and invasion properties of Yersinia enterocolitica O:3, the most frequent cause of human yersiniosis. PLoS Pathog. 2011;7:e1002117. doi: 10.1371/journal.ppat.1002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mühlenkamp M., Oberhettinger P., Leo J.C., Linke D., Schütz M.S. Yersinia adhesin A (YadA)—Beauty & beast. Int. J. Med. Microbiol. 2015;305:252–258. doi: 10.1016/j.ijmm.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Freund S., Czech B., Trülzsch K., Ackermann N., Heesemann J. Unusual, virulence plasmid-dependent growth behavior of Yersinia enterocolitica in three-dimensional collagen gels. J. Bacteriol. 2008;190:4111–4120. doi: 10.1128/JB.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leo J.C., Elovaara H., Bihan D., Pugh N., Kilpinen S.K., Raynal N., Skurnik M., Farndale R.W., Goldman A. First analysis of a bacterial collagen-binding protein with collagen Toolkits: Promiscuous binding of YadA to collagens may explain how YadA interferes with host processes. Infect. Immun. 2010;78:3226–3236. doi: 10.1128/IAI.01057-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Tahir M., Skurnik M. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 2001;291:209–218. doi: 10.1078/1438-4221-00119. [DOI] [PubMed] [Google Scholar]
- 32.Miętka K., Brzostek K., Guz-Regner K., Bugla-Płoskońska G. The mechanisms of complement activation in normal bovine serum and normal horse serum against Yersinia enterocolitica O:9 strains with different outer membrane proteins content. Pol. J. Vet. Sci. 2016;19:99–107. doi: 10.1515/pjvs-2016-0013. [DOI] [PubMed] [Google Scholar]
- 33.Mühlenkamp M.C., Hallström T., Autenrieth I.B., Bohn E., Linke D., Rinker J., Riesbeck K., Singh B., Leo J.C., Hammerschmidt S., et al. Vitronectin binds to a specific stretch within the head region of Yersinia Adhesin A and thereby modulates Yersinia enterocolitica host interaction. J. Innate Immun. 2017;9:33–51. doi: 10.1159/000449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel J., Luisi B.F. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 2011;9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieckarz M., Raczkowska A., Dębski J., Kistowski M., Dadlez M., Heesemann J., Rossier O., Brzostek K. Impact of OmpR on the membrane proteome of Yersinia enterocolitica in different environments: Repression of major adhesin YadA and heme receptor HemR. Environ. Microbiol. 2016;18:997–1021. doi: 10.1111/1462-2920.13165. [DOI] [PubMed] [Google Scholar]
- 36.Kirjavainen V., Jarva H., Biedzka-Sarek M., Blom A.M., Skurnik M., Meri S. Yersinia enterocolitica serum resistance proteins YadA and Ail bind the complement regulator C4b-binding protein. PLoS Pathog. 2008;4:e1000140. doi: 10.1371/journal.ppat.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biedzka-Sarek M., Venho R., Skurnik M. Role of YadA, Ail, and lipopolysaccharide in serum resistance of Yersinia enterocolitica serotype O:3. Infect. Immun. 2005;73:2232–2244. doi: 10.1128/IAI.73.4.2232-2244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid Y., Grassl G.A., Bühler O.T., Skurnik M., Autenrieth I.B., Bohn E. Yersinia enterocolitica Adhesin A induces production of interleukin-8 in epithelial cells. Infect. Immun. 2004;72:6780–6789. doi: 10.1128/IAI.72.12.6780-6789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai J.C., Yen M.R., Castillo R., Leyton D.L., Henderson I.R., Saier M.H., Jr. The bacterial intimins and invasins: A large and novel family of secreted proteins. PLoS ONE. 2010;5:e14403. doi: 10.1371/journal.pone.0014403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leo J.C., Grin I., Linke D. Type V secretion: Mechanism(s) of autotransport through the bacterial outer membrane. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:1088–1101. doi: 10.1098/rstb.2011.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikula K.M., Kolodziejczyk R., Goldman A. Yersinia infection tools—Characterization of structure and function of adhesins. Front. Cell. Infect. Microbiol. 2013;8:169. doi: 10.3389/fcimb.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Białas N., Kasperkiewicz K., Radziejewska-Lebrecht J., Skurnik M. Bacterial cell surface structures in Yersinia enterocolitica. Arch. Immunol. Ther. Exp. 2012;60:199–209. doi: 10.1007/s00005-012-0168-z. [DOI] [PubMed] [Google Scholar]
- 43.Deuschle E., Keller B., Siegfried A., Manncke B., Spaeth T., Köberle M., Drechsler-Hake D., Reber J., Böttcher R.T., Autenrieth S.E., et al. Role of β integrins and bacterial adhesins for Yop injection into leukocytes in Yersinia enterocolitica systemic mouse infection. Int. J. Med. Microbiol. 2016;306:77–88. doi: 10.1016/j.ijmm.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Thinwa J., Segovia J.A., Bose S., Dube P.H. Integrin-mediated first signal for inflammasome activation in intestinal epithelial cells. J. Immunol. 2014;193:1373–1382. doi: 10.4049/jimmunol.1400145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grassl G.A., Bohn E., Muller Y., Buhler O.T., Autenrieth I.B. Interaction of Yersinia enterocolitica with epithelial cells: Invasin beyond invasion. Int. J. Med. Microbiol. 2003;293:41–54. doi: 10.1078/1438-4221-00243. [DOI] [PubMed] [Google Scholar]
- 46.Wiedemann A., Linder S., Grassl G., Albert M., Autenrieth I., Aepfelbacher M. Yersinia enterocolitica invasin triggers phagocytosis via beta1 integrins, CDC42Hs and WASp in macrophages. Cell. Microbiol. 2001;3:693–702. doi: 10.1046/j.1462-5822.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- 47.Dhar M.S., Virdi J.S. Strategies used by Yersinia enterocolitica to evade killing by the host: Thinking beyond Yops. Microbes Infect. 2014;16:87–95. doi: 10.1016/j.micinf.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Deuretzbacher A., Czymmeck N., Reimer R., Trülzsch K., Gaus K., Hohenberg H., Heesemann J., Aepfelbacher M., Ruckdeschel K. Beta1 integrin-dependent engulfment of Yersinia enterocolitica by macrophages is coupled to the activation of autophagy and suppressed by type III protein secretion. J. Immunol. 2009;183:5847–5860. doi: 10.4049/jimmunol.0804242. [DOI] [PubMed] [Google Scholar]
- 49.Heroven A.K., Böhme K., Tran-Winkler H., Dersch P. Regulatory elements implicated in the environmental control of invasin expression in enteropathogenic Yersinia. Adv. Exp. Med. Biol. 2007;603:156–166. doi: 10.1007/978-0-387-72124-8_13. [DOI] [PubMed] [Google Scholar]
- 50.Raczkowska A., Brzóstkowska M., Kwiatek A., Bielecki J., Brzostek K. Modulation of inv gene expression by the OmpR two-component response regulator protein of Yersinia enterocolitica. Folia Microbiol. 2011;56:313–319. doi: 10.1007/s12223-011-0054-9. [DOI] [PubMed] [Google Scholar]
- 51.Brzostek K., Brzóstkowska M., Bukowska I., Karwicka E., Raczkowska A. OmpR negatively regulates expression of invasin in Yersinia enterocolitica. Microbiology. 2007;153:2416–2425. doi: 10.1099/mic.0.2006/003202-0. [DOI] [PubMed] [Google Scholar]
- 52.Wagner N.J., Lin C.P., Borst L.B., Miller V.L. YaxAB, a Yersinia enterocolitica pore-forming toxin regulated by RovA. Infect. Immun. 2013;81:4208–4219. doi: 10.1128/IAI.00781-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller V.L., Beer K.B., Heusipp G., Young B.M., Wachtel M.R. Identification of regions of Ail required for the invasion and serum resistance phenotypes. Mol. Microbiol. 2001;41:1053–1062. doi: 10.1046/j.1365-2958.2001.02575.x. [DOI] [PubMed] [Google Scholar]
- 54.Felek S., Krukonis E.S. The Yersinia pestis Ail protein mediates binding and Yop delivery to host cells required for plague virulence. Infect. Immun. 2009;77:825–836. doi: 10.1128/IAI.00913-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreira V.P., Pangburn M.K., Cortésa C. Complement control protein factor H: The good, the bad, and the inadequate. Mol. Immunol. 2010;47:2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bliska J.B., Falkow S. Bacterial resistance to complement killing mediated by the Ail protein of Yersinia enterocolitica. Proc. Natl. Acad. Sci. USA. 1992;89:3561–3565. doi: 10.1073/pnas.89.8.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolodziejek A.M., Sinclair D.J., Seo K.S., Schnider D.R., Deobald C.F., Rohde H.N., Viall A.K., Minnich S.S., Hovde C.J., Minnich S.A., et al. Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis. KIM Microbiol. 2007;153:2941–2951. doi: 10.1099/mic.0.2006/005694-0. [DOI] [PubMed] [Google Scholar]
- 58.Kraushaar B., Dieckmann R., Wittwer M., Knabner D., Konietzny A., Mäde D., Staruch E. Characterization of a Yersinia enterocolitica biotype 1A strain harbouring an ail gene. J. Appl. Microbiol. 2011;111:997–1005. doi: 10.1111/j.1365-2672.2011.05112.x. [DOI] [PubMed] [Google Scholar]
- 59.Söderqvist K., Boqvist S., Wauters G., Vågsholm I., Thisted-Lambertz S. Yersinia enterocolitica in sheep—A high frequency of biotype 1A. Acta Vet. Scand. 2012;54:39–45. doi: 10.1186/1751-0147-54-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye Y.W., Ling N., Han Y.J., Wu Q.P. Detection and prevalence of pathogenic Yersinia enterocolitica in refrigerated and frozen dairy products by duplex PCR and dot hybridization targeting the virF and ail genes. J. Dairy Sci. 2014;97:6785–6791. doi: 10.3168/jds.2014-8382. [DOI] [PubMed] [Google Scholar]
- 61.Cheyne B.M., Van Dyke M.I., Anderson W.B., Huck P.M. The detection and prevalence of Yersinia enterocolitica in surface water by quantitative PCR amplification of the ail and yadA genes. J. Water Health. 2010;8:487–499. doi: 10.2166/wh.2009.215. [DOI] [PubMed] [Google Scholar]
- 62.Falcão J.P., Falcão D.P., Pitondo-Silva A., Malaspina A.C., Brocchi M. Molecular typing and virulence markers of Yersinia enterocolitica strains from human, animal and food origins isolated between 1968 and 2000 in Brazil. J. Med. Microbiol. 2006;55:1539–1548. doi: 10.1099/jmm.0.46733-0. [DOI] [PubMed] [Google Scholar]
- 63.Sihvonen L.M., Hallanvuo S., Haukka K., Skurnik M., Siitonen A. The ail gene is present in some Yersinia enterocolitica biotype 1A strains. Foodborne Pathog. Dis. 2011;8:455–457. doi: 10.1089/fpd.2010.0747. [DOI] [PubMed] [Google Scholar]
- 64.Platt-Samoraj A., Syczyło K., Szczerba-Turek A., Bancerz-Kisiel A., Jabłoński A., Łabuć S., Pajdak J., Oshakbaeva N., Szweda W. Presence of ail and ystB genes in Yersinia enterocolitica biotype 1A isolates from game animals in Poland. Vet. J. 2017;221:11–13. doi: 10.1016/j.tvjl.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi A., Donaldson D.S., Erridge C., Kanaya T., Williams I.R., Ohno H., Mahajan A., Mabbott N.A. The functional maturation of M cells is dramatically reduced in the Peyer’s patches of aged mice. Mucosal Immunol. 2013;6:1027–1037. doi: 10.1038/mi.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornelis G.R. Yersinia type III secretion: Send in the effectors. J. Cell Biol. 2002;158:401–408. doi: 10.1083/jcb.200205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denecker G., Tötemeyer S., Mota L.J., Troisfontaines P., Lambermont I., Youta C., Stainier I., Ackermann M., Cornelis G.R. Effect of low- and high-virulence Yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells. Infect. Immun. 2002;70:3510–3520. doi: 10.1128/IAI.70.7.3510-3520.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgan J.M., Duncan M.C., Johnson K.S., Diepold A., Lam H., Dupzyk A.J., Martin L.R., Wong W.R., Armitage J.P., Linington R.G., et al. Piericidin A1 blocks Yersinia Ysc Type III secretion system needle assembly. mSphere. 2017;15:2. doi: 10.1128/mSphere.00030-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montagner C., Arquint C., Cornelis G.R. Translocators YopB and YopD from Yersinia enterocolitica form a multimeric integral membrane complex in eukaryotic cell membranes. J. Bacteriol. 2011;193:6923–6928. doi: 10.1128/JB.05555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartland E.L., Green S.P., Phillips W.A., Robins-Browne R.M. Essential role of YopD in inhibition of the respiratory burst of macrophages by Yersinia enterocolitica. Infect. Immun. 1994;62:4445–4453. doi: 10.1128/iai.62.10.4445-4453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marenne M.N., Journet L., Mota L.J., Cornelis G.R. Genetic analysis of the formation of the Ysc-Yop translocation pore in macrophages by Yersinia enterocolitica: Role of LcrV, YscF and YopN. Microb. Pathog. 2003;35:243–258. doi: 10.1016/S0882-4010(03)00154-2. [DOI] [PubMed] [Google Scholar]
- 72.Aili M., Isaksson E.L., Carlsson S.E., Wolf-Watz H., Rosqvist R., Francis M.S. Regulation of Yersinia Yop-effector delivery by translocated YopE. Int. J. Med. Microbiol. 2007;298:183–192. doi: 10.1016/j.ijmm.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Dave M.N., Silva J.E., Eliçabe R.J., Jeréz M.B., Filippa V.P., Gorlino C.V., Autenrieth S., Autenrieth I.B., Di Genaro M.S. Yersinia enterocolitica YopH-deficient strain activates neutrophil recruitment to Peyer’s patches and promotes clearance of the virulent strain. Infect. Immun. 2016;84:3172–3181. doi: 10.1128/IAI.00568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee W.L., Singaravelu P., Wee S., Xue B., Ang K.C., Gunaratne J., Grimes J.M., Swaminathan K., Robinson R.C. Mechanisms of Yersinia YopO kinase substrate specificity. Sci. Rep. 2017;7:39998. doi: 10.1038/srep39998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt G. Yersinia enterocolitica outer protein T (YopT) Eur. J. Cell Biol. 2011;90:955–958. doi: 10.1016/j.ejcb.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 76.Berneking L., Schnapp M., Rumm A., Trasak C., Ruckdeschel K., Alawi M., Grundhoff A., Kikhney A.G., Koch-Nolte F., Buck F., et al. Immunosuppressive Yersinia effector YopM binds DEAD Box helicase DDX3 to control ribosomal S6 kinase in the nucleus of host cells. PLoS Pathog. 2016;14:e1005660. doi: 10.1371/journal.ppat.1005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keller B., Mühlenkamp M., Deuschle E., Siegfried A., Mössner S., Schade J., Griesinger T., Katava N., Braunsdorf C., Fehrenbacher B., et al. Yersinia enterocolitica exploits different pathways to accomplish adhesion and toxin injection into host cells. Cell. Microbiol. 2015;17:1179–1204. doi: 10.1111/cmi.12429. [DOI] [PubMed] [Google Scholar]
- 78.Delor I., Kaeckenbeeck A., Wauters G., Cornelis G.R. Nucleotide sequence of yst, the Yersinia enterocolitica gene encoding the heat-stable enterotoxin, and prevalence of the gene among pathogenic and nonpathogenic Yersiniae. Infect. Immun. 1990;58:2983–2988. doi: 10.1128/iai.58.9.2983-2988.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bancerz-Kisiel A., Szweda W. Yersiniosis—A Zoonotic foodborne disease of relevance to public health. Ann. Agric. Environ. Med. 2015;22:397–402. doi: 10.5604/12321966.1167700. [DOI] [PubMed] [Google Scholar]
- 80.Tennant S.M., Skinner N.A., Joe A., Robins-Browne R.M. Homologues of insecticidal toxin complex genes in Yersinia enterocolitica biotype 1A and their contribution to virulence. Infect. Immun. 2005;73:6860–6867. doi: 10.1128/IAI.73.10.6860-6867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh I., Virdi J.S. Production of Yersinia stable toxin (YST) and distribution of yst genes in biotype 1A strains of Yersinia enterocolitica. J. Med. Microbiol. 2004;53:1065–1068. doi: 10.1099/jmm.0.45527-0. [DOI] [PubMed] [Google Scholar]
- 82.Delor I., Cornelis G.R. Role of Yersinia enterocolitica Yst toxin in experimental infection of young rabbits. Infect. Immun. 1992;60:4269–4277. doi: 10.1128/iai.60.10.4269-4277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mikulskis A.V., Delor I., Ha Thi V., Cornelis G.R. Regulation of the Yersinia enterocolitica enterotoxin Yst gene. Influence of growth phase, temperature, osmolarity, pH and bacterial host factors. Mol. Microbiol. 1994;14:905–915. doi: 10.1111/j.1365-2958.1994.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 84.Huang X., Yoshino K., Nakao H., Takeda T. Nucleotide sequence of a gene encoding the novel Yersinia enterocolitica heat-stable enterotoxin that includes a pro-region-like sequence in its mature toxin molecule. Microb. Pathog. 1997;22:89–97. doi: 10.1006/mpat.1996.0094. [DOI] [PubMed] [Google Scholar]
- 85.Bancerz-Kisiel A., Szczerba-Turek A., Platt-Samoraj A., Szweda W. Distribution of the ymoA and ystA genes and enterotoxins Yst production by Yersinia enterocolitica strains isolated from humans and pigs. Pol. J. Vet. Sci. 2012;15:609–614. doi: 10.2478/v10181-012-0096-1. [DOI] [PubMed] [Google Scholar]
- 86.Revell P.A., Miller V.L. Yersinia virulence: More than a plasmid. FEMS Microbiol. Lett. 2001;205:159–164. doi: 10.1111/j.1574-6968.2001.tb10941.x. [DOI] [PubMed] [Google Scholar]
- 87.Ellison D.W., Miller V.L. H-NS represses inv transcription in Yersinia enterocolitica through competition with RovA and interaction with YmoA. J. Bacteriol. 2006;188:5101–5112. doi: 10.1128/JB.00862-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McFeeters R.L., Altieri A.S., Cherry S., Tropea J.E., Waugh D.S., Byrd R.A. The high-precision solution structure of Yersinia modulating protein YmoA provides insight into interaction with H-NS. Biochemistry. 2007;46:13975–13982. doi: 10.1021/bi701210j. [DOI] [PubMed] [Google Scholar]
- 89.Nieto J.M., Madrid C., Miquelay E., Parra J.L., Rodriguez S., Juarez A. Evidence for direct protein-protein interaction between members of the enterobacterial Hha/YmoA and H-NS families of proteins. J. Bacteriol. 2002;184:629–635. doi: 10.1128/JB.184.3.629-635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madrid C., Nieto J.M., Juarez A. Role of the Hha/YmoA family of proteins in the thermoregulation of the expression of virulence factors. Int. J. Med. Microbiol. 2002;291:425–432. doi: 10.1078/1438-4221-00149. [DOI] [PubMed] [Google Scholar]
- 91.Dorman C.J. H-NS: A universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2004;2:391–400. doi: 10.1038/nrmicro883. [DOI] [PubMed] [Google Scholar]
- 92.Rimsky S. Structure of the histone-like protein H-NS and its role in regulation and genome superstructure. Curr. Opin. Microbiol. 2004;7:109–114. doi: 10.1016/j.mib.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Madrid C., Balsalobre C., Garcia J., Juarez A. The novel Hha/YmoA family of nucleoid-associated proteins: Use of structural mimicry to modulate the activity of the H-NS family of proteins. Mol. Microbiol. 2007;63:7–14. doi: 10.1111/j.1365-2958.2006.05497.x. [DOI] [PubMed] [Google Scholar]
- 94.Banos R.C., Pons J.I., Madrid C., Juarez A. A global modulatory role for the Yersinia enterocolitica H-NS protein. Microbiology. 2008;154:1281–1289. doi: 10.1099/mic.0.2007/015610-0. [DOI] [PubMed] [Google Scholar]
- 95.Grant T., Bennett-Wood V., Robins-Browne R.M. Identification of virulence-associated characteristics in clinical isolates of Yersinia enterocolitica lacking classical virulence markers. Infect. Immun. 1998;66:1113–1120. doi: 10.1128/iai.66.3.1113-1120.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bancerz-Kisiel A., Lipczyńska K., Szczerba-Turek A., Gospodarek E., Platt-Samoraj A., Szweda W. The use of the HRM method for identifying possible mutations in the ymoA gene region and evaluating their influence on the enterotoxic properties of Y. enterocolitica strains. BMC Vet. Res. 2014;10:207–211. doi: 10.1186/s12917-014-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grant T., Bennett-Wood V., Robins-Browne R.M. Characterization of the interaction between Yersinia enterocolitica biotype 1A and phagocytes and epithelial cells in vitro. Infect. Immun. 1999;67:4367–4375. doi: 10.1128/iai.67.9.4367-4375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh I., Virdi J.S. Interaction of Yersinia enterocolitica biotype 1A strains of diverse origin with cultured cells in vitro. Jpn. J. Infect. Dis. 2005;58:31–33. [PubMed] [Google Scholar]
- 99.Ramamurthy T., Yoshino K., Huang X., Balakrish Nair G., Carniel E., Maruyama T., Fukushima H., Takeda T. The novel heat-stable enterotoxin subtype gene (ystB) of Yersinia enterocolitica: Nucleotide sequence and distribution of the yst genes. Microb. Pathog. 1997;23:189–200. doi: 10.1006/mpat.1997.0146. [DOI] [PubMed] [Google Scholar]
- 100.McNally A., Dalton T., La Ragione R.M., Stapleton K., Manning G., Newell D.G. Yersinia enterocolitica isolates of differing biotypes from humans and animals are adherent, invasive and persist in macrophages, but differ in cytokine secretion profiles in vitro. J. Med. Microbiol. 2006;55:1725–1734. doi: 10.1099/jmm.0.46726-0. [DOI] [PubMed] [Google Scholar]
- 101.Huovinen E., Sihvonen L.M., Virtanen M.J., Haukk K., Siitonen A., Kuusi M. Symptoms and sources of Yersinia enterocolitica-infection: A case-control study. BMC Infect. Dis. 2010;10:122. doi: 10.1186/1471-2334-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]