Abstract
Recent advances in the development of immunosensors using polymeric nanomaterials and nanoparticles have enabled a wide range of new functions and applications in diagnostic and prognostic research. One fundamental challenge that all immunosensors must overcome is to provide the specificity of target molecular recognition by immobilizing antibodies, antibody fragments, and/or other peptides or oligonucleotide molecules that are capable of antigen recognition on a compact device surface. This review presents progress in the application of immobilization strategies including the classical adsorption process, affinity attachment, random cross-linking and specific covalent linking. The choice of immobilization methods and its impact on biosensor performance in terms of capture molecule loading, orientation, stability and capture efficiency are also discussed in this review.
Keywords: immunosensors, polymeric nanomaterials, immobilization methods
1. Introduction
Antibody (Ab) and antibody fragment-based biosensors or immunosensors are compact tools capable of providing sensitive and rapid detection or capture of a range of pathogens or cells of interests for further analysis. The history of biosensors dates back to 1956 when Leland C. Clark described the first biosensor which was developed to detect glucose levels in serum samples using a membrane bound biologically sensitive element [1]. Over the past few decades, numerous types of biosensors have been developed for detection of a wide variety of substrates including small molecules, proteins, oligonucleotides, cells, viruses, etc. Immunosensors, a special type of biosensor, frequently applied for the detection of specific antigens or antibodies, are analytic devices that convert the signal generated by antigen-antibody binding events into a measurable signal. To identify new and useful immunosensors, scientists must exploit recent advances in the development of nanomaterial solid supports with ideal surface properties, such as surfaces that employ antigen specific capture molecules for immunochemical reactions or antigen binding.
The fundamental basis of all immunosensors is to efficiently create stable linkages between desired capture molecules and the nanomaterial. Abs are the most extensively used antigen binding molecules due to their exquisite specificity and affinity. A vast selection of monoclonal Abs (mAbs) have been developed using hybridoma technology to provide excellent tools to detect, perturb, or enhance key components in biological systems.
Recent efforts have been made to shrink Ab-based tools through the dissociation of full size Abs into smaller antigen binding fragments [2]. Initial truncations were achieved through proteolysis and later by genetic engineering to provide mono- or multi-valent fragments. Such Ab derivatives are bona fide alternatives to full size mAbs as they retain the targeting specificity of the prototype but can be produced more economically with recombinant expression. Examples include antigen-binding (Fab) fragment, single-chain variable fragment (scFv), diabody, minibody, and variable domains derived from heavy chain-only only antibody (VHH) [3,4]. Alternative genetically engineered antibody mimetic proteins, also known as single-scaffold proteins, include designed ankryin repeat proteins (DARPins) [5] and protein A-derived affibody molecules [6]. Together with oligonucleotides-based aptamers, these new alternatives have recently entered immunosensor field [7]. In this review, progress in the development of Abs and Ab-derivative-based biosensors for disease surveillance and monitoring is reviewed, with an emphasis on recent advancement in conjugation methods for the attachment of proteins solid surfaces. The advantages and limitations of antibodies and their derivatives in the context of conjugation methods used in current immunosensors are also discussed.
2. Selection of Antigen Binding Molecules
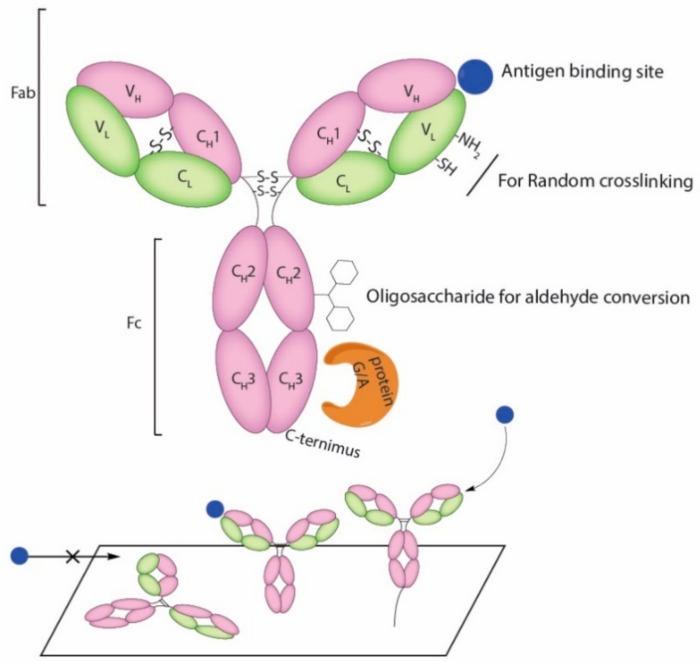
Abs or immunoglobulins (Igs) are highly soluble serum glycoproteins which can be divided into five main isotypes (IgA, IgD, IgE, IgG, and IgM) based on their heavy chain constant region sequences [8]. Polyclonal antibodies (pAb) can be easily raised in animals such as rat, rabbits, goats, and sheep. They are frequently used in immunosensors for pathogen detection. However, multiple epitopes may often be recognized by pAb since their source is a pool of Ab secreting B cells [9]. In cases where highly specific binding is required, monoclonal antibodies (mAb) are more desirable and can be generated through the use of hybridoma technology [10]. Splenocytes from an immunized animal are commonly used as the source of Ab-producing B cells for myeloma fusion. The resulting hybridoma cells are immortal cell lines secreting full size Abs [11]. The pool of hybridomas can be further screened against targeted antigens to identify suitable single cell candidate for monoclonal Ab production. Among the five main isotypes of Ig, IgG is the main type of Ab found in blood and extracellular fluid [8]. The general structure of an IgG is shown in Figure 1A, and it consists of four polypeptide chains, i.e., two heavy chains and two light chains, which are joined together by disulfide bonds. The antigen-binding (Fab) region, composed of one constant and one variable domain from a paired heavy and light chain, provides the site responsible for antigen binding (Figure 2). Conjugation reactions may result in deleterious effect on antibody avidity [12]. Thus, immobilization strategies should be carefully designed so that Fabs are left unaltered throughout the process. High degree random conjugation may inevitably results in changes in antigen binding characteristics and, in more extreme scenario, a complete loss of function [13]. In addition, different immobilization methods may lead to uniform or random orientation of Abs on solid support [14]. The ability to control the site of protein (Ab) modification is essential to avoid the destruction or steric hindrance of immobilized Fabs. In principle, modification sites should be kept far away from the Fabs to achieved the best result.
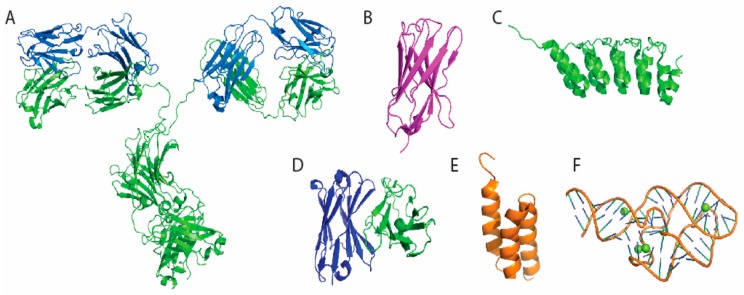
Figure 1.
Overall structure of Ab and alternative recombinant binder scaffolds used in biosensors. (A) IgG2a monoclonal antibody with two heavy chains colored in green and two light chains colored in blue (PDB:1IGT) [18]; (B) The green fluorescent protein (GFP)-VHH (PDB: 3OGO) [19]; (C) DARPin against tubulin beta chain (PDB: 4DUI) [20]; (D) anti-fluorescein ScFv (PDB: 1X9Q) [21]; (E) human epidermal growth factor receptor 2 (HER2) binding affibody (PDB: 2KZJ) [22]; (F) 5-hydroxytryptophan aptmer (PDB: 5KPY) [23]. We used PyMol to generate all the structures in this figure.
Figure 2.
Functional groups on Abs used for conjugation and the result of random and oriented immobilization onto surfaces.
Although full-size Abs generated through immunization have been widely used for immunosensors since the beginning of biosensor development, recently developed recombinant molecules generated in vitro have many advantages over conventional Abs. The advantages of these alternative scaffolds include their compact sizes, excellent thermal and chemical stabilities, as well as low production costs [4,15]. For example, to obtain useful VHHs found in camelids (Figure 1B), a cDNA library can be obtained from an immunized llama or alpaca to serve as a pool of amplified DNA fragments for generation of the phage display library [15]. Phage that bind target during panning process can then be recovered, sequenced and transferred to a periplasmic expression vector to obtain the final VHH protein from E. coli expression. VHH binders with desired properties can be recombinantly expressed with tag sequences, protein fusion partners, or artificial amino acids through covalent modification [16]. Similarly, DARPins (Figure 1C), scFv (Figure 1D), and affibody (Figure 1E) can also be screened from existing libraries as alternative binders. In addition, peptide and oligonucleotide based binding molecules, such as aptamers (Figure 1F), can be screened from a pool of random sequences to acquire molecules with desired binding specificity and affinity [17].
3. Immunosensor Types and Common Material Selection
Supporting materials of an immunosenor can be selected based on analytic needs (Table 1). Optical transducers that exploit light absorption, luminescence, and fluorescence often require optically transparent materials as support. Surface plasmon resonance (SPR) sensors rely on the unique optical properties of metallic nanostructures (gold, silver, etc.) [24]. Electrochemical immunosensors produce electrical charges for the quantitative analysis of target molecules. Many novel nanomaterials, such as carbon nanotubes, graphene, indium tin oxide, nanowire, hydrogels, and metallic nanoparticles, are employed to construct a high-performance electrode for signal output [25]. Many attempts have been made to improve the electrochemical properties of supporting material to increase electro-catalytic trait, create excellent electron transfer ability and excellent biocompatibility [25,26]. Piezoelectric immunosensors exploit the piezoelectric effect which occurs in various crystalline substances. The piezoelectric immunosensor is known to be one of the most sensitive analytical instruments with the ability to detect antigens in the picogram range [27,28].
Table 1.
Immunosensor types and common materials used.
| Immunosensor Type | Common Materials | References | |
|---|---|---|---|
| Optical | Evanescent wave | Quartz, glass, graphene oxide (GO) sheets, hydrogels | [24,29,30,31] |
| Surface plasmon resonance (SPR) | Silver, gold, copper, aluminum | ||
| Electrochemical | Conductive | Carbon, indium tin oxide, carbon nanotube, hydrogels, polythiophene | [32,33,34,35] |
| Amperometric | Graphite, Lipid, Platinum, Gold, Nickel | ||
| Piezoelectric | Bulk acoustic wave | Aluminium phosphate, aluminium nitride, zinc oxide, crystalized topaz, crystalized tourmaline, barium titanate, gallium orthophosphate, lead titanate | [27,28,36] |
| Surface acoustic wave | |||
4. Current Conjugation Methods
The performance of an immnosensor depends upon three key factors: (1) the binding affinity and specificity of antigen binding molecules; (2) the accessibility and proportion of binding sites intact after immobilization; and (3) the density of binding molecules coated on the surface of immunosensor. Different strategies for immobilization may result in different outcomes and efficiencies (Figure 2). Immobilized Abs can adopt several different orientations depending on the method applied. Adsorption method often results a “flat-on” orientation, with the Fc and Fab fragments lying flat on the surface [37]. This confirmation can result in hindrance of antigen access to antibody binding sites, which eventually lead to the decrease in antigen binding capacity [38]. Specific orientation is always ideal but not as easily achieved as adsorption, since site specific modification of antigen binding molecules commonly require incorporation of a unique reactive group. Affinity based attachment using protein A and protein G provides another solution. These proteins display multiple binding sites specific to the Fc potion of Abs and lead to a predominantly “tail-on” attachment of Abs. Further improvement can be done by introducing a linker between protein A and protein G to the system. The immobilization strategies should be also compatible with the targeted surface materials, such as gold, copper, iron, silicon, hydro-gel, carbon nanotubes, and graphene oxide. We presented the most popular conjugation methods for each different binding molecule category (Table 2).
Table 2.
A summary of popular conjugation methods.
| Type of Antigen Binding Molecules | Types of Immobilization | Functional Group | Orientation | References |
|---|---|---|---|---|
| Antibody | Adsorption | Various | Random | [39,40,41,42] |
| Affinity | Antigen-antibody reaction | Partially oriented | [42,43,44] | |
| Protein A or G (non-covalent) binding | Partially oriented | [41,45] | ||
| Radom crosslinking | Amine/carboxylic acid | Random | [43,44,46,47] | |
| Thiol group | Random | [44,46,48] | ||
| Sugar chain on CH2 | Partially Oriented | [49,50] | ||
| DNA-directed | Nucleotide Binding Site ssDNA hybridization | Uniformly oriented | [51,52] | |
| C terminus | Enzyme mediated biotinylation | Uniformly oriented | [49] | |
| VHH | C terminus | non-natural amino-acid | Uniformly oriented | [53,54] |
| C terminus | Enzyme mediated transpeptidation | Uniformly oriented | [55,56] | |
| scFv | Tag mediated | Cysteine or Histidine containing linker | Partially Oriented | [57,58,59] |
| E. coli surface displayed | Genetic fusion | Uniformly oriented | [60] | |
| DARPins | Radom crosslinking | Amine group | Random | [61,62] |
| Aptamer | Terminal modification | Thiol | Uniformly oriented | [63,64,65] |
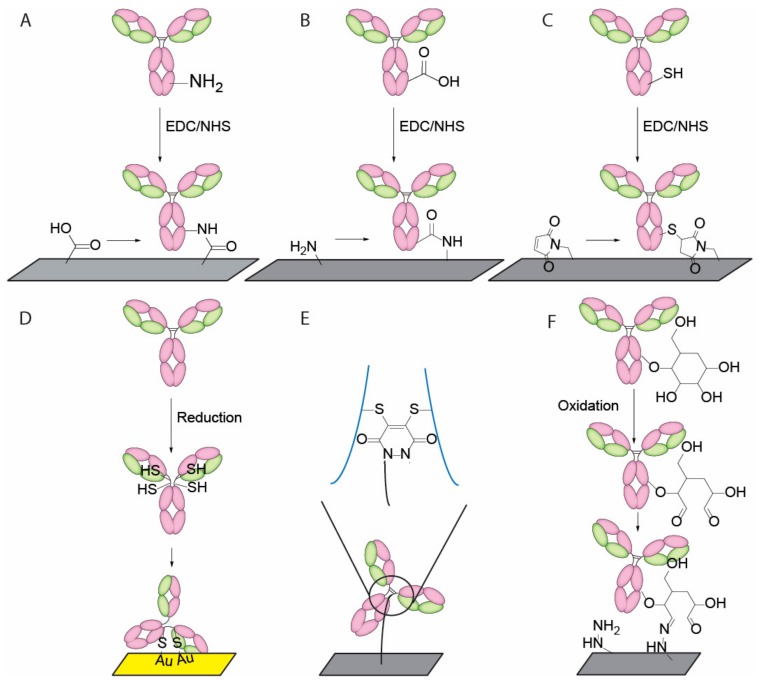
Physical adsorption of Abs onto hydrophobic surfaces such as polystyrene offers the simplest attachment. However, the process is uncontrollable in terms of orientation and stability, and often results in denaturation and detachment of protein on surface [37]. Protein G and protein A are small bacteria derived moieties bind in a specific orientation to Fc region [45]. This method offers non-covalent but mostly “tail-on” Ab attachment. Random crosslinking methods provide another mode of attachment as amine, carboxyl and thiol groups are abundant throughout the surface of antibody. Amine and carboxyl coupling is commonly achieved by using carbodiimide as a carboxyl activation reagent in combination of succinimidyl ester (NHS) for improved efficiency [46]. This method, also known as EDC/NHS coupling, can be used to robustly create covalent linkage via amide bond formation (Figure 3A,B). Reactive primary amine and carboxyl groups on Ab surface are mostly lysine, aspartic acid and glutamic acid side chains, which are usually abundant in Fab region due to its polar nature. Thus, it is impossible to control the orientation and predict the outcomes of Fab region immobilization as the immobilized product is a mixture of Abs modified at different molar equivalents and positions. Similarly, reactive thiol groups (cysteine side chains) can also be targeted for maleimide or iodoacetamide reaction (Figure 3C). Additionally, disulfide bonds can also be reduced as an alternative source of thiol groups. In addition to the classical maleimide reaction or gold surface attachment (Figure 3D), Baker et al. and Chudasama et al. reported the usage of pyridazinedone as a way to yield a more homogenous product with better retention of structural bond (Figure 3E) [48]. Several other approaches are reported to achieve specific orientations. Kang et al. reported a site specific biotinylation strategy using the sugar moiety on the Fc region (Figure 3F) [49]. Oxidation of sugar chains yield reactive aldehyde groups, which can be used to covalently link Abs in an oriented manner without disturbing the structural integrity. There are several other immobilizations methods reported recently. Bilgicer and co-workers exploited the conserved nucleotide binding site (NBS) on the Fab region to achieve site specific labeling using UV-cross linking method [51]. Boozer and others reported a DNA-directed Ab immobilization method by using ssDNA pre-conjugated to Ab to form a self-assembled monolayer on the surface coated with complementary sequence [52]. Another Ab site-specific labeling strategy is to use formylglycine-generating enzyme (FGE) to install an aldehyde tag on a specific pentapeptide sequence, which may then react with aminooxy-containing surface linkers [66].
Figure 3.
Ab immobilization scheme. (A) EDC/NHS coupling of Ab surface amine to carboxyl and (B) carboxyl groups to amine groups; (C) Sulfhydryl-reactive chemical group coupling to Ab surface thiol groups; (D) Reduction of antibody disulfides to reactive thiols for gold substrates binding; (E) Reduction of antibody disulfides for site specific pyridazinedone coupling; and (F) Oxidation of sugar chains for reactive aldehyde groups.
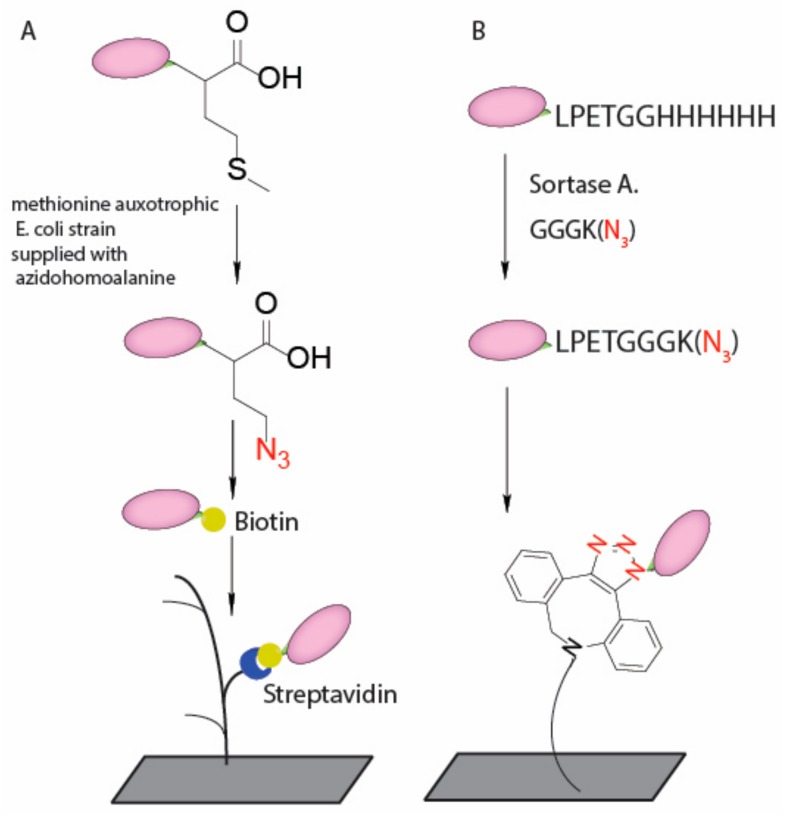
VHHs, also known as nanobodies, are recombinant, antigen-specific, single-domain, variable fragments of camelid heavy chain-only antibodies. Compared to full size IgGs, VHHs can be expressed in high yield in bacterial systems. The small size (~14 kD) provides significant advantages in medical diagnostic and therapeutic applications [67]. However, due to their small size, VHHs have a significantly higher percentage of their surface involved in binding interactions compared to full size IgGs; therefore, site-specific installation of linker is particularly important for immunosensors that use VHHs. Beekwilder et al. reported an oriented labeling method using azide functionalized VHH onto a cyclooctyne-tailored sensor surface (Figure 4A) [53]. They emphasized the importance of oriented immobilization as it increased sensor efficiency up to 800-fold compared to randomly labeling. In addition, transpeptidase can also be used to create site specific modification on VHH since a short peptide recognition sequence can be easily incorporated into recombinant expression vectors. The Ploegh group incorporated the sortase recognition motif, LPXTG, at the C terminus of VHHs, which can then be used for installation of a short GGG peptide with a biorthogonal handle (Figure 4B) [55,56,68,69,70].
Figure 4.
Oriented immobilization scheme for VHHs. (A) C-terminal N3 group introduced by artificial amino acid incorporation followed by conversion to biotin group for streptavidin binding; and (B) C-terminal N3 group attached via sortase mediated transpeptidation followed by site specific attachment on DBCO modified surface.
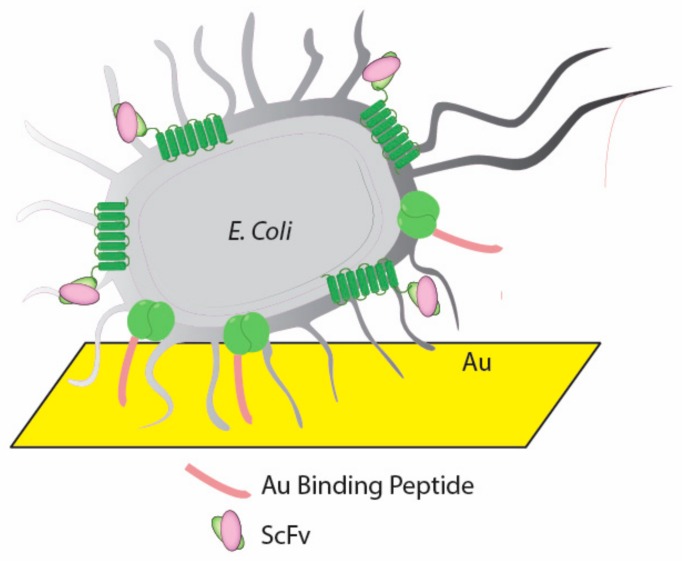
ScFvs are a type of fusion protein which contains variable regions of the heavy (VH) and light chains (VL) of Abs connected via a short peptide linker. Shen et al. optimized a 15-mer peptide linker (RGRGRGRGRSRGGGS) to increase the adsorption efficiency on anionic charged biosensor surface [57]. Chen et al. developed a cancer marker monitoring platform that constructed by a dual-expression system in E. coli which can display anti-cancer ScFvs and gold binding peptide on the surface of bacteria at the same time (Figure 5). In this case, ScFvs are fused at the C-terminus of the extracellular domain of a transmembrane protein (Lpp-OmpA) and achieve a fixed orientation display of ScFv on E. coli surface.
Figure 5.
ScFv and gold-binding peptide dual-expression system in E. coli [60].
Other novel alternatives, such as DARPins and Aptamers can also serve as the binding moiety in immunosensors. DARPins are a novel class of non-IgG scaffolds based on naturally occurring ankyrin repeats. DARPins are small (13–20 kDa) and highly soluble in aqueous solution. Deyev and others reported that DARPins can bind tightly to gold nanoparticles (GNPs) via adsorption [61].
Aptamers are single-stranded DNA or RNA (ssDNA or ssRNA) oligonucleotides or peptides engineered through repeated in vitro selection or equivalent methods. However, aptamers have distinct limitations, especially for those composed of DNA or RNA. The rapid degradation of aptamers by nucleases in biological media is a serious problem. Such degradation causes instability which is unacceptable for biosensor application. Despite such limitations, many successful attempts have been made to create aptamer-based biosensors in relatively nuclease free systems. One approach employed terminally functionalized thiol group for gold surface binding [64]. Other functional groups such as primary amine or activated carboxylic acid are also commonly used for covalent conjugation [65].
5. Conclusions
The performance of immunosensors is closely associated to the antigen binding molecules and immobilization approach. While Abs have been increasingly used as detection elements in immunosensors, recent development in antibody derivatives and other alternative binding molecules raise new opportunities and possibilities to create highly stable, efficient, and economically feasible diagnostic device. In this review, a wide range of immobilization strategies are presented for Ab and Ab alternatives and their applications on various nanomaterial surfaces are discussed. Pros and cons of each method are presented. The uniform orientation conferred by site-specific immobilization is essential for small Ab alternatives to retain their binding efficiency.
Acknowledgments
This work was supported by National Chiao Tung University (106W970 and 107W970), Ministry of Science and Technology (MOST 105-2628-B-009-001-MY3 and MOST 106-EPA-F006-001) and National Health Research Institutes (NHRI-EX107-10714EC), Taiwan.
Author Contributions
Z.L. wrote the paper; and G.-Y.C. guided and supervised the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Heineman W.R., Jensen W.B. Leland C. Clark Jr. (1918–2005) Biosens. Bioelectron. 2006;21:1403–1404. doi: 10.1016/j.bios.2005.12.005. [DOI] [Google Scholar]
- 2.Rodgers K.R., Chou R.C. Therapeutic monoclonal antibodies and derivatives: Historical perspectives and future directions. Biotechnol. Adv. 2016;34:1149–1158. doi: 10.1016/j.biotechadv.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Holliger P., Hudson P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 4.Muyldermans S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 5.Binz H.K., Amstutz P., Kohl A., Stumpp M.T., Briand C., Forrer P., Grutter M.G., Pluckthun A. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat. Biotechnol. 2004;22:575–582. doi: 10.1038/nbt962. [DOI] [PubMed] [Google Scholar]
- 6.Nord K., Gunneriusson E., Ringdahl J., Stahl S., Uhlen M., Nygren P.A. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat. Biotechnol. 1997;15:772–777. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J., Battig M.R., Wang Y. Aptamer-based molecular recognition for biosensor development. Anal. Bioanal. Chem. 2010;398:2471–2480. doi: 10.1007/s00216-010-3987-y. [DOI] [PubMed] [Google Scholar]
- 8.Vidarsson G., Dekkers G., Rispens T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipman N.S., Jackson L.R., Trudel L.J., Weis-Garcia F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J. 2005;46:258–268. doi: 10.1093/ilar.46.3.258. [DOI] [PubMed] [Google Scholar]
- 10.Singh S., Kumar N., Dwiwedi P., Charan J., Kaur R., Sidhu P., Chugh V.K. Monoclonal antibodies: A review. Curr. Clin. Pharmacol. 2017 doi: 10.2174/1574884712666170809124728. [DOI] [PubMed] [Google Scholar]
- 11.Banker D.D. Monoclonal antibodies. A review. Indian J. Med. Sci. 2001;55:651–654. [PubMed] [Google Scholar]
- 12.Vira S., Mekhedov E., Humphrey G., Blank P.S. Fluorescent-labeled antibodies: Balancing functionality and degree of labeling. Anal. Biochem. 2010;402:146–150. doi: 10.1016/j.ab.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werthen M., Nygren H. Effect of antibody affinity on the isotherm of antibody binding to surface-immobilized antigen. J. Immunol. Methods. 1988;115:71–78. doi: 10.1016/0022-1759(88)90311-0. [DOI] [PubMed] [Google Scholar]
- 14.Trilling A.K., Beekwilder J., Zuilhof H. Antibody orientation on biosensor surfaces: A minireview. Analyst. 2013;138:1619–1627. doi: 10.1039/c2an36787d. [DOI] [PubMed] [Google Scholar]
- 15.Bever C.S., Dong J.X., Vasylieva N., Barnych B., Cui Y., Xu Z.L., Hammock B.D., Gee S.J. Vhh antibodies: Emerging reagents for the analysis of environmental chemicals. Anal. Bioanal. Chem. 2016;408:5985–6002. doi: 10.1007/s00216-016-9585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Marco A. Biotechnological applications of recombinant single-domain antibody fragments. Microb. Cell Fact. 2011;10:44. doi: 10.1186/1475-2859-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray P., White R.R. Aptamers for targeted drug delivery. Pharmaceuticals. 2010;3:1761–1778. doi: 10.3390/ph3061761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris L.J., Larson S.B., Hasel K.W., McPherson A. Refined structure of an intact IgG2a monoclonal antibody. Biochemistry. 1997;36:1581–1597. doi: 10.1021/bi962514+. [DOI] [PubMed] [Google Scholar]
- 19.Kubala M.H., Kovtun O., Alexandrov K., Collins B.M. Structural and thermodynamic analysis of the GFP: GFP-nanobody complex. Protein Sci. 2010;19:2389–2401. doi: 10.1002/pro.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pecqueur L., Duellberg C., Dreier B., Wang Q., Jiang C., Pluckthun A., Surrey T., Gigant B., Knossow M. An Anti-Tubulin Darpin Caps the Microtubule Plus-End. [(accessed on 15 February 2013)]; Available online: http://www.rcsb.org/structure/4DUI.
- 21.Midelfort K.S., Hernandez H.H., Lippow S.M., Tidor B., Drennan C.L., Wittrup K.D. Substantial energetic improvement with minimal structural perturbation in a high affinity mutant antibody. J. Mol. Biol. 2004;343:685–701. doi: 10.1016/j.jmb.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Eigenbrot C., Ultsch M., Dubnovitsky A., Abrahmsen L., Hard T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc. Natl. Acad. Sci. USA. 2010;107:15039–15044. doi: 10.1073/pnas.1005025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter E.B., Polaski J.T., Morck M.M., Batey R.T. Recurrent RNA motifs as scaffolds for genetically encodable small-molecule biosensors. Nat. Chem. Biol. 2017;13:295–301. doi: 10.1038/nchembio.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabbany S.Y., Donner B.L., Ligler F.S. Optical immunosensors. Crit. Rev. Biomed. Eng. 1994;22:307–346. [PubMed] [Google Scholar]
- 25.Felix F.S., Angnes L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018;102:470–478. doi: 10.1016/j.bios.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 26.Wen W., Yan X., Zhu C., Du D., Lin Y. Recent advances in electrochemical immunosensors. Anal. Chem. 2017;89:138–156. doi: 10.1021/acs.analchem.6b04281. [DOI] [PubMed] [Google Scholar]
- 27.Zu H., Wu H., Wang Q.M. High-temperature piezoelectric crystals for acoustic wave sensor applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016;63:486–505. doi: 10.1109/TUFFC.2016.2527599. [DOI] [PubMed] [Google Scholar]
- 28.Marrazza G. Piezoelectric biosensors for organophosphate and carbamate pesticides: A review. Biosensors. 2014;4:301–317. doi: 10.3390/bios4030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akkoyun A., Bilitewski U. Optimisation of glass surfaces for optical immunosensors. Biosens. Bioelectron. 2002;17:655–664. doi: 10.1016/S0956-5663(02)00029-5. [DOI] [PubMed] [Google Scholar]
- 30.Wiederoder M.S., Kendall E.L., Han J.H., Ulrich R.G., DeVoe D.L. Flow-through microfluidic immunosensors with refractive index-matched silica monoliths as volumetric optical detection elements. Sens. Actuators B Chem. 2018;254:878–886. doi: 10.1016/j.snb.2017.07.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson G.A. Optical immunosensors. Biochem. Soc. Trans. 1991;19:18–20. doi: 10.1042/bst0190018. [DOI] [PubMed] [Google Scholar]
- 32.Liu G., Lin Y. Nanomaterial labels in electrochemical immunosensors and immunoassays. Talanta. 2007;74:308–317. doi: 10.1016/j.talanta.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munge B.S., Krause C.E., Malhotra R., Patel V., Gutkind J.S., Rusling J.F. Electrochemical immunosensors for interleukin-6. Comparison of carbon nanotube forest and gold nanoparticle platforms. Electrochem. Commun. 2009;11:1009–1012. doi: 10.1016/j.elecom.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H., Malhotra R., Peczuh M.W., Rusling J.F. Electrochemical immunosensors for antibodies to peanut allergen ara h2 using gold nanoparticle-peptide films. Anal. Chem. 2010;82:5865–5871. doi: 10.1021/ac101110q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piro B., Reisberg S. Recent advances in electrochemical immunosensors. Sensors. 2017;17:794. doi: 10.3390/s17040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hees J., Heidrich N., Pletschen W., Sah R.E., Wolfer M., Williams O.A., Lebedev V., Nebel C.E., Ambacher O. Piezoelectric actuated micro-resonators based on the growth of diamond on aluminum nitride thin films. Nanotechnology. 2013;24:025601. doi: 10.1088/0957-4484/24/2/025601. [DOI] [PubMed] [Google Scholar]
- 37.Wiseman M.E., Frank C.W. Antibody adsorption and orientation on hydrophobic surfaces. Langmuir. 2012;28:1765–1774. doi: 10.1021/la203095p. [DOI] [PubMed] [Google Scholar]
- 38.Xu H., Zhao X., Grant C., Lu J.R., Williams D.E., Penfold J. Orientation of a monoclonal antibody adsorbed at the solid/solution interface: A combined study using atomic force microscopy and neutron reflectivity. Langmuir. 2006;22:6313–6320. doi: 10.1021/la0532454. [DOI] [PubMed] [Google Scholar]
- 39.Zourob M. Recognition Receptors in Biosensors. Springer; New York, NY, USA: London, UK: 2010. p. xvi.863p [Google Scholar]
- 40.Ligler F.S., Taitt C.A.R. Optical Biosensors: Present and Future. 1st ed. Elsevier; Amsterdam, The Netherlands: New York, NY, USA: 2002. p. viii.607p [Google Scholar]
- 41.Yang V.C.-M., Ngo T.T. Biosensors and Their Applications. Kluwer Academic/Plenum Publishers; New York, NY, USA: 2000. p. xviii.360p [Google Scholar]
- 42.Rasooly A., Herold K.E. Biosensors and Biodetection: Methods and Protocols. Humana Press; New York, NY, USA: 2009. [DOI] [PubMed] [Google Scholar]
- 43.Hermanson G.T. Bioconjugate Techniques. 3rd ed. Elsevier/AP; London, UK: Waltham, MA, USA: 2013. p. xvii.1146p [Google Scholar]
- 44.Watson R.R., Preedy V.R. Genetically Modified Organisms in Food: Production, Safety, Regulation and Public Health. Elsevier Science/Academic Press; Amsterdam, The Nertherlands: Boston, MA, USA: 2016. p. xxi.494p [Google Scholar]
- 45.Choe W., Durgannavar T.A., Chung S.J. Fc-binding ligands of immunoglobulin G: An overview of high affinity proteins and peptides. Materials. 2016;9:994. doi: 10.3390/ma9120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spicer C.D., Davis B.G. Selective chemical protein modification. Nat. Commun. 2014;5:4740. doi: 10.1038/ncomms5740. [DOI] [PubMed] [Google Scholar]
- 47.Howard G.C., Bethell D.R. Basic Methods in Antibody Production and Characterization. CRC Press; Boca Raton, FL, USA: 2001. p. 271. [Google Scholar]
- 48.Lee M.T.W., Maruani A., Richards D.A., Baker J.R., Caddick S., Chudasama V. Enabling the controlled assembly of antibody conjugates with a loading of two modules without antibody engineering. Chem. Sci. 2017;8:2056–2060. doi: 10.1039/C6SC03655D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang J.H., Choi H.J., Hwang S.Y., Han S.H., Jeon J.Y., Lee E.K. Improving immunobinding using oriented immobilization of an oxidized antibody. J. Chromatogr. A. 2007;1161:9–14. doi: 10.1016/j.chroma.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 50.Makaraviciute A., Ramanaviciene A. Site-directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron. 2013;50:460–471. doi: 10.1016/j.bios.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 51.Alves N.J., Champion M.M., Stefanick J.F., Handlogten M.W., Moustakas D.T., Shi Y., Shaw B.F., Navari R.M., Kiziltepe T., Bilgicer B. Selective photocrosslinking of functional ligands to antibodies via the conserved nucleotide binding site. Biomaterials. 2013;34:5700–5710. doi: 10.1016/j.biomaterials.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 52.Boozer C., Ladd J., Chen S., Yu Q., Homola J., Jiang S. DNA directed protein immobilization on mixed ssdna/oligo(ethylene glycol) self-assembled monolayers for sensitive biosensors. Anal. Chem. 2004;76:6967–6972. doi: 10.1021/ac048908l. [DOI] [PubMed] [Google Scholar]
- 53.Trilling A.K., Harmsen M.M., Ruigrok V.J., Zuilhof H., Beekwilder J. The effect of uniform capture molecule orientation on biosensor sensitivity: Dependence on analyte properties. Biosens. Bioelectron. 2013;40:219–226. doi: 10.1016/j.bios.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 54.Rush J.S., Bertozzi C.R. New aldehyde tag sequences identified by screening formylglycine generating enzymes in vitro and in vivo. J. Am. Chem. Soc. 2008;130:12240–12241. doi: 10.1021/ja804530w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen G.Y., Li Z., Theile C.S., Bardhan N.M., Kumar P.V., Duarte J.N., Maruyama T., Rashidfarrokh A., Belcher A.M., Ploegh H.L. Graphene oxide nanosheets modified with single-domain antibodies for rapid and efficient capture of cells. Chemistry. 2015;21:17178–17183. doi: 10.1002/chem.201503057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G.Y., Li Z., Duarte J.N., Esteban A., Cheloha R.W., Theile C.S., Fink G.R., Ploegh H.L. Rapid capture and labeling of cells on single domain antibodies-functionalized flow cell. Biosens. Bioelectron. 2017;89:789–794. doi: 10.1016/j.bios.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen Z., Yan H., Zhang Y., Mernaugh R.L., Zeng X. Engineering peptide linkers for scFv immunosensors. Anal. Chem. 2008;80:1910–1917. doi: 10.1021/ac7018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen Z., Mernaugh R.L., Yan H., Yu L., Zhang Y., Zeng X. Engineered recombinant single-chain fragment variable antibody for immunosensors. Anal. Chem. 2005;77:6834–6842. doi: 10.1021/ac0507690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falco C.N., Dykstra K.M., Yates B.P., Berget P.B. Scfv-based fluorogen activating proteins and variable domain inhibitors as fluorescent biosensor platforms. Biotechnol. J. 2009;4:1328–1336. doi: 10.1002/biot.200900075. [DOI] [PubMed] [Google Scholar]
- 60.Ng F.-L., Lan K.-C., Chang C.-Y., Hsu C.-H., Ho T.-Y., Hsieh M.-H., Kuo T.-H., Wang Y.-Y., Lin R.-H., Hsu W.-H., et al. E.Cotector: The fluorescent E. coli with surface displayed anti-cancer marker scFv to detect specific cancer markers. [(accessed on 15 March 2018)];PLoS Collect. 2016 Available online: http://blogs.plos.org/blog/2016/10/15/igem-research-article-e-cotector-the-fluorescent-e-coli-with-surface-displayed-anti-cancer-marker-scfv-to-detect-specific-cancer-markers/ [Google Scholar]
- 61.Deyev S., Proshkina G., Ryabova A., Tavanti F., Menziani M.C., Eidelshtein G., Avishai G., Kotlyar A. Synthesis, characterization, and selective delivery of darpin-gold nanoparticle conjugates to cancer cells. Bioconjug. Chem. 2017;28:2569–2574. doi: 10.1021/acs.bioconjchem.7b00410. [DOI] [PubMed] [Google Scholar]
- 62.Jost C., Pluckthun A. Engineered proteins with desired specificity: Darpins, other alternative scaffolds and bispecific igGs. Curr. Opin. Struct. Biol. 2014;27:102–112. doi: 10.1016/j.sbi.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 63.Na W., Liu X., Wang L., Su X. Label-free aptamer biosensor for selective detection of thrombin. Anal. Chim. Acta. 2015;899:85–90. doi: 10.1016/j.aca.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 64.Eissa S., Zourob M. Aptamer- based label-free electrochemical biosensor array for the detection of total and glycated hemoglobin in human whole blood. Sci. Rep. 2017;7:1016. doi: 10.1038/s41598-017-01226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farokhzad O.C., Karp J.M., Langer R. Nanoparticle-aptamer bioconjugates for cancer targeting. Expert Opin. Drug Deliv. 2006;3:311–324. doi: 10.1517/17425247.3.3.311. [DOI] [PubMed] [Google Scholar]
- 66.York D., Baker J., Holder P.G., Jones L.C., Drake P.M., Barfield R.M., Bleck G.T., Rabuka D. Generating aldehyde-tagged antibodies with high titers and high formylglycine yields by supplementing culture media with copper(ii) BMC Biotechnol. 2016;16:23. doi: 10.1186/s12896-016-0254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Meyer T., Muyldermans S., Depicker A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014;32:263–270. doi: 10.1016/j.tibtech.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Bardhan N.M., Kumar P.V., Li Z., Ploegh H.L., Grossman J.C., Belcher A.M., Chen G.Y. Enhanced cell capture on functionalized graphene oxide nanosheets through oxygen clustering. ACS Nano. 2017;11:1548–1558. doi: 10.1021/acsnano.6b06979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang T., Duarte J.N., Ling J., Li Z., Guzman J.S., Ploegh H.L. Structurally defined alphaMHC-ii nanobody-drug conjugates: A therapeutic and imaging system for B-cell lymphoma. Angew. Chem. Int. Ed. Engl. 2016;55:2416–2420. doi: 10.1002/anie.201509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Z., Theile C.S., Chen G.Y., Bilate A.M., Duarte J.N., Avalos A.M., Fang T., Barberena R., Sato S., Ploegh H.L. Fluorophore-conjugated holliday junctions for generating super-bright antibodies and antibody fragments. Angew. Chem. Int. Ed. Engl. 2015;54:11706–11710. doi: 10.1002/anie.201505277. [DOI] [PMC free article] [PubMed] [Google Scholar]