Highlights
-
•
The half-life of BaP is 3–4 days and then it metabolized in the liver.
-
•
BaP concentration in the muscle of treated fish reached a maximum level after 4 days.
-
•
Exposure of fish to BaP resulted in a decrease in T3 and T4 plasma levels up to day 4.
-
•
Exposure of fish to BaP resulted in an increase in TSH plasma level up to day 4.
Abbreviations: BaP, benzo[a]pyrene; EDC, endocrine disrupting chemical; PAH, polycyclic aromatic hydrocarbon; PCB, polychlorinated biphenyl; ROPME, Regional Organization for the Protection of the Marine Environment; RSA, ROPME sea area; T3, triiodothyronine (3,5,3-triiodo-l-thyroinine); T4, thyroxine (3,5,3,5-tetraiodo-l-thyronine); TSH, thyroid-stimulating hormone
Keywords: Polycyclic aromatic hydrocarbon, Fish, Triiodothyronine, Thyroxine
Abstract
Benzo[a]Pyrene (BaP) is a ubiquitous polycyclic aromatic hydrocarbon (PAH) that has been shown to disrupt the metabolism of thyroid hormone. Then, the present investigation aimed to study the effects of BaP on thyroid function in Liza abu. Fish were injected with 2, 10 and 25 mg/kg-bw of BaP. Samples were taken from blood, thyroid and muscle tissues at days 1, 2, 4, 7, and 14. Blood was evaluated for changes in the plasma levels of TSH, T3 and T4. Also, BaP bioaccumulation in the fish muscle was measured. Thyroid tissues were processed for routine histology. BaP concentration in the muscle of treated fish reached a maximum level after 4 days. Exposure of fish to BaP resulted in a significant decrease in T3 and T4 plasma level and increase in TSH concentration up to day 4. Also some pathological alterations were observed in BaP-exposed fish such as hemorrhage and increased number of large follicles with squamous epithelium. In conclusion, according to the results of the present investigation, short term exposure to sublethal concentrations of BaP significantly affected thyroid function in fish. The results revealed BaP ability to alter thyroid function.
1. Introduction
Thyroid follicular cells synthesize and secrete the thyroid hormones after activation of the hypothalamo-pituitary-thyroid (HPT) axis. The hypothalamus induces the pituitary gland to secrete thyroid-stimulating hormone (TSH) which activates synthesis of thyroxine (T4; 3,5,3,5-tetraiodo-l-thyronine) and triiodothyronine (T3; 3,5,3_-triiodo-l-thyroinine) in the thyroid gland. The level of T4 is generally higher than T3 in the blood circulation. T4 acts as a prohormone that can be converted into T3 by 5-iodothyronine deiodinases in target tissues [1]. Thyroid hormones are essential for regulating development, growth, morphogenesis, basal metabolism, reproduction, osmoregulatory properties, and behavior in fishes [1].
Different environmental contaminants disturb the thyroid system at many levels. Polycyclic aromatic hydrocarbons (PAHs) have two or more fused aromatic rings. They are classified as a group of organic pollutants [2] that arise from anthropogenic activities, such as vehicle exhausts, oil shipping and refineries. These organic chemicals presented in the priority pollutant list of the United States Environmental Protection Agency because of their mutagenic, carcinogenic and immunosuppressive properties [3]. These compounds have adverse effects on development and function of the thyroid gland in mammals [2]; however, there are few studies on the effects of PAHs on thyroid development or function in fish. 3-Methylcholanthrene is a carcinogen PAHs which adversely affected thyroid function in rat [4]. Teles et al. [5] reported that naphthoflavone, an aryl hydrocarbon receptor prototype ligand, significantly decreased plasma T4 levels, whereas TSH, T3 and plasma cortisol remained constant in adult Anguilla anguilla. Also as it seems, many PAH compounds are able to bind to transthyretin (TTR), in vitro [6]. According to Sun et al. [7], 1- naphthol and 2-naphthol, two hydroxylated PAHs, inhibit the TRβ1-mediated transcription in vitro. Totally, PAHs decrease the circulating and tissue levels of thyroid hormones through at least three mechanisms: 1. PAHs may directly interfere with thyroid gland function and then change the structure of thyroid gland that lead to disruption of hormone synthesis [8]. 2. PAHs can target the metabolism of thyroid hormone. They may affect 5′-iodothyronine deiodinases (enzymes that convert T4 to T3 in target tissues). 3. PAHs may attach to thyroid hormone binding proteins in the blood stream [8].
Benzo[a]Pyrene (BaP) is an ubiquitous PAH that could be considered as one of the most mutagenic and carcinogenic pollutants. It has been shown to be a CYPlA inducer. BaP resulted from incomplete combustion of fossil fuels and identified in ambient air, surface water, drinking water, and wastewater, and in char-broiled foods (I.A.R.C. 1983). BaP is primarily released to the air and removed from the atmosphere by photochemical oxidation and dry deposition on land or water. The main route of excretion is hepatobiliary followed by bowel elimination. BaP has evoked much interest due to its carcinogenic properties [8]. Existence of detergents or liquid hydrocarbons in the water bodies, simplify BaP solution in the sewage and surface water leading to its infiltration to the plant and animal cells [9]. BaP entrance to the organism, could priming a chain of oxidative processes mediated by the mixed-function oxygenase (MFO) system (phase I). By-products of the first phase may affected by conjugative enzymes (phase II), which will make them more readily excretable. BaP would cause immune changes in fish such as those observed in mammals.
The objective of the present study was to evaluate the effects of BaP on the tissue structure of thyroid gland and plasma levels of T3, T4 and TSH in the abu mullet (L. abu) under laboratory conditions. L. abu was selected due to its commercial importance in the ROPME (Regional Organization for the Protection of the Marine Environment) Sea Area. BaP was used in the present study because of its endocrine disruptive properties.
2. Material and methods
Benzo [a] pyrene was obtained from Aldrich (USA), with a purity of greater than 97%. All other chemicals were of analytical grade and were obtained from Merck (Germany). Hanks balanced salt solution (HBSS) was bought from Sigma (USA).
The range finding tests were required to conduct before running the experiment, to determine the adequate concentrations. Based on the primary range finding experiments, a 14 day BaP LC50 of 48 mg/kg was determined and then high nominal concentration and two low nominal concentrations were set at 25 mg/kg and 2 and 10 mg/kg, respectively. BaP solutions were injected with nominal amounts of 2, 10 and 25 mg/kg-bw in 1 mL coconut oil into the peritoneal cavity.
500 L. abu (150 ± 7.9gr mean body weight and 15.8 ± 0.2 cm mean body length) were collected from Bahrakan Creek located at the north west of the Persian Gulf during October 2011. To study the effects of BaP, 300 L. abu were randomly placed in fifteen 300 L tanks containing 200L running, UV-treated, aerated seawater and 20 L. abu were maintained in each tank. Firstly, fish were adapted to the experimental condition for 10 days. The tanks were then divided into five experimental groups, with each group run in triplicate: (1) control, (2) solvent (tested only with coconut oil), (3) low concentration of BaP (2 mg/kg-bw), (4) medium concentration of BaP (10 mg/kg-bw), and (5) high concentration of BaP (25 mg/kg-bw). Fish were captured with a hand-held net and were injected intraperitoneally (IP) with BaP (2, 10 and 25 mg/kg-bw) in I ml coconut oil. The control fish received no injection or handling. The fish were kept under the experimental conditions for 2 weeks. 70% of the water in the tanks was renewed each day. Fish were not fed during the experiment [10]. During the experiment the chemical characteristics of water were as follows: Water temperature 26 ± 1 °C, environmental temperature 29 ± 1 °C, pH 8.8 ± 0.1 and salinity 50 ± 1 ppt.
5 fish per group were sampled at days 1, 2, 4, 7 and 14 following the injection. The fish were euthanized with 2- phenoxy ethanol (0.35 mL/l) and the blood samples were then collected from the caudal vein into heparinized syringes. The blood samples were centrifuged for 10 min and plasma was separated and frozen at −20 °C for further thyroid hormones analysis. Also, the tissue samples were taken from the fish muscle to measure BaP concentration in the fish muscle. Then the muscle samples were stored in aluminum foil at −20 °C. For histological study of thyroid gland, the fish jaws were cut to expose pharyngeal region, and all tissues between the gills were fixed in Bouin’s solution for 72 h.
Extraction of BaP was carried out using Moopam [11] method. Samples of the fish muscles were moved to pre-washed glass jars to freeze-dry using freeze drier apparatus (Zirbus, Germany) for 4–5 days. Then the samples were weighed again to verify dry/wet ratio and powdered into a porcelain mortar. The BaP extraction procedure was performed using soxhlet apparatus (a soxhlet extractor). For hydrocarbon measurement, the extract was loaded onto a silica/alumina column. The silica and alumina were first soxhlet extracted with the mixture of n-hexane and di-chloromethane for 8 h and then activated at 200 °C within 4 h. They partially deactivated with 5% water. In the end, 1 g sodium sulfate was added to the surface of column to prevent disturbance of the top layer when decanting the solvent. Finally two fractions (F1 and F2) were separated. F2 was concentrated to about 10–15 mL using rotary evaporator and then clean and dried F2 was concentrated to about 1-1.5 mL under moderate nitrogen flow [11]. To analyze the BaP using High-Performance Liquid Chromatography (HPLC), samples were dried under a pure nitrogen flow, and 20 μL acetonitrile was added to the concentrated F2. F2 then was injected into the High-Performance Liquid Chromatography (HPLC) System (Knaeur, Germany) [11] outfitted with double piston pump(K-1001), UV detector (K-2600) and reverse phase column (25 cm length and 4.6 mm internal diagonal) (Eurospher 100-5C18). The limit of detection of HPLC System was higher than 0.07 mg/kg-bw BaP.
The L. abu were dissected to expose the internal organs and the jaws were cut to expose pharyngeal region. All the pharyngeal tissues were fixed in Bouin’s fixative for 72 h and then stored in 70% ethanol. Tissues were dehydrated through an ethanol series and embedded in paraffin. The samples were then sectioned at 5–6 μm and were stained with hematoxylin and eosin (H&E). The height of the thyroid epithelium and diagonal of thyroid follicles were measured in a total of 15 follicles per fish. Measurements were made at four points within each follicle at 90∘ to one another.
The concentration of thyroid hormones (T3 and T4) was measured in the plasma samples by radioimmunoassays (RIAs) method using commercial T3 and T4 RIA kits (Immunotech, Beckman Culture Company, France), as previously described by Morgado et al. [12]. TSH was also measured using radioimmunoassay method according to Forest et al. [13].
Results are reported as mean±SE. Analysis was carried out using One-way ANOVA with SPSS 16.0 software. The data were processed by post hoc test and P≤0.05 was accepted as statistically significant.
3. Results
No significant difference was observed in all studied parameters between the control and solvent control groups.
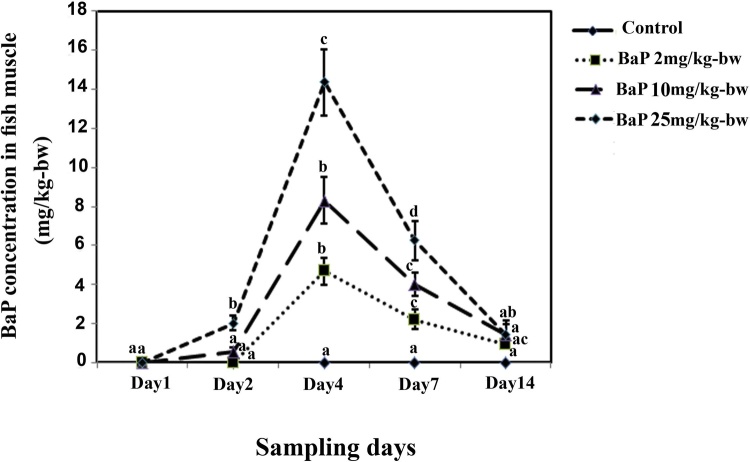
BaP concentration in the muscle of control groups were undetectable across all sampling days and then considered 0 (Fig. 1). BaP concentration was also undetectable in all treatments on the first sampling day. BaP accumulation in the muscle samples from all treatments during the experiment is presented in Fig. 1. BaP concentration in the muscle of BaP- treated fish reached a maximum level after 4 days (0.36-14.37 mg/kg-bw). It then decreased until the end of the experiment. There were significant differences in BaP accumulation between treatments in both sampling days (days 4 and 7) (P < 0.05; Fig. 1).
Fig. 1.
BaP concentrations in control and treated fish during the exposure period.
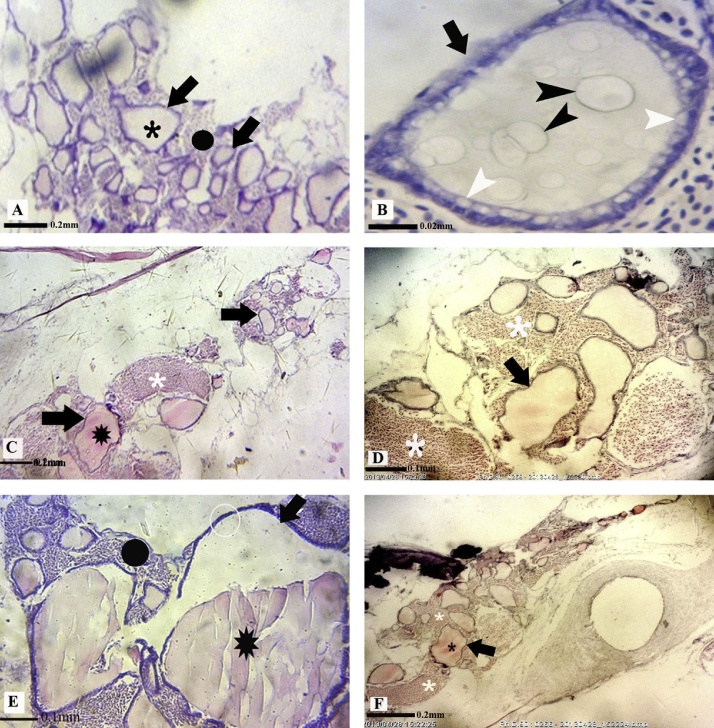
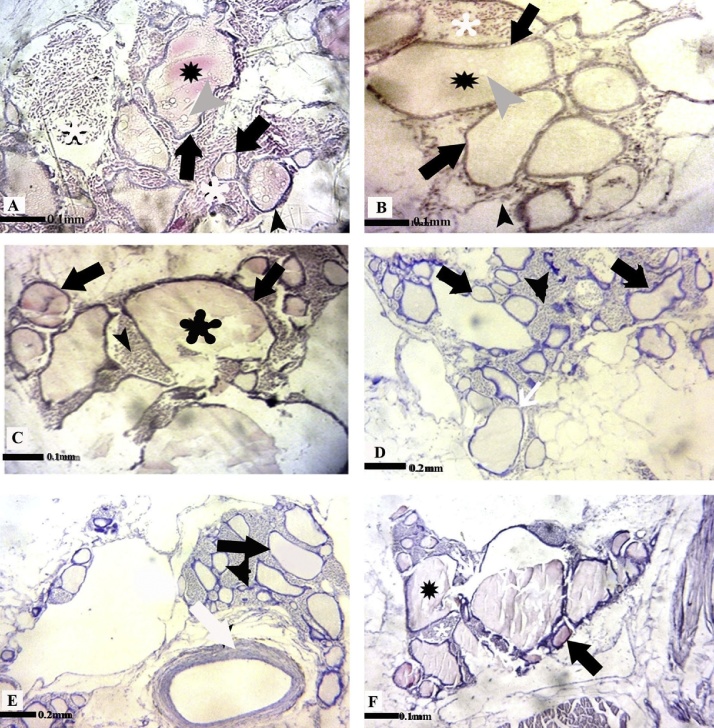
According to the results, the thyroid gland of L. abu is not encapsulated. It consisted of follicles distributed all over the pharyngeal region along the dorsal surface of ventral aorta and bronchial arteries. The wall of spherical thyroid follicles in the control groups was lined with one layer of cuboidal epithelial cell that surrounded a central lumen full of colloid fluid. Interstitial connective tissue existed among the thyroid follicles (Fig. 2). No alteration was detected in the tissue structure of thyroid gland in L. abu exposed to different concentrations of BaP on the first sampling day. The tissue damages increased dose-dependently in all treatments on day 4 of experiment compared to controls. The most tissue changes observed in tissue samples taken from the BaP-treated fish on day 4 included the increase in size of follicles lined by squamous epithelial cells and decrease in interstitial connective tissue (Fig. 2). Moreover, hemorrhage and thyroid gland disorganization were also detected in BaP treated fish in this day (Fig. 2). It was followed by a decrease of tissue damages in the thyroid gland of BaP- exposed fish up to the end of the experiment (Fig. 3).
Fig. 2.
Representative photomicrograph of thyroid tissue structure in control (A and B) and BaP treated L. abu (C: 2 mg/kg-bw; D: 10 mg/kg-bw; E and F: 25 mg/kg-bw) on day 4 of experiment. Thyroid follicle (black arrows), interstitial connective tissue (black●), colloid fluid (black ), vacuolated cuboidal epithelial cell (white arrowhead), vacuoles in colloid fluid (black arrowhead), Hemorrhage (white
), vacuolated cuboidal epithelial cell (white arrowhead), vacuoles in colloid fluid (black arrowhead), Hemorrhage (white ). A,F (H&E; 290X); B (H&E; 2900X); C,D,E (H&E; 725X).
). A,F (H&E; 290X); B (H&E; 2900X); C,D,E (H&E; 725X).
Fig. 3.
Representative photomicrograph of thyroid tissue structure in BaP treated L. abu on day 7 (A: 2 mg/kg-bw; B: 10 mg/kg-bw; C: 25 mg/kg-bw) and day 14 of experiment (D: 2 mg/kg-bw; E: 10 mg/kg-bw; F: 25 mg/kg-bw). Thyroid follicle (black arrows), interstitial connective tissue (black arrowhead), colloid fluid (black ), vacuoles in colloid fluid (grey arrowhead), Hemorrhage (white
), vacuoles in colloid fluid (grey arrowhead), Hemorrhage (white ), bronchial artery (white arrow), interstitial connective tissue (black arrowhead). A,B,C,F (H&E; 725X); D,E (H&E; 290X).
), bronchial artery (white arrow), interstitial connective tissue (black arrowhead). A,B,C,F (H&E; 725X); D,E (H&E; 290X).
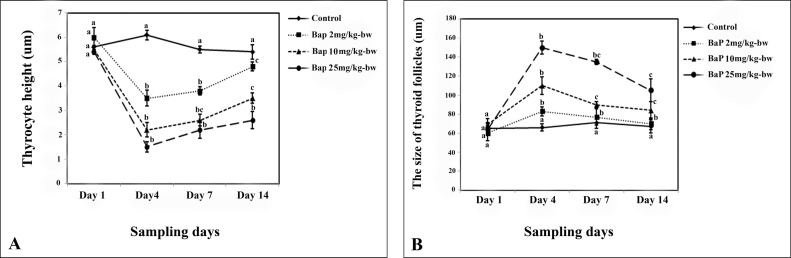
The minimum height of thyrocytes (follicular epithelial cells) was recorded in the fish exposed to 25 mg/kg-bw of BaP on day 4 of experiment compared to control (5.6±0.2 μm) (P < 0.05) (Fig. 4A). Totally, the height of thyrocytes were decreased dose-dependently up to day 4 and then gradually increased until the end of experiment (Fig. 4A). There was no significant difference in the size of thyroid follicles among different groups on the first sampling day (P > 0.05). The size of follicles increased dose dependently up to 4th day of experiment and then decreased up to day 14 (the end of the experiment) (Fig. 4B). The largest thyroid follicles were observed in fish exposed to 25 mg/kg-bw of BaP on day 4 of experiment compared to control.
Fig. 4.
The height of thyrocytes (A) and the size of thyroid follicles (B) in control and BaP treated fish during the exposure period. Data are represented as mean±SE. The letters indicate the significant difference between controls and BaP treated groups (P < 0.05).
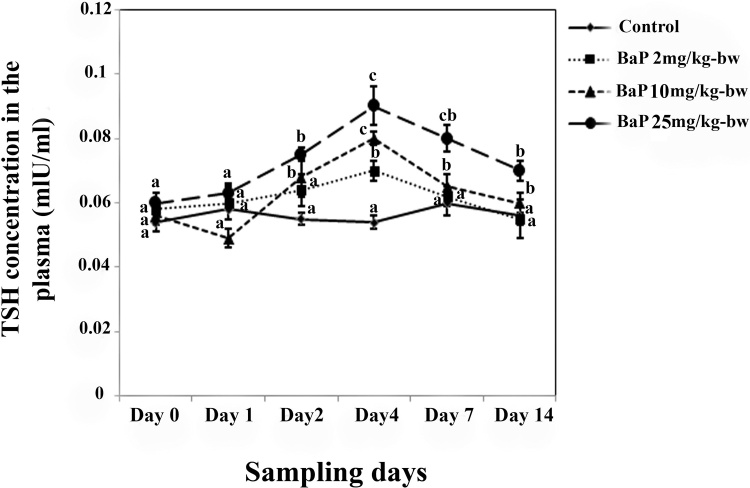
The plasma levels of TSH, T4 and T3 in L. abu exposed to different concentrations (2, 10 and 25 mg/kg-bw) of BaP didn’t show significant alterations on the first sampling day when compared to control (Fig. 5). Plasma TSH levels were increased dose-dependently in all treatments from the first day of experiment up to day 4 compared to controls (P < 0.05; Fig. 5). It was followed by a significant decrease in TSH levels in all treated fish up to the end of the experiment. However, no significant difference was found in the hormone level between fish treated with 2 and 10 mg/kg-bw of BaP on day 14 (P > 0.05).
Fig. 5.
The effect of sub lethal concentrations of BaP on TSH concentration (mean±SE) in L. abu during the experiment. The letters indicate the significant difference between controls and BaP treated groups (P < 0.05).
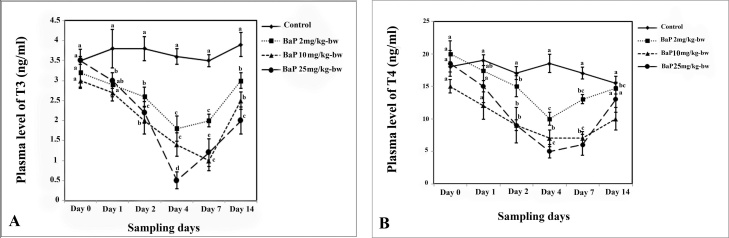
Altered T3 plasma level in L. abu affected by different BaP concentrations is presented in Fig. 6A. The concentration of T3 decreased dose dependently in the plasma of fish exposed to BaP up to 4th day. The T3 level was significantly lower in fish treated with 25 mg/kg-bw of BaP than other treatments on all sampling days (P < 0.05). Then, T3 levels increased in all BaP- exposed fish up to the end of the experiment; however, these amounts were significantly lower than those recorded in controls (P < 0.05). The changes in plasma T4 level in L. abu affected by sublethal concentrations of BaP are presented in Fig. 6B. T4 was found to be decreased dose dependently in treated fish after 1-day exposure up to day 4. The level of this hormone significantly decreased in fish treated with the highest concentration of BaP (25 mg/kg-bw) compared to other treatments (P < 0.05) on this day. After 4 days, the level of T4 increased in all treatments up to the end of the experiment (day 14). However, no significant difference was found in the T4 concentration between groups treated with 10 and groups treated with 25 mg/kg-bw of Bap (P > 0.05) on days 7 and 14 (Fig. 6B).
Fig. 6.
The effect of sub lethal concentrations of BaP on the plasma levels of T3 (A) and T4 (B) in L. abu during the experiment. Data are represented as mean±SE. The letters indicate the significant difference between controls and BaP treated groups (P < 0.05).
4. Discussion
According to the results of the present study, the thyroid gland was not encapsulated in L. abu. It consisted of round follicles with the interstitial connective tissue scattered along the ventral aorta. Einarsdottir et al. [14] also reported that the thyroid gland of Atlantic halibut composed of spherical follicles dispersed throughout the mandible. Geven et al. [15] also observed thyroid follicles scattered in subpharyngeal region in tilapia, Oreochromis mossambicus. Thyroid follicles distributed in connective tissue along the ventral aorta and gill arches in Danio rerio [16]. The size of thyroid follicles and the height of follicular epithelium could be used as indices of secretory activity of this gland. Small thyroid follicles lined with high cuboidal or columnar epithelial cells have more secretory activity than large follicles with squamous epithelium. Fish exposure to EDC compounds may lead to thyroid tissue changes [1]. Changes in thyroid tissue have been rarely reported in feral fish; however, enlarged thyroid gland and signs of hypothyroidism were observed in Coho salmon, Oncorhynchus kisutch, caught from lakes polluted with huge amounts of EDCs [17]. In the present investigation, many large thyroid follicles lined by short epithelium with partly depleted colloid and a few small follicles were observed in BaP treated L. abu. Other pathological alterations including hemorrhage and decrease in interstitial connective tissue also were detected in L. abu treated with high concentrations of Zhou et al. [18] also reported the same alterations in the thyroid gland of Fundulus heteroclitus collected from Piles Creek in New Jersey, USA. Piles Creek is a very contaminated station enclosed by many industrial centers and a major highway in Linden, New Jersey, USA. The histological structure of thyroid gland in BaP-treated fish in the present study returned to an approximately normal condition up to the end of experiment. BaP accumulates in animal tissues because of its lipophilic nature [10]. In the present study, high concentration of BaP was detected in the fish muscle on day 4. McCarthy et al. [19] reported a half-life of 4 days for BaP accumulation in the muscle of fathead minnows. The constant decrease in BaP from day 4 to the end of the experiment (day 14) might be due to BaP metabolism by the fish liver [10]. Thus fish recovery in this study after 4 days of experiment was possibly due to BaP metabolism.
According to the results of the present study, exposure of L. abu to sublethal concentrations of BaP caused drastic changes in the plasma levels of TSH, T4 and T3. Exposure to 2, 10, and 25 mg/kg-bw of BaP led to a significant increase in the plasma TSH levels in all treatments up to day 4. Oliveira et al. [20] recorded an elevated level of TSH in Liza aurata colected from contaminated stations in Ria de Aveiro, Portugal. They stated that increase in TSH level would be expected upon decreased plasma T4 level. In the present study, a lower T4 level was recorded in BaP- treated fish on day 4. A high TSH level was also measured in zebra fish (Danio rerio) exposed to triadimefon [21]. Increase in TSH levels may be expected upon a decrease of plasma levels of T4 and/or lower level of thyroid hormone production by the pituitary [20]. On the other hand, in the present study, BaP might disrupt the synthesis of the circulating thyroid hormones or their secretion and T4 to T3 conversion in treatments. Thyroid hormones play important roles in multiple physiological functions in aquatic animals. Several investigators reported a decrease in T4 plasma level in Aaguilla anguilla treated with chromium and copper [22], in Carassius auratus exposed to extracted microcystins [23], in Sparus aurata exposed to diethylstilbestrol, ioxynil and propilthy-ouracil [24], and in Zebrafish larvae treated with pentachlorophenol [25]. A significant decrease in the plasma T4 levels also was reported in zebrafish exposed to hexaconazole and tebuconazole (fungicides) [26]. Hallgren and Darnerud [27] stated that a decreased level of T4 is probably due to an increase in UDP-glucuronosyl transferase (UDP-GT) activity that responsible for glucuronidation and clearance of T4.
In the present investigation, the level of T3 also reduced in fish exposed to BaP, up to day 4. In fish, like other vertebrates, T3, the active form of thyroid hormones, is mostly produced by the peripheral enzymatic monodeiodination of T4 mainly in the liver and other tissues. Leatherland & Farbridge [28] suggested that decrease in T3 plasma concentration is probably due to an inability of the organism to produce the optimal level T3 or hypothalamus, pituitary and ovary interaction Ruby et al., 1993. Iodothyronine deiodinases play an important role in thyroid hormones biotransformation in extra-thyroidal tissues [29]. Moreover, the level of the deiodinase mRNA is highly sensitive to numerous contaminants in fish [30]. Also according to Zhang et al. [29], decrease in the circulating T3 level in the Carassius auratus exposed to different concentrations of monocrotophos (0.01 and 0.10 mg/l) may be resulted from higher stimulated metabolism of T3. Hea et al. [31] studied the thyroid development of Sebastiscus marmoratus embryos affected by pyrene. They reported that decrease in T3 plasma level and Deio1 gene expression in pyrene −treated fish was probably due to pyrene which affected the conversion from T4 or accelerated the T3 metabolism. Deio1 has a major role in iodine recovery, thyroid hormones degradation and homeostasis [30]. This gene removes iodine from the outer ring of T4 and then converts T4 to T3 [30].
Generally, a reduction of the plasma T3 levels is mainly due to a drop in the T4 production and secretion. In the present study, increased plasma levels of thyroid hormones after 4 days of the experiment correlated with BaP metabolism in the liver. The steady decrease of BaP from day 4 to the end of the experiment (day 14) in the present investigation could indicate a progressive metabolism of BaP. On the other hand, the elevated plasma levels of T3 and T4 was accompanied by decreased plasma concentration of TSH after day 7 due to an altered feedback mechanism in the pituitary or hypothalamus.
Simon et al. [32] demonstrated the effects of BaP on the RPTEC/TERT1 cell line derived from renal proximal tubule epithelial cells (RPTEC) of a healthy human donor. RPTEC/TERT1 cells represented a significant increase in the expression of genes coding for CYP1A1 and CYP1B1 in a dose and time dependent manner at 3, 6, and 24 h post exposure to BaP.
Simon et al. [33] also exhibited the RPTEC/TERT1 cell line treated with different concentrations of BaP were able to produce BPDE metabolites that react with DNA.
5. Conclusion
In conclusion, our study on fish exposed to different concentrations of BaP ranging from 2 to 25 mg/kg-bw showed drastic changes in the structure and secretions of thyroid tissue. According to the results of the present investigation, short term exposure to sublethal concentrations of BaP significantly affected thyroid function in fish. Such research may provide us with a better understanding of the effects of xenobiotics on animals and how they adapt to environmental contaminants in natural systems.
Conflict of interest
There is no conflict of interest.
Acknowledgment
This work was supported by the Khorramshahr University of Marine Science and Technology [grant numbers 123].
References
- 1.Brown S.B., Adams B.A., Cyr D.G., Eales J.G. Contaminant effects on the teleost fish thyroid. Environ. Toxicol. Chem. 2004;23:1680–1701. doi: 10.1897/03-242. [DOI] [PubMed] [Google Scholar]
- 2.Porazzi P., Calebiro D., Benato F., Tiso N., Persani L. Thyroid gland development and function in the zebrafish model. Mol. Cell. Endocrinol. 2009;312:14–23. doi: 10.1016/j.mce.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Khaniyan M., Salamat N., Safahieh A., Movahedinia A. Detection of benzo[a]pyrene-induced immunotoxicity in orange spotted grouper (Epinephelus coioides) Environ. Toxicol. 2014 doi: 10.1002/tox.22047. [DOI] [PubMed] [Google Scholar]
- 4.Newman W.C., Moon R.C. Altered thyroxine metabolism resulting from chemical carcinogen 3-methylcholanthrene. Endocrinology. 1967;80:896. doi: 10.1210/endo-80-5-896. [DOI] [PubMed] [Google Scholar]
- 5.Teles M., Oliveira M., Pacheco M., Santos M.A. Endocrine and metabolic changes in Anguilla anguilla L. following exposure to betanaphthoflavone −a microsomal enzyme inducer. Environ. Int. 2005;31:99–104. doi: 10.1016/j.envint.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Bekki K., Takigami H., Suzuki G., Tang N., Hayakawa K. Evaluation of toxic activities of polycyclic aromatic hydrocarbon derivatives using in vitro bioassays. J. Health Sci. 2009;55:601–610. [Google Scholar]
- 7.Sun H., Shen O.X., Xu X.L., Song L., Wang X.R. Carbaryl, 1-naphthol and 2- naphthol inhibit the beta-1 thyroid hormone receptor-mediated transcription in vitro. Toxicology. 2008;249:238–242. doi: 10.1016/j.tox.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Boas M., Feldt-Rasmussen U., Skakkebaek N.E., Main K.M. Environmental chemicals and thyroid function. Eur. J. Endocrinol. 2006;154:599–611. doi: 10.1530/eje.1.02128. [DOI] [PubMed] [Google Scholar]
- 9.Janikowska G., Wardas W. Concentration of benzo(a)pyrene in Chorella BB cells. Pol. J. Environ. Stud. 2002;11:345–348. [Google Scholar]
- 10.Boleas S., Fernandez C., Beyer J., Tarazona V., Gokssoyr A. Accumulation and effects of benzo(a)pyrene on cytochrome P450 1A in waterborne exposed and intraperitoneal injected juvenile turbot (Scophthalmus maximus) Mar. Environ. Res. 1998;46:17–20. [Google Scholar]
- 11.MOOPAM (Manual of Oceanographic Observations and Pollutant Analyses) 3rd edition. 1999. Regional Organization for the Protection of the Marine Environment. (Kuwait) [Google Scholar]
- 12.Morgado I., Santos C.R.A., Jacinto R., Power D.M. Regulation of transthyretin by thyroid hormones in fish. Gen. Comp. Endocrinol. 2007;152:189–197. doi: 10.1016/j.ygcen.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Forest J., Massem J., Lane A. Evaluation of the analytical performance of the Boehringer Mannheim Elecsys 2010 immunoanalyzer. Clin. Biochem. 1998;31:81–88. doi: 10.1016/s0009-9120(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 14.Einarsdottir I.E., Nadia S.D., Power M., Heiddis S.B. Thyroid and pituitary gland development from hatching through metamorphosis of a teleost flatfish, the Atlantic halibut. Anat. Embryol. 2006;211:47–60. doi: 10.1007/s00429-005-0055-z. [DOI] [PubMed] [Google Scholar]
- 15.Geven E., Nguyen N.K., van den Boogaart M., Spanings F.A., Flik G., Klaren P.H. Comparative thyroidology: thyroid gland location and iodothyronine dynamics in Mozambique tilapia (Oreochromis mossambicus Peters) and common carp (Cyprinus carpio L.) J. Exp. Biol. 2007;210:4005–4015. doi: 10.1242/jeb.010462. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt F., Braunbec K.T. Alterations along the hypothalamic- pituitary-thyroid axis of the zebrafish (Danio rerio) after exposure to propylthiouracil. J. Thyroid Res. 2011 doi: 10.4061/2011/376243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leatherland J.F., Sonstegard R.A. Seasonal changes in thyroid hyperplasia, serum thyroid hormone and lipid concentrations, and pituitary gland structure in Lake Ontario coho salmon, Oncorhynchus kisutch Walbaum and a comparison with coho salmon from Lakes Michigan and Erie. J. Fish Biol. 1980;16(5):539–562. [Google Scholar]
- 18.Zhou T., John-Alder H., Weis P., Weis J. Thyroid status of mummichogs (Fundulus heteroclitus) from a polluted versus a reference habitat. Environ. Toxicol. Chem. 1999;18(12):2817–2823. [Google Scholar]
- 19.McCarthy J.F., Burrus L.W., Tolbert V.R. Bioaccumulation of benzo(a)pyrene from sediment by fathead minnows: effects of organic content, resuspension and metabolism. Arch. Environ. Contam. Toxicol. 2003;45:364–370. doi: 10.1007/s00244-003-2148-0. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira M., Maria V., Ahmad I., Serafim A., Bebianno M., Pacheco M., Santos M. Contamination assessment of a coastal lagoon (Ria de Aveiro, Portugal) using defense and damage biochemical indicators in gill of Liza aurata – an integrated biomarker approach. Environ. Pollut. 2009;157:959–967. doi: 10.1016/j.envpol.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Liu S.Y., Chang J.H., Zhao Y., Zhu G.N. Changes of thyroid hormone levels and related gene expression in zebrafish on early life stage exposure to triadimefon. Environ. Toxicol. Pharmacol. 2011;32:472–477. doi: 10.1016/j.etap.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Teles M., Santos M.A., Pacheco M. Physiological and genetic responses of European eel (Anguilla anguilla L.) to short-term chromium or copper exposure—influence of pre exposure to a PAH-like compound. Environ. Toxicol. 2005;20:92–99. doi: 10.1002/tox.20082. [DOI] [PubMed] [Google Scholar]
- 23.Li D.P., Xie P., Zhang X.Z. Changes in plasma thyroid hormones and cortisol levels in crucian carp (Carassius auratus) exposed to the extracted microcystins. Chemosphere. 2008;74:13–18. doi: 10.1016/j.chemosphere.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 24.Morgado I., Campinho M.A., Costa R., Jacinto R., Power D.M. Disruption of the thyroid system by diethylstilbestrol and ioxynil in the sea bream (Sparus aurata) Aquat. Toxicol. 2009;92(4):271–280. doi: 10.1016/j.aquatox.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y., Zhou B. Thyroid endocrine system disruption by pentachlorophenol: an in vitro and in vivo assay. Aquat. Toxicol. 2013;142:138–145. doi: 10.1016/j.aquatox.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Yu L., Chen M., Liu Y., Gui W., Zhu G. Thyroid endocrine disruption in zebrafish larvae following exposure to hexaconazole and tebuconazole. Aquat. Toxicol. 2013;138–139:35–42. doi: 10.1016/j.aquatox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Hallgren S., Darnerud P.O. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177:227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- 28.Leatherland J.F., Farbridge K.J. Chronic fasting reduces the response of the thyroid to growth hormone and TSH, andalters the growth hormone-related changes in hepatic5-monodeiodinase activity in rainbow trout, Oncorhynchus mykiss. Gen. Comp. Endocrinol. 1992;87:342–353. doi: 10.1016/0016-6480(92)90040-q. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X., Tian H., Wang W., Ru S. Exposure to monocrotophos pesticide causes disruption of the hypothalamic–pituitary–thyroid axis in adult male goldfish (Carassius auratus) Gen. Comp. Endocrinol. 2013;193:158–166. doi: 10.1016/j.ygcen.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Yu L.Q., Deng J., Shi X.J., Liu C.S., Yu K., Zhou B.S. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic–pituitary–thyroid axis of zebrafish larvae. Aquat. Toxicol. 2010;97:226–233. doi: 10.1016/j.aquatox.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Hea C., Zuo Z., Shi X., Sun L., Wang C. Pyrene exposure influences the thyroid development of Sebastiscus marmoratus embryos. Aquat. Toxicol. 2012;124–125:28–33. doi: 10.1016/j.aquatox.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Simon B.R., Wilson M.J., Wickliffe J.K. The RPTEC/TERT1 cell line models key renal cell responses to the environmental toxicants, benzo[a]pyrene and cadmium. Toxicol. Rep. 2014;1:231–242. doi: 10.1016/j.toxrep.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon B.R., Wilson M.J., Blake D.A., Yu H., Wickliffe J.K. Cadmium alters the formation of benzo[a]pyrene DNA adducts in the RPTEC/TERT1 human renal proximal tubule epithelial cell line. Toxicol. Rep. 2014;1:391–400. doi: 10.1016/j.toxrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]