Graphical abstract
Keywords: Cadmium, Liver injury, Vernonia amygdalina leaf, Oral LD50, Liver function biomarkers, Oxidative stress indicators, Wistar rats
Highlights
-
•
Cadmium induced inflammatory response and disseminated steatosis in the liver.
-
•
The oral LD50 of acetonic extract of Vernonia amygdalina (Del.) leaf is >5000 mg/kg.
-
•
AEVAL beneficially elicited cytoplasmic, cytosolic and tissue-regenerative mechanisms.
-
•
The extract’s pharmacological activities are conferred by its essential phytochemicals.
Abstract
Exposure to cadmium (Cd), even at low doses, is of serious health concern because it does not undergo metabolic degradation to less toxic metabolite. Liver injury/disease, with a world-wide increasing incidence, is one of the consequences of exposure to Cd toxicity. This study aimed at determining the effects of acetonic extract of Vernonia amygdalina leaf (AEVAL) in a Wistar rat model of Cd-induced liver injury. Phytochemical screening of the extract was carried out and its oral LD50 was determined to guide the choice of therapeutic doses. Thereafter, thirty male Wistar rats were recruited for this study. The experimental groups received 4 weeks oral graded doses of the extract (100, 200 and 400 mg/kg) following Cd-induced liver injury. Cd-induced liver injury (5 mg/kg i.p for 5 consecutive days) was characterized by deleterious alterations in the levels of AST, ALT, ALP, total bilirubin and hepatic total protein (p ˂ 0.05). Also, deleterious alteration of oxidative stress indicators (GSH, SOD and CAT) and lipid peroxidation index (TBARS) was observed in the liver homogenates. Histopathological examination showed evidence of degenerated hepatocytes as well as inflammation with disseminated steatosis. These conditions were significantly attenuated (p ˂ 0.05) following treatment with graded doses of the extract, with the highest dose expressing least therapeutic effects. This study concluded that AEVAL attenuated Cd-induced liver injury and is, potentially, a suitable option in adjuvant therapy for heavy metal toxicity.
1. Introduction
The liver, one of the vital organs in the body that performs homeostatic function through detoxification mechanisms, is a natural chemical factory which neutralizes toxins and aids in the anabolism of complex molecules from simple substances that are absorbed from the gastro-intestinal tract [[1], [2]]. Adverse disturbances of liver function can have deteriorating health consequences which sometimes lead to terminal illness [3] due to biological build-up of toxins. Liver injury can result from exposure to various kinds of exogenous compounds or chemicals, either through job demands or way of life [4].
Cadmium (Cd), one of the known environmental toxins that are detrimental to liver function, is a ubiquitous heavy metal that has found its relevance in several industrial processes such as electroplating, manufacturing of paint pigments, plastic, dyes as well as its use in agriculture for the production of fertilizers [[5], [6], [7]]. Exposure to Cd is of serious health concern because it does not undergo metabolic degradation to less toxic metabolites [8]. Human exposure to this heavy metal is majorly by two main routes, inhalation and ingestion [9]. It is both an environmental and an occupational toxin. Its emission from industrial processes can cause atmospheric, soil, water and food pollution [[10], [11]]. Food consumption is a major source of its exposure because it is readily absorbed by the roots of plants in contaminated soils [[12], [13]]. Through cigarette smoke, Cd is readily absorbed in the body by inhalation [14], thereby exerting its deleterious effects in both active and passive smokers. Unlike most heavy metals, its exposure can induce deleterious health effects at relatively lower doses once it is absorbed in the body [[15], [16], [17]]. It is known to exert its toxic effects by inducing reacting oxygen species (ROS) generation through oxidative damage [18]. These ROS (H2O2 and OH+) initiate reactions with cellular biomolecules, causing lipid peroxidation with consequent disruption of the antioxidant system as well as membrane protein damage [[18], [19]]. We, therefore, hypothesized that a potent antioxidant may inhibit, retard or beneficially alter this basic mechanism of Cd-induced deleterious alterations and possibly ameliorate its toxic effects on hepatic function. Established models of therapy for heavy metal toxicity (such as the use of dimercaptosuccinic acid) are often very expensive, not readily available and burdened with undesirable side effects [20]. Therefore, this hypothesis was tested using ethno-botanical approach, since plant-derived medicines (besides their easy availability, being relatively cheaper and affordable to the common man) are safe [21] and has been used as a source of inspiration for the development of novel drugs [22].
Vernonia amygdalina, commonly called “Bitter leaf” because of its bitter taste, is a member of the Asteraceae family; a small shrub that grows in tropical Africa [23]. In various regions of Africa, particularly in Nigeria, the plant is a common homestead farming vegetable and is being used as an ingredient to prepare several delicacies [[24], [25]]. Its usage as a medicinal herb is being explored by scientists to date. Vernonia amygdalina is reputed to have several medicinal properties such as antibacterial [[26], [27]], anti-parasitic [[28], [29]], anti-plasmodial [[30], [31]], anti-mutagenic [32], anti-inflammatory [[33], [34]], anti-nephrotoxic [35] as well as anti-oxidant [[36], [37]] activities.
Although literatures exist on the experimental evaluation of Vernonia amygdalina activity in several models of xenobiotic-induced hepatic injury [[38], [39], [40], [41]], our literature survey revealed that (to date) there is dearth of experimental evidence of its effects on liver function in models of heavy metal toxicity. This study aimed at bridging this gap in knowledge by providing information on the effects of its acetonic extract in a Wistar rat model of Cd-induced liver injury.
2. Materials and methods
2.1. Plant material, chemicals and biochemical kits
Vernonia amygdalina leaves were harvested from a garden at Ife-Ibadan area of Ile-Ife, Osun state, Nigeria and certified by a Taxonomist (Mr. A. Gabriel) at the Department of Botany, Obafemi Awolowo University (OAU), Ile-Ife, Osun state, Nigeria.
Cadmium sulphate (CdSO4) was purchased from Guangzhou Fischer Chemical Co., Ltd, Guangdong – China while acetone (analytical grade) was procured from Crescent Chemical Co. Inc., New York, United States.
Standard laboratory kits (Randox products) for assaying the biomarkers of liver function were purchased from Randox Laboratory Ltd., United Kingdom.
2.2. Extraction process
Acetonic extraction of Vernonia amygdalina (Del.) leaves was carried out as follows; Fresh leaves of the plant were air-dried and pulverized with an electric pulverizer (DIK-2910, Daiki Rika Kogyo Co. Ltd, Tokyo – Japan) and, thereafter, weighed (W1). The pulverized leaves were further crushed in 80% acetone (1:2 w/v) using a Waring blender. The resulting mixture was homogenized in a Polyron Homogenizer (Glen Mills Inc., Clifton, NJ) for about 3 min and the homogenate was filtered under vacuum using Buchner funnel and Whatman number 2 filter paper (Whatman PLC, Middlesex, UK). The filtrate was concentrated under vacuum using a Rotary Evaporator (Hahnshin Scientific, HS-2005-N) and freeze-dried in a Lyophilizer (Ilshin Lab. Co. Ltd., Seoul, Republic of Korea). The yield obtained (after the extraction process) was weighed (W2) and kept in a desiccator until when needed. This yield was the acetonic extract of Vernonia amygdalina leaf (AEVAL).
The percentage yield of AEVAL was calculated as follows;
2.3. Detection and quantification of phytochemicals in AEVAL
The presence of alkaloids was qualitatively determined by the method of Halilu et al. [42] and quantified as described by Harbone [43]. Flavonoids were also qualitatively determined by the method of Halilu et al. [42] but quantified by the method of Obadomi and Ochuko [44]. The presence of Tannins was qualitatively determined by the method of Halilu et al. [42] and quantified by the method of Allen et al. [45]. Saponins were qualitatively determined using Froth test as described by Benmehdi et al. [46] and thereafter quantified by the method of Obadoni and Ochuko [44]. Keller-Kiliani test as described by Anjali and Sheetal [47] was used to qualitatively determine cardiac glycosides while it was quantified by the method of Harbone [43].
2.4. Determination of oral lethal dose (LD50) of AEVAL
The oral LD50 of AEVAL was determined by a modification of Lorke’s method [48]. The modification was the use of 8 rats in the second phase of study, rather than 4 rats as proposed by Lorke. Lorke’s method proposed the use of a total number of 13 animals; 9 animals for the first phase and 4 animals for the second phase. However, a total number of 17 adult Wistar rats were used for this study. In the first phase of study, 9 rats were divided into 3 groups of 3 rats each and were administered AEVAL at graded doses of 10, 100 and 1000 mg/kg, orally. The rats were observed for 24 h after which the first phase of study was terminated. In the second phase of study, 8 rats were divided into 4 groups of 2 rats each and were administered AEVAL at 750, 1500, 3000 and 6000 mg/kg, orally. They were also observed for 24 h after which the oral LD50 of the extract was determined by the formula below;
| LD50 = √ a x b |
Where a = least dose that killed a rat; and
b = highest dose that did not kill any rat.
2.5. Preparation of AEVAL and Cd solutions
The choice of therapeutic doses that was adopted for this study was guided by the (determined) oral LD50 of AEVAL. These doses were taken to be less than 10% of the oral LD50. Hence, doses of 100, 200 and 400 mg/kg of AEVAL were prepared as follows;
1 g of AEVAL was dissolved in 20 ml of distilled water to make a stock solution of 100 mg/kg of AEVAL. Stock solutions of 200 and 400 mg/kg AEVAL were prepared by dissolving 2 g and 4 g of AEVAL each in 20 ml of distilled water, respectively. The rats, therefore, received 0.2 ml/100 g of AEVAL, orally throughout the study period. Fresh samples were prepared every 48 h while left-overs were stored in a deep-freezer after use.
Cd in the form of CdSO4 was administered to the rats at 5 mg/kg for five consecutive days via intraperitoneal route (i.p.). 50 mg of the salt was dissolved in 20 ml of distilled water in order to prepare a stock solution of 5 mg/kg. Therefore, each rat received 0.2 ml/100 g of Cd, intraperitoneally for a period of 5 days.
2.6. Animal management and experimental protocol
All experimental protocols were in strict compliance with the guidelines for animal research, as detailed in the NIH Guidelines for the Care and Use of Laboratory Animals [49] and approved by local institutional Research Committee. Thirty (30) male Wistar rats of about 2–3 months of age, weighing 120–150 g, were used for this study. They were purchased from the Animal Holdings Unit of the College of Health Sciences, OAU, Ile-Ife, Osun State, Nigeria where the study was carried out. They were housed in plastic cages under natural light and dark cycle and allowed access to standard laboratory rat chow (Caps Feed PLC, Osogbo – Nigeria) and water ad libitum.
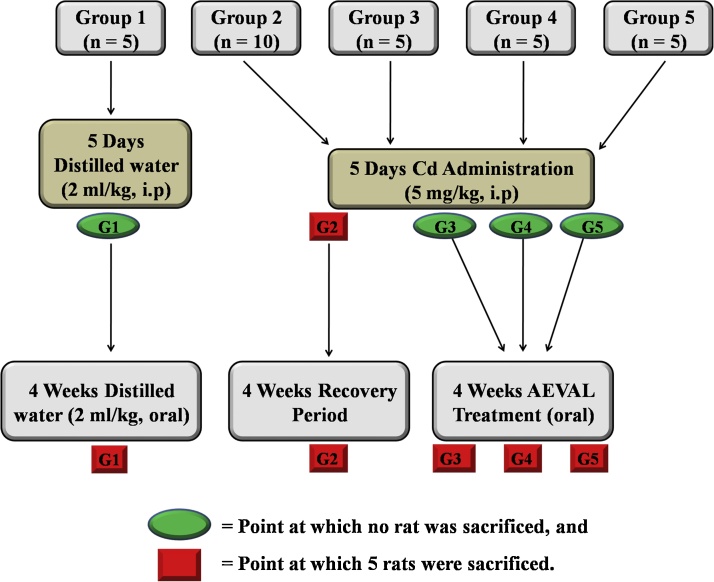
The rats were divided into five groups as follows; Group 1 (control) consisted of 5 rats that received distilled water (2 ml/kg) intraperitoneally for 5 consecutive days and thereafter received oral distilled water (2 mg/kg) for 4 weeks before they were sacrificed. Group 2 (toxic) consisted of 10 rats that received Cd (5 mg/kg) intraperitoneally for 5 consecutive days after which 5 rats were sacrificed. The remaining 5 rats were left for a recovery period of 4 weeks, after which they were also sacrificed. Groups 3, 4 and 5 each consisted of 5 rats that were pre-treated as group 2 after which they received graded doses of AEVAL at 100, 200 and 400 mg/kg respectively, orally, for 4 weeks after which they were also sacrificed (Fig. 1). At the end of the study, the rats were euthanized and their blood samples were collected by cardiac puncture into separate EDTA bottles.
Fig. 1.
Experimental Protocol.
G1 = Group 1 (2 ml/kg of distilled water, both i.p and orally); G2 = Group 2 (First 5 rats = 5 mg/kg Cd; Remaining 5 rats = 5 mg/kg Cd + Recovery); G3 = Group 3 (Cd + 100 mg/kg AEVAL); G4 = Group 4 (Cd + 200 mg/kg AEVAL); G5 = Group 5 (Cd + 400 mg/kg AEVAL); n = number of rats in the group.
Blood samples were centrifuged at 4000 rpm for 15 min at −4 °C using a cold centrifuge (Centurium Scientific, Model 8881). The plasma obtained was decanted into separate plain bottles using sterile syringes, for the assessment of biochemical markers of liver function. About 1 g of the liver of each rat was excised and kept in a cooler for the preparation of tissue homogenates for the assessment of markers of oxidative stress and lipid peroxidation while the other portion of the liver were fixed in 10% formal-saline solution for histopathological examination using Hematoxylin − Eosin (H & E) staining technique.
2.7. Measurement of body and organ weight
The assessment of weekly body weight was carried out using Hanson digital weighing balance (Hanson, China) while organ weight was determined using Camry sensitive weighing balance (Camry, China). The percentage weight change as well as relative liver weight was determined using the formulae below;
| [50,51] |
| [50,51] |
2.8. Assessment of biochemical markers of liver function
Assessment of biochemical markers of liver function such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and total bilirubin in the plasma was carried out using Randox standard laboratory kits. The procedures for these tests were as provided in their respective kits. However, total protein was assayed in the liver homogenate according to the method of Lowry and co-workers [52].
2.9. Assessment of indicators of oxidative stress and lipid peroxidation
With the aid of an electric homogenizer (S1601001), 10% homogenate in phosphate buffer (100 mM) was prepared with the tissues at pH of 7.4. The homogenates were centrifuged at 3000 rpm for 20 min and the supernatants were collected for the assessment of the following indicators of oxidative stress and lipid peroxidation;
Reduced glutathione (GSH) level was determined by the method of Beutler and co-workers [53], Super-oxide dismutase (SOD) by the method of McCord and Fridovich [54], catalase by the method of Sinha [55] while Thiobarbituric acid reactive substances (TBARS), an index of lipid peroxidation, was determined as described by Ohkawa et al. [56].
2.10. Histopathological examination
The liver of each rat was fixed in 10% formal- saline solution. Thereafter, they were dehydrated in graded alcohol and embedded in paraffin wax. Sections taken (7–8 μm thick) were stained using H & E technique. Photomicrographs were taken using a Leica DM 750 camera microscope at ×400 magnification.
2.11. Statistical analysis
Data obtained were expressed as mean ± standard error of mean using one-way analysis of variance (for multiple comparisons) and thereafter subjected to Tukey’s post-hoc test at p < 0.05. Student’s t-test was used to determine differences between two variables. Data were analysed using Graph Pad Prism 5.03 (Graph Pad Software Inc., CA, USA).
3. Results
3.1. Percentage yield (%), phytochemical screening and oral LD50 of AEVAL
After three different extraction processes, the percentage yield of AEVAL is as presented in Table 1.
Table 1.
Percentage Yield of AEVAL (%).
| Extraction Processes | Weight of Pulverized Leaves (g) | Yield (g) | Percentage Yield (%) |
|---|---|---|---|
| 1st Extraction | 300 | 24.50 | 8.17 |
| 2nd Extraction | 300 | 24.80 | 8.27 |
| 3rd Extraction | 300 | 24.20 | 8.07 |
Result shows that the percentage yield of AEVAL is 8.17 ± 0.06% (where n = 3).
Important phytochemicals that are present in the extract (AEVAL) are as presented in Table 2.
Table 2.
Phytochemical Screening and Quantification of AEVAL.
| Phytochemical Constituent | Status | Quantification/Percentage Composition (g/100 g) |
|---|---|---|
| Alkaloids | + | 10.09 ± 0.38 |
| Flavonoids | + | 32.54 ± 0.25 |
| Tannins | + | 16.62 ± 0.69 |
| Saponins | + | 3.97 ± 0.11 |
| Cardiac glycosides | − | nil |
Each value (n = 3) is expressed as mean ± standard error of mean; + = present; − = absent.
The determined oral lethal dose (LD50) of AEVAL is as presented in Table 3.
Table 3.
Acute Oral Toxicity Test (LD50) of AEVAL.
| No of rats | Dose (mg/kg) | Mortality |
|---|---|---|
| 1ST PHASE | ||
| 3 | 175 | 0/3 |
| 3 | 350 | 0/3 |
| 3 | 700 | 0/3 |
| 2ND PHASE | ||
| 2 | 625 | 0/2 |
| 2 | 1250 | 0/2 |
| 2 | 2500 | 0/2 |
| 2 | 5000 | 0/2 |
Least dose that killed a rat = nil; Highest dose that did not kill any rat = 5000 mg/kg. Therefore, oral LD50 of AEVAL is >5000 mg/kg in Wistar rats.
3.2. Effects of AEVAL on percentage weight change (%) and relative liver weight (%) of Wistar rats exposed to Cd toxicity
The AEVAL-treated groups 3, 4 and 5 showed significant restoration of percentage weight change to physiological levels when compared with the control, toxic and toxic recovery groups (Table 4).
Table 4.
Effects of AEVAL on Percentage Weight Change and Relative Liver Weight of Wistar Rats Exposed to Cd Toxicity.
| Groups | Percentage Weight Change (%) | Relative Liver Weight (%) |
|---|---|---|
| [1] Control | 19.80 ± 0.55 | 3.25 ± 0.09 |
| [2a] Cd | −18.40 ± 0.60e | 5.10 ± 0.11e |
| [2b] Cd + Recovery | −7.20 ± 0.48e,a | 4.65 ± 0.20e |
| [3] Cd + 100 mg/kg AEVAL | 9.20 ± 0.45e,a,b | 3.55 ± 0.11a,b |
| [4] Cd + 200 mg/kg AEVAL | 13.30 ± 0.50e,a,b,c | 3.40 ± 0.09a,b |
| [5] Cd + 400 mg/kg AEVAL | 4.50 ± 0.38e,a,b,c,d | 3.75 ± 0.13a,b |
Each value represents mean ± standard error of mean (p < 0.05).
Significantly different from Cd group.
Significantly different from Cd + recovery group.
Significantly different from Cd + 100 mg/kg AEVAL.
Significantly different from Cd + 200 mg/kg AEVAL.
Significantly different from control group.
Also, the Cd-induced significant increase in relative kidney weight was significantly restored to physiological levels in the AEVAL-treated groups 3, 4 and 5 when compared with the control, toxic and toxic recovery groups (Table 4).
3.3. Effects of AEVAL on hepatic GSH (μg/mg protein), SOD (mM/mg protein), CAT (μmol/min/mg protein) and TBARS (nmol/mg protein) activities in wistar rats with Cd-induced liver injury
Cd-induced reduction in hepatic GSH, SOD and CAT levels was significantly normalized following AEVAL administration. The AEVAL-treated groups 3, 4 and 5 showed no significant difference in these indicators of oxidative stress (at the end of the study) when compared with the control, but the levels were recorded to be significantly higher than both the toxic and toxic recovery groups (Table 5).
Table 5.
Effects of AEVAL on Hepatic Indicators of Oxidative Stress and Lipid Peroxidation in Wistar Rats with Cd-Induced Liver Injury.
| Indicators of Oxidative Stress |
Indicator of Lipid Peroxidation | |||
|---|---|---|---|---|
| Groups | GSH (μg/mg protein) | SOD (mM/mg protein) | CAT (μmol/min/mg protein) | TBARS (nmol/mg protein) |
| [1] Control | 5.68 ± 0.30 | 1.15 ± 0.08 | 2.60 ± 0.10 | 35.65 ± 0.80 |
| [2a] Cd | 1.07 ± 0.25c | 0.32 ± 0.07c | 1.08 ± 0.09c | 84.90 ± 1.20c |
| [2b] Cd + Recovery | 2.00 ± 0.29c | 0.66 ± 0.07c,a | 1.49 ± 0.09c,a | 62.25 ± 1.10c,a |
| [3] Cd + 100 mg/kg AEVAL | 4.87 ± 0.18a,b | 1.01 ± 0.06a,b | 2.37 ± 0.07a,b | 36.55 ± 1.00a,b |
| [4] Cd + 200 mg/kg AEVAL | 5.35 ± 0.28a,b | 1.07 ± 0.05a,b | 2.43 ± 0.10a,b | 32.33 ± 0.77a,b |
| [5] Cd + 400 mg/kg AEVAL | 4.60 ± 0.35a,b | 0.98 ± 0.04a,b | 2.20 ± 0.10a,b | 38.63 ± 1.10a,b,d |
Each value represents mean ± standard error of mean (p < 0.05).
Significantly different from Cd group.
Significantly different from Cd + recovery group.
Significantly different from control group.
Significantly different from Cd + 200 mg/kg AEVAL.
The significantly elevated level of TBARS that accompanied Cd administration was significantly lowered following AEVAL administration. The AEVAL-treated groups 3, 4 and 5 showed significant reductions when compared with both the toxic and toxic recovery groups, but recorded no significant difference when compared with the control (Table 5).
3.4. Effects of AEVAL on plasma AST (U/L), ALT (U/L) and ALP (U/L) levels in wistar rats with Cd-induced liver injury
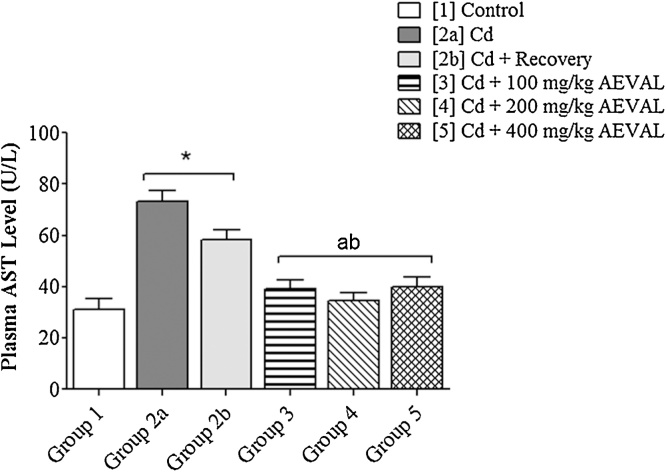
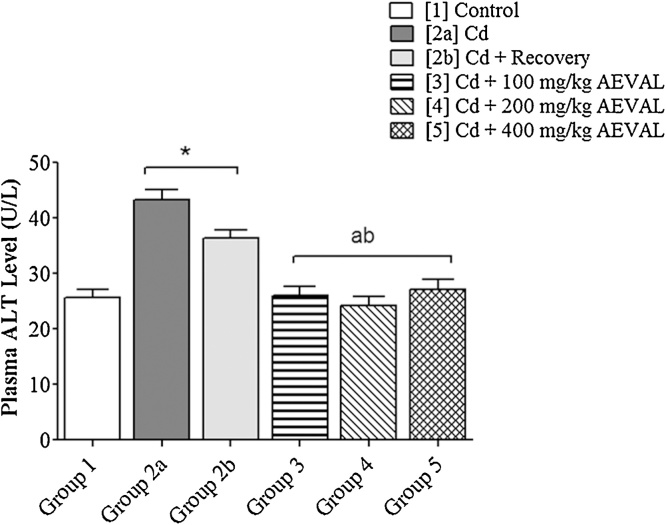
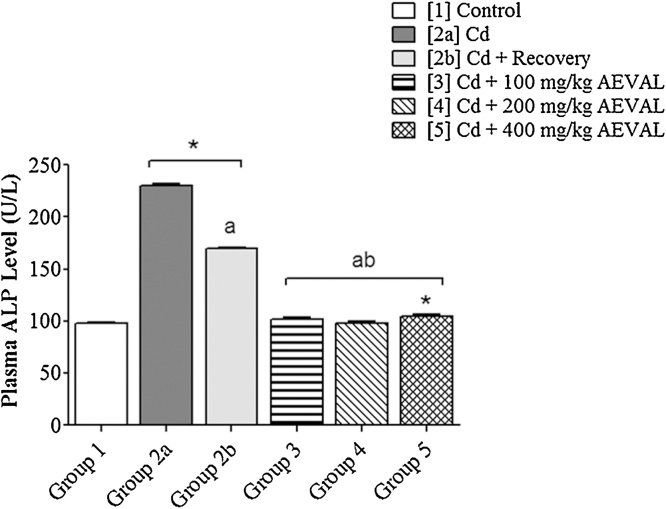
The plasma levels of AST, ALT and ALP were significantly elevated following Cd exposure. These levels were, however, restored to physiological levels in the AEVAL-treated groups 3, 4 and 5 when compared with the control, toxic and toxic recovery groups (Fig. 2, Fig. 3, Fig. 4).
Fig. 2.
Graph Showing the Effects of AEVAL on Plasma AST Level of Wistar Rats with Cd-induced Liver Injury.
Each bar represents mean ± standard error of mean (p < 0.05).
* = significantly different from control group;
a = significantly different from Cd group; and
b = significantly different from Cd + recovery group.
Fig. 3.
Graph Showing the Effects of AEVAL on Plasma ALT Level of Wistar Rats with Cd-induced Liver Injury.
Each bar represents mean ± standard error of mean (p < 0.05).
* = significantly different from control group;
a = significantly different from Cd group; and
b = significantly different from Cd + recovery group.
Fig. 4.
Graph Showing the Effects of AEVAL on Plasma ALP Level of Wistar Rats with Cd-induced Liver Injury.
Each bar represents mean ± standard error of mean (p < 0.05).
* = significantly different from control group;
a = significantly different from Cd group; and
b = significantly different from Cd + recovery group.
3.5. Effects of AEVAL on plasma total bilirubin (μmol/L) and hepatic total protein (mg/mL) levels in wistar rats with Cd-induced liver injury
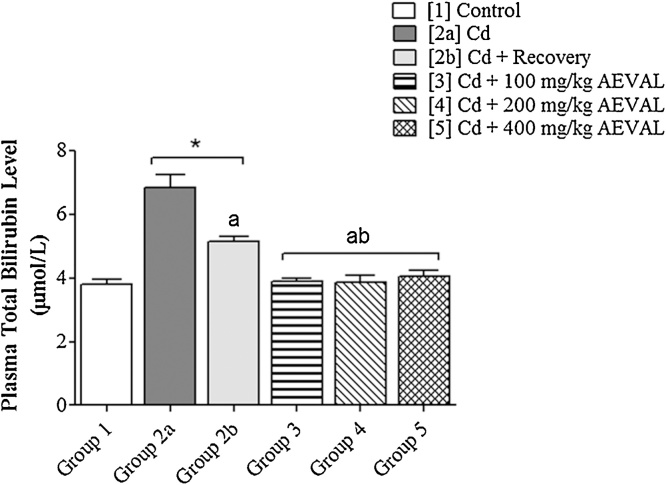
Cadmium administration was associated with significantly elevated level of plasma total bilirubin level. The total bilirubin levels were significantly lowered in the AEVAL-treated groups 3, 4 and 5 when compared with both the toxic and toxic recovery groups with no recorded significant difference when these treated groups were compared with the control group (Fig. 5).
Fig. 5.
Graph Showing the Effects of AEVAL on Plasma Total Bilirubin Level of Wistar Rats with Cd-induced Liver Injury.
Each bar represents mean ± standard error of mean (p < 0.05).
* = significantly different from control group;
a = significantly different from Cd group; and
b = significantly different from Cd + recovery group.
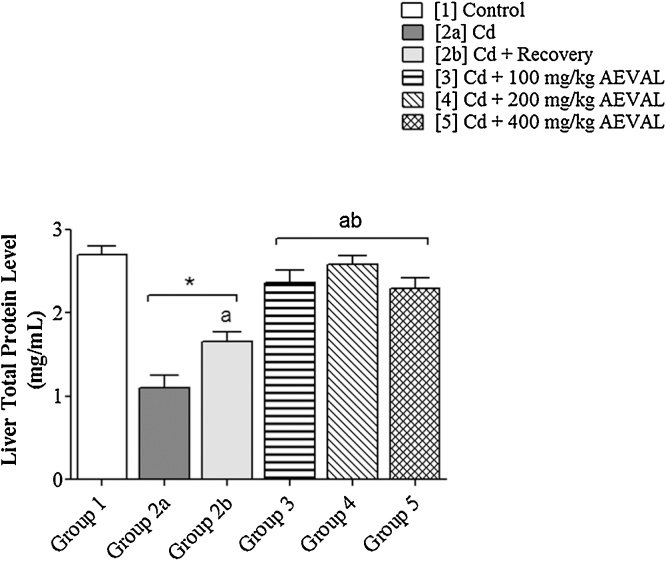
Following exposure to Cd, hepatic total protein level was significantly lowered. AEVAL treatment significantly restored hepatic total protein levels to physiological levels when compared with the control, toxic and toxic recovery groups (Fig. 6).
Fig. 6.
Graph Showing the Effects of AEVAL on Liver Total Protein Level of Wistar Rats with Cd-induced Liver Injury.
Each bar represents mean ± standard error of mean (p < 0.05).
* = significantly different from control group;
a = significantly different from Cd group; and
b = significantly different from Cd + recovery group.
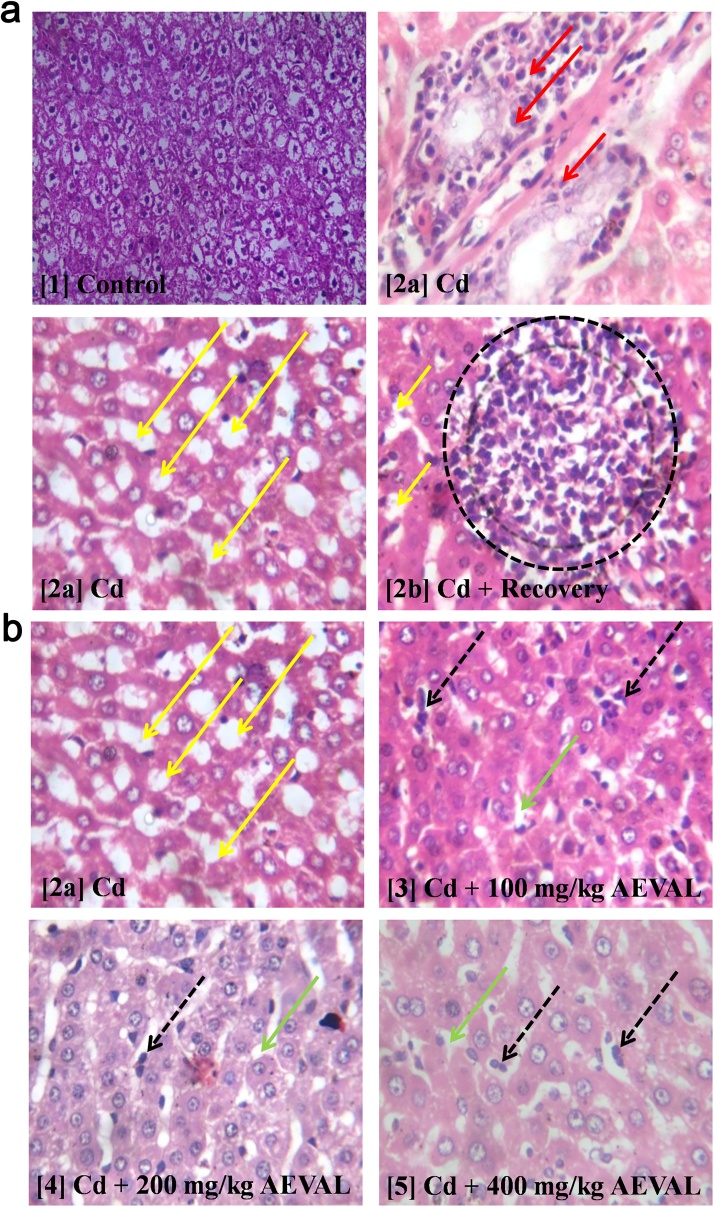
3.6. Histological effects of AEVAL on the liver of Wistar rats exposed to Cd toxicity
The photomicrograph of the control group [Plate 1] showed evidence of apparently normal liver histoarchitecture that was characterised by intact lamellar pattern of the hepatocytes and Kupffer cells. However, the toxic group [Plate 2a] showed evidence of hepatocyte and kupffer cell degeneration with peri-portal infiltration by inflammatory cells. The abnormal morphological disruption was also characterised by well disseminated focal areas of steatosis. The toxic recovery group [Plate 2b] also showed evidence of steatosis with aggregate of inflammatory cells. The AEVAL-treated groups [Plates 3, 4 and 5] showed evidence of ameliorative effects against the Cd-induced disruption in liver histoarchitecture. This was characterised by a milder disseminated steatosis, fewer distribution of inflammatory cells and apparent restoration of the lamellar pattern of both hepatocytes and Kupffer cells when compared with Plates 2a and 2b (Fig. 7).
Fig. 7.
Photomicrographs Showing the Histological Effects of AEVAL on the Liver of Wistar Rats Exposed to Cd Toxicity.
Red arrow = peri-portal infiltration by inflammatory cells; Yellow arrow = focal area of steatosis; Black dotted circle = aggregate of inflammatory cells; Black broken arrow = mild distribution of inflammatory cells; Green arrow = mild disseminated steatosis.
4. Discussion
This study investigated the hepatic effects of four weeks oral administration of acetonic extract of Vernonia amygdalina (Del.) leaf (AEVAL) in a Wistar rat model of cadmium-induced liver injury. Based on the indices that were assayed, the overall finding of this study showed that AEVAL administration at graded doses restored homeostasis of the antioxidant system as well as liver function biomarkers. However, these effects were not dose-dependent; the least effective dose being the highest dose of the extract (400 mg/kg). Also, administration of the extract was associated with improved liver histoarchitecture following Cd-induced injury.
The associated decrease in percentage weight change (PWC) that followed exposure to Cd is consistent with the findings of Merali and Singhal [57]. By way of physical examination, the rats became lethargic and ate less after exposure to Cd toxicity. Consequently, their feed was left, almost, untouched. Therefore, the reduction in body weight can be attributed to the reduction in food consumption since weight gain or loss is determined by a balance between dietary intake and energy expenditure [58]. Furthermore, the hypothalamus is known to be the “key controller” for the maintenance of energy homeostasis within neural circuitry [58]. Cadmium has been reported in literature to bioaccumulate in the brain by easily crossing the blood-brain barrier [[59], [60]] and exerting undesirable effects on the hypothalamus [[60], [61]]. This study suggests that Cd administration is associated with the suppression or inhibition of hypothalamic centre for food consumption and energy expenditure. This also explains the decreased desire for food consumption with a corresponding decrease in body weight. Following exposure to Cd toxicity, the administration of the extract may have modulated the hypothalamic centre for food consumption to bring about normal homeostasis in feeding pattern with a consequent increase in body weight. This study, therefore, suggests a possible appetite-stimulating effect of the extract; demonstrating its potential as an adjuvant Cd therapy.
A significant difference in the organ weight of experimental animals without any apparent morphological changes can be a sensitive indicator of the effect of a chemical agent [62]. However, in order to avoid any complication that may arise as a result of differences in the body weight between experimental groups, the ratio of body weight to organ weight (generally described as relative organ weight) is commonly used for analysis [62]. The observed Cd-induced increase in relative liver weight (RLW) may have resulted from inflammatory response by the liver. Micrographic evidence of the toxic group showed features of inflammation with well disseminated focal areas of steatosis. Although the liver is reputed to have a self-regenerating potential (as supported by the finding of this study), self-restoration to its physiological size was impossible without the intervention of a potent agent (like AEVAL). Tannins and flavonoids, important phytochemicals of the extract, are reputed for their anti-inflammatory and anti-oxidant properties [[63], [64], [65]]. Apparently, this study demonstrated an anti-inflammatory potential of the extract as micrographic evidence of the AEVAL-treated groups showed features of milder distribution of inflammatory cells, scanty disseminated steatosis and an apparent restoration of the lamellar pattern of hepatocyte when compared with that of the toxic and toxic recovery groups. This explains the resulting restorative effect of the extract against Cd-induced increase in relative liver weight. The finding of this study, therefore, suggests that AEVAL potentiates restoration of relative liver weight to physiological levels through anti-oxidant and anti-inflammatory mechanisms.
While GSH is a non-enzymatic index of oxidative stress [[50], [51], [66]], both SOD [67] and CAT [[68], [69]] are important enzymatic indices of oxidative stress. On the other hand, TBARS is an important index of lipid peroxidation [[35], [50], [51], [65]]. Increased activity of TBARS is in direct proportion to the increasing degree of injury in biological tissues; hence, it is an index of lipid peroxidation [[35], [50]]. The deleterious reduction in the activities of GSH, SOD and CAT in the liver following exposure to Cd toxicity was an indication of oxidative stress due to generation of free radicals. This can be attributed to an increased use of these enzymatic and non-enzymatic biomarkers by the liver to scavenge free radicals in an attempt to restore homeostasis of the antioxidant system and (or) reduced ability of the liver to sustain these lines of defence following ROS generation due to Cd toxicity. These culminated in increased TBARS level which was reflective of a high degree of Cd-induced liver injury. The AEVAL-treated groups demonstrated attenuating effects against the Cd-induced oxidative stress and lipid peroxidation. This shows that the plant is a good source of potent antioxidant activities; a finding that supports existing literatures [[35], [36], [37], [50]]. It therefore implies that AEVAL restores homeostasis of the antioxidant system by conjugating and excreting toxic cellular molecules, detoxification of ROS, sustenance of cellular integrity as well as a possible enhancement of tissue regeneration (as depicted by the micrographic evidences). This scientific assertion was further buttressed with the fact that the group that was left to self-recover could not reverse the alterations in the antioxidant system to physiological levels, although some measures of recovery was recorded. These pharmacological activities of the extract can be attributed to the presence of mostly flavonoids, tannins and alkaloids (reputed for their anti-oxidant and anti-inflammatory properties). The potent anti-oxidant and anti-inflammatory capacities of the extract may be the basis for its pharmacological activities since increased ROS generation and inducing cellular inflammation are the basic biological mechanisms of Cd-induced cellular injury.
The assay for the activities of blood levels of AST, ALT and ALP as important biomarkers of liver function is of clinical relevance [[70], [71], [72]]. These enzymes are located in the liver cells and are released into the plasma in response to liver cell injury or damage. Following Cd administration, the activities of these liver enzymes were significantly elevated beyond physiological levels. AST and ALT enzymes are greatly concentrated in the liver [73]. Whereas AST is both mitochondrial (about 80% of total activity) and cytosolic (about 20% of total activity) in location, ALT is solely cytoplasmic [73]. Therefore, the expressed potential of the extract to attenuate the Cd-induced derangements in both AST and ALT activities is suggestive of its cytoplasmic, cotosolic and mitochondrial effects to bring about the prevention of membrane fragility and reduced leakage of hepatic enzymes into the blood. Since ALP is a cholestatic index [[73], [74]], the AEVAL-enhanced attenuating effects against elevated ALP levels can be attributed to the extract’s potential to reverse cholestatic mechanism(s). This is because cholestasis (obstruction of bile flow) enhances both synthesis and release of hepatic ALP from cell surfaces [[73], [74]].
Total bilirubin is used as an index of bile duct lesion [75] or hepatic injury due to bile duct obstruction [76]. This study recorded an elevated level of plasma total bilirubin after exposure to Cd toxicity. The apparent normalization of the total bilirubin level that was associated with AEVAL treatment can be attributed to the potential of the extract to ameliorate bile duct obstruction or lesions, possibly, via its antioxidant and tissue regeneration mechanisms; a potential that may have been enhanced by the synergetic effects of its important phytochemical constituents.
A contributory self-healing mechanism to liver regeneration process is the stimulation of protein synthesis [77]. A chemical agent can induce adverse changes in the process of protein synthesis; hence, the level of hepatic total protein content can be an important index for the determination of chemically-induced liver injury or dysfunction [74]. Although the self-recovery group showed increased level of total protein level when compared with the toxic group, this level was not within the physiological range. The extract’s potential to restore physiological levels of liver total protein is indicative of its tissue-regenerative ability (as supported by their micrographic evidences); an ability that was least expressed in the group that received the highest dose of the extract.
Worthy of note is the fact that the attenuating effects of the extract were not dose-dependent. Almost all the measured parameters showed least therapeutic effects of the extract at the highest dose (400 mg/kg). A note of caution should, therefore, be taken during administration of the extract or during a further experimental evaluation of the acetonic extract of this plant as this study indicates a possible high risk profile of liver dysfunction at high doses. A limitation of this study, worthy of further investigation, is the assessment of blood level of Cd during/after treatment with an intervention; since this heavy metal does not undergo metabolic degradation to less toxic metabolite. Another possible limitation of this study is the low number of rats that was used in appraising the extract’s therapeutic effects. It is not unlikely to pool larger data which may provide more information about the extract’s therapeutic effects if a larger number of rats were recruited for the study. In addition, this study recommends that cadmium-exposed subject should resort to prompt and efficacious treatment/management therapy. This is because the group that were left untreated (self-recovery), although showed some measure of regenerative and self-healing attributes, did not restore the biomarkers of liver function as well as indicators of oxidative stress to physiological levels.
5. Conclusion
It was concluded that acetonic extract of Vernonia amygdalina (Del.) leaf attenuates cadmium-induced liver injury through antioxidant, anti-inflammatory, membrane-stabilizing as well as tissue-regenerating mechanisms. The pharmacological activities of the extract, attributed to its important phytochemical constituents, potentially make it a suitable option in adjuvant heavy metal therapy.
Acknowledgements
The authors wish to acknowledge the members of staff of the Central Science Laboratory, Obafemi Awolowo University (OAU), Ile-Ife, Osun State, Nigeria as well as Dr Obuotor’s Laboratory, Department of Biochemistry, OAU, Ile-Ife, for their kind support and technical assistance.
References
- 1.Palanive M.G., Rajkapoor B., Kumar R.S. Hepatoprotective and antioxidant effect of Pisonia aculeate L. Against CCl4-induced hepatic damage in rats. Sci. Pharm. 2008;76:203–215. [Google Scholar]
- 2.Buraimoh A.A., Bako I.G., Ibrahim F.B. Hepatoprotective effect of ethanolic leaves extract of Moringa Oleifera on the histology of paracetamol induced liver damage in wistar rats. Int. J. Anim. Vet. Adv. 2011;3:10–13. [Google Scholar]
- 3.Subramoniam A., Pushpangadan P. Development of phytomedicine for liver diseases. Indian J. Pharmacol. 1999;31:166–175. [Google Scholar]
- 4.Olukiran S.O., Akomolafe R.O., Bamitale K.D., Ajayi A.O., Okonji R.E., Bejide R.A. Protective and curative effects of Livolin forte® on carbon tetrachloride-induced liver damage in Wistar rats. J. Exp. Integr. Med. 2013;4:57–65. [Google Scholar]
- 5.WHO (World Health organization) 2nd edition. WHO, Regional Office for Europe; Copenhagen, Denmark: 2000. Cadmium Air Quality Quide Lines. [Google Scholar]
- 6.Newairy A.A., El-Sharaky A.S., Badreldeen M.M., Eweda S.M., Sheweita S.A. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology. 2007;242:23–30. doi: 10.1016/j.tox.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Renugadevi J., Prabu S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. 2009;256:128–134. doi: 10.1016/j.tox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Sethi P.K., Khandelwal D.J. Cadmium exposure: health hazards of silver cottage industry in developing countries. Med. Toxicol. 2006;2:14–25. doi: 10.1007/BF03161007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mead M.N. Cadmium confusion: do consumers need protection? Environ. Health Perspect. 2010;118:528–534. doi: 10.1289/ehp.118-a528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinar M. Cadmium and effects at biological system. Veterinarium. 2003;14:79–84. [Google Scholar]
- 11.Kaplan O., Yildirim N.C., Yildirim N., Cimen M. Toxic elements in animal products and environmental health. Asian Anim. Vet. Adv. 2011;6:228–232. [Google Scholar]
- 12.IPCS (International Programme on Chemical Safety); Geneva: 1992. Cadmium – Environmental Aspects. 135. [Google Scholar]
- 13.WHO (World Health Organization); Geneva, Switzerland: 2010. Preventing Disease Through Healthy Environments, Exposure to Cadmium: A Major Public Health Concern. [Google Scholar]
- 14.Lewis G.P., Coughlin L.L., Jusko W.J., Hartz S. Contribution of cigarette smoking to cadmium accumulation in man. Lancet. 1972;1:291–292. doi: 10.1016/s0140-6736(72)90294-2. [DOI] [PubMed] [Google Scholar]
- 15.Järup L., Berglund M., Elinder C.G., Nordberg G., Vahter M. Health effects of cadmium exposure – a review of the literature and a risk estimate. Scand. J. Work Environ. Health. 1998;24:1–51. [PubMed] [Google Scholar]
- 16.Bernard A. Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals. 2004;17:519–523. doi: 10.1023/b:biom.0000045731.75602.b9. [DOI] [PubMed] [Google Scholar]
- 17.Nordberg G., Nogawa K., Nordberg M., Friberg L. Handbook on Toxicology of Metals. 3rd edition. Academic Press; New York: 2007. Cadmium; pp. 65–78. [Google Scholar]
- 18.Stohs S.J., Bagchi D., Hassoun E., Bagchi M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2001;20:77–88. [PubMed] [Google Scholar]
- 19.Stohs S.J., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 20.Doaa El-Nager M., Bader A.A. Effect of corn oil, flax seed oil and black seed oil on testicular damage induced by lead acetate in Albino Mice: a histological study. Pak. J. Zool. 2013;45:1083–1089. [Google Scholar]
- 21.Iwu M.M., Duncan A.R., Okunji C.O. New anti-microbials of plant origin. In: Janick J., editor. Prospectives on New Crops and New Uses. ASHS Press; Alexandria, VA: 1999. pp. 457–462. [Google Scholar]
- 22.Robbers J., Speedie M., Tyler V. 3rd edition. Williams, Wilkins; Baltimore: 1996. Pharmacognosy and Pharmaco-biotechnology. [Google Scholar]
- 23.Ijeh I.I., Ejike C.E.C. Current perspectives on the medicinal potential of Vernonia amygdalina Del. J. Med. Plants Res. 2011;5:1051–1061. [Google Scholar]
- 24.Onabanjo O.O., Oguntona C.R.B. Iron, zinc, copper and phytate content of standardized Nigerian dishes. J. Food Compos. Anal. 2003;16:669–676. [Google Scholar]
- 25.Ndaeyo N.U. Assessing the contributions of homestead farming to food security in a developing economy: a case study of Southeastern Nigeria. J. Agric. For. Soc. Sci. 2007;3:11–16. [Google Scholar]
- 26.Newbold C.J., El-Hassan S.M., Wang J., Ortega M.E., Wallace R.J. Influence of foliage from African multipurpose trees on activity of rumen protozoa and bacteria. Br. J. Nutr. 1997;78:237–249. doi: 10.1079/bjn19970143. [DOI] [PubMed] [Google Scholar]
- 27.Kambizi L., Afolayan A.J. An ethnobotanical study of plants used for the treatment of sexually transmitted disease (njovher) in Guruve District Zimbabwe. J. Ethnopharmacol. 2001;77:5–9. doi: 10.1016/s0378-8741(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 28.Moundipa P.F., Flore K.G., Bilong C.F., Bruchhaus I. In vitro amoebicidal activity of some medicinal plants of the Bamun region (Cameroon) Afr. J. Tradit. Complement. Altern. Med. 2005;2:113–121. [Google Scholar]
- 29.Orisajo S.B., Dongo L.N. Nematicidal potential of some indigenous plant extracts against root-knot nematode on cacao. Afr. Acad. Sci. 2005;6:129–134. [Google Scholar]
- 30.Tona L., Cimanga R.K., Mesia K., Musuamba C.T., Bruyne T.D., Apers S., Hernans N., Miert S.V., Pieters L., Totte J., Vlietinck A.J. In vitro antiplasmodial activity of extracts and fractions from seven medicinal plants used in the Democratic Republic of Congo. J. Ethnopharmacol. 2004;93:27–32. doi: 10.1016/j.jep.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Froelich S., Onegi B., Kakooko A., Schubert C., Jenette-Siems K. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected east African plants used for the treatment of malaria. Planta Med. 2006;72:001–015. [Google Scholar]
- 32.Obaseiki-Ebor E.E., Odukoya K., Telikepalli H., Mitscher L.A., Shankel D. Antimutagenic activity of extracts of leaves of four common edible vegetable plants in Nigeria (West Africa) Mutat. Res. Lett. 1993;302:109–117. doi: 10.1016/0165-7992(93)90012-k. [DOI] [PubMed] [Google Scholar]
- 33.Koko W.S., Mesaik M.A., Yousaf S., Galal M., Choudhary M.I. In vitro immunomodulating properties of selected Sudanese medicinal plants. J. Ethnopharmacol. 2008;118:26–34. doi: 10.1016/j.jep.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Iroanya O., Okpuzor J., Mbagwu H. Anti-nociceptive and antiphlogistic actions of a polyherbal decoction. Int. J. Pharmacol. 2010;6:31–36. [Google Scholar]
- 35.Imafidon C.E., Akomolafe R.O., Abubakar S.A., Ogundipe D.J., Olaoluwa S.O., Oladele A.A. Amelioration of cadmium-induced nephropathy using polyphenol-rich extract of Vernonia amygdalina (Del.) leaves in rat model. Open Access Maced. J. Med. Sci. 2015;3:567–577. doi: 10.3889/oamjms.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erasto P., Grierson D.S., Afolayan A.J. Antioxidant constituents in Vernonia amygdalina leaves. Pharm. Biol. 2007;45:195–199. [Google Scholar]
- 37.Ayoola G.A., Coker H.A.B., Adesegun S.A., Adepoju-Bello A.A., Obaweva K., Ezennia E.C., Atangbayila T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008;7:1019–1024. [Google Scholar]
- 38.Arhoghro E.M., Ekpo K.E., Anosike E.O., Ibeh E.O. Effect of aqueous extract of bitter leaf (Vernonia amygdalina Del) on carbon tetrachloride (CCl4) induced liver damage in albino Wistar rats. Eur. J. Sci. Res. 2009;26:122–130. [Google Scholar]
- 39.Lolodi O., Eriyamremu G.E. Effects of methanolic extract of Vernonia amygdalina (Common bitter leaf) on lipid peroxidation and antioxidant enzymes in rats exposed to cycasin. Pak. J. Biol. Sci. 2013;16:642–646. doi: 10.3923/pjbs.2013.642.646. [DOI] [PubMed] [Google Scholar]
- 40.Momoh J., Longe A.O., Damazio A.O., Eleyowo O.O. Hepatoprotective effects of ethanolic extract of Vernonia amygdalina and Azadiracha indica against acetaminophen-induced hepatotoxicity in Sprague-Dawley male albino rats. Am. J. Pharmacol. Sci. 2015;3:79–86. [Google Scholar]
- 41.Iwo M.I., Sjahlim S.L., Rahmawati S.F. Effect of Vernonia amygdalina Del. Leaf ethanolic extract on intoxicated male Wistar rat liver. Sci. Pharm. 2017;85:1–7. doi: 10.3390/scipharm85020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halilu M.E., Abubakar A., Garbar M.K., Isah A.A. Antimicrobial and preliminary phytochemical studies of methanol extract of root bark of Crossopteryx febrifuga (Rubiaceae) J. Appl. Pharm. Sci. 2012;2:066–070. [Google Scholar]
- 43.Harborne J.B. 2nd edition. Chapman and Hall; London: 1980. Phytochemical Methods; pp. 288–293. [Google Scholar]
- 44.Obadoni B.O., Ochuko P.O. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Global J. Pure Appl. Sci. 2002;8:203–208. [Google Scholar]
- 45.Allen S.E., Grinshaw H.M., Parkinson J.A., Quarmbay C. 1st edition. Blackwell Scientific Publication; London: 1973. Chemical Analysis of Ecological Materials. [Google Scholar]
- 46.Benmehdi H., Hasnaoui O., Benali O., Salhi F. Phytochemical investigation of leaves and fruit extracts of Chamaerops humilis L. J. Mater. Environ. Sci. 2012;3:320–337. [Google Scholar]
- 47.Anjali S., Sheetal S. Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J. Pharmacogn. Phytochem. 2013;2:22–29. [Google Scholar]
- 48.Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 49.8th edition. 2011. Guide for the Care and Use of Laboratory Animals. https://grants.nih.gov/grants/./Guide-for-the-Care-and-use-of-laboratory-animals.pdf (Access date: 5 October 2015) [Google Scholar]
- 50.Imafidon C.E., Olatoye T.R., Bamidele F.S., Ojo O.E., Ademoye K.A. Cadmium-induced testicular toxicity, oxidative stress and histopathology in Wistar rats: sustained effects of polyphenol-rich extract of Vernonia amygdalina (Del.) leaf. J. Interdiscip. Histopathol. 2016;4:54–62. [Google Scholar]
- 51.Ayoka A.O., Ademoye A.K., Imafidon C.E., Ojo O.E., Oladele A.A. Aqueous extract of Allium sativum (Linn.) bulbs ameliorated pituitary-testicular injury and dysfunction in Wistar rats with Pb-induced reproductive disturbances. Open Access Maced. J. Med. Sci. 2016;4:200–212. doi: 10.3889/oamjms.2016.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lowry O.H., Nira J.R., Farr L.A., Rose J.R. Protein measurement with the folinphenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 53.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood Glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 54.McCord J.M., Fridovich I. Superoxide dismutase, an enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 55.Sinha K.A. Colorimetric assay of catalase. Anal. Biochem. 1971;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 56.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues bythiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 57.Merali Z., Singhal R.L. Prevention by zinc of cadmium-induced alterations in pancreatic and hepatic functions. Br. J. Pharmacol. 1976;57:573–579. doi: 10.1111/j.1476-5381.1976.tb10387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katherine A.S., Niamh M.M., Steve R.B. Hypothalamic regulation of apetite. Expert Rev. Endocrinol. Metab. 2008;3:577–592. doi: 10.1586/17446651.3.5.577. [DOI] [PubMed] [Google Scholar]
- 59.Pretto A., Loro V.L., Morsch V.M., Moraes B.S., Menezes C., Clasen B., Hoehne L., Dressler V. Acetylcholinesterase activity, lipid peroxidation, and bioaccumulation in Silver catfish (Rhamdia quelen) exposed to cadmium. Arch. Environ. Contam. Toxicol. 2010;58:1008–1014. doi: 10.1007/s00244-009-9419-3. [DOI] [PubMed] [Google Scholar]
- 60.Rossana F., Giovanni C., Maria C.G., Salvatore D.B., Massimo L., Ida F. vol. 27. Taylor and Francis; 2011. pp. 39–46. (Bioaccumulation of cadmium and its cytotoxic effect on zebrafish brain). [Google Scholar]
- 61.Farombi E.O., Adedara I.A., Akinrinde S.A., Ojo O.O., Eboh A.S. Protective effects of kolaviron and quercetin on cadmium-induced testicular damage and endocrine pathology in rats. Andrologia. 2012;44:273–284. doi: 10.1111/j.1439-0272.2012.01279.x. [DOI] [PubMed] [Google Scholar]
- 62.Steven A.B., Robert H.Z., Richard W.P. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol. Pathol. 2004;32:448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- 63.2016. Health Benefits of Plant Tannins. http://www.medibiztv.com/articles/health-tannins (Access date: 3 January 2016) [Google Scholar]
- 64.2016. Health Benefits of Flavonoids. http://www.livestrong.com/article/492244-whatare-the-health-benefits-of-flavonoids/ (Access date: 5 April 2016) [Google Scholar]
- 65.Ayoka A.O., Ojo O.E., Imafidon C.E., Ademoye K.A., Oladele A.A. Nuero-endocrine effects of aqueous extract of Amaranthus viridis (Linn.) leaf in male Wistar rat model of cyclophosphamide-induced reproductive toxicity. J. Toxicol. Rep. 2016;3:608–619. doi: 10.1016/j.toxrep.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sunitha S., Nagaraj M., Varalakshmi P. Hepatoprotective effect of lupeol and lupeol linoleate on tissue antioxidant defence system in cadmium-induced hepatotoxicity in rats. Fitoterapia. 2001;72:516–523. doi: 10.1016/s0367-326x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 67.Bowler C., Montagu M.V., Irize D. Superoxide Dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992;43:83–116. [Google Scholar]
- 68.Zamocky M., Furtmuller P.G., Obinger C. Evolution of catalases from bacteria to humans. Antioxid. Redox Signal. 2008;10:1527–1548. doi: 10.1089/ars.2008.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heck D.E., Shakarjian M., Kim H.D., Laskin J.D., Vetrano A.M. Mechanisms of oxidant generation by catalase. Ann. N. Y. Acad. Sci. 2010;1203:120–125. doi: 10.1111/j.1749-6632.2010.05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitra S.K., Venkataranganna M.V., Sundaram R., Goupmadhavan S. Protective effects of HD-03, herbal formulation, against various hepatotoxic agents in rats. J. Ethnopharmacol. 1998;63:181–186. doi: 10.1016/s0378-8741(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 71.Atta A.H., Elkoly T.A., Mouneir S.M., Gehan K., Alwabel N.A., Shaimaa Z. Hepatoprotective effects of methanol extracts of Zingiber officinale and Cichorium intybus. Indian J. Pharm. Sci. 2010;72:564–570. doi: 10.4103/0250-474X.78521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kazeem M.I., Bankole H.A., Fatai A.A. Protective effect of ginger in normal and carbon-tetrachloride induced hepatotoxic rats. Ann. Biol. Res. 2011;2:1–8. [Google Scholar]
- 73.Thapa B.R., Anuj W. Liver function tests and their interpretation. Indian J. Pediatr. 2007;74:663–671. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- 74.Motawi T.K., Hamed M.A., Shabana M.H., Hashem R.M., Aboul-Naser A.F. Zingiber officinale acts as a nutraceutical agent against liver fibrosis. Nutr. Metab. 2011;8:1–11. doi: 10.1186/1743-7075-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leonard T.B., Neptun D.A., Popp J.A. Serum gamma glutamyl transferase as a specific indicator of bile duct lesions in the rat liver. Am. J. Pathol. 1984;116:262–269. [PMC free article] [PubMed] [Google Scholar]
- 76.Bun S.S., Bun H., Guedon D., Rosier C., Ollivier E. Effect of green tea extract on liver functions in Wistar rats. Food Chem. Toxicol. 2006;44:1108–1113. doi: 10.1016/j.fct.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 77.Sharma N., Shukla S. Hepatoprotective potential of aqueous extract of Butea monosperma against CCl4 -induced damage in rats. Exp. Toxicol. Pathol. 2011;63:671–676. doi: 10.1016/j.etp.2010.05.009. [DOI] [PubMed] [Google Scholar]