Graphical abstract
Johar_Illustration 1
Chemicals compounds studied in this article: Alloxan; Daidzein (4′,7-dihydroxyisoflavone); Daidzin (7,4′-dihydroxyisoflavone); Genistein (4′,5,7-trihydroxyisoflavone); Genistin (5,7,7′-trihydroxyisoflavone); Glycitein (7, 4′-dihydroxy-6-methoxyisoflavone); Lignan; Nitroblue tetrazolium; Streptozotocin; Thiobarbituric acid
Keywords: Isoflavones, Lignans, Germination, Reactive oxygen species, Alloxan, Rats
Highlights
-
•
Soybean and whole-wheat have beneficial effects on the oxidative status of AD rats more than broadbean.
-
•
Feeding dried wheat is effective in improving MDA, GSH and α-T levels.
-
•
Germination is favorable than drying and moistened heat. Germination enhances the effect of soybeans on TAGs and in the case of soy and wheat enhanced the effect on total cholesterol.
-
•
Diabetic patients, beside controlling their hyperglycaemia with medication, are recommended to include whole foods containing naturally occurring phytochemicals to ameliorate their oxidative status.
-
•
Possible protective factors in the diet such as flavonoids, lutein, lycopene, lignans, and saponins, may provide new strategies to enhance diet and health of diabetic patients.
Abstract
Background
The importance of whole-food antioxidants in terms of promoting antioxidant recycling in the body in complex human diseases is not fully understood. We aim to discuss the benefits of whole-food antioxidants in ameliorating the diabetic complications in vivo and to address the effect of germination versus heat processing or drying on the potential therapeutic effect of whole grains and legumes. We studied the antioxidant status of alloxan-diabetic (AD) male Spargue Dawley rats, injected intraperitoneally with alloxan dose of 150 mg/kg body weight, and fed on experimental diets based on the flour of soybean, broadbean and whole-wheat for five weeks.
Results
Diabetes-induced oxidative stress in liver was manifested by significant increase in hepatic malondialdehyde (MDA), erythrocytes superoxide dismutase (eSOD) and plasma alpha-tocopherol (α-T) levels, reduction in hepatic glutathione (GSH) levels and catalase (CAT) activity. Consumption of soybean and whole-wheat both had beneficial effects on the oxidative status of AD rats more than broadbean. Feeding dried wheat was effective in improving MDA, GSH and α-T levels. Soybeans and wheat lowered triacylglycerols (TAGs) and tended to lower total cholesterol. Germination enhanced the effect of soybeans on TAGs and in the case of soy and wheat enhanced the effect on total cholesterol.
Conclusion
Whole foods containing naturally occurring phytochemicals and antioxidant vitamins such as legumes and whole grains are recommended, alongside medication, for controlling hyperglycaemia, blood lipids and oxidative status in diabetes.
1. Introduction
Diabetes mellitus (DM) is a major clinical and public health problem worldwide [[1], [2], [3]] with equal rates in both women and men [3]. DM is a clinically and genetically heterogeneous group of disorders characterized by abnormally high levels of glucose in the blood. Hyperglycaemia is due to deficiency of insulin secretion or to resistance of the body’s cells to the action of insulin, or a combination of these. Often there are also disturbances of carbohydrates, fat, and protein metabolism [8]. The criteria for the classification and diagnosis of DM are as follows:
Type I: caused by β-cell destruction, often immune mediated, that leads to loss of insulin secretion and absolute insulin deficiency.
Type II: caused by a combination of genetic and sporadic factors that result in insulin resistance and insulin deficiency. The specific genes are unknown but have been under intense investigation. Sporadic factors include aging, high caloric intake, overweight, central adiposity, sedentary lifestyle, and low birth weight.
Other specific types: These comprise a heterogeneous etiologic group that include cases in which the causes are uncovered or partially known. The later includes known genetic defects affecting β-cell function or insulin action, diseases of exocrine pancreas, endocrinopathies, drug-or chemical-induced pancreatic changes, diseases and conditions in which the incidence of diabetes is substantially elevated but a precise aetiology has not been established.
Gestational diabetes: caused by insulin resistance and relative insulin deficiency associated with pregnancy [9].
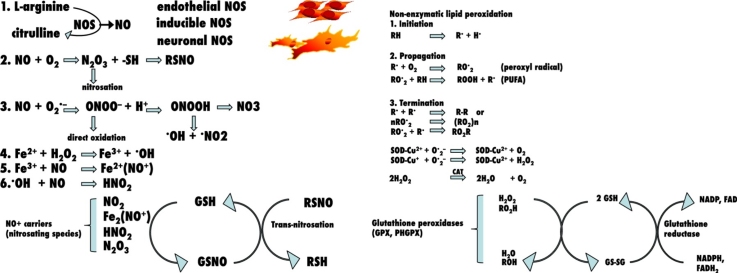
Oxidative stress is a pathogenetic mechanism in diabetic complications [[4], [5], [6], [7]]. Long-term effects of diabetes include glycation of proteins, increased risk of cardiovascular diseases, atherosclerosis, retinopathy, nephropathy, and neurological dysfunctions [[4], [5], [6], [7]]. Hyperglycaemia is a widely known cause of enhanced free radical concentration. The generation of reactive oxygen species (ROS) has shown increment in DM patients [10].
Although streptozotocin (STZ) is preferable than alloxan in induction of DM, alloxan-induced diabetes is very widely used and acceptable disease model [11]. The procedure for chemical induction of DM proved to be effective [12]. Alloxan-induced DM experimental model adds to various others [[13], [14]] and can be used as alternative model in studies carried out in several fields such as plastic surgery [15]. Accumulating evidence showed that “alloxan is comparable to STZ” [16]. Evidence for upregulated oxidative stress responses in diabetes includes observations of decreased plasma antioxidant concentrations in both diabetic subjects [17] and animal models [18]. Mechanisms that contribute to increased oxidative stress in diabetes have been reviewed [[4], [19], [20]]. In addition, glycation in DM modifies lipids, lipoproteins as in the case of apo B and low density lipoprotein (LDL) [21], and increases the susceptibility of LDL to oxidation [22].
Tissue GSH and thiols (-SH) are the ultimate bastion against oxidative stress and tissue injury, and are maintained in the reduced state by the concerted action of tissue tocopherols and other reducing factors such as urates [23].
GSH is the most abundant non-protein thiol in mammalian cells [24]. It is a tripeptide, γ-glutamylcycteinylglycine. It has two structural features responsible for much of its biochemical functions. They are its thiol group (-SH due to cysteine) and the γ-glutamyl bond (linking cysteine to glutamate). The −SH is responsible for most of the catalytic and reactive properties of GSH. It confers of GSH the ability to participate in both oxidation-reduction reactions. Thus it occurs both in reduced GSH and oxidized GSSG forms [25].
GSH is the major intracellular redox buffer in almost all cell types [[26], [27]]. A relatively high concentration of GSH is present in β-cells [28]. GSH plays a central role in the cellular defence against chemical and ROS injury [29], because of its role in xenobiotic detoxification and free radical metabolism. i.e. GSH serves as a substrate for the GSH-S-transferase that catalyzes the addition of −SH group of GSH to the activated intermediate of various xenobiotics, thereby facilitating their excretion from the cell [30]. Additionally, GSH is used by GSH peroxidase to reduce H2O2 and other hyderoperoxides to less destructive metabolites [31]. Furthermore, GSH is a cosubstrate in destroying hyderoperoxide fatty acids located in phospholipids [32].
GSH is also a scavenger of •OH radical and singlet O2, in the case of •OH radical, the thiyl radical is generated [33]. In addition, serum −SH groups act as an important extracellular scavengers of peroxides and are therefore helpful in protecting the surrounding tissues [34]. Thiol compounds also could prevent Millard reaction in vitro [35]. The GSH redox cycle is a major determinant of the antioxidative capacity of plasma and its constituents [36]. A decrease in the levels of both plasma and intracellular antioxidants such as GSH, vitamin E, −SH and ascorbate has been demonstrated in both types of DM [37]. GSH has beneficial effects against diabetes-induced nerve damages and was shown to decrease in nerves [22]. Blood GSH was significantly reduced by 25% at the first two years of diagnosis of type II diabetes. Moreover, a progressive decline of GSH was associated with the increased peroxidation [38], while plasma GSH was reduced by 50% [36]. This was also found in chronic diabetes for longer periods [[39], [40]].
Isoflavones are a subclass of the more ubiquitous flavonoids. The primary isoflavones in soybeans are genistein and daidzein and their respective glycosides, genistin and daidzin [55].
In western countries, beans play only a minor dietary role despite the fact that they are low in fat and are excellent sources of protein, dietary fibers, and a variety of micronutrients and phytochemicals. Dry beans and soy foods offer benefits in the prevention of diabetes and in the clinical management of established diabetes [41]. Cereal fibers have long attracted attention in relation to the prevention of chronic diseases [42]. Whole grains and wheat germ are the richest dietary sources of α-Tocopherols [43].
Heat processing, enzymatic hydrolysis, and fermentation, can significantly alter the isomeric distribution of the three major isoflavones in soy and broadbean [[44], [45], [46]]. Germination is an alternative process for the improvement of protein quality of legumes. Although antioxidants supplementation may be of benefit to diabetic patients, it may be associated with risks i.e.: excessive antioxidant treatment may impair vascular function in small mesenteric arteries of STZ-diabetic rats [47]. For this reason, considerable attention has been focused on possible protective factors in the diet. We aim to assess the impact of naturally occurring phytochemicals after being processed by germination, heat-processing and drying, on the oxidative metabolism in AD rats.
2. Materials and methods
Seeds were prepared either by germination, heat treatment or drying. After soaking in water for 8 h [48], soybean was germinated for 3 days at 25 °C while broadbean for 2 days at 20 °C [49]. Heat treatment for soybean and broadbean was carried out in a water bath at 90 °C for 10 min[50]. Dried wheat was used directly without treatment. All seeds were then dehulled, dried at 50 °C, and ground into a fine powder. The six types of flours were conducted to proximate analysis of major neutrients (total proteins and fats). Table 3 shows the chemical constituents of the experimented seeds (g/100g). Total fat content was determined by Soxhelt method [51], protein content of dry base was determined by macro Kjeldahl method [52], by multiplying protein nitrogen (N) × 6.25 in case of soybean and broadbean, while (N) × 5.7 in case of wheat. Ash and fiber were determined according to [53]. The antioxidant composition of the experimental legumes and seeds is published [[46], [54], [55]]. Most of the isoflavones (99%) occurs as 7-0-monoglucosides, of which genistin, daidzin and glycitin accounts approximately for 64%, 23%, and 13% respectively [[56], [57]]. Song et al. [58] found that the total isoflavone content in soygerm equal 23201 μg/g. Also, they referred to that soybeans and soy products contain about 1–3 mg isoflavones/g protein. Whole grain cereals antioxidant concertation was compared with the standard antioxidant Trolox and was expressed as Trolox equivalents (as 1 mM) concentration [59]. Each 100 g of cereals were found to contain 2200–3500 Trolox equivalents. The most recently studied class is lignin.
Table 3.
The chemical constituents of the experimented seeds (g/100g).
| Seed type and treatment | Moisture | Protein | Fat | Carbohydrates (by difference) | Ash | Fiber |
|---|---|---|---|---|---|---|
| Heat-treated soy | 33.235 | 26.75 | 16.38 | 17.23 | 3.45 | 2.93 |
| Germinated soy | 58.32 | 16.7 | 10.22 | 10.77 | 2.16 | 1.83 |
| Heat-treated broadbean | 18.17 | 23.35 | 1.25 | 44.21 | 2.78 | 4.67 |
| Germinated broadbean | 62.75 | 10.63 | 0.63 | 22.44 | 1.41 | 2.13 |
| Germinated wheat | 49.2 | 7.19 | 1.06 | 41.33 | 0.03 | 1.19 |
| Dried wheat | 10.27 | 12.7 | 1.87 | 73.01 | 0.05 | 2.10 |
2.1. Experimental design
All protocols were approved by Ain Shams University at Cairo, Egypt. Adult male albino rats (Sprague-Dawely) weighing 200–250 g were used. Rats were housed individually in mesh bottom metallic cages and were fed a control “basal” diet, Table 1, for 1 week as an adaptation period. Antioxidants content in soy is in the oil portion. All diets contained 10% oil and 10% protein at the expense of starch. Diabetes was induced by intraperitoneal injection of freshly prepared alloxan monohydrate solution in saline at a dose level of 150 mg/kg body weight [60]. Five days after alloxan injection, blood samples were collected by amputation of the tail tip of all surviving animals and the blood glucose levels were determined using a Haemo-Gluco test and a glucometer (Boehringer Mannheim). Rats with blood sugar levels >200 mg/dl were considered diabetic and were employed in the study. Diabetic rats were then divided into 7 groups as shown in Table 2 . Body weight of all rats and food intake were recorded at weekly intervals to monitor the average body weight changes, Fig. 1(A).
Table 1.
Composition of control and experimental diets.
| Constituents | Diet | Experimental diets |
|||||
|---|---|---|---|---|---|---|---|
| (g/100 g diet) | Control dietb | 1 | 2 | 3 | 4 | 5 | 6 |
| Casein | 13 | – | – | – | – | – | – |
| Germinated soybeana | – | 60 | – | – | – | – | – |
| Heat-processed soybeana | – | – | 37 | – | – | – | – |
| Germinated broadbean | – | – | – | 94 | – | – | |
| Heat-processed broadbean | – | – | – | – | 43 | – | – |
| Germinated whole wheat | – | – | – | – | – | 139 | – |
| Dried whole wheat | – | – | – | – | – | – | 79 |
| Sunflower oil (ml) | 10 | 4 | 4 | 10 | 10 | 10 | 10 |
| Salt mixturec | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Vitamin mixtured | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cellulose | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Cornstarch | 67 | 26 | 49 | – | 37 | – | 1 |
Choline chloride (0.5 ml of 20% solution/100 g diet) was added to all diets. Cod liver oil to supply 2000 IU vitamin A, 200 IU vitamin D were added to all diets at the expense of sunflower oil.
All the ingredients of the vitamin mixture were purchaced from Sigma.
Soy flours used were full fat.
According to Campbell et al. (1963).
According to Hegsted et al. (1941).
According to AOAC. The Association of Official Analytical Chemists Incorporation. Arlington, Virginia, USA [53].
Table 2.
Experimental groups.
| G1 (7) | Normal control rats fed 10% protein (casein) | G5 (7) | Fed 10% protein (germinated broadbean) |
| G2 (7) | Diabetic control rats fed 10% protein (casein) | G6 (12) | Fed 10% protein (heat-processed broadbean) |
| G3 (7) | Fed 10% protein (germinated soybean) | G7 (6) | Fed 10% protein (germinated whole wheat) |
| G4 (8) | Fed 10% protein (heat-processed soybean) | G8 (6) | Fed 10% protein (dried whole wheat) |
G = Group. Number of rats per group is presented between parentheses.
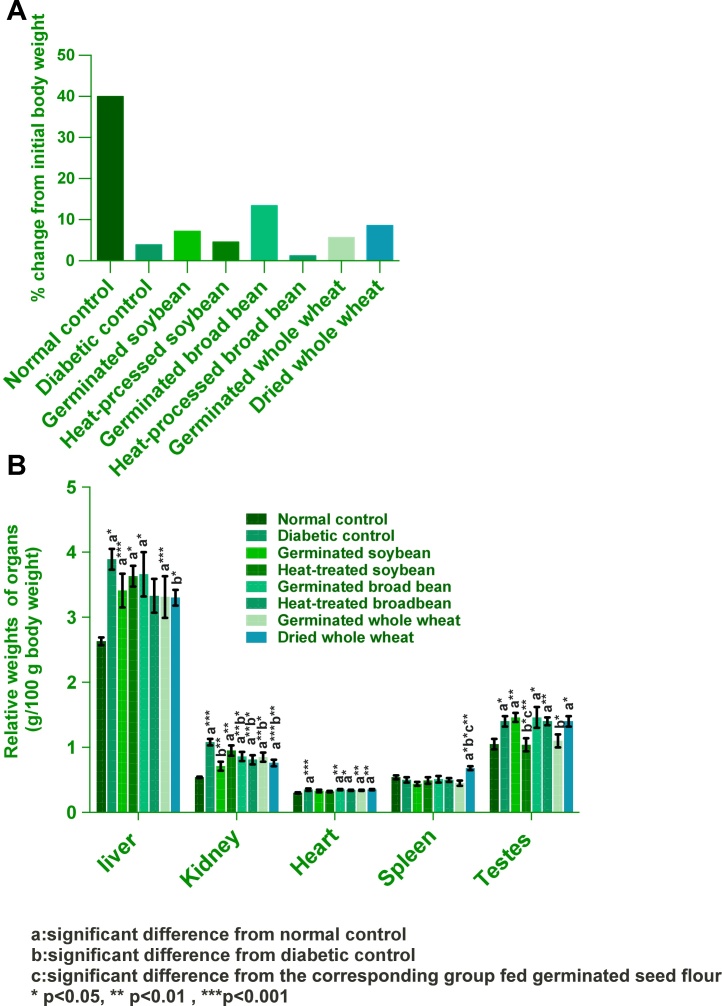
Fig. 1.
Effect of experimental diets on: A) body weight changes; B) relative organs weights (mean ± SEM).
2.2. Biochemical analysis
At the end of the experimental period, animals were fasted overnight, anesthetized with diethyl ether and sacrificed. Blood samples were collected from posterior vena cava in heparinized and non-heparinized tubes. Immediately after sacrificing, organs (liver, kidney, heart, spleen and testes) were plotted free of adhering blood, washed with cold saline, dried between filter papers and weighed. Plasma and serum were separated by centrifugation at 3000 r.p.m. for 15 mins. Fresh serum was used for the determination of serum glucose and protein thiol. Plasma aliquots were frozen at −10°С until further determinations of other parameters. After separation of plasma the red blood cells of 2 ml blood were washed twice with cold saline and an aliquot of packed cells were diluted with cold distilled water. An ethanol-chloroform extract was prepared for the assay of SOD activity as will be described later.
Non-heparinized blood was centrifuged and the separated serum was used for estimation of uric acid (UA), and protein −SH content after [[61], [62], [63]] respectively. Heparinized blood was centrifuged at 3000 r.p.m and plasma was used for α-T determination using High Pressure Liquid Chromatography (HPLC) after [64]. Erythrocytes Cu-Zn SOD was assayed in saline-washed red blood cells using the method described by [65]. Immediately after sacrificing rats, their livers were excised, plotted free of adhering blood and washed with cold saline. Liver tissue preparation: Three tiny weighed portions (≈1 g) were homogenized separatly. The first portion was homogenized in 1.15% KCl, the second in M/15 phosphate buffer pH 7, and the third portion in 3% ice-cold metaphosphoric acid to make 10% W/V liver homogenate which was used for the determination of MDA according to [66], CAT according to [67], and GSH according to [68]. Protein content of the liver was estimated by the method of [69]. Plasma was used for the determination of TAGs [70], HDL [71], LDL [72] and total cholesterol [73] levels using enzymatic kits provided by bioMérieux, France.
2.3. Determination of MDA in the liver homogenate
Lipohydroperoxides or MDA precursor reacts with thiobarbituric acid (TBA) in an acid medium containing phosphoric acid (at pH 2) to produce colored TBA complex that is measured colorimetrically from the difference in the readings at two wavelengths 535 and 520 nm, according to Uchiyama and Mihara [66].
2.4. Determination of fasting serum glucose
The pink color formed in the enzymatic determination of glucose depends on the reaction described by Trinder [61]. The kit was provided from bioMérieux, Sa, France. The absorbance of sample was read against the blank at 505 nm within 30 mins.
2.5. Determination of protein thiol in serum
Protein-SH groups react with 5,5′-dithiobis-(2-nitrobenzoic acid) [DTNB-Ellman's reagent] at pH 7.4 to give a colored product, 5-thio-2-nitrobenzoic acid (NBA), which is measured colorimetrically at 412 nm. The method was described by Koster et al. [63].
2.6. Determination of serum UA
The pink color formed in the enzymatic determination of UA depends on two step reactions: in the first step allantoin is formed by the effect of uricase on UA, and in the second step 3,5 dichloro-2-hydroxybenzene-sulfonic acid reacts with 4-aminoantipyrine in the presense of peroxidase to form chromogen. The absorbance of the latter is read against the blank at 520 nm within 30 mins., according to Artiss and Entwistle [62]. The kit was provided from bioMérieux, Sa, France.
2.7. Determination of GSH in the liver homogenate
Determination of reduced GSH and non-protein-SH groups is based on the development of stable yellow color when 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB) is added to sulfhydril compounds. This color is related to the amount of GSH. The method is described by Beutler et al. [68].
2.8. Determination of protein in the liver homogenate
The reduction of phosphomolybdic-phosphotungestic reagent by the copper-treated protein in alkaline medium at room temperature, results in the formation of blue color that is measured spectrophotometrically at 500 nm. The method was described by Lowry et al. [69].
2.9. Determination of CAT activity/g protein in the liver homogenate
The ultraviolet absorbtion of H2O2 solution is easily measured between 230 and 250 nm. On decomposition of H2O2 with CAT, the absorption decreases with time and from this decrease the enzyme activity can be calculated. This method can only be used with enzyme solutions which do not absorb strongly at 230–250 nm. The method was described by Maehly and Chance [67]. One unit of CAT activity is the amount of enzyme which liberates half the peroxide oxygen from an H2O2 solution of any concentration in 100 s at 25°С.
2.10. Determination of eSOD activity
The determination of eSOD activity is based on the ability of the enzyme to inhibit the reduction of nitroblue tetrazolium (NBT), (i.e. to inhibit formazan formation), by the superoxides generated in the reaction of photoreduced riboflavin and oxygen. The method was described by Winterbourn et al. [65].
2.11. Determination of plasma α-T by HPLC
The method was described by Bieri et al. [64]. Plasma was deprotinized with ethanol, and the lipid was extracted with hexane. After an aliquot of the solvent phase was evaporated, the residue was dissolved in ethanol. A portion of the solution was injected into a C18 reversed-phase chromatographic column, and the absorbance of the vitamins and standard were measured at 285 nm. α-T is quantified by applying the peak area ratio method.
2.11.1. Liquid chromatograph
Model 204 with model 6000-solvent delivery system, a U6K universal injector, and model 440-absorbance detector (all from Waters Associates, Inc., Milford, Massachusetts).
2.11.2. Chromatographic column
Reversed-phase U Bondapk C18
2.11.3. Standard curve and calculation
Constant amount of α-tocopheryl acetate was combined with variable amount of the corresponding alcohol form of the vitamin to give solutions with a three-fold range of weight ratios. These solutions were chromatographed and the peak-area ratios were recorded.
2.12. Determination of plasma TAGs concentration
The pink color formed in the enzymatic determination of TAGs depends on the reaction described by Fossati and Prencipe [70]. The kit was provided from bioMérieux, Sa, France. The absorbance of sample was read against the blank at 505 nm within 30 mins.
2.13. Determination of plasma total cholesterol
The pink color formed in the enzymatic determination of total cholesterol is based on the reaction described by Allain et al. [73]. The test kit was provided from bioMérieux, Sa, France. The absorbance of sample was read against the blank at 500 nm within 30 mins.
2.14. Determination of plasma HDL cholesterol
The chylomicrons and lipoproteins of very low density (VLDL) and low density (LDL) contained in the sample are precipitated by the addition of phosphotungestic acid in the presence of magnesium ions. The supernatant obtained after centrifugation contains HDL, from which cholesterol can be determined enzymatically as was described by Burstein et al. [71]. The absorbance of sample was read against the blank at 500 nm within 30 mins. The kit was provided from bioMérieux, Sa, France.
2.15. Calculation of plasma LDL cholesterol concentration
The method used for determining LDL involves an equation in which the VLDL is estimated from the TAGs concentration in plasma. The equation used was developed by Friedewald et al. [72] where the TAGs value is divided by 5 when measurements are in units of mg/dl to give VLDL. The LDL is then estimated by subtraction from total cholesterol concentration as follows:
| LDL − cholesterol (mg/dl) = Total cholesterol − (HDL − cholesterol + TAGs/5). |
2.16. Statistical analysis
Statistical analysis of data was accomplished using the Statistical Package for Social Sciences (SPSS) for Microsoft Windows release 8.0 statistical software package. The Bonferroni t-test was used for multiple comparison at a significance level of Alpha p < 0.05, p < 0.01, p < 0.001. All values are expressed as Mean ± Standard Error of the Mean (SEM).
3. Results
As compared with initial body weight, final body weight of normal control group was increased, while that of all diabetic control group was decreased, Fig. 1(A). Normal and diabetic rats did not score difference in food intake, Fig. 1(B).
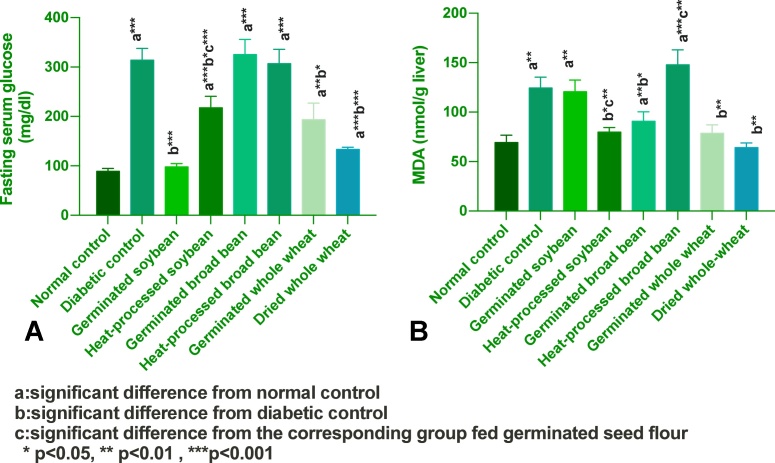
As shown in Fig. 2(A), germinated soybean induced the most predominant effect in normalizing fasting serum glucoe level of diabetic rats. Serum glucose levels of germinated soy or whole wheat-fed groups were significantly lower as compared to the diabetic control group. The elevated hepatic MDA of diabetic rats decreased significantly in groups fed heat-treated soybean, germinated broadbean and wheat-based diets compared to the diabetic control. The most favorable effect in reducing hepatic MDA levels was achived by whole-wheat and heat processed soy, Fig. 2(B).
Fig. 2.
Effect of experimental diets on: A) fasting serum glucose; B) hepatic MDA levels (mean ± SEM).
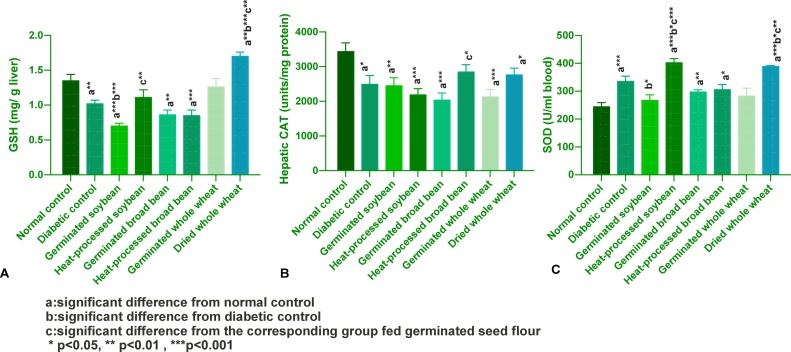
Whole wheat restored the diminshed hepatic GSH content of the diabetic control rats and most favorably by dried wheat, Fig. 3(A). The rest of the experimental diets induced similar reduction in hepatic GSH from the normal and diabetic controls. Hepatic CAT activity was significantly decreased in all diabetic groups as compared to the normal control group, Fig. 3(B). The tested diets have no significant effect on CAT activity compared to the diabetic control group. All the experimental diets restored eSOD levels to baseline values and even above such values Fig. 3(C). Heat-processed soybean and dried wheat induced the most favorable increase in eSOD compared to the diabetic control group.
Fig. 3.
Effect of experimental diets on: A) hepatic GSH levels; B) hepatic CAT; C) eSOD activities (mean ± SEM).
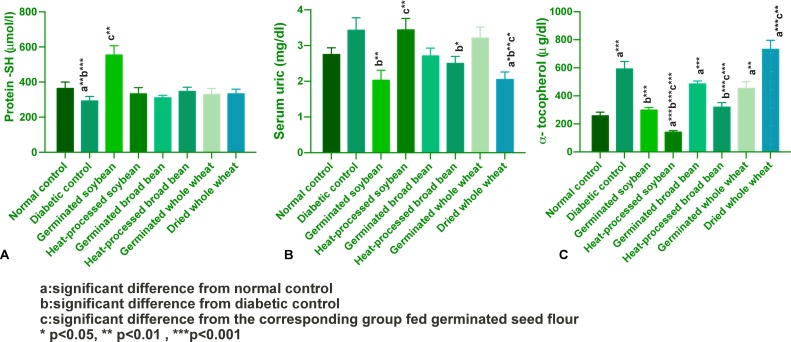
Fig. 4(A) showed that diabetes induced significant reduction in protein −SHs levels compared to normal control. Such reduction was restored to normal levels by all experimental diets. Our results showed that diabetes did not change serum UA levels from normal, Fig. 4(B). Germinated soybean, heat-processed broadbean and dried wheat-based diets reduced serum UA compared to the diabetic control significantly, whereas dried wheat normalized such level. Diabetes induced a significant elevation of plasma α-T levels compared to normal control, and the most predominant increase above the baseline value was induced by dried wheat. Such elevation was reduced significantly compared to diabetic control group by soybean and heat-treated broadbean.
Fig. 4.
Effect of experimental diets on: A) serum protein–SH; B) serum UA; C) plasma α-T (Mean ± SEM).
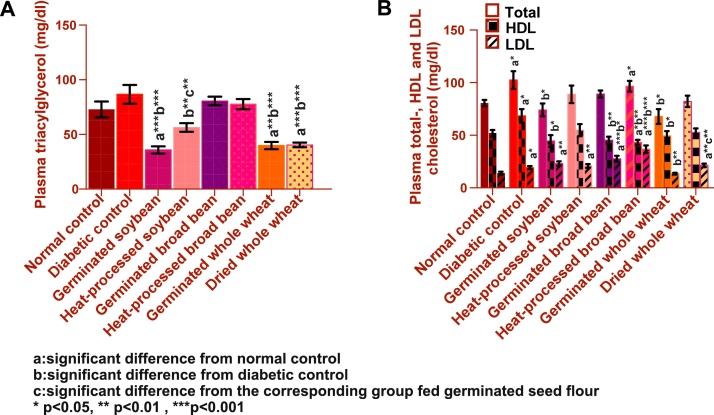
Diabetes did not change TAGs levels compared to normal, Fig. 5(A). Comparing to normal control, plasma total-, HDL- and LDL-cholesterol levels of diabetic control were significantly elevated by 27%, 32%, and 38% respectively, Fig. 5(B). This increase tended to decline significantly by the experimental diets compared to diabetic control.
Fig. 5.
Effect of experimental diets on: A) plasma TAGs; B) plasma total, HDL- and LDL-cholesterol of normal and diabetic rats (means ± SEM).
Soybeans and whole wheat had a strong lowering effect on TAGs and even normalized TAGs levels. Germinated soy caused the most favorable TAGs-lowering effect than heat-processed soy. Germinated and dried wheat induced similar effects on lowering TAGs. Broadbeans did not have such effect.
Soybeans and wheat also had a significant lowering effect on total cholesterol, but only if germinated. Significant reduction was observed in plasma total cholesterol in germinated soy group (by 28%) and germinated wheat group (by 34%) as compared to the diabetic control group.
The experimental diets did not improve plasma HDL-cholesterol compared to diabetic control rats, and induced HDL-cholesterol levels comparable to normal, except heat-processed broadbean that reduced HDL- significantly from both normal and diabetic control groups. Germinated wheat induced the most favorable effect in reducing LDL-cholesterol than the diabetic control group.
4. Discussion
We found that food intake did not change in diabetic and normal controls. Final body weights of diabetic rats were decreased, except for the group fed dried wheat as compared to their initial weights. This may result from impaired utilization of nutrients. The reduction in body weight of diabetic rats agrees with the findings of [[47], [74], [75]]. The liver, kidney, heart, and testes hypertrophied in most diabetic groups compared to normal control, and this was more evident in the kidney relative weights. Similar findings have been reported in several studies [[76], [77], [78]] which support the present data. This may be attributed to the polydipsia. Our data showed normalized kidney and liver relative weights of all experimental groups compared to the diabetic control group. Substituting soy protein for animal protein in diabetic patients diets was shown to be effective in reversing or slowing the progression of established kidney disease. This results in less hyperfiltration and glomerular hypertension and, therefore, resultant protection from diabetic nephropathy [79]. This may explain the noticed improvement in the kidney relative weights of animals fed germinated soybean, since thier serum glucose level was normalized. Limited evidence suggests that drybeans-induced protection may also have renal protective effects [80]. More investigation of this area is required.
Although measuring the antioxidant content would have been informative of the level at which ameliorative effects are achieved, we confirm that feeding the whole food reduced the diabetic hyperglycaemia in our study to variable levels and in a diet-dependent manner. The dietary intervention used in this study improved serum glucose of diabetic rats being more evident in germinated soybean and wheat, heat-processed soybean and dried wheat groups. The normalization of serum glucose level observed in diabetic rats fed germinated soybean diet is consistent with the findings of [[81], [82], [83], [84]]. In this study, a germinated soybean diet is more effective than boiled soybean in ameliorating serum glucose levels of diabetic rats. Whole wheat-based diets improved hyperglycaemia in the present study. This effect may be related to the wide range of nutrients and phytochemicals in the whole grain that may work synergistically to optimize animal health [85], and improve insulin sensitivity [86].
lipid peroxidation is a free radical-related process that in biologic systems may occure under enzymatic control, or non-enzymatically. This latter form is associated mostly with cellular damage as a result of oxidative stress [87]. The primary targets for attack by oxygen free radicals are the polyunsaturated fatty acids (PUFAs) of membrane phospholipids. However, attack of LDL-PUFAs should be also considered [33]. The reaction then proceeds through 3 stages as depicted in Slater [88].
The enzymes lipoxygenase, cyclooxygenase and peroxidase promote the controlled peroxidation of fatty acids to generate hydroperoxides and endoperoxides. Hydroperoxides degrade to various secondary products including hydroxy-fatty acids, epoxides and scission products such as aldehydes, including malondialdehydes, ketons, and lactones, many of which are toxic [89]. Other products are alkenals and hydroxy alkenals such as 4-hydroxynonenal [90] and hydrocarbon-expressing genes as pentane [91]. As well as 8-isoprostaglandin F2α [92]. The consequences of the peroxidation of membrane lipids include loss of PUFAs, decreased lipid fluidity, alterations in membrane permeability and membrane-associated enzymes, ion transport, substance release from the subcellular compartment and the generation of cytotoxic metabolites of lipid hydroperoxides [93].
MDA is a widely used marker of lipid peroxidation [94]. Kędziora-kornatowska et al. [95] reported that MDA content was significantly elevated in erythroctyes of type II diabetic patients versus the control group. In addition, lipid peroxidation data presented by TBA reactive substrates (TBARS) in plasma were found to be elevated by 80% in the early stages of diabetes in human, with time-dependent progressive increases [96].
The present data revealed alloxan-induced marked oxidative impact. The changes observed in the parameters measured in the liver are in accordance with other studies [[47], [96], [97], [98]]. In our study, the observed increment in hepatic MDA was normalized in dried wheat fed rats, and partially improved by heat-processed soybean, germinated broadbean and dried wheat. The reported effects might be due to the reduced hyperglycaemia-induced oxidative stress in such groups. Astuti [99] found that tempeh (a fermented soy product) was able to inhibit lipid peroxidation, suggested to be through the direct antioxidant effect of isoflavonoid in tempeh and through iron binding capability of isoflavonoids into chelate complexes, which then inhibit iron catalyzing effect in lipid peroxidation.
Nutritional status may infleunce tissue GSH levels. This could be a possible cause of the reduced GSH in some groups in our study as the experimental diets were difficient in the amino acid cysteine, the rate limiting amino acid for GSH synthesis. The present data also showed that hepatic GSH was restored by wheat flour-based diets. Similar improving effects on blood and tissue redox status were obtained after feeding bioactive polyphenolic compounds from winery wastes to broilers [100], also the antioxidative capacity was improved by feeding byproducts from olive mill wastwater to chickens [101], or to piglets [102].
Results of activities of antioxidant enzymes in the course of DM are equivocal and seem to depend on the duration and the control of diabetes [103]. In the present study, the inhibition of hepatic CAT activity in all diabetic rats versus healthy control could be attributed to hyperglycaemia-induced oxidative stress. Several studies support our data and demonstrate that alloxan-induced reduction in antioxidant enzymes activites during the course of type I DM in the liver, pancreas and testes [[97], [103], [104]].
Increased eSOD activity in diabetic control rats have been shown by this study and confirms others’ findings in animals in vivo [105] and in human [106]. The increased diabetic control eSOD activity reported in our study might be a manifestation of an adaptive response. The effect of tested diets on eSOD level was variable. As well, might be related to the increased absorption of zinc as reported by [[96], [107]]. Zbronska et al. [108] reported a close correlation between eSOD activity and its cofactors, both Cu and Zn. Riddoch et al. [109] reported that germination increases ascorbic acid content of seeds, and eventually may reduce oxidative stress-mediated eSOD increment in erythrocytes of diabetic animals. Sundaram et al. [96] suggested that the inactivation of eSOD by glycosylation may be a dominant factor in the loss of eSOD activity among diabetic patients due to the absence of protein synthesizing machinery in the erythrocytes.
The present study demonstrated no difference in plasma −SH groups between healthy and diabetic controls. This goes in accordance with Ceriello et al. [110] in his study on diabetic patients, and confilcted with [111] who demonstrated unaltered −SH level in STZ-diabetic rats, which could be attributed to the difference in the assay method. Another possible cause of the diminshed −SH groups in our study is the oxidized methionine levels that are likely to increase further with diabetes [22].
Plasma α-T level was significantly higher in diabetic control versus healthy control groups. The dietary intervention reduced the increased levels compared to the diabetic control. Our results agree with [[111], [112]] and contradict with [[110], [113]]. The discripancies among reports are likely to rely on the experimental conditions such as duration and stage of diabetes.
The significant elevation of plasma α-T reported in our diabetic control rats is consistent with the findings of [[74], [114], [105]]. The diminshed plasma α-T levels in most experimental rats could be due to improved lipid pattern, evidenced in our data, Fig. 5. In addition to the increased consumption of α-T in reducing the elevated oxidative stress as evidenced by reduced hepatic MDA marker in such groups.
Önning et al. [115] studied the effect of oatmilk, soymilk, and cow’s milk on plasma lipid, glucose, insulin and antioxidant status of 24 healthy men and women. Consumption of (0.75–1 l/day of oatmilk and soymilk for 4 weeks each) resulted in decreased total and plasma LDL-cholesterol with no effect on HDL-cholesterol. Also a higher glucose value was observed for women but lower for men consuming soymilk. Insulin values tended to increase when the subjects consumed soymilk compared to oat milk. However, no effect of soymilk on serum antioxidant status was observed.
In the present study, feeding whole wheat-based diets improved the deteriorated oxidative status of AD rats. This effect was manifested by marked decrease in hepatic MDA accompanied by an increase in hepatic GSH content, plasma α-T and eSOD levels. Such effect could be attributed to a reduction in hyperglycaemia-induced oxidative stress and/or the efficient antioxdative action of total carotenoids and tocopherol that exist in wheat grain and in the germ oil [116]. Others' findings support our suggestion [[117], [97]]. Whole wheat diets induced an increase in hepatic GSH content, which may enhance the GSH/GSSG ratio, decrease hepatic lipid peroxidation and improve serum glucose regulation.
The available studies indicate that soy isoflavones have antioxidant effects in vitro [79], as well as in vivo [118]. Those studies suggest that soy isoflavones are transported in LDL and act like vitamin E to inhibit in vivo oxidation. In this study, the antioxidative properties of soy isoflavones were apparent only in the group fed heat-processed soybean diet, since hepatic MDA was significantly decreased accompanied by alterations in the antioxidant measures. This is inconsistent with the results of [41], suggesting that the in vitro antioxidative effects of soy isoflavones are somewhat unclear.
Our findings reveals that diabetes induced the expected changes in the lipid profile while soybeans and wheat lowered TAGs and tended to lower total cholesterol. Germination enhanced the effect of soybeans on TAGs and in the case of soy and wheat, enhanced the effect on total cholesterol, Fig. 5. Although the effectiveness of whole wheat flour in digestive fermentation and lipid metabolism has not been established, rats fed the whole wheat flour diets had the fecal excretion of the total steroids markedly enhanced [119].
Several studies, as well as the present one, have shown that chronic sever DM in rats [[120], [121]] and in human [122] results in hyperlipidemia [123]. Our data shows that the elevated plasma total cholesterol was significantly decreased only in germinated soybean, germinated wheat or dried wheat fed groups. Similarly, plasma TAGs levels were significantly lowered even below the normal control level in groups fed soybean or whole wheat diets. The exact mechanism of the hypocholesterolemic effect of soy protein remains elusive and is almost certain to be multifactorial. Soybean protein has no cholesterol, it has the effect of increasing fecal bile acid excretion and altering bile acid synthesis rates, one of the primary mechanisms responsible for the regulation of cholesterol homeostasis, hepatic cholesterol secretion is also increased [124].
In our study, the observed effect of wheat on serum lipids is consistent with the findings of other authors [[119], [125]] who attributed this effect to the fiber and protein content of wheat.
Our results revealed no difference in UA between healthy and diabetic controls. Our findings agrees with [111] on STZ-diabetic rats and in diabetic patients [126]. Several studies have shown an association between hyperuricaemia and diabetes and suggested that UA increases insulin reistance [[82], [127]]. Our data showed that the tested diets-induced reduction in UA levels, which could be traced to decreased synthesis of UA due to the diminshed intake of purine nucleotides, or to the marked improvement in blood glucose by most of the experimental diets.
The present data also showed that a germinated broadbean diet induced partial improvement in the elevated hepatic MDA content of diabetic rats, associated with insignificant alterations in the other antioxidants measured. However, a heat-processed broadbean diet brought about significant decrease in UA and α-T levels. Unlike soybean, broadbean is poor in phytochemicals and its fat content averages only 1% by weight, with unsaturated fatty acids predominating [128]. Thus, the mildly ameliorative effects on the oxidative status of diabetic rats may be related to the various components present in the broadbean such as fibers, saponins and phytosterols.
5. Limitations
We were limited by the available resources of conducting a histopathological analysis of the oxidative stress markers in the studied tissues, of measuring the average water intake by animals and of measuring glucose tolerance, insulin tolerance and the exact lean and fat mass composition before and after serving the experimental diets. Including an insulin-treated control group would have addressed the glycemic effects in general, but would not have helped tracing such curative effect to soy proteins or to phytochemicals. The same applies to the other experimental diets we used in our study model. We could not determine glycosylated hemoglobin HbA1c, a diabetes marker, for financial concerns. Our extended current research will take such valuable comments into account.
6. Significance and conclusion
This study is of considerable implication. Consumption of soybean and whole wheat beneficially affect the antioxidative status and lipid pattern of AD rats. Based on such findings, we recommend that diabetic patients, beside controlling their hyperglycaemia with medications, may include whole foods containing naturally occurring phytochemicals to ameliorate their oxidative status.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Ain Shams University, Cairo, Egypt.
Consent for publication
Not applicable.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
Authors declare no competing interests.
Funding
Authors declare no honorarium, grant, or other form of payment was given to anyone to produce the manuscript. DJ supported the animal model, chemicals and materials, kits, publication and software charges of this work.
Author's contribution
All authors contributed to conception, design, acquisition of data, the analysis and writing the manuscript. The authors have seen and approved the manuscript being submitted. The authors affirm that the article is the authors original work and has not received prior publication and is not under consideration for publication elsewhere.
Conflict of interest
The authors declare no conflict of interest exists.
Contributor Information
Dina Johar, Email: umjohar@myumanitoba.ca, dinajohar@gmail.com.
Ahmed Maher, Email: ahmedmaher2020@gmail.com.
Omnia Aboelmagd, Email: omnia_aboelmagd@yahoo.com.
Ali Hammad, Email: ali.m.hamaad@students.kasralainy.edu.eg.
Mahmoud Morsi, Email: mah.atef93@gmail.com.
Hamdy F. Warda, Email: hfarag246@gmail.com.
Hamdy I. Awad, Email: hamdy.ibrahim50@gmail.com.
Taha A. Mohamed, Email: taha.a.mohamed@students.kasralainy.edu.eg.
Samy Zaky, Email: samyzs55@yahoo.com.
References
- 1.Shi Y., Hu F.B. The global implications of diabetes and cancer. Lancet. 2014;383(9933):1947–1948. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- 2.Melmed S., Polonsky K.S., Larsen P.R., Kronenberg H.M. 12 edn. Elsevier Saunders; Philadelphia: 2011. Williams Textbook of Endocrinology; pp. 1371–1435. [Google Scholar]
- 3.Vos T., Flaxman A.D., Naghavi M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baynes J.W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 5.Giugliano D., Ceriello A., Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19(3):257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A. Acute hyperglycaemia and oxidative stress generation. Diabetic Med. 1997;14(Suppl 3):S45–49. doi: 10.1002/(sici)1096-9136(199708)14:3+<s45::aid-dia444>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Kakkar R., Kalra J., Mantha S.V., Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell. Biochem. 1995;151(2):113–119. doi: 10.1007/BF01322333. [DOI] [PubMed] [Google Scholar]
- 8.Harris M.I. Definition and classification of diabetes mellitus and the new criteria for diagnosis. In: Le Roith D., Taylor S.I., Olefsky J.M., editors. Diabetes Mellitus: A Fundamental and Clinical Text. 2nd edn. Lippincott Williams and Wilkins; USA: 2000. pp. 326–327. [Google Scholar]
- 9.ADA. American Diabetes Association Expert Committee Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 10.Wolff S.P.J., Jiang Z.Y., Hunt J.V. Protein glycation and oxidative stress in diabetes mellitus and aging. Free Radic Biol. Med. 1991;10:339–352. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- 11.Eliziane N., Nestor A.S., Lydia M.F. Experimental model of induction of diabetes mellitus in rats. Acta Cir. Bras. 2003;(18 no) (spe São Paulo) [Google Scholar]
- 12.Ahrén R., Sundkvist G. Long term effects of aloxan in mice. Int. J. Pancreatol. 1995;2:197–201. doi: 10.1007/BF02788539. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya S.K. Activity of shilajit on aloxan induced hypoglicaemia in rats. Fitoterapia. 1995;116(4):328–332. [Google Scholar]
- 14.Oi K., Komori H., Kajimura H. Changes in plasma glucose, insulin, glucagon, cathecolmine, and glicogen contentes in tissue during development of alloxan diabetes in rats. Biochem. Mol. Med. 1997;62:70–75. doi: 10.1006/bmme.1997.2622. [DOI] [PubMed] [Google Scholar]
- 15.Dunn J.S., Mcletchie N. Experimental alloxan diabetes in the rat. Lancet. 1943;245:484–487. [Google Scholar]
- 16.Covington D.S., Xue H., Pizzini R., Lally K., Andrassy R.J. Streptozotocin and aloxan are comparable agents in the diabetic model of impaired wound healing. Diabetic Res. 1993;23:47–53. [PubMed] [Google Scholar]
- 17.Karpen C.W., Cataland S., O'Dorisio T.M., Panganamala R.V. Production of 12-hydroxyeicosatetraenoic acid and vitamin E status in platelets from type I human diabetic subjects. Diabetes. 1985;34(6):526–531. doi: 10.2337/diab.34.6.526. [DOI] [PubMed] [Google Scholar]
- 18.McLennan S., Yue D.K., Fisher E. Deficiency of ascorbic acid in experimental diabetes: relationship with collagen and polyol pathway abnormalities. Diabetes. 1988;37(3):359–361. doi: 10.2337/diab.37.3.359. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura N., Ookawara T., Suzuki K., Konishi K., Mino M., Taniguchi N. Increased glycated Cu,Zn-superoxide dismutase levels in erythrocytes of patients with insulin-dependent diabetis mellitus. J. Clin. Endocrinol. Metab. 1992;74(6):1352–1354. doi: 10.1210/jcem.74.6.1592880. [DOI] [PubMed] [Google Scholar]
- 20.Kashiwagi A., Asahina T., Ikebuchi M. Abnormal glutathione metabolism and increased cytotoxicity caused by H2O2 in human umbilical vein endothelial cells cultured in high glucose medium. Diabetologia. 1994;37(3):264–269. doi: 10.1007/BF00398053. [DOI] [PubMed] [Google Scholar]
- 21.Hunt J.V., Smith C.C., Wolff S.P. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39(11):1420–1424. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- 22.Bloomgarden Z.T. Antioxidants and diabetes. Diabetes Care. 1997;20(4):670–673. doi: 10.2337/diacare.20.4.670. [DOI] [PubMed] [Google Scholar]
- 23.Liu P.T., Ioannides C., Symons A.M., Parke D.V. Role of tissue glutathione in prevention of surgical trauma. Xenobiotica. 1993;23(8):899–911. doi: 10.3109/00498259309059417. [DOI] [PubMed] [Google Scholar]
- 24.Bray T.M., Ho E., Levy M.A. Glutathione, sulphur amino acids and chemical detoxication. In: Loannides C., editor. Nutrition and Chemical Toxicity. John Wiley & Sons; England: 2018. p. 183. [Google Scholar]
- 25.Orlowski M., Karkowsky A. Glutathione metabolism and some possible functions of glutathione in the nervous system. Int. Rev. Neurobiol. 1976;19:75–121. doi: 10.1016/s0074-7742(08)60702-3. [DOI] [PubMed] [Google Scholar]
- 26.Meister A. The biochemistry of glutathione. In: Taniguchi N., Higashi T., Sakamoto Y., Meister A., editors. Glutathione Centennial: Molecular Perspectives and Clinical Implications. Academic press; New York: 1989. pp. 3–22. [Google Scholar]
- 27.Staal F.J., Anderson M.T., Staal G.E., Herzenberg L.A., Gitler C. Redox regulation of signal trasnduction: tyrosine phosphorylation and calcium influx. Proc. Nat. Inst. Sci., U. S. A. 1994;91:3619–3622. doi: 10.1073/pnas.91.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grankvist K., Marklund S., Sehlin J., Taljedal I.B. Superoxide dismutase, catalase and scavengers of hydroxyl radical protect against the toxic action of alloxan on pancreatic islet cells in vitro. Biochem. J. 1979;182(1):17–25. doi: 10.1042/bj1820017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellomo G., Palladini G., Vairetti M. Intranuclear distribution, function and fate of glutathione and glutathione-S-conjugate in living rat hepatocytes studied by fluorescence microscopy. Microsc. Res. Tech. 1997;36(4):243–252. doi: 10.1002/(SICI)1097-0029(19970215)36:4<243::AID-JEMT3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 30.Habig W.H., Jakoby W.B. Assays for differentiation of glutathione-S-transferases. Methods Enzymol. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 31.Eklow L., Moldeus P., Orrenius S. Oxidation of glutathione during hydroperoxide metabolism A study using isolated hepatocytes and the glutathione reductase inhibitor 1,3-bis(2-chloroethyl)-1-nitrosourea. Eur. J. Biochem. 1984;138:459–463. doi: 10.1111/j.1432-1033.1984.tb07938.x. [DOI] [PubMed] [Google Scholar]
- 32.Parke D.V. Nutritional antioxidants and disease prevention: mechanisms of action. In: Basu T.K., Temple N.J., Garg M.L., editors. Antioxidants in Human Health and Disease. 1st edn. CABI; Wallingford, UK: 1999. p. 4. [Google Scholar]
- 33.Diplock A.T. Antioxidants and free radical scavengers. In: Rice-Evans C.A., Burdon R.H., editors. 1st edn. vol. 28. Elsevier; Netherlands: 1994. pp. 131–133. (New Comprehensive Biochemistry: Free Radical Damage and Its Control). [Google Scholar]
- 34.Gandy S.E., Buse M.G., Crouch R.K. Protective role of superoxide dismutase against diabetogenic drugs. J. Clin. Invest. 1982;70(3):650–658. doi: 10.1172/JCI110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaanane A., Labuza T.P. The Millard reaction in food. In: Baynes J.W., Monnier V.M., editors. The Millard Reaction in Aging, Diabetes and Nutrition. Liss; New York: 1989. [Google Scholar]
- 36.Zaltzberg H., Kanter Y., Aviram M., Levy Y. Increased plasma oxidizability and decreased erythrocyte and plasma antioxidative capacity in patients with NIDDM. Isr. Med. Assoc. J. 1999;1(4):228–231. [PubMed] [Google Scholar]
- 37.Collier A., Wilson R., Bradley H., Thomson J.A., Small M. Free radical activity in type 2 diabetes. Diabet. Med. 1990;7(1):27–30. doi: 10.1111/j.1464-5491.1990.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 38.Sundaram R.K., Bhaskar A., Vijayalingam S., Viswanathan M., Mohan R., Shanmugasundaram K.R. Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin. Sci. 1996;90:255–260. doi: 10.1042/cs0900255. [DOI] [PubMed] [Google Scholar]
- 39.Loven D.P.S., Wilson H., Daabees T.T., Stegnik I.D., Diekus M., Oberley L. Effect of insulin and oral glutathione on glutathione levels and superoxide dismutase activities in organs of rats with streptozotocin induced diabetes. Diabetes. 1986;35:403–407. doi: 10.2337/diab.35.5.503. [DOI] [PubMed] [Google Scholar]
- 40.Wohaieb S.A., Godin D.V. Alterations in free radical tissue defense mechanisms in streptozotocin induced diabetes in rat. Effects of insulin treatment. Diabetes. 1987;36:1014–1018. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 41.Anderson J.W., Gowri M.S., Turner J. Antioxidant supplementation effects on low-density lipoprotein oxidation for individuals with type 2 diabetes mellitus. J. Am. Coll. Nutr. 1999;18(5):451–461. doi: 10.1080/07315724.1999.10718883. [DOI] [PubMed] [Google Scholar]
- 42.Burkitt D.P., Walker A.R., Painter N.S. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972;2(7792):1408–1412. doi: 10.1016/s0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- 43.VERIS . Vitamin E Research & Information Service corporation; South Ninth Avenue, La Grange, Illinois, USA: 1993. Vitamin E Research Summary. [Google Scholar]
- 44.Dostálová J., Pokorný J. Plant phenols. In: Davídek J.J., editor. Natural Toxic Compounds of Foods. Formation and Change During Processing and Storage. CRC press; London: 1995. [Google Scholar]
- 45.Grzeskowiak B.B.E. Inhaltsstoffe von leguminosen und leguminosen produkten mit antioxidativen eigenschaften. Ernährung/Nutrition. 1986;10:291–296. [Google Scholar]
- 46.Wang H., Murphy P.A. Mass balance study of isoflavones during soybean processing. J. Agric. Food Chem. 1996;44:2377–2383. [Google Scholar]
- 47.Palmer A.M., Thomas C.R., Gopaul N. Dietary antioxidant supplementation reduces lipid peroxidation but impairs vascular function in small mesenteric arteries of the streptozotocin-diabetic rat. Diabetologia. 1998;41(2):148–156. doi: 10.1007/s001250050883. [DOI] [PubMed] [Google Scholar]
- 48.Jimenez M.J., Elias L.G., Bressani R., Navarrete D.A., Gomez-Brenes R., Molina M.R. Biochemical and nutritional studies of germinated soybean seeds. Arch. Latinoam. Nutr. 1985;35(3):480–490. [PubMed] [Google Scholar]
- 49.ISTA Proceeding of the international seed testing association. Seed Sci. Technol. 1986;13:153–157. [Google Scholar]
- 50.Anderson R.L., Wolf W. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J. Nutr. 1999;125(S):581–588. doi: 10.1093/jn/125.3_Suppl.581S. [DOI] [PubMed] [Google Scholar]
- 51.Soxhlet F. Die gewichtsanalytische Bestimmung des Milchfettes. Dingler's Polytechnisches J. 1879;232:461–465. (in German) [Google Scholar]
- 52.Kjeldahl J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern: New method for the determination of nitrogen in organic substances. Zeitschrift für analytische Chemie. 1883;22(1):366–383. [Google Scholar]
- 53.AOAC . In: Official Methods Of Analysis. 16th edn. Helrich K., editor. The Association of Official Analytical Chemists, INC.; Arlington, Virginia, USA: 1995. [Google Scholar]
- 54.Berghofer E., Grzeskowiak B., Mundigler N., Sentall W.B., Walcak J. Antioxidative properties of faba bean-, soybean- and oat tempeh. Int. J. Food Sci. Nutr. 1998;49:45–54. [Google Scholar]
- 55.Messina M.J. Legumes and soybeans: overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999;70(3 Suppl):439S–450S. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- 56.Hammerschmidt P.A., Pratt D.E. Phenolic antioxidants of dried soybeans. J. Food Sci. 1978;43:556–559. [Google Scholar]
- 57.Pratt D.E., Birac P.M. Source of antioxidant activity of soybeans and soy products. J. Food Sci. 1979;44:1720–1722. [Google Scholar]
- 58.Song H.K., Suh S.W. Kunitz-type soybean trypsin inhibitor revisited: refined structure of its complex with porcine trypsin reveals an insight into the interaction between a homologous inhibitor from Erythrina caffra and tissue-type plasminogen activator. J. Mol. Biol. 1998;275(2):347–363. doi: 10.1006/jmbi.1997.1469. [DOI] [PubMed] [Google Scholar]
- 59.Rice-Evans C.A., Miller N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996;24(3):790–795. doi: 10.1042/bst0240790. [DOI] [PubMed] [Google Scholar]
- 60.Lazarow A., Palay S.L. The production and course of alloxan diabetes in the rat. J. Lab. Clin. Med. 1946;31:1004–1015. [PubMed] [Google Scholar]
- 61.Trinder P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J. Clin. Pathol. 1969;22(2):246. doi: 10.1136/jcp.22.2.246-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Artiss J.D., Entwistle W.M. The application of a sensitive uricase-peroxidase couple reaction to a centrifugal fast analyser for the determination of uric acid. Clinica Chimica Acta. 1981;116(3):301–309. doi: 10.1016/0009-8981(81)90049-8. [DOI] [PubMed] [Google Scholar]
- 63.Koster J.F., Biemond P., Swaak A.J. Intracellular and extracellular sulphydryl levels in rheumatoid arthritis. Ann. Rheum. Dis. 1986;45(1):44–46. doi: 10.1136/ard.45.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bieri J.G., Tolliver T.J., Catignani G.L. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am. J. Clin. Nutr. 1979;32(10):2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 65.Winterbourn C.C., Hawkins R.E., Brian M., Carrell R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975;85(2):337–341. [PubMed] [Google Scholar]
- 66.Uchiyama M., Mihara M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 67.Maehly A.C., Chance B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- 68.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 69.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 70.Fossati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28(10):2077–2080. [PubMed] [Google Scholar]
- 71.Burstein M., Scholnick H.R., Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 1970;11(6):583–595. [PubMed] [Google Scholar]
- 72.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 73.Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 74.Sun F., Iwaguchi K., Shudo R. Change in tissue concentrations of lipid hydroperoxides, vitamin C and vitamin E in rats with streptozotocin-induced diabetes. Clin. Sci. 1999;96(2):185–190. [PubMed] [Google Scholar]
- 75.El-Sawalhy M. Faculty of Pharmacy, Cairo University; Cairo, Egypt: 2001. Role of Antioxidant Vitamins and Trace Elements in Ameliorating Certain Biochemical Changes in Erythrocytes and Kidney of Diabetic Rats. PhD Thesis. [Google Scholar]
- 76.Loven D., Schedl H., Wilson H. Effect of insulin and oral glutathione on glutathione levels and superoxide dismutase activities in organs of rats with streptozocin-induced diabetes. Diabetes. 1986;35(5):503–507. doi: 10.2337/diab.35.5.503. [DOI] [PubMed] [Google Scholar]
- 77.Wohaieb S.A., Godin D.V. Alterations in free radical tissue-defense mechanisms in streptozocin-induced diabetes in rat. Effects of insulin treatment. Diabetes. 1987;36(9):1014–1018. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 78.Chen V., Downing S.E. Amelioration of hyperlipidemia by low fat diets in chronically streptozotocin-diabetic rats. Life Sci. 1991;49(12):857–864. doi: 10.1016/0024-3205(91)90170-g. [DOI] [PubMed] [Google Scholar]
- 79.Anderson J.W., Blake J.E., Turner J., Smith B.M. Effects of soy protein on renal function and proteinuria in patients with type 2 diabetes. Am. J. Clin. Nutr. 1998;68(6 Suppl):1347S–1353S. doi: 10.1093/ajcn/68.6.1347S. [DOI] [PubMed] [Google Scholar]
- 80.Jibani M.M., Bloodworth L.L., Foden E., Griffiths K.D., Galpin O.P. Predominantly vegetarian diet in patients with incipient and early clinical diabetic nephropathy: effects on albumin excretion rate and nutritional status. Diabet. Med. 1991;8(10):949–953. doi: 10.1111/j.1464-5491.1991.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 81.Barakat M., Motawi T.M.K., El-Aser A.A., Osman A. vol. 27. Bull. Fac. Pharm.; Cairo Univ: 1989. pp. 1–5. (Effect of Soy Bean Feeding with and Without Zinc Supplement on Carbohydrate Metabolism in Alloxan-diabetic Rats). [Google Scholar]
- 82.Modan M., Halkin H., Karasik A., Lusky A. Elevated serum uric acid–a facet of hyperinsulinaemia. Diabetologia. 1987;30(9):713–718. doi: 10.1007/BF00296994. [DOI] [PubMed] [Google Scholar]
- 83.Jenkins D.J., Wolever T.M., Taylor R.H. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981;34(3):362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 84.Fujita H., Yamagami T., Ohshima K. Long-term ingestion of a fermented soybean-derived Touchi-extract with alpha-glucosidase inhibitory activity is safe and effective in humans with borderline and mild type-2 diabetes. J. Nutr. 2001;131(8):2105–2108. doi: 10.1093/jn/131.8.2105. [DOI] [PubMed] [Google Scholar]
- 85.Slavin J.L., Jacobs D., Marquart L. Grain processing and nutrition. Crit. Rev. Food Sci. Nutr. 2000;40(4):309–326. doi: 10.1080/10408690091189176. [DOI] [PubMed] [Google Scholar]
- 86.Pereira M.A., Jacobs D.R., Jr., Pins J.J. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am. J. Clin. Nutr. 2002;75(5):848–855. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- 87.Romero F.J., Bosch-Morell F., Romero M.J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998;106(Suppl 5):1229–1234. doi: 10.1289/ehp.98106s51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Slater T.F. Free-radical mechanisms in tissue injury. Biochem. J. 1984;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halliwell B., Betteridge D.J. Relationships between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetologia. 1997;40:647–653. doi: 10.1007/s001250050729. [DOI] [PubMed] [Google Scholar]
- 90.Esterbauer H. In: Free Radicals in Liver Injury. Poli G., Cheeseman K., Dianzani M.U., Slater T., editors. IRL press; Oxford: 1985. pp. 29–47. [Google Scholar]
- 91.Lemoyne M., Gossum A.V., Kurian R., Ostro M., Axler J., Jeejeebhoy K.N. Breath pentane analysis as an index of lipid peroxidation: a functional test of vitamin E status. Am. J. Clin. Nutr. 1987;46:267–272. doi: 10.1093/ajcn/46.2.267. [DOI] [PubMed] [Google Scholar]
- 92.Laight D.W., Desai K.M., Gopaul N.K., Anggard E.E., Carrier M.J. F2-isoprostane evidence of oxidant stress in the insulin resistant, obese Zucker rat: effects of vitamin E. Eur. J. Pharmacol. 1999;377(1):89–92. doi: 10.1016/s0014-2999(99)00407-0. [DOI] [PubMed] [Google Scholar]
- 93.Rice-Evans C.A. Formation of free radicals and mechanisms of action in normal biochemical processes and pathological states. In: Rice-Evans C.A., Burdon R.H., editors. 1st edn. vol. 28. Elsevier; Netherlands: 1994. pp. 131–151. (New Comprehensive Biochemistry: Free Radical Damage and Its Control). [Google Scholar]
- 94.Janero D.R. Malondialdehyde thioparbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 95.Kędziora-kornatowska K.Z., Luciak M., Blaszczyk J., Pawlak W. Lipid peroxidation and activities of antioxidant enzymes in erythrocytes of patients with non-insulin dependent diabetes with or without diabetic nephropathy. Nephrol. Dial. Transplant. 1998;13:2829–2832. doi: 10.1093/ndt/13.11.2829. [DOI] [PubMed] [Google Scholar]
- 96.Sundaram R.K., Bhaskar A., Vijayalingam S., Viswanathan M., Mohan R., Shanmugasundaram K.R. Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin. Sci. 1996;90(4):255–260. doi: 10.1042/cs0900255. [DOI] [PubMed] [Google Scholar]
- 97.Soto C.P., Perez B.L., Favari L.P., Reyes J.L. Prevention of alloxan-induced diabetes mellitus in the rat by silymarin comparative biochemistry and physiology Part C. Pharmacol., Toxicol. Endocrinol. 1998;119(2):125–129. doi: 10.1016/s0742-8413(97)00198-9. [DOI] [PubMed] [Google Scholar]
- 98.El-Missiry M.A., El-Gindy A.M. Amelioration of alloxan induced diabetes mellitus and oxidative stress in rats by oil of Eruca sativa seeds. Annals of Nutr. Metab. 2000;44(3):97–100. doi: 10.1159/000012829. [DOI] [PubMed] [Google Scholar]
- 99.Astuti M. The role of tempe on lipid profile and lipid peroxidation. Am. J. Clin. Nutr. 1998;68(suppl):1519–1523. [Google Scholar]
- 100.Makri S., Kafantaris I., Stagos D. Novel feed including bioactive compounds from winery wastes improved broilers' redox status in blood and tissues of vital organs. Food Chem. Toxicol. 2017;102:24–31. doi: 10.1016/j.fct.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 101.Gerasopoulos K., Stagos D., Petrotos K. Feed supplemented with polyphenolic byproduct from olive mill wastewater processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015;86:319–327. doi: 10.1016/j.fct.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Gerasopoulos K., Stagos D., Kokkas S. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015;82:42–49. doi: 10.1016/j.fct.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 103.Asayama K., Hayashibe H., Dobashi K., Niitsu T., Miyao A., Kato K. Antioxidant enzyme status and lipid peroxidation in various tissues of diabetic and starved rats. Diabetes Res. 1989;12(2):85–91. [PubMed] [Google Scholar]
- 104.El-Missiry M.A. Enhanced testicular antioxidant system by ascorbic acid in alloxan diabetic rats Comparative biochemistry and physiology Part C. Pharmacol., Toxicol. Endocrinol. 1999;124(3):233–237. doi: 10.1016/s0742-8413(99)00070-5. [DOI] [PubMed] [Google Scholar]
- 105.Kakkar R., Mantha S.V., Radhi J., Prasad K., Kalra J. Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin. Sci. 1998;94(6):623–632. doi: 10.1042/cs0940623. [DOI] [PubMed] [Google Scholar]
- 106.Godin D.V., Wohaieb S.A., Garnett M.E., Goumeniouk A.D. Antioxidant enzyme alterations in experimental and clinical diabetes. Mol. Cell. Biochem. 1988;84(2):223–231. doi: 10.1007/BF00421057. [DOI] [PubMed] [Google Scholar]
- 107.Craft N.E., Failla M.L. Zinc, iron, and copper absorption in the streptozotocin-diabetic rat. Am. J. Physiol. 1983;244(2):E122–128. doi: 10.1152/ajpendo.1983.244.2.E122. [DOI] [PubMed] [Google Scholar]
- 108.Zbronska H., Grzeszczak W., Jendryczko A., Zbronski R., Kuzniewicz R. Activity of superoxide dismutase in erythrocytes and leukocytes and levels of zinc and copper in blood of patients with diabetes. Effect of diabetic treatment on examined parameters. Pol. Arch. Med. Wewn. 1995;94(3):228–234. [PubMed] [Google Scholar]
- 109.Riddoch C.H., Mills C.F., Duthie G.G. An evaluation of germinating beans as a source of vitamin C in refugee foods. Eur. J. Clin. Nutr. 1998;52:115–118. doi: 10.1038/sj.ejcn.1600524. [DOI] [PubMed] [Google Scholar]
- 110.Ceriello A., Bortolotti N., Motz E. Meal-generated oxidative stress in type 2 diabetic patients. Diabetes Care. 1998;21(9):1529–1533. doi: 10.2337/diacare.21.9.1529. [DOI] [PubMed] [Google Scholar]
- 111.Asayama K., Nakane T., Uchida N., Hayashibe H., Dobashi K., Nakazawa S. Serum antioxidant status in streptozotocin-induced diabetic rat. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1994;26(7):313–315. doi: 10.1055/s-2007-1001693. [DOI] [PubMed] [Google Scholar]
- 112.Nourooz-Zadeh J., Tajaddini-Sarmadi J., McCarthy S., Betteridge D.J., Wolff S.P. Elevated levels of authentic plasma hydroperoxides in NIDDM. Diabetes. 1995;44(9):1054–1058. doi: 10.2337/diab.44.9.1054. [DOI] [PubMed] [Google Scholar]
- 113.Young I.S., Torney J.J., Trimble E.R. The effect of ascorbate supplementation on oxidative stress in the streptozotocin diabetic rat. Free Radical Biol. Med. 1992;13(1):41–46. doi: 10.1016/0891-5849(92)90164-c. [DOI] [PubMed] [Google Scholar]
- 114.Mooradian A.D., Morley J.E. Micronutrient status in diabetes mellitus. Am. J. Clin. Nutr. 1987;45(5):877–895. doi: 10.1093/ajcn/45.5.877. [DOI] [PubMed] [Google Scholar]
- 115.Onning G., Akesson B., Oste R., Lundquist I. Effects of consumption of oat milk, soya milk, or cow's milk on plasma lipids and antioxidative capacity in healthy subjects. Ann. Nutr. Metab. 1998;42(4):211–220. doi: 10.1159/000012736. [DOI] [PubMed] [Google Scholar]
- 116.Souci S., Fachmann H.J., Kraut A. Food composition and nutrition tables. Stuttgart Med. Pharm. Sci. Pub. 1999 [Google Scholar]
- 117.Krings U., Berger R.G. Antioxidant activity of some roasted foods. Food Chem. 2001;72:223–229. [Google Scholar]
- 118.Wiseman H., O'Reilly J.D., Adlercreutz H. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am. J. Clin. Nutr. 2000;72(2):395–400. doi: 10.1093/ajcn/72.2.395. [DOI] [PubMed] [Google Scholar]
- 119.Adam A., Levrat-Verny M.A., Lopez H.W., Leuillet M., Demigne C., Remesy C. Whole wheat and triticale flours with differing viscosities stimulate cecal fermentations and lower plasma and hepatic lipids in rats. J. Nutr. 2001;131(6):1770–1776. doi: 10.1093/jn/131.6.1770. [DOI] [PubMed] [Google Scholar]
- 120.Chen V., Ianuzzo C.D. Metabolic alterations in skeletal muscle of chronically streptozotocin-diabetic rats. Arch. Biochem. Biophys. 1982;217(1):131–138. doi: 10.1016/0003-9861(82)90486-6. [DOI] [PubMed] [Google Scholar]
- 121.Friedman M.I., Ramirez I., Edens N.K., Granneman J. Food intake in diabetic rats: isolation of primary metabolic effects of fat feeding. Am. J. Physiol. 1985;249(1 Pt 2):R44–51. doi: 10.1152/ajpregu.1985.249.1.R44. [DOI] [PubMed] [Google Scholar]
- 122.Kesavulu M.M., Giri R., Kameswara Rao B., Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 2000;26(5):387–392. [PubMed] [Google Scholar]
- 123.Popper D.A., Shiau Y.F., Reed M. Role of small intestine in pathogenesis of hyperlipidemia in diabetic rats. Am. J. Physiol. 1985;249(2 Pt 1):G161–167. doi: 10.1152/ajpgi.1985.249.2.G161. [DOI] [PubMed] [Google Scholar]
- 124.Duane W.C. Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J. Lipid Res. 1997;38(6):1120–1128. [PubMed] [Google Scholar]
- 125.Vuksan V., Jenkins D.J., Vidgen E. A novel source of wheat fiber and protein: effects on fecal bulk and serum lipids. Am. J. Clin. Nutr. 1999;69(2):226–230. doi: 10.1093/ajcn/69.2.226. [DOI] [PubMed] [Google Scholar]
- 126.Vucic M., Rocic B., Bozikov V., Ashcroft S.J. Plasma uric acid and total antioxidant status in patients with diabetes mellitus. Hormone Metab. Res.=Hormon- und Stoffwechselforschung=Hormones et metabolisme. 1997;29(7):355–357. [PubMed] [Google Scholar]
- 127.Tuomilehto J., Zimmet P., Wolf E., Taylor R., Ram P., King H. Plasma uric acid level and its association with diabetes mellitus and some biologic parameters in a biracial population of Fiji. Am. J. Epidemiol. 1988;127(2):321–336. doi: 10.1093/oxfordjournals.aje.a114807. [DOI] [PubMed] [Google Scholar]
- 128.Haytowitz D., Matthews R. US Department of Agriculture; Washington: 1986. Legumes and Legume Products; pp. 1–156. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.