Graphical abstract
Αbbreviations: DPPH•, 2,2-diphenyl-1-picrylhydrazyl; ABTS•+, 2,2΄-Azino-bis-(3-ethyl-benzthiazoline-sulphonic acid; ROS, reactive oxygen species; GSH, glutathione; TBARS, thiobarbituric acid-reactive substances
Keywords: Goji berry, Antioxidant, Polyphenols, Muscle cells, Glutathione, Lipid peroxidation, Protein oxidation
Highlights
-
•
Goji bery extracts scavenged at low concentrations free radicals.
-
•
Extracts protected at low concentrations peroxyl radical-induced DNA damage.
-
•
Extracts increased GSH levels in C2C12 muscle cells.
-
•
Extracts decreased lipid peroxidation and protein oxidation in C2C12 muscle cells.
Abstract
The aim of this study was to assess the antioxidant and antimutagenic activities of ultrasound assisted aqueous extracts from dry goji berry fruits cultivated in Greece. The extracts’ free radical scavenging activity was assessed by the DPPH• and ABTS•+ assays. The results from both assays demonstrated that the extracts exhibited strong radical scavenging activity with IC50 values ranging from 1.29 to 3.00 mg/ml for DPPH• and from 0.39 to 1.10 mg/mL for ABTS•+ assay. The investigated extracts also inhibited free radical-induced DNA damage induced by peroxyl (ROO•) radicals with IC50 ranging from 0.69 to 6.90 mg/mL. Τhe antioxidant activity of the goji berry extract exhibited the highest potency in the above assays was also examined in muscle cells. In particular, muscle C2C12 cells were treated with the selected extract at non cytotoxic concentrations for 24 h and four oxidative stress markers were measured: total reactive oxygen species (ROS), glutathione (GSH), lipid peroxidation and protein carbonyl levels. The results showed that the extract at 25 and 100 μg/mL increased GSH levels up to 189.5% and decreased lipid peroxidation and protein carbonyls by 21.8 and 29.1% respectively. The present study was the first on the antioxidant effects of ultrasound assisted aqueous extracts from goji berry fruits in muscle cells.
1. Introduction
The production of reactive oxygen species (ROS) occurs physiologically in living organisms. ROS are useful molecules at low concentrations, since they regulate growth, differentiation, proliferation, and apoptosis. However, when there is an excess production of ROS, a pathological condition called oxidative stress, then several diseases (e.g. cardiovascular, cancer and neurodegenerative) may be caused [[1], [2], [3]].
One of the tissues that are especially susceptible to oxidative stress is skeletal muscle [4]. In skeletal, muscle, overproduction of ROS may occur even under physiological processes such as exercise [5]. During exercise there is a high rate of O2 consumption in skeletal muscles, that may cause incomplete O2 reduction and electron leakage from the electron transfer chain, leading to the generation of ROS and oxidative stress [5]. In turn, oxidative stress results in cell damage and muscle fatigue [6]. Thus, antioxidant supplementation has been suggested for counteracting oxidative stress-induced damage to skeletal muscles [7].
Moreover, it is well documented that several traditional herb and plant extracts have antioxidant properties and are potential candidates for the prevention and treatment of ROS-induced diseases [[8], [9], [10]]. One of those herbs is goji berry (Lycium barbarum), whose extracts have been shown to protect from damage caused by ROS [11]. For example, goji berry’s extracts have been reported to prevent oxidative stress-induced disorders (e.g. neurological) and pathological conditions (e.g. aging) [12].
Both in vitro and in vivo studies have provided evidence for these antioxidant effects of goji berry’s extracts. For instance, the antioxidant capacity of L. barbarum polysaccharides (LBPs) has been demonstrated by in vitro methods including superoxide, DPPH• and ABTS•+ radical scavenging activity and reducing power [13]. In addition, other studies with rats and mice have reported that LBPs protected from DNA damage and inhibited lipid peroxidation and damage in hepatic and renal tissues caused by chronic hyperglycemia-induced oxidative stress in a high-fat diet [14,15]. Moreover, LBPs protected against oxidative damage in skeletal muscle, caused by exhaustive exercise [16]. Apart from polysaccharides, other antioxidant compounds found in goji berry are carotenoids (e.g. zeaxanthin) and polyphenols. It is believed that the antioxidant properties of the polysaccharides are likely attributed to low molecular weight phenolic substances that bind to them during the extraction process [17].
Most studies on goji berry’s extracts have used plants from cultivations in China, which is the main supplier of this fruit. In the present study, we used extracts from L. barbarum and L. chinensis dry fruits from Greek cultivations and investigated their antioxidant and antimutagenic activity using molecular and cell culture methods. It is known that the climatic and soil conditions may affect the chemical composition of a plant, and so the bioactivity of its different parts [18,19]. The extracts were produced by an ultrasound assisted extraction process using water as solvent, optimized for giving extracts with maximum antioxidant activity as we have described previously [20].
2. Materials and methods
2.1. Chemicals
Folin Ciocalteu, sodium carbonate, gallic acid, ethanol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), methanol, 2,2΄-Azino-bis-(3-ethyl-benzthiazoline-sulphonic acid) (ABTS), horseradish peroxidase enzyme (HRP), hydrogen peroxide (H2O2), 2,7-dichlorofluorescein diacatete (DCF-DA) and mercury orange, were purchased from Sigma Aldrich (St Louis, MO, USA). Dulbescco’s modified Eagle medium (DMED), fetal bovine serum (FBS) and phosphate buffered saline (PBS) of analytical grade were purchased from Gibco (UK). Cell proliferation kit II (XTT) was purchased from Roche Diagnostics (Mannheim, Germany).
2.2. Goji berry fruits
Goji berry fruits from L. barbarum and L. chinensis were collected from a 3 year old experimental plantation, located in the region of Thessaly, and they were subsequently sun-dried. The moisture content of all samples was measured after fruits were drying to constant weight at 105 °C following the AOAC 935.29 official method. The samples were then stored under refrigeration (0–4 °C) for further analysis.
2.3. Extraction process
To optimize the extraction process the extraction conditions were varied as previously described [20]. In particular, dried whole fruits of goji berry were frozen by liquid nitrogen and were pulverized in mortar. From each sample, 2.5 g were extracted with distilled water (using 50, 75, 93, 99 or 100 mL of water,) in a 250 mL glass beaker. The samples were preheated to the selected experimental temperature (45, 55, 57 or 65 °C), and then they were introduced to the ultrasound extraction device (Hielscher UP400 S). The ultrasound power was set at 138.0, 220.0, 253.0, 360.0, 366.7 or 368.0 W/cm2, while the extraction time was set at 23, 30, 33.4, 35 or 40 min, according to the chosen experimental conditions. The obtained extracts were centrifuged at 12,000 rpm for 15 min and the supernatant was collected and kept in a freezer (−20 °C) until further use.
2.4. Determination of the total carbohydrate content of the extracts
Phenol/sulfuric method was used for the determination of total carbohydrates and was carried out according to Dubois et al. [21]. Briefly, 1 mL of each extract was mixed with 0.5 mL of 4% phenol and 2.5 mL of 95% sulfuric acid, and after 10 min incubation the optical density was measured at 490 nm. The total carbohydrate content was calculated on the basis of a calibration curve of D-glucose (concentration range: 0.01–0.1 mg/L; R2 = 0.991) and were expressed as g of carbohydrates/L of extract. The assay was repeated three times.
2.5. Determination of the total polyphenolic content (TPC) of the extracts
The method of Singleton et al. [22] modified by Waterhouse [23] was used to determine the TPC. Briefly, 20 μL of each extract was mixed with 1.58 mL water, and then with 100 μL of Folin- Ciocalteu reagent (0.2 N). Subsequently, 300 μL of Na2CO3 solution (200 g/L) was added and after 120 min incubation in dark, the optical density was measured at 765 nm. TPC was calculated on the basis of a calibration curve of gallic acid (concentration range: 50–500 mg/L, R2 = 0.991) and expressed as gallic acid equivalents (GAE)/mg of dried extract. The assay was repeated three times.
2.6. DPPH• radical scavenging activity assay
The DPPH• radical scavenging activity of extracts was assessed as described previously by Spanou et al., [24]. Briefly, 1 mL of freshly made methanolic solution of DPPH• radical (100 μM) was mixed with the tested goji berry extracts dissolved in distilled water at different concentrations and after 20 min incubation in dark, the optical density was measured at 517 nm. The percentage of radical scavenging capacity (RSC) of the tested extracts was calculated according to the following equation:
| % DPPH• radical scavenging activity = [(Αbscontrol − Abssample)/Abscontrol] Χ 100 |
where Abs control and Abs sample are the absorbance values of the control and the tested sample respectively. Moreover, in order to compare the radical scavenging efficiency of the extracts, the IC50 value showing the concentration caused 50% scavenging of DPPH• radical was estimated. All measurements were carried out in triplicate and at least in two independent experiments.
2.7. ABTS•+ radical scavenging activity assay
ABTS•+ radical scavenging activity was measured as described by Kerasioti et al., [25]. The RSC percentage of the ABTS•+ radical scavenging activity and the IC50 values were determined as described above for the DPPH• method. All measurements were carried out in triplicate and at least in two independent experiments.
2.8. Peroxyl radical-induced DNA plasmid strand cleavage
The assay was performed as described previously by Priftis et al., [26]. The preventive activity of the tested extracts against peroxyl radical-induced DNA strand breakage was based on the inhibition of the conversion of supercoiled form to the open-circular. The analysis was performed using an AlphaImager EC photodocumentation system and the amounts of supercoiled and open-circular forms were analyzed with the Alpha View software (AlphaInnotech, CA, USA).
The percentage inhibition was calculated using the following formula:
| % inhibition = [(S − So)/(Scontrol − So)] × 100 |
where Scontrol is the percentage of supercoiled DNA of the negative control sample (plasmid DNA alone), So is the percentage of super-coiled plasmid DNA of the positive control sample (without tested extracts but in the presence of the radical initiating factor), and S is the percentage of supercoiled plasmid DNA of the sample with the tested extracts and the radical initiating factor. Each experiment was carried out three times.
2.9. Total solid assay
Total solids of extract no. 5 were measured according to the method of Symons and Morey [27]. The extract was well-mixed and evaporated in a weighed dish and dried to constant weight in an oven at 103–105 °C.
The calculation of the total solid was performed using the following formula:
| mg of total solids/L = (A − B)×1000/sample volume in mL |
where: A is the weight of dried residue plus the weight of the dish in mg, and B is the weight of the dish in mg.
2.10. Cell culture
C2C12 muscle cells were cultured in normal Dulbecco’s modified Eagle’s medium (DMEM, Gibko, UK), containing 10% (v/v) fetal bovine serum, 2 mM L-glutamine (Gibko, UK), 100 units/ml of penicillin, and 100 units/ml of streptomycin (Gibko, UK) in plastic disposable tissue culture flasks at 37 °C in 5% CO2.
2.11. Cell viability assay
Cell viability was assessed using the XTT assay kit (Roche, Germany), as described by Goutzourelas et al. [1]. All experiments were carried out in triplicate and at least on two separate occasions.
2.12. Assessment of GSH and ROS levels by flow cytometry
The levels of GSH and ROS in C2C12 cells were assessed using mercury orange and 2,7-dichlorofluorescein diacetate (DCF-DA), respectively. The flow cytometry methodology was made as described by Goutzourelas et al., [1]. The fluorescent of mercury orange binds directly to GSH. For ROS assessment, the fluorescent of DCF (produced by esterases via the diacetylation of DCF-DA and ROS presentation) was estimated. The cells were analyzed at a flow rate of 1000 events/sec. Analyses were performed on 10,000 cells per sample, and the fluorescence intensities were measured on a logarithmic scale. Data were analyzed using BD Cell Quest software (Becton-Dickinson). Each experiment was repeated at least three times.
2.13. Assessment of TBARS levels by spectrophotometry
A spectrophotometric assay was performed as described previously by Kerasioti et al., for the determination of TBARS [25]. The assay requires a minimum of 30 μg of protein in the test sample (protein determination was performed using Bradford assay). TBARS were expressed as equivalents of malondialdehyde (MDA) and measured at 530 nm. Calculation of TBARS concentration was based on the molar extinction coefficient of malondialdehyde. Each experiment was repeated at least three times.
2.14. Assessment of protein carbonyl levels
Protein carbonyl determination was based on the spectrophotometric method of Patsoukis et al., [28]. According to this assay, the formation of carbonyls is detected by their reaction with DNPH (2,4-dinitriphenylhydrazine) to form 2,4-dinitrophenylhydrazone measured at 375 nm. The assay requires 30 μg of protein in the test sample (protein determination was performed using Bradford assay). Each experiment was repeated at least three times.
2.15. Statistical analysis
The optimal extraction parameters were determined by mathematical model based on Box Behnken designed experiment as described previously [20]. Data from all methods were analyzed by one-way ANOVA followed by Tukey's test for post-hoc analysis. The level of statistical significance was set at p < .05. For all statistical analyses, SPSS software, version 13.0 (SPSS Inc., Chicago, IL, USA) was used. Data are presented as the means ± SEM.
3. Results
3.1. Polyphenolic and carbohydrate content of goji berry extracts
In Table 1, TPC and total carbohydrate content of the goji berry extracts are presented. TPC ranged from 234.3 to 394.3 mg GAE/L of extract (Table 1). The highest level of polyphenols was found in extract 5 from L. barbarum variety (Table 1). The highest value of TPC in L. chinensis variety was 371.3 mg GAE/L of extract 9 (Table 1).
Table 1.
Extraction parameters, TPC and carbohydrate content of each extract as well as their antioxidant activity as assessed by DPPH, and ABTS•+ assays and ROO•− −induced DNA plasmid breakage.
| Samples | Variety | Ratio of water to dry goji berry (ml/gr) | Extraction temperature (°C) | Ultrasonic power (W/cm2) | Extraction time (min) | Total carbohydrate (g/L of extract) | TPCa(mg GAE/L) | DPPH•b | ABTS•+b | ROO•b |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L. barbarum | 30.0 | 55.0 | 138.0 | 40.0 | 0.958 | 272.3 | 2.33 ± 0.03 | 0.67 ± 0.01 | 1.80 ± 0.05 |
| 2 | L. barbarum | 40.0 | 45.0 | 253.0 | 30.0 | 1.155 | 296.3 | 2.10 ± 0.02 | 0.85 ± 0.02 | 1.90 ± 0.01 |
| 3 | L. barbarum | 39.4 | 50.0 | 335.7 | 33.4 | 1.201 | 323.3 | 1.90 ± 0.01 | 0.75 ± 0.03 | 4.60 ± 0.05 |
| 4 | L. barbarum | 40.0 | 65.0 | 138.0 | 30.0 | 1.079 | 234.3 | 2.15 ± 0.02 | 0.77 ± 0.01 | 4.30 ± 0.04 |
| 5 | L. barbarum | 20.0 | 57.0 | 220.0 | 23.0 | 1.191 | 394.3 | 1.45 ± 0.02 | 1.10 ± 0.04 | 0.69 ± 0.02 |
| 6 | L. barbarum | 20.0 | 45.0 | 360.0 | 35.0 | 1.181 | 351.3 | 1.29 ± 0.01 | 0.42 ± 0.03 | 1.75 ± 0.03 |
| 7 | L. barbarum | 30.0 | 65.0 | 368.0 | 30.0 | 1.010 | 303,3 | 2.80 ± 0.04 | 1.05 ± 0.04 | 1.40 ± 0.03 |
| 8 | L. barbarum | 36.3 | 56.4 | 224.4 | 22.9 | 1.054 | 283.3 | 3.00 ± 0.03 | 0.65 ± 0.02 | 6.90 ± 0.04 |
| 9 | L. chinensis | 20.0 | 57.0 | 220.0 | 23.0 | 0.941 | 371.3 | 2.20 ±0.04 | 0.64 ± 0.02 | 2.40 ± 0.03 |
| 10 | L. chinensis | 20.0 | 45.0 | 360.0 | 35.0 | 0.916 | 297.3 | 1.70 ± 0.03 | 0.39 ± 0.01 | 1.95 ± 0.02 |
TPC: total polyphenolic content.
IC50 values (mg/ml) shown as mean ± SD of at least three independent experiments.
The total carbohydrate content of the extracts ranged from 0.916 to 1.201 g/L. The highest carbohydrate content was exhibited by extract 3 from L. barbarum variety, while extract 9 had the highest value (0.941 mg GAE/L) from L. chinensis extracts (Table 1).
3.2. Assessment of the free radical scavenging activity using the DPPH•and ABTS•+assays
All the extracts exhibited scavenging activity against DPPH• and ABTS•+ radicals. In DPPH assay, the IC50 values ranged from 1.29 to 3.00 mg/ml. The highest antioxidant activity was shown by the extract 6 of L. barbarum variety, while the extract 8 (L. barbarum) had the lowest activity (Table 1). Extract 10 was the most potent extract from L. chinensis variety with IC50 1.70 mg/ml (Table 1).
In ABTS•+ assay, the most potent activity was exhibited by extract 10 from L. chinensis variety with IC50 0.39 mg/ml and the lowest activity by extract 5 from L. barbarum variety with IC50 1.10 mg/ml (Table 1). Among L. barbarum extracts, no. 6 exhibited the highest antioxidant activity with IC50 0.42 mg/ml (Table 1).
3.3. Protective activity against free radical-induced DNA damage
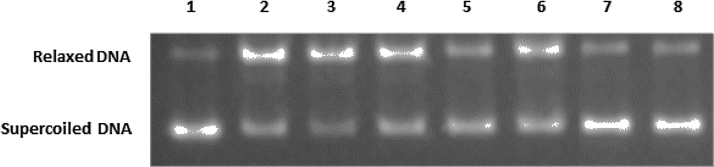
All the extracts protected from ROO•-induced DNA damage with IC50 values ranging from 0.69 to 6.90 mg/ml (Table 1). The most potent was the extract 5 (L. barbarum variety), while the extract 8 (L. barbarum variety) had the lowest activity (Fig. 1). The most potent from L. chinensis extracts was the 10 with IC50 1.95 mg/ml (Table 1).
Fig. 1.
Representative photo of the protective activity of extract 5 against ROO•- induced DNA damage. Lane 1, plasmid DNA without any treatment; lane 2, plasmid DNA exposed to AAPH; lanes 3–7, plasmid DNA exposed to AAPH in the presence of 0.06, 0.12, 0.24, 0.48 and 1.92 mg/ml of extract respectively; lane 8, plasmid DNA exposed to 1.92 mg/ml of extract alone.
3.4. Effects of goji berry extract on viability of C2C12 cells
The extract 5 from L. barbarum variety exhibited on average the highest antioxidant activity in free radical scavenging assays and ROO•-induced DNA damage. Thus, it was selected for examining its antioxidant effects in C2C12 muscle cells. However, at first, extract’s effect on cell viability was assessed using the XTT assay, in order to use non-cytotoxic concentrations.
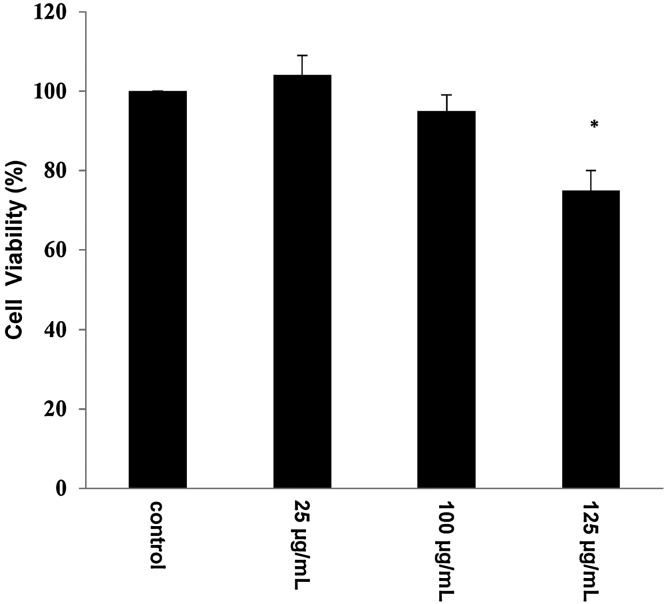
The results showed that the extract had cytotoxicity at concentration >125 μg/ml (Fig. 2). Thus, the non-cytotoxic concentrations used for the examination of the extract’s antioxidant activity were 25 and 100 μg/ml.
Fig. 2.
Cell viability of C2C12 cells after treatment with the goji berry extract. The results represent the mean ± SEM of three independent experiments carried out in triplicate. *p < .05: significantly different from the control value.
3.5. Antioxidant effects of goji berry extract in C2C12 muscle cells
For assessing the antioxidant effects of extract 5 in C2C12 cells, four oxidative stress markers were used, ROS, GSH, TBARS and CARB levels (Fig. 3).
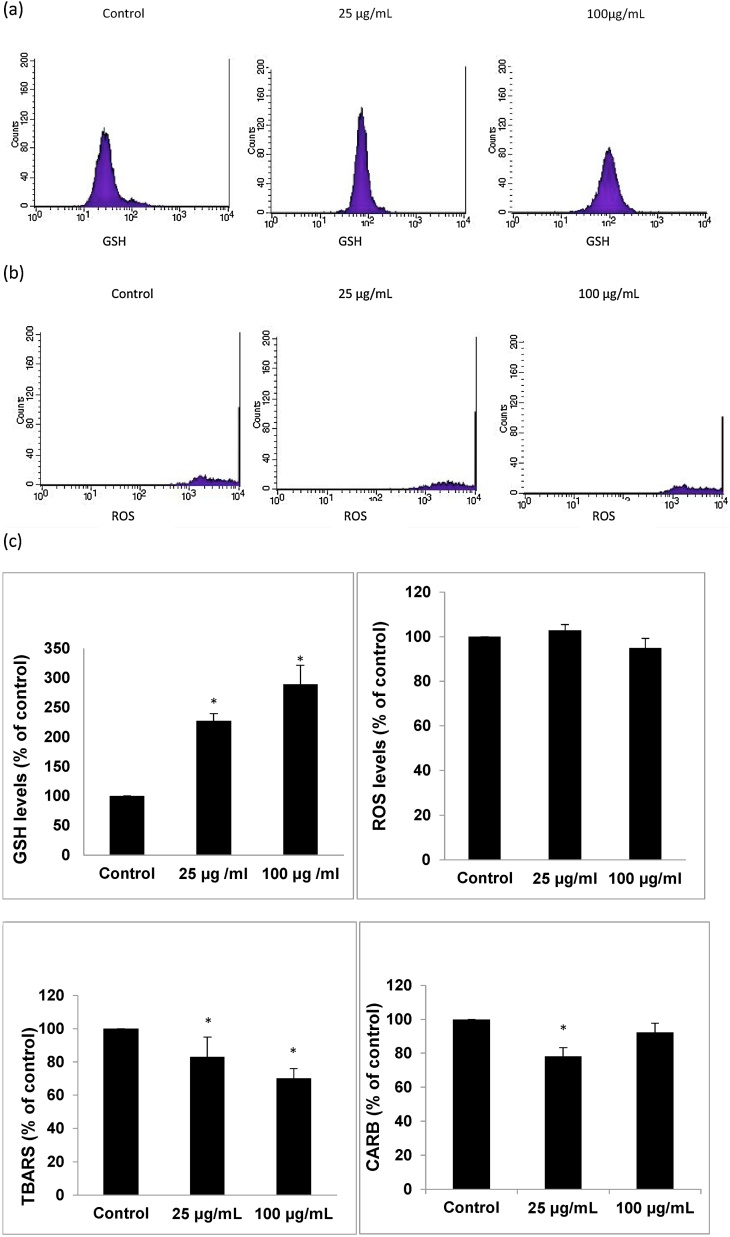
Fig. 3.
Effects of the goji berry extract 5 after treatment for 24 h on oxidative stress markers in C2C12 cells. (a) The histograms of cell counts versus fluorescence of 10,000 cells analyzed using flow cytometer for the detection of GSH. The histograms represent the detection of fluorescence using 488 and 580 nm as the excitation and emission wavelengths, respectively. (b) The histograms show the cell counts versus fluorescence of 10,000 cells analyzed using flow cytometer for the detection of ROS. The histograms represent the detection of fluorescence using 488 and 530 nm as the excitation and emission wavelengths, respectively. For ROS and GSH measurement, C2C12 cells were incubated with 10 mM DCF-DA and 40 mM mercury orange respectively, for 30 min at 37 °C. The cells were then washed, suspended in PBS, and analyzed using flow cytometry, as described in Materials and methods section. (c) Effects of extract 5 on ROS, GSH, TBARS and CARB are shown. All values are presented as the mean ± SEM of three independent experiments. *p < 0.05: significantly different from the control value.
GSH levels were assessed by flow cytometry. The findings showed that extract 5 increased GSH levels by 127.5 and 189.5% at 25 and 100 μg/mL respectively, compared to control (Fig. 3C).
However, flow cytometry analysis demonstrated that treatment of cells with extract 5 did not affect ROS levels compared to control (Fig. 3C).
Regarding TBARS levels, a marker of lipid peroxidation, it was found that cell treatment with extract 5 decreased them by 21.8 and 9.4% at 25, and 100 μg/mL, respectively, compared to control (Fig. 3C).
Finally, extract 5 decreased CARB levels, a marker of protein oxidation, by 26.8 and 29.9% at 25, and 100 μg/mL, respectively, compared to control (Fig. 3C).
4. Discussion
L. barbarum fruits exhibit a number of beneficial health effects and are used widely in the Chinese traditional medicine [29]. Since L. barbarum fruits cultivated in many areas differ in their chemical composition, the potency of their bioactivities may also be different [30,31]. In a previous study, we have identified the optimal parameters for the UAE in order to receive extracts with high polyphenol and polysaccharide content from commercial imported goji berry fruits as well as from fruits cultivated in Greece [20]. UAE is an advanced green extraction method which uses ultrasound to increase the frequency and speed of molecular movement, solvent penetrating power and dissolution rate, and thus to reduce extraction time and efficiency of extraction. Thus, this method is very effective for increasing the production yield and the quality of bioactive extracts from natural materials [32]. In the present study, we investigated the antioxidant and antimutagenic activities of UAE extracts of goji berry from L. barbarum and L. chinensis varieties cultivated in the region of Thessaly in Greece.
The results demonstrated that the extracts from the variety of L. barbarum had higher concentrations in total carbohydrate and TPC compared with those of L. chinensis using the same extraction parameters, confirming the notion that L. barbarum fruits have higher nutritional quality [29]. Moreover, the results of the total carbohydrate and TPC of the extracts were in accordance with the results of our previous study [20] as well as of other studies [33,34]. Small differences in the total carbohydrate and TPC between different studies are probably due to the different conditions of the cultivated regions and cultivation seasons of the goji berry fruits [34].
Goji berry’s carbohydrates are mainly “arabinogalactan-proteins” (AGPs), but they are mostly referred in the Chinese literature as “L. barbarum polysaccharide” (LBP). Matsumura et al., [35] pointed out that the galacturonic acid-containing pectin showed a strong free radical scavenging activity, probably due to the bond breakage between galacturonic acid and glucoside, resulting in pectin degradation. In general, goji berry’s carbohydrates may play a major role in its antioxidant properties and exhibit several pharmacological and biological functions and are used widely in the Chinese traditional medicine [29].
The extract 5 exhibited the second higher total carbohydrate content and the highest TPC. These results were in accordance with its antioxidant activity, since it exhibited on average the highest antioxidant potency in DPPH• and ABTS•+ assays and ROO•-induced DNA damage. The IC50 values of the tested extracts in the DPPH assay were similar with those presented for aqueous extracts by Kosar et al., [33]. However, Benchennouf et al., [36] found higher IC50 values than ours for the scavenging of DPPH• from a water fraction from goji berry fruits cultivated in the Greek island of Creta and acquired by a Soxhlet extraction. Probably, the different soil and climatic conditions as well as the different extraction method and parameters (e.g. extraction temperature) led to these differences in the IC50 results.
Moreover, the extracts showed to protect effectively at very low concentrations from free radical-induced DNA damage. To the best of our knowledge, this is the first study to report the protective effects of L. barbarum and L. chinensis UAE extracts against DNA damage induced by ROO• radicals. Likewise, Ceccarini et al., [37] have demonstrated that L. barbarum berries cultivated in Umbria (Italy) exerted antigenotoxic activity in human hepatocellular carcinoma cells. The extract 5 exhibited the highest protective activity against ROO• radical-induced DNA damage (2-fold higher than the second best protective activity). Importantly, as mentioned, extract 5 had also the second total carbohydrate content and the highest TPC which may account for this potent protective activity. For example, Zhao et al., [38] have shown that the highest antioxidant activity of L. barbarum extracts was attributed to their high carbohydrate content. Moreover, other studies have shown that carbohydrates from L. barbarum protected from DNA damage [39,40].
Furthermore, our results showed that treatment of muscle cells C2C12 with goji berry extract 5 resulted in the enhancement of their antioxidant status. Specifically, there was an increase in GSH levels, one of the most crucial antioxidant molecules, which exerts its antioxidant activity either through formation of S-conjugates or by serving as an electron donor from its sulfhydryl group (—SH) [41]. This finding suggested a crucial role of the increased GSH levels in the observed antioxidant activity of the goji berry extract in muscle cells. Our results were also in accordance with those from the study of Yi et al., [42] who used an orthogonal design to examine the UAE technology for the extraction of L. barbarum polysaccharides (LBP). Specifically, they demonstrated that LBP can increase superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) levels. In addition, administration of carbohydrates from L. barbarum has been shown to increase glutathione transferase (GST) and GSH levels activity in aging rats [43]. The observed increase in GSH levels by the goji berry extract treatment may be attributed to the activation of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Nrf2 is a transcription factor regulating the expression of several antioxidant and cytoprotective enzymes and consists one of the major antioxidant responses to oxidative stress [44]. Moreover, Nrf2 regulates the expression of synthase gamma-glutamylcysteine (g-GCS) and glutathione reductase (GR) enzymes playing important role in the determination of GSH levels in cells [44,45]. Several studies in cell lines and in vivo have demonstrated that L. barbarum polysaccharides enhance antioxidant activity through induction of Nrf2 mechanism [[45], [46], [47]].
The increase of GSH in muscle cells was in accordance with the reduction in TBARS levels, a marker of lipid peroxidation. Lipid peroxidation is a self-propagating chain reaction which may cause damage to integrity of cell, endoplasmic reticulum and nucleus membranes, leading to a wide array of primary and secondary oxidation products such as conjugated dienes, lipid hydroperoxides, lipid aldehydes (e.g. MDA) and alkanes. In vivo studies have also shown that LBP administration reduces MDA levels after exercise-induced oxidative stress in rats [48]. Other in vivo studies have also demonstrated that the polysaccharides extracted from L. barbarum inhibited the formation of MDA in aged mice and increased antioxidant enzymes (SOD, GSH-Px, CAT), total antioxidant capacity (TAC) and immune functions [49,50].
Apart from reduction in lipid peroxidation, treatment of C2C12 muscle cells with L. barbarum extract 5 resulted in decrease in protein oxidation as shown by the reduction in CARB levels. This effect is important, since oxidative stress-induced carbonylation of proteins leads to the loss of their physiological function [51]. It is also believed that there is an association between lipid and protein oxidation [52]. Our results were consistent with those of Li et al., [8], who have demonstrated that carbohydrates from L. barbarum decreased protein oxidation damage in aged rats.
Although the treatment with the extract 5 from L. barbarum increased antioxidant mechanism and decreased lipid peroxidation and protein oxidation in muscle cells, it did not decrease ROS levels. In previous studies of our research group on other plant extracts, changes in ROS levels were also not accompanied by protection against oxidative stress-induced damage or increase in antioxidant mechanism levels. This may be explained by the fact that the increased antioxidant mechanisms (e.g. GSH) may be able to protect macromolecules from free radical-induced damage but not to scavenge the ‘free forms’ of these radicals [25].
5. Conclusion
The findings of the present study indicated for the first time that UAE extracts of L. barbarum cultivated in Greece exhibited potent free radical scavenging activity and protected against DNA damage induced by free radicals. Moreover, it was shown for the first time that one of these extracts increased the antioxidant mechanism of GSH and decreased lipid peroxidation and protein oxidation in muscle cells. Thus, the findings suggested that L. barbarum extracts may be used as a food supplement to reduce the adverse effects of oxidative stress, especially after intensive exercise of athletes.
References
- 1.Goutzourelas N., Stagos D., Demertzis N., Mavridou P., Karterolioti H., Georgadakis S., Kerasioti E., Aligiannis N., Skaltsounis L., Statiri A., Tsioutsiouliti A., Tsatsakis A., Hayes A., Kouretas D. Effects of polyphenolic grape extract on the oxidative status of muscle and endothelial cells. Hum. Exp. Toxicol. 2014;33:1099–1112. doi: 10.1177/0960327114533575. [DOI] [PubMed] [Google Scholar]
- 2.Ji L.L. Antioxidant signaling in skeletal muscle: a brief review. Exp. Gerontol. 2007;42:582–593. doi: 10.1016/j.exger.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Gerasopoulos K., Stagos D., Kokkas S., Petrotos K., Kantas D., Goulas P., Kouretas D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015;82:42–49. doi: 10.1016/j.fct.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Nikolaidis M.G., Jamurtas A.Z., Paschalis V., Fatouros I.G., Koutedakis Y., Kouretas D. The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: magnitude and time-course considerations. Sports Med. (Auckland, N.Z.) 2008;38:579–606. doi: 10.2165/00007256-200838070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Hood D.A., Uguccioni G., Vainshtein A., D’souza D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: implications for health and disease. Comp. Physiol. 2011;1:1119–1134. doi: 10.1002/cphy.c100074. [DOI] [PubMed] [Google Scholar]
- 6.Phaneuf S., Leeuwenburgh C. Apoptosis and exercise. Med. Sci. Sports Exercise. 2001;33:393–396. doi: 10.1097/00005768-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Kerasioti E., Kiskini A., Veskoukis A., Jamurtas A., Tsitsimpikou C., Tsatsakis A.M., Koutedakis Y., Stagos D., Kouretas D., Karathanos V. Effect of a special carbohydrate-protein cake on oxidative stress markers after exhaustive cycling in humans. Food. Chem. Toxicol. 2012;50:2805–2810. doi: 10.1016/j.fct.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Li X.M., Ma Y.L., Liu X.J. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J. Ethnopharmacol. 2007;111:504–511. doi: 10.1016/j.jep.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Leontopoulos S.V., Skenderidis P., Anatolioti V., Kokkora M.I., Tsilfoglou S., Petrotos K.B., Vagelas I. Antifungal activity of azadirachta indica aqueous and non-aqueous extracts on colletotrichum gloeosporioides botryodiplodia theobromae and fusarium solani. A First Approach. J. Food Biol. Eng. 2017;6(1):38–50. [Google Scholar]
- 10.Leontopoulos S., Skenderidis P., Kalorizou H., Petrotos K. Bioactivity Potential of polyphenolic compounds in human health and their effectiveness against various food borne and plant pathogens. A Review. J. Food Bio. Eng. 2017;7:1–19. [Google Scholar]
- 11.Chu P.H.W., Li H.Y., Chin M.P., So K.F., Chan H.H.L. Effect of Lycium Barbarum (Wolfberry) Polysaccharides on preserving retinal function after partial optic nerve transection. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q., Du X., Xu Y., Dang L., Xiang L., Zhang J. The effects of Gouqi extracts on Morris maze learning in the APP/PS1 double transgenic mouse model of Alzheimer’s disease. Exp. Ther. Med. 2013;5:1528–1530. doi: 10.3892/etm.2013.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X.M. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 2007;40:461–465. doi: 10.1016/j.ijbiomac.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Wu H.T., He X.J., Hong Y.K., Ma T., Xu Y.P., Li H.H. Chemical characterization of lycium barbarum polysaccharides and its inhibition against liver oxidative injury of high-fat mice. Int. J. Biol. Macromol. 2010;46:540–543. doi: 10.1016/j.ijbiomac.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Li X.M. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats l. Int. J. Bio Macromol. 2007;40:461–465. doi: 10.1016/j.ijbiomac.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 16.jun Niu A., mei Wu J., hai Yu D., Wang R. Protective effect of Lycium barbarum polysaccharides on oxidative damage in skeletal muscle of exhaustive exercise rats. Int. J. Biol. Macromol. 2008;42:447–449. doi: 10.1016/j.ijbiomac.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Bucheli P., Gao Q., Redgwell R., Karine V., Wang J., Zhang W., Nong S., Cao B. 2013. Chapter 14 Wolfberry Biomolecular and Clinical Aspects of Chinese; pp. 1–17. [Google Scholar]
- 18.Zhang J., Li M., Zheng G. Effect of stand age on soil microbial community structure in wolfberry (Lycium barbarum L.) fields. Act. Ecol. Sin. 2017;37:10–17. [Google Scholar]
- 19.Wen-Ping M.A., Zhi-jinga Ni, Heb Li, Min Chen. Changes of the main carotenoid pigment contents during the drying processes of the different harvest stage fruits of lycium barbarum l. Agric. Sci. China. 2008;7:363–369. [Google Scholar]
- 20.Skenderidis P., Petrotos K., Giavasis I., Hadjichristodoulou C., Tsakalof A. Optimization of ultrasound assisted extraction of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Proc. Eng. 2016 [Google Scholar]
- 21.DuBois M., a. Gilles K., Hamilton J.K., a. Rebers P., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 22.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998;299:152–178. [Google Scholar]
- 23.Waterhouse A.L. John Wiley & Sons, Inc.; 2001. Determination of Total Phenolics, In: Current Protocols in Food Analytical Chemistry. [Google Scholar]
- 24.Spanou C., Stagos D., Tousias L., Angelis A., Aligiannis N., Skaltsounis A.L., Kouretas D. Assessment of antioxidant activity of extracts from unique Greek varieties of Leguminosae plants using in vitro assays. Anticancer Res. 2007;27:3403–3410. [PubMed] [Google Scholar]
- 25.Kerasioti E., Stagos D., Priftis A., Aivazidis S., Tsatsakis A.M., Hayes A.W., Kouretas D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014;155:271–278. doi: 10.1016/j.foodchem.2014.01.066. [DOI] [PubMed] [Google Scholar]
- 26.Priftis A., Stagos D., Konstantinopoulos K., Tsitsimpikou C., Spandidos D.A., Tsatsakis A.M., Tzatzarakis M.N., Kouretas D. Comparison of antioxidant activity between green and roasted coffee beans using molecular methods. Mol. Med. Rep. 2015;12:7293–7302. doi: 10.3892/mmr.2015.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Symons G.E., Morey B. The effect of drying time on the determination of solids in sewage and sewage sludges. Sewage Works J. 1941;13:936. [Google Scholar]
- 28.Patsoukis N., Zervoudakis G., Panagopoulos N.T., Georgiou C.D., Angelatou F., Matsokis N.A. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 2004;357:83–86. doi: 10.1016/j.neulet.2003.10.080. [DOI] [PubMed] [Google Scholar]
- 29.Cheng J., Zhou Z.-W., Sheng H.-P., He L.-J., Fan X.-W., He Z.-X., Sun T., Zhang X., Zhao R.J., Gu L., Cao C., Zhou S.-F. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des. Dev. Ther. 2015;9:33–78. doi: 10.2147/DDDT.S72892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Z., Xiao J., Fan H., Yu Y., He R., Feng X., Kurihara H., So K., Yao X., Gao H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017;214:644–654. doi: 10.1016/j.foodchem.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 31.Dong Jing Z. Analysis on the main active components of Lycium barbarum fruits and related environmental factors. J. Med. Plants Res. 2012;6:2276–2283. [Google Scholar]
- 32.Picó Y. Ultrasound-assisted extraction for food and environmental samples. Trends Anal. Chem. 2013;43:84–99. [Google Scholar]
- 33.Kosar M., Altintas A., Kirimer N., Baser K.H.C. Determination of the free radicals scavenging activity of Lycium extracts. Chem. Nat. Comp. 2003;39:439–442. [Google Scholar]
- 34.Zheng G.Q., Zheng Z.Y., Xu X., Hu Z.H. Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill. of different regions and varieties. Biochem. Syst. Ecol. 2010;38:275–284. [Google Scholar]
- 35.Matsumura Y., Egami M., Satake C., Maeda Y., Takahashi T., Nakamura A., Mori T. Inhibitory effects of peptide-bound polysaccharides on lipid oxidation in emulsions. Food Chem. 2003;83:107–119. [Google Scholar]
- 36.Benchennouf A., Grigorakis S., Loupassaki S. Phytochemical analysis and antioxidant activity of Lycium barbarum (Goji) cultivated in Greece. Pharm. Biol. 2017;55:596–602. doi: 10.1080/13880209.2016.1265987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceccarini M.R., Vannini S., Cataldi S., Moretti M., Villarini M., Fioretti B., Albi E., Beccari T., Codini M. In vitro protective effects of Lycium barbarum berries cultivated in Umbria (Italy) on human hepatocellular carcinoma cells. BioMed Res. I. 2016;2016:7529521. doi: 10.1155/2016/7529521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H., Alexeev A., Chang E., Greenburg G., Bojanowski K. Lycium barbarum glycoconjugates: effect on human skin and cultured dermal fibroblasts. Phytomedicine. 2005;12:131–137. doi: 10.1016/j.phymed.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Gan L., Wang J., Zhang S. Inhibition the growth of human leukemia cells by Lycium barbarum polysaccharide. J. Hyg. Res. 2001;30:333–335. [PubMed] [Google Scholar]
- 40.Lin N.-C., Lin J.-C., Chen S.-H., Ho C.-T., Yeh A.-I. Effect of goji (Lycium barbarum) on expression of genes related to cell survival. J. Agric. Food Chem. 2011;59:10088–10096. doi: 10.1021/jf2021754. [DOI] [PubMed] [Google Scholar]
- 41.Yu B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 42.Yi R., Liu X.-M., Dong Q. A study of Lycium barbarum polysaccharides (LBP) extraction technology and its anti-aging effect. Afr. J. Tradit. Comp. Altern. Med. 2013;10:171–174. doi: 10.4314/ajtcam.v10i4.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jing L., Qin O., Jie S. 2018. Study of Effect of Lycium Barbarum Polysaccharides on Protein Oxidation Damage in D-gal-induced Aging Rats; pp. 2384–2385. Available at http://europepmc.org/abstract/CBA/648268. [Google Scholar]
- 44.Vomund S., Schafer A., Parnham M.J., Brune B., von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao S., Du J., Hei Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp. Ther. Med. 2017;14:4919–4927. doi: 10.3892/etm.2017.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H., Li Z., Peng L., Jiang N., Liu Q., Zhang E., Liang B., Li R., Zhu H. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Rad. Res. 2017;51:200–210. doi: 10.1080/10715762.2017.1294755. [DOI] [PubMed] [Google Scholar]
- 47.He M., Pan H., Chang R.C.-C., So K.-F., Brecha N.C., Pu M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS One. 2014;9:e84800. doi: 10.1371/journal.pone.0084800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Changbo Duan. Supplementation of Lycium barbarum polysaccharides protection of skeletal muscle from exercise-induced oxidant stress in mice. Afr. J. Pharm. Pharmacol. 2012;6:643–647. [Google Scholar]
- 49.Liang B., Jin M., Liu H. Water-soluble polysaccharide from dried Lycium barbarum fruits: isolation, structural features and antioxidant activity. Carbohydr. Polym. 2011;83:1947–1951. [Google Scholar]
- 50.Li X.M., Li X.L., Zhou A.G. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007;43:488–497. [Google Scholar]
- 51.Beal M.F. Oxidatively modified proteins in aging and disease. Free Radical Biol. Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 52.Vasil’ev Y.V., Tzeng S.-C., Huang L., Maier C.S. Protein modifications by electrophilic lipoxidation products: adduct formation, chemical strategies and tandem mass spectrometry for their detection and identification. Mass Spectrom. Rev. 2014;33:157–182. doi: 10.1002/mas.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]