Abstract
BACKGROUND
The reproducibility of transcranial Doppler (TCD) ultrasound measurements in Sturge-Weber syndrome (SWS) and TCD’s ability to predict neurologic progression is unknown.
METHODS
In fourteen SWS patients, TCD measured mean flow velocity, pulsatility index, peak systolic velocity (PSV), and end diastolic velocity (EDV) in the middle (MCA), posterior (PCA) and anterior cerebral arteries (ACA) of the affected and unaffected hemisphere. TCD was performed either once (n=5) or twice in one day (n=9). We assessed the reproducibility of the measurements performed twice on the same day on subjects and compared the TCD measurements to previously published age-matched controls. Clinically obtained neuroimaging was scored for extent and severity of SWS brain involvement. Patients were prospectively assigned SWS neuroscores.
RESULTS
MCA velocity (r=0.79, p=0.04, n=7), PCA velocity (r=0.90, p=0.04, n=5), and ACA pulsatility index (r=0.82, p=0.02, n=7) were reproducible TCD measurements comparing same-day percent side-to-side differences. In subjects with SWS, affected and unaffected mean PSV and EDV velocities in the MCA, PCA, and ACA were globally lower compared to age-matched controls. Subjects with the lowest affected MCA velocity had the greatest worsening in total neurologic score between time 1 and 2 (r=−0.73, p=0.04, n=8) and the most severe MRI involvement of the affected frontal lobe (r=−0.82, p=0.007, n=9).
CONCLUSIONS
TCD is suggested as a reliable measure with potential clinical value, indicating blood flow may be globally decreased in SWS patients with unilateral brain involvement.
Keywords: transcranial Doppler ultrasound, Sturge-Weber syndrome, peak systolic velocity, end-diastolic velocity
Introduction
Sturge-Weber syndrome (SWS) is a neurocutaneous syndrome consisting of leptomeningeal angioma of the brain, a facial capillary malformation (port-wine birthmark), and capillary venous malformation of the eye [1]. SWS is caused by a R183Q somatic mutation in GNAQ occurring during fetal development [2, 3]. Individuals with SWS often suffer from seizures and stroke-like episodes [1]. The extent of brain involvement is formally assessed using a magnetic resonance imaging (MRI) of the brain with and without intravenous contrast. The contrast specifically highlights the stereotypical leptomeningeal angiomatosis enhancement in affected individuals. However, low sensitivity of neuroimaging in infancy makes it challenging to confirm early SWS brain involvement. Furthermore, MRIs of the brain at this point frequently require conscious sedation and intravenous contrast administration, hindering the accessibility of the MRI as a biomarker for monitoring patient response to treatment. Early suspicion of brain involvement can be assessed using electroencephalograms (EEGs), though in some cases the EEG and subsequent MRI are not concordant, or may not correlate with neurologic symptoms [4]. The SWS community is in need of an additional, early detection tool to screen for SWS brain involvement and a non-invasive biomarker to evaluate treatment response.
Research has demonstrated that individuals with SWS brain involvement have decreased cerebral blood flow in involved regions of the brain [5, 6]. Transcranial Doppler (TCD) ultrasound is a non-invasive vascular procedure that measures velocity of blood flow throughout the brain's blood vessels. TCD has been used successfully in individuals with neurovascular disorders (specifically stroke prevention in sickle cell disease) to predict neurologic progression [7]. To test the utility of TCD in assessing cerebral blood flow in patients with SWS brain involvement, Jordan and colleagues (2008) evaluated eight children with unilateral brain involvement by TCD performed once to assess non-angle-corrected mean flow values (cm/sec) for the velocity, depth, and pulsatility index (PI) [8]. Those with SWS had lower middle cerebral artery (MCA)-velocity and posterior cerebral artery (PCA)-velocity TCD, as well as higher MCA PI values on the affected hemisphere compared to the unaffected hemisphere. It is therefore concluded that TCD was a promising tool for monitoring abnormal blood flow in SWS. A larger number of subjects were needed to seek correlations with neurologic status and additional comparisons with age-matched controls were also required. Therefore, the goals of this study are three-fold: 1) assess reproducibility of TCD ultrasound measurements in subjects with SWS, 2) determine whether there is an association in changes in TCD with clinical change and 3) compare TCD results with age-matched normal subjects.
Methods
The Johns Hopkins Institutional Review Board approved this study and the subjects or their legal guardian signed informed consent for their participation.
Participants with a diagnosis of SWS unilateral brain involvement as defined on neuroimaging were eligible for this study. Participants were recruited from the Kennedy Krieger Institute (Baltimore, Maryland) as patients of A.M.C and came from Maryland and the neighboring states. Participants were recruited prospectively. No subjects were excluded. Age-matched control data for mean flow velocity, PSV (peak systolic velocity), and EDV (end-diastolic velocity) were obtained from Bode and Wais (1988) [9].
TCD was performed by a clinical ultrasonographer, M. R. D., on an ATL/Philips Model 5000 (Bothell, Washington) and a Siemens Antares (Malvern, Pennsylvania) for the participants who received TCD once (n=5) and on an ATL/Philips Model 5000 (Bothell, Washington) and a Philips IU-22 (Bothell, Washington) for the participants who received TCD twice in one day (n=9). No repeat TCD data are available for the participants reported in the Jordan et al., 2008 study [8]. Standard clinical procedures were used with three different clinical models that supported TCD to avoid participants waiting for the unit to be available; all models had preventative maintenance every six months. Using previously described methods, the non-angle corrected mean, PSV and EDV (cm/sec), and the PI were measured for the MCA, PCA, and ACA using a 2 MHz probe during approximately 90 minute sessions [8]. Not all TCD measurements were able to be collected for each participant due to subject cooperation (see Supplemental Table 1 for details). For participants with multiple velocities, the highest velocity per session for each hemisphere of each TCD vessel was used in the analyses. TCD measurements were done blinded to both MRI scores and SWS clinical severity scores.
Average differences and average percent side-to-side differences of TCD values were calculated to compare the first TCD session to the second. Reproducibility of the TCD measures was evaluated using Spearman’s correlation with two measurements done on the same day on the same subjects (n=9). For the participants that had more than one session, the average TCD value for the sessions was calculated for each hemisphere; similarly, an average percent difference between the affected and unaffected hemisphere was calculated for the two sessions.
SWS clinical severity scores were collected prospectively (A.M.C.), on average, at the time of the TCD and twelve months later. Differences were calculated for each subcategory of the clinical severity scores between the two assessment dates. A SWS clinical severity score contained frequency of seizures, severity of hemiparesis, assessment of visual field cut, degree of cognitive functioning with a total score ranging from 0–15 as previously published (see Supplemental Table 2) [10]. Correlations of TCD values with the SWS clinical severity scores were evaluated using Spearman’s rho. TCD values of those with two sessions were averaged together. Percent side-to-side differences (PSSDs) of TCD values were calculated between the affected and unaffected side of the brain as below:
MCA, PCA, and ACA velocities were compared between affected hemispheres and unaffected hemispheres and also compared to previously published age matched normal values using Wilcoxon matched-pairs signed rank tests [9]. For brevity, the authors only report significant results.
All subjects had prior brain MRI with and without contrast imaging done for clinical reasons. The time between MRIs and the date of TCD ranged from 7 months to 10 years and 6 months with a median time range of 1 year and 3 months. Each MRI was rated by D.D.M.L and A.M.C., blinded to the TCD and clinical severity scores. The raters scored MRI scans individually and then came to consensus on discrepancies. A Likert scale of 1 – 4 (1 = “no asymmetry”, 2 = “mild asymmetry” (atrophy or angiomatosis only), 3 = “moderate asymmetry” (angiomatosis and mild atrophy), and 4 = “severe asymmetry” (angiomatosis and severe atrophy)) was used to assign a score for the frontal, parietal, occipital, and temporal lobes of the brain in both hemispheres (adaptation from Jansen et al., 2002) [11]. Correlations of MRI severity scores with TCD values and clinical severity scores were evaluated using Spearman’s rho.
Non-parametric analyses were used for correlations due to the non-continuous and semi-quantitative nature of the scales and/or non-normal and small datasets. Inter-rater reliability was evaluated using Cohen’s weighted kappa (κw). P-values less than 0.05 for two-tailed analyses were used as the threshold for determining significance. All analyses were conducted using IBM SPSS Statistics 23.0 and 24.0 software.
Results
Demographics and Clinical Information
TCD ultrasound was performed either once (n=5, 2F, 8 mos-9 yrs) or twice in one day (n=9, 5F, 3 yrs-20 yrs). Seventy-one percent of the participants identified as Caucasian (6F, 4M). Six participants had brain involvement on the left hemisphere compared to the eight with right hemisphere brain involvement. SWS eye involvement was present in five participants unilaterally and in three participants bilaterally. Six participants did not have eye involvement. Seven participants had SWS skin involvement on one side of their face and three had involvement on both sides. Four participants did not have any skin involvement. More individual-specific demographics and clinical information can be found in Table 1.
Table 1.
Demographics at Time of TCD.
| ID | Age | Sex | Race / Ethnicity |
BI | SI | EI | TCD Sessions * |
Hgb | ASP | Epilepsy | AED | Developmental and cognitive delays |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 mos | M | C | L - F, T | L | - | One | 13.0 | N | Y | OCBZ, LVT | Developmental delays |
| 2 | 10 mos | F | C | R - O, P, T | R | R | One | 14.3 | Y | N | - | No cognitive delays |
| 3 | 3 yrs 5 mos | M | C | R - F, O, P, T | - | - | One | - | N | Y | CBZ | Mild cognitive impairment |
| 4 | 3 yrs 1 mos | F | C | L - F, P, O, T | B | L | Two | 11.6 | Y | Y | LVT, OCBZ | No cognitive delays |
| 5 | 7 yrs 9 mos | F | C | R - O, P, T | R | R | One | - | Y | Y | OCBZ | No cognitive delays |
| 6 | 7 yrs 8 mos | M | C | R - P, O, T | B | B | Two | 13.2 | Y | Y | LVT, OCBZ | Borderline intellectual abilities |
| 7 | 7 yrs 6 mos | M | ASAM | L - F, P, T | L | - | Two | 12.8 | Y | Y | OCBZ | Learning disabilities |
| 8 | 9 yrs 4 mos | M | C | L - O, P | - | - | One | - | N | Y | TPM | Learning disabilities |
| 9 | 9 yrs 2 mos | M | AA | L - P, O | L | L | Two | 12.9 | Y | Y | OCBZ | Borderline intellectual abilities and ADHD |
| 10 | 10 yrs 5 mos | F | C | R - F, P, O, T | R | B | Two | 13.8 | N | Y | CBZ | Mild cognitive impairment |
| 11 | 11 yrs 2 mos | F | C | R - F | B | B | Two | 13.7 | N | N** | - | ADD and learning disabilities |
| 12 | 13 yrs 10 mos | F | ASAM | R - P, O | - | - | Two | 13.4 | Y | Y | OCBZ | No cognitive delays |
| 13 | 17 yrs 3 mos | M | AA | R - P, O | - | - | Two | 14.9 | Y | Y | LMT, TPM, OCBZ | Borderline intellectual abilities |
| 14 | 20 yrs 6 mos | F | C | L - F, P, O, T | L | R | Two | 12.8 | Y | Y | CBZ, TPM | ADD |
BI – Brain Involvement
ASP – Aspirin (3 – 5 mg/kg/day)
C – Caucasian
B – Bilateral
P – Parietal lobe
CBZ – Carbamazepine
ADHD – Attention Deficit Hyperactivity Disorder
SI – Skin Involvement
AED – Anticonvulsants
ASAM – Asian American
F – Frontal lobe
Y – Yes N – No
TPM – Topiramate
EI – Eye Involvement
yrs – years mos – months
AA – African American
T – Temporal lobe
OCBZ – Oxcarbazepine
LMT – Lamotrigine
ADD – Attention Deficit Disorder
Hgb – Hemoglobin (g/dL)
M – Male F – Female
L – Left R – Right
O – Occipital lobe
LVT – Levetiracetam
Nine subjects had two TCD sessions in the same day. Five subjects had one TCD session.
Patient had seizures at one month of age and was weaned off Phenobarbital at 2 ½ year of age. No seizures since
MRI Location and Severity of Brain Involvement
Eleven out of fourteen participants had neuroimaging available for scoring. There was moderate agreement between the two raters, with the following values: total MRI severity score κw = 0.43 and total affected MRI severity score κw = 0.46. Consensus total MRI severity scores ranged from 9 to 19 with a median total score of 13. For those with left-sided brain involvement, the median total MRI severity score was 10.50 (6–14). Participants with right-sided brain involvement had a median total score of 9 (5–15). On the affected hemisphere, six out of these eleven participants had unaffected frontal lobes, four participants had unaffected temporal lobes, and one participant had an unaffected parietal lobe. Zero participants had an unaffected occipital lobe on their affected hemisphere.
MRI severity score correlations with TCD and clinical severity
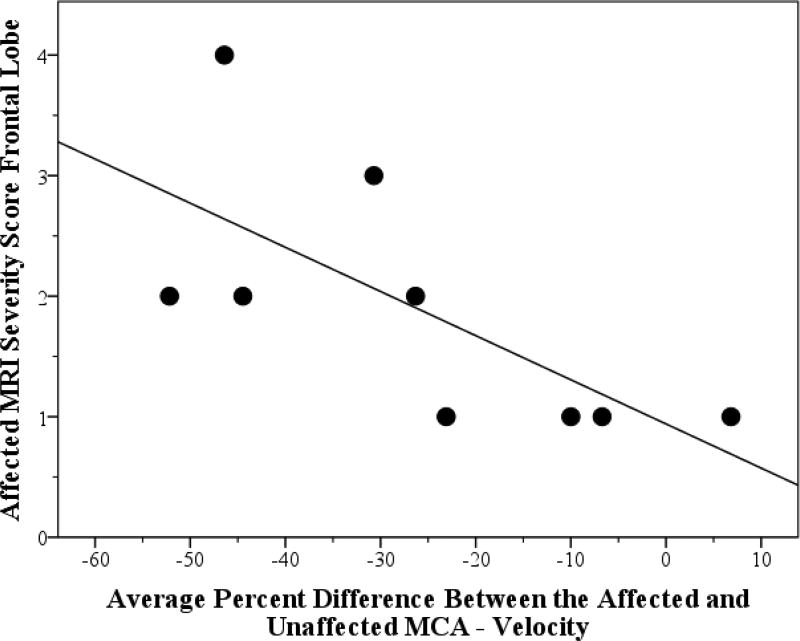
The average percent side-to-side difference in MCA mean flow velocity negatively correlated with total affected MRI severity in the frontal lobe (Spearman, r=−0.82, p=0.007, n=9); thus, lower MCA velocity on the affected hemisphere correlated with increased MRI severity scores in the frontal lobe of the affected hemisphere (see Figure 1). The total affected MRI severity score positively correlated with a change in total SWS neuroscore (Spearman, r=0.69, p=0.04, n=9); therefore, increased MRI severity scores on the affected hemisphere correlated with worsening neurologic status over a year (see Table 2).
Figure 1.
Correlation of TCD Values with MRI Severity. Figure 1 depicts the correlation and trend line between the total affected MRI severity score with the difference in total SWS severity scores from time 1 to time 2.
Table 2.
Correlation (p-value) of MRI Severity Scores with Changes in Clinical Severity Scores.
| Lobe | Seizure Score Changes r (p-value) |
Hemiparesis Score Changes r (p-value) |
Visual Field Cut Score Changes r (p-value) |
Cognitive Functioning Score Changes r (p-value) |
Total Neuroscore Changes r (p-value) |
|---|---|---|---|---|---|
| Affected Frontal | 0.42 (0.27) | 0.35 (0.36) | 0.50 (0.17) | 0.33 (0.39) | 0.83 (0.006) |
| Affected Temporal | −0.02 (0.97) | 0.03 (0.94) | 0.54 (0.14) | 0.65 (0.06) | 0.62 (0.08) |
| Affected Parietal | −0.34 (0.37) | 0.00 (1.00) | 0.66 (0.05) | 0.64 (0.06) | 0.55 (0.13) |
| Affected Occipital | −0.22 (0.57) | −0.17 (0.67) | 0.58 (0.10) | 0.72 (0.03) | 0.49 (0.18) |
| Affected Total MRI | −0.10 (0.80) | 0.08 (0.84) | 0.64 (0.06) | 0.66 (0.05) | 0.69 (0.04) |
The difference of clinical severity scores obtained between baseline and twelve months later.
All values are presented as Spearman’s rho (r) correlation coefficient (two-tailed p-value significance).
Reproducibility of TCD Measurements
Reproducible Percent Side- to- Side Differences
Five out of seven, five out of five, and two out of seven subjects had decreased MCA, PCA, and ACA mean velocity on the affected side compared to the unaffected side, respectively. Six out of seven, five out of five, and five out of seven subjects had increased MCA, PCA, and ACA pulsatility index on the affected side compared to the unaffected side, respectively (first session of TCD data for those with two sessions, for full data see Supplemental Table 4). MCA velocity percent side-to-side difference was significantly correlated from session-to-session (n=7) as were PCA velocity percent side-to-side differences (n=5). ACA pulsatility index percent side-to-side differences were also reproducible from session-to-session (n=7), but PCA and ACA pulsatility indices were not (see Table 3). The nine participants with TCD twice in one day were used for these analyses; though not all had successful measurements for each vessel (see Supplemental Table 1 for details).
Table 3.
Reproducibility of TCD Data.
| Vessel | Velocity r (p-value) | Pulsatility Index r (p-value) |
|
| ||
| MCA Percent Side-to-Side Difference | 0.79 (0.04) | −0.25 (0.59) |
|
| ||
| PCA Percent Side-to-Side Difference | 0.90 (0.04) | −0.30 (0.62) |
|
| ||
| ACA Percent Side-to-Side Difference | 0.54 (0.22) | 0.82 (0.02) |
|
| ||
| Vessel | PSV (peak systolic velocity) r (p-value) | EDV (end-diastolic velocity) r (p-value) |
|
| ||
| MCA Affected | 0.60 (0.21) | 0.77 (0.07) |
| MCA Unaffected | 0.77 (0.07) | 0.26 (0.62) |
|
| ||
| PCA Affected | 0.90 (0.04) | 0.60 (0.30) |
| PCA Unaffected | 0.70 (0.19) | 0.90 (0.04) |
|
| ||
| ACA Affected | 0.10 (0.87) | 0.20 (0.75) |
| ACA Unaffected | 1.00 (0.01) | 0.87 (0.05) |
r – Spearman’s rho correlation coefficient
p-value – two-tailed significance value
Reproducible Velocities
The first TCD session’s MCA unaffected PSV trended towards reproducibility with the second session’s values (n=6). PSV and EDV of the MCA on the affected hemisphere of the MCA also trended towards reproducibility (n=6). These reproducibility correlations for MCA, PCA, and ACA measurements are summarized in Table 3.
Correlation of Clinical Severity scores with TCD
SWS clinical severity scores at the time of the TCD ranged from a total score of 1 to 8 with a median total score of 4. Scores from 7 to 17 months after TCD ranged from a total score of 0 to 8 with a median total score of 4 (see Supplemental Table 3).
MCA correlations with clinical severity
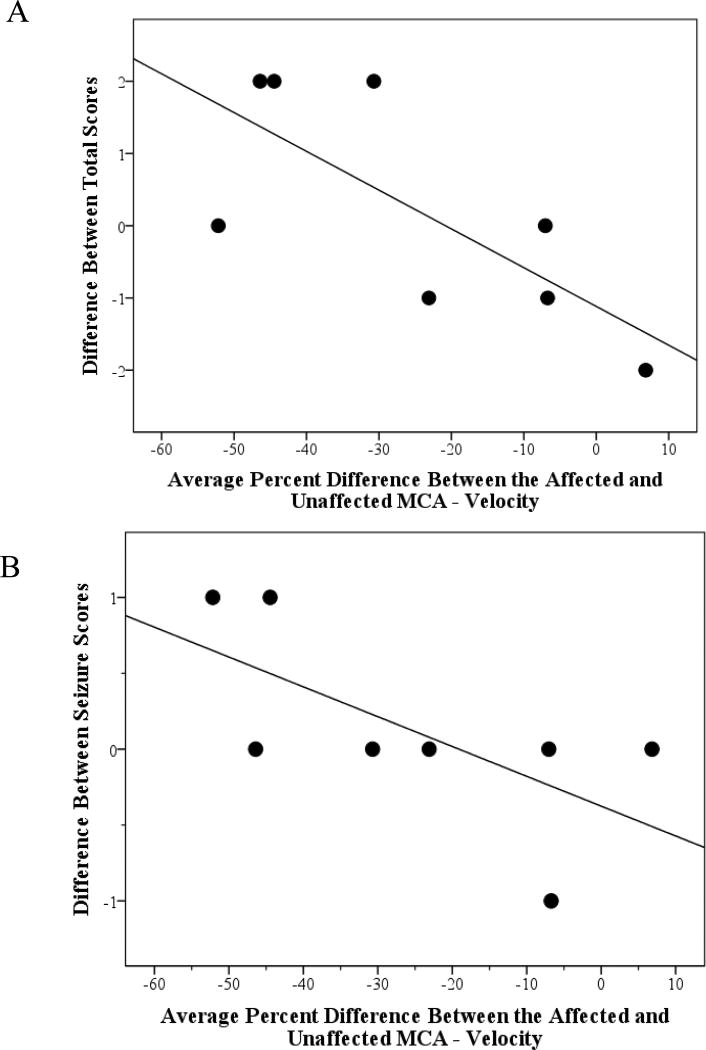
Percent difference in MCA velocity was negatively correlated with a change in total SWS neuroscore (Spearman, r=−0.73, p=0.04, n=8); therefore, lower velocity in the MCA on the affected side correlated with worsening neurologic status over a year (see Figure 2 Panel A). A trend was also noted for a decrease in MCA mean flow velocity on the affected side (compared to the unaffected side) correlated with a worsening in seizure scores from time 1 to time 2 (Spearman, r=−0.69, p=0.06, n=8, see Figure 2 Panel B). There were no significant correlations between the percent difference in MCA velocity and the initial SWS clinical severity score.
Figure 2.
Correlation of TCD Values with SWS Clinical Severity Scores. Panel A depicts the correlation and trend line between the average percent difference between the affected and unaffected MCA velocity with the difference in total SWS severity scores from time 1 and time 2. Panel B depicts the correlation and trend line between the average percent difference between the affected and unaffected MCA velocity with difference in seizure scores from time 1 to time 2. Only participants with both time 1 and time 2 SWS clinical severity scores were included. A greater decrease in MCA velocity on the affected side (negative percent difference) was associated with a decline in function (Figure 2A) and a worsening of seizures (Figure 2B) from time 1 to time 2.
Comparison with age-matched normal data
MCA velocities
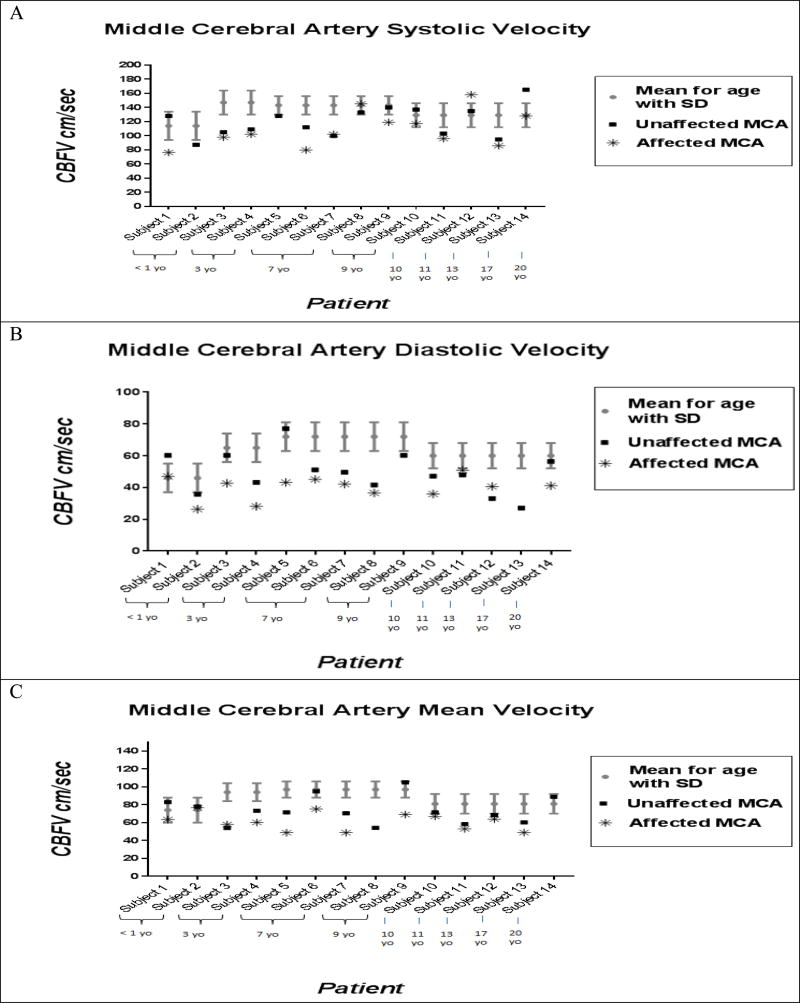
The PSV, EDV, and mean flow velocity for the affected and unaffected MCA were significantly lower compared to that of age-matched controls. Unaffected MCA values were significantly lower compared to affected values for the EDV and mean flow velocity (see Figure 3 Panel A, B, and C and Supplemental Table 5).
Figure 3.
Comparison of MCA flow velocities in SWS patients compared to previously published age matched controls. Panel A: MCA peak systolic velocities for each patient are shown for the affected and unaffected hemispheres compared to previously published age-matched controls. Panel B: MCA diastolic velocities for each patient are shown for the affected and unaffected hemispheres compared to previously published age-matched controls. Panel C: MCA mean velocities for each patient are shown for the affected and unaffected hemispheres compared to previously published age-matched controls. Subjects are arranged by age in all three panels of Figure 3.
PCA velocities
The PSV, EDV, and mean flow velocity for the affected PCA were significantly lower compared to that of age-matched controls. The EDV and mean flow velocity for the unaffected PCA were significantly lower compared to controls. Unaffected PCA values were significantly lower compared to affected values for the PSV, EDV, and mean flow velocity (see Supplemental Figure 1 Panel A, B, and C and Supplemental Table 5).
ACA velocities
The EDV for the affected ACA was significantly lower compared to that of age-matched controls. The PSV, EDV, and mean flow velocity for the unaffected ACA was also significantly lower than age-matched controls (see Supplemental Figure 2 Panel A, B, and C and Supplemental Table 5). Unaffected ACA values were lower compared to affected values for the PSV, EDV, and mean flow velocity, though not significant.
Discussion
A novel finding in this study was that the mean flow, PSV and EDV on the unaffected hemisphere of these subjects with SWS were significantly lower than expected for age. All subjects recruited for this study had unilateral brain involvement diagnosed on neuroimaging performed after one year of age with the exception of one subject (subject 10) whose imaging was obtained at birth. Therefore, these data suggest that even unilateral SWS brain involvement has the potential to negatively impact the putatively unaffected hemisphere. It is unknown at this time whether the decrease in flow velocities on the unaffected side is secondary to decreased metabolic demand and tissue changes resulting from seizures and strokes on that or the other side, or alternatively suggests a more global underlying disruption in cerebral hemodynamics in SWS than previously suspected.
Decreases in EDV on the unaffected side, compared to normal controls, is particularly interesting as it likely reflects impaired venous drainage [12, 13]. Decreased cerebral perfusion due to impaired venous drainage has been demonstrated in SWS by magnetic resonance (MR) perfusion imaging and therefore these results are consistent with SWS being a neurovascular disorder of venous impairment [14]. These results suggest that global impaired venous drainage is present in patients with a unilateral leptomeningeal angioma.
In the affected hemisphere, the relative mean flow velocities in the MCA and PCA were significantly lower, compared to the contralateral unaffected hemisphere. The mean flow velocities, PSV, and EDV of the MCA and PCA in the affected hemisphere were also significantly lower than expected for age. These findings are consistent with prior studies that have shown decreased blood flow in individuals with SWS on the affected hemisphere using various different techniques [5, 6, 15]. Chiron and colleagues (1989) used single photon emission computed tomography (SPECT) to assess regional cerebral blood flow (rCBF) in thirteen patients with SWS [5]. They noted a 32–72% decrease in rCBF in computerized tomography (CT) scan-confirmed brain regions related to SWS [5]. Pinto (1997) also detected decreased rCBF in the affected hemispheres of twenty-two babies with diagnosed SWS using SPECT imaging [6]. Riela, Stump, Roach, McLean, and Garcia (1985) used a non-invasive Xenon-133 inhalation technique to track rCBF in four patients with SWS [15]. Vasomotor dysfunction, indicating decreased rCBF, was discovered in the affected brain regions of all patients in this study [15]. Miao and colleagues (2011) identified correlates between more severe brain atrophy in individuals with low perfusion in the affected white matter tissue of the brain with lower cerebral blood flow and lower cerebral blood volume [16]. Clinically, a longer duration of epilepsy was also associated with lower cerebral blood flow and lower cerebral blood volume. Decreased perfusion on the affected hemisphere was found to be correlated with more frequent seizures. Aylett and colleagues (1999) also reported mean flow velocity decreased by 29–62% on the affected hemisphere compared to the unaffected in two of the three infants studied [17]. Based on these perfusion imaging studies, decreased mean flow velocities on the affected hemispheres, relative to the unaffected side and compared to normal controls, are expected.
One would also anticipate a greater MCA and PCA (compared to ACA) relative decrease in mean flow velocity in the affected hemisphere, compared to the unaffected hemisphere, since SWS brain involvement is more commonly in posterior occipital, parietal, and temporal regions of the brain [18], as was seen in our cohort of subjects. The frontal lobe, perfused in part by the ACA, is the least likely region to be affected by SWS brain involvement [19]. The increased relative mean flow velocity in the ACA on the affected side compared to the unaffected side in our data suggests that some compensatory increased flow through the anterior circulation may be occurring.
Increased relative pulsatility index in the MCA and PCA on the affected side compared to the unaffected (noted in the first TCD session of most subjects) was previously noted in our Jordan et al., 2008 study [8]. However, pulsatility index was not reproducible from session to session of the current study. It may be that a larger number of subjects are needed to determine if pulsatility index is sufficiently reproducible to be clinically useful in SWS; further studies are needed.
Clinical correlates with reproducible TCD values suggest that mean flow velocities of the MCA may be predictive of clinical worsening in patients with SWS. If confirmed in larger studies and clinical trials, TCD may prove to be a useful biomarker for monitoring and predicting neurologic progression in SWS. With this cross-sectional study, it is not possible to determine whether the associations between lower relative mean flow velocities in the MCA on the affected hemispheres are causative of the worsening in clinical symptoms or reflective of existing injury. While cerebral flow velocities are not a direct measure of cerebral blood flow, the lower relative flow velocities (affected side compared to the unaffected side) may relate to decreased cerebral perfusion and ongoing ischemia, which may explain why decreased relative MCA mean velocity was associated with worsening clinical measures. Lower MCA mean flow velocity on the affected hemisphere correlation with increased MRI severity scores in the frontal lobe of the affected hemisphere further supports a relationship between TCD measurements and extent of brain involvement. This decrease in relative mean flow velocity may be reflective of an arterial abnormality or rather may potentially be due to elevated venous resistance, which is the more likely scenario with SWS. Alternatively, the relative flow velocities are lower, on the affected side compared to the unaffected side, because the already ischemic/injured posterior regions of the brain have lower metabolic demand.
The MR perfusion study by Miao and colleagues (2011) argued that cerebral hyperfusion actually precedes brain atrophy in young children with SWS and that cerebral blood flow later evolves to decreased cerebral perfusion [16]. Thus the lower cerebral flow velocities (both relative and compared to normal) may be surrogate markers of the degree of cerebral injury and thus disease severity. It is also possible, even likely, that the underlying causes of the TCD measurement abnormalities are different in a young infant compared to an older child or adult with SWS. TCD in an older patient is more likely to reflect atrophy and compensatory changes than in a young infant. We postulate that a longitudinal TCD study of young patients with SWS may further clarify the significance of these findings. While the data in this study did not show age-related differences in TCD measurements, a future study of serial TCD measurements over the first year of life is needed to determine the early cerebral flow velocities as measured by TCD.
This study also sought to determine the reproducibility of TCD measures in subjects with SWS. The percent difference in relative MCA mean flow velocity, PCA mean flow velocity, and ACA pulsatility index were reproducible across sessions. A majority of the PSV and EDV for the MCA, PCA, and ACA were also found to be reproducible between sessions, most consistently on the unaffected hemisphere. Based on these results, it is concluded that cerebral blood flow measurement by TCD in SWS is feasible and reproducible. The reproducibility of pulsatility index may require further study.
Limitations of the study
SWS is a rare disorder and the relatively small sample size for this study is a limitation. A larger number of subjects will enable additional correlations with MRI scores and other clinical outcomes, as well as greater certainty regarding the reproducibility and value of pulsatility measurements. This was a cross-sectional TCD study, and therefore no conclusions can be drawn regarding changes in TCD measurements in SWS over time. Serial TCDs in infants with port-wine birthmarks need to be done in order to determine to what extent decreases in blood flow by TCD occur early in the progression of SWS and to determine whether TCD is useful for screening infants with facial port-wine birthmark for brain involvement. TCD has technical limitations in uncooperative participants that can, at times, prevent successful measurements of all the cerebral vessels. Nevertheless, the bilateral decreases in velocities in both PCA and MCA vessels demonstrated on both the affected and the unaffected sides of most of the subjects provides confidence that even an incomplete study is likely to be useful. Also, given the safety and wide availability of TCD, these results justify further TCD work in patients with SWS. With further development, TCD may be considered as a clinical outcome for future clinical trials to gauge progression of SWS.
In conclusion, the correlations between mean flow velocity on TCD in SWS patients and clinical outcomes suggest that TCD has the potential to predict neurologic progression of SWS and aid in clinical care. TCD may also be useful in assessing treatment response and screening for the need for more aggressive treatment. Further study, particularly of MCA measurements, is needed to demonstrate the clinical utility of TCD in SWS.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) (National Institutes of Health [NIH] U54NS065705) (to Dr. Lawton; Sturge-Weber Project PI Dr. Comi) and from Celebrate Hope Foundation (to Dr. Comi). The Brain Vascular Malformation Consortium (U54NS065705) is a part of the NIH Rare Diseases Clinical Research Network (RDCRN), supported through the collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS) and the NINDS.
References
- 1.Comi A. Current Therapeutic Options in Sturge-Weber Syndrome. Seminars in Pediatric Neurology. 2015 Dec;22(4):295–301. doi: 10.1016/j.spen.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comi AM, Marchuk DA, Pevsner J. A needle in a haystack: Sturge-Weber syndrome gene discovery. Pediatric Neurology. 2013 Dec;49(6):391–2. doi: 10.1016/j.pediatrneurol.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Shirley MD, Tang H, Gallione CJ, Baugher JD, Freline LP, Cohen B, North PE, Marchuk DA, Comi AM, Pevsner J. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. The New England Journal of Medicine. 2013 May 23;368(21):1971–9. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kossoff EH, Bachur CD, Quain AM, Ewen JB, Comi AM. EEG evolution in Sturge-Weber syndrome. Epilepsy Research. 2014 May;108(4):816–9. doi: 10.1016/j.eplepsyres.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiron C, Raynaud C, Tzourio N, Diebler C, Dulac O, Zilbovicius M, Syrota A. Regional cerebral blood flow by SPECT imaging in Sturge-Weber disease: an aid for diagnosis. Journal of Neurology, Neurosurgery, & Psychiatry. 1989 Dec;52(12):1402–9. doi: 10.1136/jnnp.52.12.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinton F, Chiron C, Enjolras O, Motte J, Syrota A, Dulac O. Early single photon emission computed tomography in Sturge-Weber syndrome. Journal of Neurology, Neurosurgery, & Psychiatry. 1997 Nov;63(5):616–21. doi: 10.1136/jnnp.63.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, Abboud M, Gallagher D, Kutlar A, Nichols FT, Bonds DR, Brambilla D. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. The New England Journal of Medicine. 1998 Jul 2;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 8.Jordan LC, Wityk RJ, Dowling MM, DeJong MR, Comi AM. Transcranial Doppler ultrasound in children with Sturge-Weber syndrome. Journal of Child Neurology. 2008 Feb;23(2):137–43. doi: 10.1177/0883073807307079. [DOI] [PubMed] [Google Scholar]
- 9.Bode H, Wais U. Age dependence of flow velocities in basal cerebral arteries. Archives of Disease in Childhood. 1988 Jun;63(6):606–11. doi: 10.1136/adc.63.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley TM, Hatfield LA, Lin DD, Comi AM. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. Journal of Child Neurology. 2005 Nov;20(11):867–70. doi: 10.1177/08830738050200110201. [DOI] [PubMed] [Google Scholar]
- 11.Jansen FE, van Huffelen AC, Witkamp T, Couperus A, Teunissen N, Wieneke GH, van Nieuwenhuizen O. Diazepam-enhanced beta activity in Sturge Weber syndrome: its diagnostic significance in comparison with MRI. Clinical Neurophysiology. 2002 Jul;113(7):1025–9. doi: 10.1016/s1388-2457(02)00105-0. [DOI] [PubMed] [Google Scholar]
- 12.Meila D, Lisseck K, Jacobs C, Lanfermann H, Brassel F, Feldkamp A. Cranial Doppler ultrasound in Vein of Galen malformation. Neuroradiology. 2015 Feb;57(2):211–9. doi: 10.1007/s00234-014-1455-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Duan YY, Zhou HY, Yuan LJ, Zhang L, Wang W, Li LH, Li L. Middle cerebral arterial flow changes on transcranial color and spectral Doppler sonography in patients with increased intracranial pressure. Journal of Ultrasound in Medicine. 2014 Dec;33(12):2131–6. doi: 10.7863/ultra.33.12.2131. [DOI] [PubMed] [Google Scholar]
- 14.Lin DD, Barker PB, Hatfield LA, Comi AM. Dynamic MR perfusion and proton MR spectroscopic imaging in Sturge-Weber syndrome: correlation with neurological symptoms. Journal of Magnetic Resonance Imaging. 2006 Aug;24(2):274–81. doi: 10.1002/jmri.20627. [DOI] [PubMed] [Google Scholar]
- 15.Riela AR, Stump DA, Roach ES, McLean WT, Jr, Garcia JC. Regional cerebral blood flow characteristics of the Sturge-Weber syndrome. Pediatric Neurology. 1985 Mar-Apr;1(2):85–90. doi: 10.1016/0887-8994(85)90042-6. [DOI] [PubMed] [Google Scholar]
- 16.Miao Y, Juhász C, Wu J, Tarabishy B, Lang Z, Behen ME, Kou Z, Ye Y, Chugani HT, Hu J. Clinical correlates of white matter blood flow perfusion changes in Sturge-Weber syndrome: a dynamic MR perfusion-weighted imaging study. American Journal of Neuroradiology. 2011 Aug;32(7):1280–5. doi: 10.3174/ajnr.A2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aylett SE, Neville BG, Cross JH, Boyd S, Chong WK, Kirkham FJ. Sturge-Weber syndrome: cerebral haemodynamics during seizure activity. Developmental Medicine & Child Neurology. 1999 Jul;41(7):480–5. [PubMed] [Google Scholar]
- 18.Alkonyi B, Miao Y, Wu J, Cai Z, Hu J, Chugani HT, Juhász C. A perfusion-metabolic mismatch in Sturge-Weber syndrome: a multimodality imaging study. Brain & Development. 2012 Aug;34(7):553–62. doi: 10.1016/j.braindev.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comi AM, Fischer R, Kossoff EH. Encephalofacial angiomatosis sparing the occipital lobe and without facial nevus: on the spectrum of Sturge-Weber syndrome variants? Journal of Child Neurology. 2003 Jan;18(1):35–8. doi: 10.1177/08830738030180010601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.