Abstract
Objective
To examine whether experimentally-induced weight gain raises ambulatory blood pressure (BP) in healthy subjects and identify any relationship between changes in BP and changes in regional fat distribution.
Patients and Methods
Twenty-six normal weight subjects were randomized to 8 weeks of weight gain through overfeeding (n=16; age 30.4±6.6 years) or to weight maintenance (controls; n=10; age 27.1±7.7 years) between July, 2004, and August, 2010. Measures of body composition via dual energy X-ray absorptiometry and computed tomography, circulating biomarkers, and 24-hour ambulatory BP were obtained at baseline and after the 8-week experimental phase.
Results
Overfeeding resulted in 3.7 kg (95% CI 2.9, 4.5) increase in body weight in weight gainers, with increments in total (46.2 cm2, 95% CI 27.6, 64.9), visceral (13.8 cm2, 95% CI 5.8, 21.9), and subcutaneous fat (32.4 cm2, 95% CI 13.5, 51.3). No changes occurred in the maintenance group. Increases in 24-hour systolic BP (4 mmHg, 95% CI 1.6, 6.3), mean BP (1.7 mmHg, 95% CI 0.3, 3.3), and pulse pressure (2.8 mmHg, 95% CI 1.1, 4.4) were evident following weight gain in the experimental group, while BP remained unchanged in controls. Changes in mean BP correlated only with changes in visceral fat (rho=0.45, P=.02), but not with changes in other body composition measures.
Conclusion
Modest weight gain causes elevation in 24-hour BP in healthy subjects. The association between increased BP and abdominal visceral fat accumulation suggests that visceral deposition of adipose tissue may contribute specifically to the enhanced risk of hypertension associated with weight gain.
Keywords: ambulatory blood pressure, hypertension, obesity, visceral fat, weight gain
Excess body weight is widely recognized as a leading contributor to morbidity and mortality,1,2 being associated with enhanced vulnerability to a variety of cardiovascular and non-cardiovascular diseases including hypertension. Current estimates on prevalence of hypertension show that 36 to 47% of the obese population suffers from high blood pressure (BP), compared to 20% of normal weight individuals,3,4 and prospective studies have consistently identified increased body weight as a determinant of BP elevation and new-onset hypertension.5–7 Nevertheless, there is substantial variability in the disease risk conferred by excess weight when individuals are stratified solely based on their body mass index (BMI). In spite of its conventional use as a surrogate for total body adiposity, BMI does not discriminate between lean mass and fat mass, nor does it take into account fat partitioning among various depots. In this regard, growing evidence indicates that the anatomic location of fat accumulation is a key feature in determining risk status,8–10 suggesting that inter-individual differences in disease propensity may be partially ascribed to the heterogeneity in regional adiposity distribution existing at any given BMI. Specifically, abdominal visceral obesity has recently emerged as the obesity-phenotype conveying the most unfavorable health profile.
In comparison to total and subcutaneous adiposity, visceral fat is more closely related to cardiometabolic risk factors, such as fasting glucose, lipids, and endothelial function,11–13 as well as to the presence of overt diseases such as coronary atherosclerosis and stroke.14 In addition, visceral adiposity has been showed to perform better than other anthropometric measures as a predictor of cardiovascular and all-cause deaths.15,16
The critical role of visceral fat in obesity-related hypertension is increasingly apparent. Several population-based studies, including the Framingham and the Jackson cohorts, have linked visceral fat deposition to heightened BP values and greater prevalence of hypertension,11,12,17,18 with these associations being independent of total body weight and subcutaneous adiposity. More recently, observational longitudinal data on the impact of visceral fat accumulation on incident hypertension have also been reported.17,19–21
Nevertheless, unlike the relative abundancy of observational evidence connecting visceral fat deposition to high BP, there is a paucity of interventional, mechanistic studies addressing the effects of experimental fat gain, and specifically of increases in visceral fat, on BP in human subjects.
Building upon these considerations, we conducted a randomized, controlled study to examine whether experimental weight gain raises 24-hour ambulatory blood pressure in healthy individuals (primary outcome) and to define the relative contribution of changes in regional fat distribution (secondary outcome). We hypothesized that overfeeding-induced weight gain would increase ambulatory BP and that fat deposition in the visceral compartment would be preferentially associated with larger BP increments.
Patients and Methods
Study Population
Twenty-six nonobese, healthy individuals (16 male; mean±SD age: 29.1±7.1 years; BMI: 23.6±3.1 kg/m2) were recruited as part of a larger project on the effects of weight gain on cardiometabolic health.22–24 Eligible subjects had to be sedentary, nonsmokers, free of overt medical or psychiatric diseases and not taking any medications aside from the birth control pill for women. Absence of undiagnosed medical conditions was confirmed by physical examination, collection of medical history, and polysomnography to rule out sleep disordered breathing. A negative pregnancy test was required for women.
The protocol was approved by the Mayo Clinic Institutional Review Board and informed consent was obtained from all participants. These studies were conducted between July, 2004, and August, 2010.
Study Design
Subjects underwent an initial 3-day period of weight maintenance, during which they adhered to a dietary regimen consisting of 40% carbohydrate, 40% fat, and 20% protein. The individual calorie intake required for weight stability was determined by research dieticians after consultation with each participant.
Following baseline evaluation, enrolled subjects were randomized to an 8-week experimental protocol of either moderate weight gain (5% increase in body weight) or weight maintenance. Subjects assigned to the weight gain group (n=16) were instructed to increase their habitual food intake by increasing their portion sizes or by consuming 400–1200 extra kcal/day via dietary supplements. Available supplements were chocolate bars (king-size Snickers bar, 510 kcal; Mars Inc), ice-cream shakes (402 kcal), and nutritional energy drinks (Boost Plus, 360 kcal/8 oz; Nestle Nutrition). Participants randomized to weight maintenance (n=10) were instructed to continue with their normal diet for the 8-week experimental phase. Weight measures were obtained during the study ≥5 times/week and caloric intakes were adjusted to achieve the targeted increase in body weight in gainers. Adherence to usual diet was reinforced in maintainers if they exhibited ±2% changes in body weight. All subjects were advised to maintain their usual lifestyle routines throughout the study period with the exception of avoiding caffeine and alcohol consumption for 24-hour prior and on the day scheduled for measurements. Body composition, blood pressure and blood specimen measures were taken at study entry and after completion of the 8-week experimental protocol.
Measures
Body composition measures
Height and weight were measured by an electronic scale and a stadiometer, respectively, and BMI was calculated as weight in kilograms divided by height in meters squared. Waist and hip circumferences were measured by nonelastic tape.
Whole-body dual energy X-ray absorptiometry (DPX-IQ, Lunar Radiation, Madison, WI) was used to obtain total body fat mass and fat-free mass.
Single-slice abdominal computed tomographic scans were collected at three inter-vertebral spaces (L2–3, L3–4 and L4–5). Total, visceral, and subcutaneous fat areas were quantified by a blinded, trained observer as described elsewhere,25, and the average values from the three slices were derived.
Blood pressure measures
Resting supine systolic and diastolic BP (SBP/DBP) measurements were obtained using an automatic sphygmomanometer (Dinamap 8100, Critikon, Tampa FL). Ambulatory blood pressure monitors (Spacelabs 90207; Spacelabs Medical, Inc., Redmond WA) with cuffs of appropriate size were fitted to each subject’s arm. Readings were taken at 30 min intervals during the day and every 60 min at night (10 pm to 6 am). Participants were instructed to fill out a diary to record their activity when wearing the monitors. Artefactual readings were removed according to the manufacturer’s setting.
Twenty-four hour averages were computed for SBP, DBP, mean BP (MAP, mmHg), pulse pressure (PP, mmHg), and heart rate (HR, bpm). Mean daytime and nighttime values were estimated based on diary entries. Blood pressure variability was estimated as standard deviation of SBP and DBP for each 24-hour period.
Biochemical measures
Fasting morning blood samples for determination of lipid profile (high-density lipoprotein, HDL; low-density lipoprotein, LDL; total cholesterol; triglycerides), insulin, glucose, high-sensitivity C-reactive protein (hs-CRP), leptin and adiponectin were collected and processed as previously reported.23
Statistical Analyses
Data are presented as counts for categorical variables and means±SD for continuous variables. Changes from baseline to follow-up are expressed as means (95% confidence intervals, CI). Due to limited sample size and unmet assumptions for general linear models, non-parametric tests were applied. Subject characteristics at study entry were compared by group using Wilcoxon rank-sum and Chi-squared tests when appropriate. Wilcoxon signed-rank tests were applied to assess changes from baseline. To identify body composition parameters related to variations in BP, we ran Spearman’s rank correlations on the pooled sample. Multivariable regression models were also explored to assess independence of observed associations. JMP Pro 9.0 (SAS Institute Inc.) was used for statistical analyses, with the significance level set a priori at P<.05.
Results
Subject characteristics at study entry and at follow-up are listed in Table 1. Baseline demographic, anthropometric and body composition measures were comparable between groups. Glucose was higher in weight gainers while CRP was higher in weight maintainers (both P=.03) at study entry. There were no other group differences in baseline laboratory measures.
Table 1.
| Weight Gainers (n=16) | Controls (n=10) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | Weight Gain |
Change (95% CI) |
Baseline |
Weight Maintenance |
Change (95% CI) |
|
| Age, years | 30.4±6.6 | - | 27.1±7.7 | - | ||
| Male, n | 10 | - | 6 | - | ||
| Weight, kg | 71.9±12.9 | 75.6±13.4*** | 3.7 (2.9, 4.5) | 74.2±16.5 | 74.3±16.1 | 0.1 (−0.6, 0.8) |
| BMI, kg/m2 | 23.5±3.5 | 24.8±3.6*** | 1.3 (0.9, 1.5) | 23.6±2.7 | 23.7±2.6 | 0.1 (−0.2, 0.3) |
| Waist circumference, cm | 84.9±9.4 | 88.5±9.7*** | 3.6 (1.9, 5.2) | 80.1±10.8 | 80.6±11.1 | 0.5 (−0.1, 1.1) |
| Hip circumference, cm | 98.5±6.2 | 101.1±6.5** | 2.6 (1.3, 4) | 98.6±7.8 | 98.8±7.7 | 0.2 (−1.5, 1.7) |
| Total body fat mass, kg | 21.9±8.2 | 25.2±8.6*** | 3.3 (1.9, 4.4) | 20.4±6.7 | 21±6.7 | 0.6 (0, 1.1) |
| Total body fat-free mass, kg | 46.9±9.3 | 47.3±9.6 | 0.4 (−0.4, 1.3) | 50.6±12.1 | 50.2±12 | −0.4 (−1.4, 0.5) |
| % Body fat | 31.5±8.9 | 34.4±8.7*** | 2.9 (1.4, 4.3) | 28.7±6.7 | 29.4±6.6 | 0.7 (−0.1, 1.5) |
| Abdominal total fat area, cm2 | 197.1±99.3 | 243.3±105*** | 46.2 (27.6, 64.9) | 171.8±77.5 | 176±76.3 | 4.2 (−9.5, 17.8) |
| Abdominal visceral fat area, cm2 | 61.6±32.7 | 75.5±30.9** | 13.9 (5.8, 21.9) | 46.6±34.2 | 47.2±35.7 | 0.6 (−6.5, 7.7) |
| Abdominal subcutaneous fat area, cm2 | 135.5±77.4 | 167.9±82.9*** | 32.4 (13.5, 51.3) | 125.2±53.1 | 128.7±52.3 | 3.5 (−5.1, 12.1) |
| Insulin, U/mL | 5.2±2.6 | 6.6±3.1 | 1.4 (−0.7, 3.4) | 5.5±3.6 | 4.9±2.6 | −0.6 (−1.9, 0.8) |
| Glucose, mg/dL | 93.5±4.9 | 98.9±10.4 | 5.4 (−1.1, 11.8) | 85.8±6.4† | 87.3±3.8 | 1.5 (−4.4, 7.4) |
| Total Cholesterol, mg/dL | 162.9±20.7 | 163.1±35.3 | 0.2 (−15.9, 16.5) | 166.8±17.9 | 164.8±13.6 | −2 (−15.8, 11.8) |
| HDL, mg/dL | 45.1±15.5 | 45±9.5 | −0.1 (−8.6, 8.5) | 43.9±8.3 | 43.1±7.2 | −0.8 (−6.2, 4.8) |
| LDL, mg/dL | 102.4±19.4 | 101.6±29.7 | −0.8 (−14, 12.5) | 106.9±13.5 | 106.6±9.9 | −0.3 (−8.1, 7.6) |
| Triglycerides, mg/dL | 77.1±28.8 | 86±46.5 | 8.9 (−11.4, 29.3) | 80.8±22.8 | 69.1±5.9 | −11.7 (−37.5, 14.1) |
| hs-CRP, mg/L | 0.04±0.03 | 0.3±0.7 | 0.3 (−0.1, 0.7) | 0.3±0.6† | 0.07±0.04 | −0.2 (−0.9, 0.4) |
| Leptin, ng/mL | 7.3±4.6 | 11.7±5.9*** | 4.4 (2.6, 6.3) | 5.6±3 | 6.9±4.5 | 1.3 (−0.7, 3.3) |
| Adiponectin, ng/mL | 7324±3874 | 8986±5463* | 1662 (−19, 3343) | 7508±2076 | 8009±2024 | 501 (−492, 1495) |
Values are means±SD. Changes from baseline to follow-up are expressed as mean (95% CI).
P<.05,
P<.01,
P<.001 for within-group comparisons as determined from Wilcoxon signed rank tests.
P<.05 for between-group comparisons are determined from Wilcoxon rank-sum tests.
BMI = body mass index; HDL = high-density lipoprotein; hs-CRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein.
SI conversion factors: To convert insulin to pmol/L, multiply by 6.945; to convert glucose to mmol/L, multiply by .0555; to convert total cholesterol, HDL, and LDL to mmol/L, multiply by .0259; to convert triglycerides to mmol/L, multiply by .0113; to convert leptin to µg/L, multiply by 1.0; to convert adiponectin to µg/mL, multiply by .001
As per study design, body weight increased by 3.7 kg (95% CI 2.9, 4.5) in weight gainers after 8 weeks of overfeeding (P<.001), while it remained unchanged in maintainers (P=.79). Waist (P=.001) and hip circumferences (P=.002) increased in weight gainers in response to excess calorie consumption.
Analysis of imaging body composition measures showed that in the experimental group weight gain occurred through increments in fat mass (P<.001), as the fat-free mass did not vary (P=.40). Both subcutaneous (P<.001) and visceral fat areas (P=.003) increased in weight gainers, leading to enlarged total abdominal fat area following overfeeding (P<.001). All body composition metrics were stable in subjects assigned to weight maintenance (all P>.23).
Insulin and lipid profile did not change appreciably after the dietary intervention (all P>.24), while leptin (P<.001) and adiponectin (P=.03) were significantly elevated as compared to baseline in weight gainers. No other changes were seen in either group.
Blood pressure measures are summarized in Table 2. Office measurements of BP and HR did not vary significantly after the 8-week experimental phase in either the experimental group or control group. However, increases in 24-hour SBP (P=.009), 24-hour MAP (P=.02), and 24-hour PP (P=.003) were evident following weight gain. No significant changes occurred in 24-hour DBP (P=.10) or 24-hour HR (P=.53) after weight gain.
Table 2.
| Weight Gainers (n=16) | Controls (n=10) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | Weight Gain |
Change (95% CI) |
Baseline |
Weight Maintenance |
Change (95% CI) |
|
| Office BP | ||||||
| Office SBP, mmHg | 115.9±12.3 | 117.6±15.3 | 1.7 (−6.5, 9.8) | 113.4±9.8 | 114.8±7.8 | 1.4 (−4.6, 7.4) |
| Office DBP, mmHg | 73.6±11.6 | 70.6±11.1 | −3 (−9.5, 3.6) | 68.3±5.4 | 69.6±9.4 | 1.3 (−6.6, 9.2) |
| Office HR, bpm | 65±8.8 | 66.8±8.2 | 1.8 (−3, 6.5) | 67.5±15.3 | 66.1±12.9 | −1.4 (−6.1, 3.3) |
| 24-h BP | ||||||
| SBP, mmHg | 113.7±8 | 117.7±7.9** | 4 (1.6, 6.3) | 115.6±7 | 116±7 | 0.4 (−2.5, 3.4) |
| DBP, mmHg | 70.7±4.3 | 71.9±4.4 | 1.2 (−0.2, 2.6) | 68.6±3.7 | 68.9±4.3 | 0.3 (−1.9, 2.7) |
| MAP, mmHg | 85.1±4.9 | 86.8±5.1* | 1.7 (0.3, 3.3) | 84.1±2.9 | 84.4±3.5 | 0.3 (−1.9, 2.6) |
| PP, mmHg | 43±5 | 45.8±5** | 2.8 (1.1, 4.4) | 47±7.7 | 47.1±7.7 | 0.1 (−1.5, 1.6) |
| HR, bpm | 71±8.8 | 72±7.8 | 1 (−2.9, 5) | 69.8±9.2 | 72.7±9.3* | 2.9 (0.9, 5) |
| Daytime BP | ||||||
| SBP, mmHg | 117.7±8.4 | 121.5±8.9** | 3.8 (1.3, 6.2) | 118±7.2 | 118.7±6.9 | 0.7 (−1.9, 3.4) |
| DBP, mmHg | 74.4±4.5 | 75.6±5 | 1.2 (−0.1, 2.4) | 71.3±3.7 | 71.7±4.7 | 0.4 (−2.2, 3) |
| MAP, mmHg | 88.7±5.2 | 90.4±5.9* | 1.7 (0.3, 3) | 86.7±3.1 | 87.3±3.8 | 0.6 (−1.8, 3) |
| PP, mmHg | 43.3±5.2 | 45.9±5.3** | 2.6 (0.7, 4.6) | 46.7±8.1 | 47±7.6 | 0.3 (−1.1, 1.9) |
| HR, bpm | 74.3±9.7 | 74.8±8.1 | 0.5 (−3.9, 4.9) | 71.9±9.7 | 75.9±9.5** | 4 (1.6, 6.4) |
| Nighttime BP | ||||||
| SBP, mmHg | 100.9±7 | 105.8±6.6* | 4.9 (1.3, 8.6) | 106.6±8.2 | 106.7±9 | 0.1 (−5.8, 6) |
| DBP, mmHg | 58.3±4.5 | 59.7±5.1 | 1.4 (−1.4, 4.3) | 58.1±4.8 | 59.7±4.5 | 1.6 (−1, 4.1) |
| MAP, mmHg | 73.1±4.5 | 75.3±4.8 | 2.2 (−0.7, 5.2) | 74.4±4.3 | 75±4.3 | 0.6 (−2.9, 4.1) |
| PP, mmHg | 42.6±5.5 | 46.1±6* | 3.5 (0.8, 6.3) | 48.5±8.1 | 47.1±7.9 | −1.4 (−5.1, 2.3) |
| HR, bpm | 60.4±7 | 62.8±8.4 | 2.4 (−1.8, 6.6) | 61.2±8.9 | 62±10.3 | 0.8 (−2.7, 4.2) |
| BP Variability | ||||||
| SBP, mmHg | 11.4±2.4 | 11.1±3.1 | −0.3 (−1.6, 1.1) | 10.1±2 | 11.5±2.8 | 1.4 (−0.7, 3.4) |
| DBP, mmHg | 10.7±1.9 | 10.7±1.8 | 0 (−1, 1.1) | 10.1±2.2 | 10.4±2.3 | 0.3 (−1.3, 2) |
Values are means±SD. Changes from baseline to follow-up are expressed as mean (95% CI).
P<.05,
P<.01 for within-group comparisons as are determined from Wilcoxon signed-rank tests.
BP = blood pressure; DBP = diastolic blood pressure; HR = heart rate; MBP = mean blood pressure; PP = pulse pressure; SBP = systolic blood pressure.
Evaluation of the diurnal profile showed that SBP was higher during both daytime (P=.009) and nighttime (P=.01) in weight gainers after overfeeding. A similar pattern was seen in PP, which was higher in both the daytime (P=.009) and nighttime (P=.02), while MAP was significantly more elevated during daytime only (P=.03). There were no significant changes in daytime and nighttime readings of DBP and HR (all P>.12). Ambulatory BP measures remained unchanged in weight maintainers (all P>.13) but they exhibited higher 24-hour (P=.01) and daytime HR (P=.002) at follow-up. Blood pressure variability was stable in both groups (all P>.19).
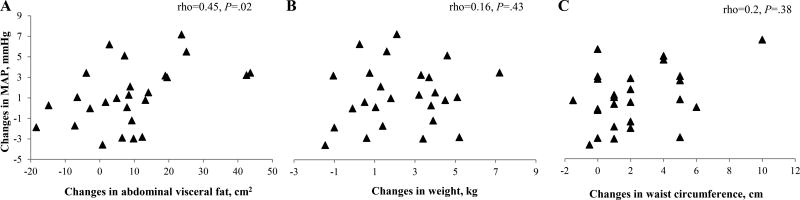
Spearman’s rank correlations run on the pooled sample showed that the magnitude of changes in MAP were significantly related to the magnitude of changes in abdominal visceral fat (rho=0.45, P=.02) (Figure 1, Panel A). Elevation in MAP was not related to increases in body weight (rho=0.16, P=.43; Figure 1, Panel B) or waist circumference (rho=0.2, P=.38; Figure 1, Panel C). No associations were seen with total body fat (rho=0.02, P=.92) or subcutaneous body fat (rho=0.16, P=.44) either. Changes in visceral fat remained significantly associated with changes in BP even when controlling for weight gain (R2=0.21, P=.03; visceral fat estimate=0.1, P=.03), and when controlling for changes in total adiposity (R2=0.29, P=.02; visceral fat estimate=0.13, P=.005). None of these variables was associated with increases in BP (weight estimate=−0.1, P=.73; total adiposity estimate=−0.0005, P=.1).
Figure 1.
Relation between changes in MAP and changes in abdominal visceral fat (Panel A), weight (Panel B), and waist circumference (Panel C) in the pooled sample (N=26). MAP= mean arterial pressure.
Discussion
Our data show that the exposure of lean, healthy individuals to short-term, overfeeding-induced modest weight gain caused significant elevation in 24-hour ambulatory BP, and that this pressor response was selectively associated with visceral fat expansion. This is the first randomized, controlled study of experimental weight gain in humans to demonstrate the role of visceral fat in contributing to increased 24-hour mean BP, independently of generalized weight gain or fat accumulation.
Twenty four-hour SBP, MAP and PP increased after 8 weeks of excess calorie intake, while neither DBP nor HR varied significantly. The increments in ambulatory SBP and PP observed after 5% weight gain occurred during both daytime and nighttime, while MAP was significantly higher during daytime. Notably, the average magnitude of elevation we recorded, while modest, is prognostically significant, as a 2 mmHg increase in SBP is associated with 7% risk of ischemic heart disease and 10% risk of stroke on a population level.26
It is now established that ambulatory BP outperforms standard office measures, with compelling evidence attesting to its clinical and prognostic significance. Twenty-four hour BP predicts fatal and non-fatal cardiovascular events independent of conventional covariates including office BP,27–29 and correlates more closely with target organ damage than do clinic readings.30–32
In addition, by delineating the BP patterning over the 24-hour period, ambulatory BP monitoring enables characterization of daytime vs nighttime BP separately. In this regard, the predictive power of nighttime BP exceeds that of office and even 24-hour BP for subclinical organic injury30,33 and adverse cardiovascular outcomes including cardiovascular mortality,27–29 thus further supporting the superiority of BP measures derived from ambulatory BP monitoring. Previous investigations using similar experimental approaches have examined the impact of overfeeding-induced weight gain on BP,34–37 although only a few of them included ambulatory recordings.34,37 While Gentile et al35 reported higher ambulatory and office BP following a 5 kg-increment in body weight, Gupta et al36 found that 5–10% weight gain raised 24-hour ambulatory BP in absence of significant variations in resting measurements. Importantly, those studies lacked a control group of weight maintainers, and the increased body weight resulted from increments in both fat mass and fat-free mass.34–37 In our study higher body weight was achieved through fat accumulation only, as the fat-free mass remained unchanged; therefore the effects we found can be unequivocally attributed to augmented adiposity.
The observed increase in the pulsatile component of BP, as reflected by amplification of PP, in combination with heightened SBP (and unchanged DBP), suggests reduced vascular distensibility and compliance in response to overfeeding. When interpreted in the context of our prior findings of impaired endothelial function after moderate weight gain,23 these data are indicative of increased arterial stiffness. Such a hypothesis is consistent with a prior report of higher arterial stiffness and diminished arterial compliance secondary to weight gain,37 and corroborates the idea of early vascular damage induced by fat accumulation.
Further insights into the mechanisms involved in the pressor response to excess calorie intake arise from the association found between BP increases and expanded visceral adipose tissue depot. Although the pathophysiology of obesity-related hypertension is likely multifactorial, a growing body of research indicates that visceral fat may be a key contributor.17,19–21 Our findings are in line with this construct, as we observed, for the first time, that the elevation in BP following overfeeding was not related to changes in body weight or total body fat but specifically to increases in visceral fat.
A number of mechanisms have been implicated in the BP surge associated with visceral fat accumulation. Visceral adiposity has been proposed as a link between obesity and sympathetic upregulation,38,39 a key mediator of comorbid hypertension in the obese population.40,41 Central sympathetic outflow, as measured from muscle sympathetic nerve activity, is higher in visceral obese than in subcutaneous obese38 and does not differ between subcutaneously obese and nonobese men with similar levels of visceral adiposity.39 Similarly, cardiac sympathetic activity was found to be higher in visceral obese than in subcutaneous obese,42 and more closely related to visceral than to subcutaneous fat depots.43 Since the renin-angiotensin system (RAS) is overexpressed in the visceral compartment,44,45 expansion of this fat depot could elicit angiotensinogen and angiotensin II production, which would then increase BP. Support for this idea arises from prior research showing activation of systemic35 and adipose tissue46 RAS in response to overfeeding. However, we cannot exclude the possibility that visceral fat gain does not play a causative role in the BP elevation observed in our study, and a third factor may drive changes in both variables. In this regard, RAS activation secondary to excess calorie consumption, and particularly increased angiotensin II, may both raise BP and favor visceral fat accumulation, as suggested by an in-vitro study reporting stimulation of human visceral adipocyte proliferation by angiotensin II.47 Another mechanism potentially responsible for the observed effects involves overfeeding-induced upregulation of the hypothalamic–pituitary–adrenal axis, which may mediate both BP elevation48 and predisposition to visceral fat accumulation.49 Further research is needed to directly address these hypotheses.
Strengths of our work include the application of a randomized, controlled, interventional, longitudinal study design; inclusion of only healthy, nonobese individuals without prior risk factors; and a control group of weight maintainers. Furthermore, to determine body composition, we used gold standard imaging techniques that yield quantitative and qualitative characterization of body fat compartments. Nonetheless, several limitations have to be recognized, including the modest sample size which precludes us from assessing whether the impact of weight gain varied based on gender or age. The changes in BP we observed occurred in response to a relatively short intervention period and it is plausible that adaptation may occur following prolonged overfeeding and increased body weight. Hence, larger studies and longer periods of follow-up are warranted. Furthermore, our study was not designed to identify the biological mechanisms underlying changes in BP in response to excess calorie intake nor the role of individual macronutrients.
The early pressor response observed following 5% increase in body weight highlights the detrimental impact of even modest weight gain. Our findings are in accord with previous research documenting that even moderate increments in body weight raise vulnerability to cardiovascular disease, and particularly to hypertension.6,50,51 A longitudinal analysis of the Framingham cohort found that a 5% increment in body weight, which aligns to the magnitude of weight gain sought in our study, was associated with a 20–30% enhanced likelihood of future hypertension.51 Importantly, the health consequences of modest weight gain are likely exacerbated when fat is primarily accumulated in the abdominal visceral region.
In terms of preventive and therapeutic strategies for BP management, the antihypertensive effects of weight control are well-established52 and clinically relevant benefits can be achieved with moderate weight loss.53,54 Mirroring the differential impact of regional fat depots on disease risk, evidence suggests that selective reductions in visceral fat are more effective in improving the cardiometabolic profile, including lowering BP, compared to reduced subcutaneous fat depots and generalized weight loss.55–57 Therefore, efforts to identify weight loss and weight management interventions preferentially facilitating visceral fat mobilization should be undertaken.
Conclusion
Our research shows that moderate weight gain in healthy individuals leads to elevation in blood pressure, with the rise in mean BP being selectively related to increases in visceral adipose tissue. These findings have important epidemiological and clinical implications, suggesting that healthy subjects who are predisposed to visceral fat gain are also predisposed to accompanying increases in blood pressure. Therefore, the accumulation of abdominal visceral fat may be a critical determinant in the high risk of hypertension associated with weight gain and obesity. Furthermore, even modest weight gain can contribute to an increase in blood pressure if the fat accumulation is predominantly visceral.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health (NIH) grants RO1 HL73211 and R21 DK81014 to V.K.S. N.C. is supported by American Heart Association grant 16SDG27250156. P.S. is supported by CCaTS Early Stage Investigator Award. V.K.S is supported by NIH grants RO1 HL065176, RO1 HL114024, RO1 HL114676, RO1 HL134808, and RO1 HL134885. This publication was made possible by the Mayo Clinic CCaTS through grant number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS), a component of the NIH.
V.K.S. served as a consultant for Respicardia, ResMed, Sorin Inc., U-Health, Philips, Ronda Grey and Glaxo Smith Kline and is working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease. F.H.S.K. is a full-time employee of Philips Respironics.
Abbreviations
- BMI
Body Mass Index
- BP
Blood Pressure
- DBP
Diastolic Blood Pressure
- HDL
High-Density Lipoprotein
- HR
Heart Rate
- hs-CRP
High-Sensitivity C-Reactive Protein
- LDL
Low-Density Lipoprotein
- MAP
Mean Arterial Pressure
- PP
Pulse Pressure
- RAS
Renin-Angiotensin System
- SBP
Systolic Blood Pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was previously presented in Abstract form at the High Blood Pressure Research 2014 Scientific Sessions of the American Heart Association. (Hypertension. 2014;64:A029).
Disclosures
The other authors report no conflicts.
References
- 1.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 3.Saydah S, Bullard KM, Cheng Y, et al. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999–2010. Obesity (Silver Spring) 2014;22(8):1888–1895. doi: 10.1002/oby.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 5.Droyvold WB, Midthjell K, Nilsen TI, Holmen J. Change in body mass index and its impact on blood pressure: a prospective population study. Int J Obes (Lond) 2005;29(6):650–655. doi: 10.1038/sj.ijo.0802944. [DOI] [PubMed] [Google Scholar]
- 6.Juhaeri J, Stevens J, Chambless LE, et al. Associations between weight gain and incident hypertension in a bi-ethnic cohort: the Atherosclerosis Risk in Communities Study. Int J Obes Relat Metab Disord. 2002;26(1):58–64. doi: 10.1038/sj.ijo.0801846. [DOI] [PubMed] [Google Scholar]
- 7.Shihab HM, Meoni LA, Chu AY, et al. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation. 2012;126(25):2983–2989. doi: 10.1161/CIRCULATIONAHA.112.117333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med. 2015;163(11):827–835. doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162(18):2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 10.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab. 2002;282(5):E1023–1028. doi: 10.1152/ajpendo.00467.2001. [DOI] [PubMed] [Google Scholar]
- 11.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh NI, Keyes MJ, Larson MG, et al. Visceral and subcutaneous adiposity and brachial artery vasodilator function. Obesity (Silver Spring) 2009;17(11):2054–2059. doi: 10.1038/oby.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30(7):850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14(2):336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 17.Seven E, Thuesen BH, Linneberg A, Jeppesen JL. Abdominal adiposity distribution quantified by ultrasound imaging and incident hypertension in a general population. Hypertension. 2016;68(5):1115–1122. doi: 10.1161/HYPERTENSIONAHA.116.07306. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Boyko EJ, Leonetti DL, et al. Visceral adiposity and the prevalence of hypertension in Japanese Americans. Circulation. 2003;108(14):1718–1723. doi: 10.1161/01.CIR.0000087597.59169.8D. [DOI] [PubMed] [Google Scholar]
- 19.Chandra A, Neeland IJ, Berry JD, et al. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol. 2014;64(10):997–1002. doi: 10.1016/j.jacc.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan CA, Kahn SE, Fujimoto WY, Hayashi T, Leonetti DL, Boyko EJ. Change in intra-abdominal fat predicts the risk of hypertension in Japanese Americans. Hypertension. 2015;66(1):134–140. doi: 10.1161/HYPERTENSIONAHA.114.04990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi T, Boyko EJ, Leonetti DL, et al. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann Intern Med. 2004;140(12):992–1000. doi: 10.7326/0003-4819-140-12-200406150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Adachi T, Sert-Kuniyoshi FH, Calvin AD, et al. Effect of weight gain on cardiac autonomic control during wakefulness and sleep. Hypertension. 2011;57(4):723–730. doi: 10.1161/HYPERTENSIONAHA.110.163147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, et al. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J Am Coll Cardiol. 2010;56(8):662–666. doi: 10.1016/j.jacc.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh P, Somers VK, Romero-Corral A, et al. Effects of weight gain and weight loss on regional fat distribution. Am J Clin Nutr. 2012;96(2):229–233. doi: 10.3945/ajcn.111.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potretzke AM, Schmitz KH, Jensen MD. Preventing overestimation of pixels in computed tomography assessment of visceral fat. Obes Res. 2004;12(10):1698–1701. doi: 10.1038/oby.2004.210. [DOI] [PubMed] [Google Scholar]
- 26.Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. The Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 27.Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999;282(6):539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 28.Kikuya M, Ohkubo T, Asayama K, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality. Hypertension. 2005;45(2):240–245. doi: 10.1161/01.HYP.0000152079.04553.2c. [DOI] [PubMed] [Google Scholar]
- 29.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality. Hypertension. 2005;46(1):156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 30.Benhamou PY, Halimi S, Gaudemaris RD, et al. Early disturbances of ambulatory blood pressure load in normotensive type I diabetic patients with microalbuminuria. Diabetes Care. 1992;15(11):1614–1619. doi: 10.2337/diacare.15.11.1614. [DOI] [PubMed] [Google Scholar]
- 31.Opsahl JA, Abraham PA, Halstenson CE, Keane WF. Correlation of office and ambulatory blood pressure measurements with urinary albumin and N-acetyl-βD-glucosaminidase excretions in essential hypertension. Am J Hypertens. 1988;1(3 Pt 3):117S–120S. doi: 10.1093/ajh/1.3.117s. [DOI] [PubMed] [Google Scholar]
- 32.Su TC, Lee YT, Chou S, Hwang WT, Chen CF, Wang JD. Twenty-four-hour ambulatory blood pressure and duration of hypertension as major determinants for intima-media thickness and atherosclerosis of carotid arteries. Atherosclerosis. 2006;184(1):151–156. doi: 10.1016/j.atherosclerosis.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 33.Cuspidi C, Facchetti R, Bombelli M, et al. Nighttime blood pressure and new-onset left ventricular hypertrophy: findings from the Pamela population. Hypertension. 2013;62(1):78–84. doi: 10.1161/HYPERTENSIONAHA.111.00682. [DOI] [PubMed] [Google Scholar]
- 34.Gentile CL, Orr JS, Davy BM, Davy KP. Cardiorespiratory fitness influences the blood pressure response to experimental weight gain. Obesity (Silver Spring) 2007;15(12):3005–3012. doi: 10.1038/oby.2007.358. [DOI] [PubMed] [Google Scholar]
- 35.Gentile CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1834–1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- 36.Gupta AK, Johnson WD, Johannsen D, Ravussin E. Cardiovascular risk escalation with caloric excess: a prospective demonstration of the mechanics in healthy adults. Cardiovasc Diabetol. 2013;12:23. doi: 10.1186/1475-2840-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orr JS, Gentile CL, Davy BM, Davy KP. Large artery stiffening with weight gain in humans: role of visceral fat accumulation. Hypertension. 2008;51(6):1519–1524. doi: 10.1161/HYPERTENSIONAHA.108.112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106(20):2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287(1):H414–H418. doi: 10.1152/ajpheart.01046.2003. [DOI] [PubMed] [Google Scholar]
- 40.Kalil GZ, Haynes WG. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens Res. 2012;35(1):4–16. doi: 10.1038/hr.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48(5):787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 42.Gao YY, Lovejoy JC, Sparti A, Bray GA, Keys LK, Partington C. Autonomic activity assessed by heart rate spectral analysis varies with fat distribution in obese women. Obesity (Silver Spring) 1996;4(1):55–63. doi: 10.1002/j.1550-8528.1996.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 43.Hillebrand S, Mutsert R, Christen T, et al. Body fat, especially visceral fat, is associated with electrocardiographic measures of sympathetic activation. Obesity (Silver Spring) 2014;22(6):1553–1559. doi: 10.1002/oby.20709. [DOI] [PubMed] [Google Scholar]
- 44.Giacchetti G, Faloia E, Mariniello B, et al. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens. 2002;15(5):381–388. doi: 10.1016/s0895-7061(02)02257-4. [DOI] [PubMed] [Google Scholar]
- 45.Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta. 2000;1500(1):88–96. doi: 10.1016/s0925-4439(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 46.Alligier M, Meugnier E, Debard C, et al. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J Clin Endocrinol Metab. 2012;97(2):E183–E192. doi: 10.1210/jc.2011-2314. [DOI] [PubMed] [Google Scholar]
- 47.Sarzani R, Marcucci P, Salvi F, et al. Angiotensin II stimulates and atrial natriuretic peptide inhibits human visceral adipocyte growth. Int J Obes (Lond) 2008;32(2):259–267. doi: 10.1038/sj.ijo.0803724. [DOI] [PubMed] [Google Scholar]
- 48.Grassi G, Seravalle G, Dell’Oro R, et al. Participation of the hypothalamus-hypophysis axis in the sympathetic activation of human obesity. Hypertension. 2001;38(6):1316–1320. doi: 10.1161/hy1201.096117. [DOI] [PubMed] [Google Scholar]
- 49.Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production rates, free cortisol, and 11β-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab. 2009;296(2):E351–E357. doi: 10.1152/ajpendo.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Z, Willett WC, Manson JE, et al. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128(2):81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 51.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358(9294):1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 52.Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension. 2016;67:733–739. doi: 10.1161/HYPERTENSIONAHA.115.06853. [DOI] [PubMed] [Google Scholar]
- 53.Engeli S, Böhnke J, Gorzelniak K, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45(3):356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 54.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134(1):1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 55.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48(4):839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 56.Kanai H, Tokunaga K, Fujioka S, Yamashita S, Kameda-Takemura K, Matsuzawa Y. Decrease in intra-abdominal visceral fat may reduce blood pressure in obese hypertensive women. Hypertension. 1996;27(1):125–129. doi: 10.1161/01.hyp.27.1.125. [DOI] [PubMed] [Google Scholar]
- 57.Miyatake N, Takahashi K, Wada J, et al. Daily exercise lowers blood pressure and reduces visceral adipose tissue areas in overweight Japanese men. Diabetes Res Clin Pract. 2003;62(3):149–157. doi: 10.1016/s0168-8227(03)00176-1. [DOI] [PubMed] [Google Scholar]