Abstract
Although people with HIV infection (PLWH) are at higher risk of polypharmacy and substance use, there is limited knowledge about potential harms associated with polypharmacy such as falls and fractures in this population. The study objective was to determine whether polypharmacy, as measured by the number and type of medication, is associated with falls and fractures among PLWH and DSM-IV substance dependence in the past year or ever injection drug use (IDU). We identified the number of medications by electronic medical record review in the following categories: i) systemically active, ii) non-antiretroviral (non-ARV), iii) sedating, iv) non-sedating as well as any opioid medication and any non-opioid sedating medication. Outcomes were self-reported 1) fall/accident requiring medical attention and 2) fracture in the previous year. Separate logistic regression models were fitted for medications in each category and each outcome. Among 250 participants, the odds of a fall requiring medical attention were higher with each additional medication overall (odds ratio [OR] 1.12, 95% Confidence Interval [CI]=1.05, 1.18), each additional non-ARV medication (OR 1.13, 95%CI=1.06, 1.20), each additional sedating medication (OR 1.36, 95%CI=1.14, 1.62), and a non-opioid sedating medication (OR 2.89, 95%CI=1.06, 7.85) but not with an additional non-sedating medication or opioid medication. In receiver operating characteristic (ROC) curve analyses, optimal cutoffs for predicting falls were: ≥8 overall and ≥2 sedating medications. Odds ratios for fracture in the previous year were OR 1.05, 95%CI=0.97, 1.13 for each additional medication overall and OR 1.11, 95%CI=0.89, 1.38 for each additional sedating medication. In PLWH and substance dependence or ever IDU, a higher number of medications was associated with greater odds of having a fall requiring medical attention. The association appeared to be driven largely by sedating medications. Future studies should determine if reducing such polypharmacy, particularly sedating medications, lowers the risk of falls.
BACKGROUND
An increasingly important challenge for the treatment of HIV infection is management of comorbid chronic health conditions (Deeks, Lewin, & Havlir, 2013; Greene, Justice, Lampiris, & Valcour, 2013). Care for PLWH who experience durable HIV viral suppression has shifted from acute care of AIDS-related infections to management of HIV-associated chronic conditions such as coronary artery disease (Freiberg et al., 2013), chronic obstructive pulmonary diseases (Gingo et al., 2014), and diabetes mellitus (Butt et al., 2009). It is estimated that about half of the population with HIV in the United States is 50 or older (Greene et al., 2013). As a result, the number of medications prescribed to PLWH has increased even as antiretroviral medication (ARV) pill burden has decreased (Edelman et al., 2013; Greene et al., 2013). Compared with HIV-uninfected populations, PLWH have a higher burden of chronic pain, mental health disorders, and falls and injuries (K. M. Erlandson et al., 2016) that may lead to prescription of opioid or other sedating medications (Vijayaraghavan, Freitas, Bangsberg, Miaskowski, & Kushel, 2014). While the potential impact of polypharmacy on adherence and medication interactions is well-recognized (Cantudo-Cuenca, Jiménez-Galán, Almeida-Gonzalez, & Morillo-Verdugo, 2014; Edelman et al., 2013), little is known about the impact of polypharmacy on other adverse outcomes such as falls and fractures despite recognition as significant problems in the elderly (Fried et al., 2014; Munson et al., 2016).
Each year 2.8 million older adults are treated in emergency departments for fall-related injuries (Centers for Disease Control and Prevention, 2015). Falls among the elderly cause the majority of hip fractures, which can lead to long-term functional impairments (Wolinsky, Fitzgerald, & Stump, 1997). HIV infection is associated with a higher risk of falls (Erlandson et al., 2012) due to multiple factors including premature frailty (Brothers et al., 2014; Erlandson et al., 2013), lower bone and muscle mass, imbalance symptoms (Erlandson et al., 2016), and substance use. One study found that fall risk among middle-aged PLWH paralleled the risk of falls in the HIV-uninfected elderly population (Erlandson et al., 2012). PLWH are more likely to have low bone mineral density and a higher risk of fracture (Dong, Cortés, Shiau, & Yin, 2014; Güerri-Fernandez et al., 2013). Factors contributing to low bone mineral density in this population include tobacco use - highly prevalent among PLWH (Pacek and Cioe, 2015) - and commonly prescribed HIV ARV medications such as tenofovir (Grant & Cotter, 2016).
Falls, injuries, and fractures are also common among people who use illicit drugs and excessive amounts of alcohol (Miller, Lestina, & Smith, 2001). People with unhealthy substance use are overrepresented among PLWH (Green et al., 2010; Vijayaraghavqn et al., 2014) and PLWH are particularly susceptible to harms related to substance use such as peripheral neuropathy and depression (Nicholas et al., 2007; Sullivan, Goulet, Justice & Fiellen, 2011). Binge alcohol use has increased among older adults with HIV (Han et al., 2017). Yet, prior studies on falls and fractures omit substance use data (Cantudo-Cuenca et al., 2014) or have relied on imprecise clinical data to detect and assess substance use (Erlandson et al., 2012).
Another challenge with quantifying risks of polypharmacy is that the commonly used definition of polypharmacy - 5 or more - was established in elderly populations (Gnjidic et al., 2012) and may not be applicable to PLWH given that HIV viral suppression generally requires ≥3 ARV medications. A higher prevalence of SUDs among PLWH may increase the risk of falls and fractures associated with polypharmacy. In addition, it is unclear if the number of medications, medication type, or both are of importance.
In this study, we assessed the associations between number of medications and falls and fractures in PLWH. We defined polypharmacy empirically by determining the best cutoffs for the number of medications associated with falls and fractures. We tested three hypotheses. First, a greater number of systemically active medications is associated with greater risk of fall and fracture. Second, excluding ARV medications from the medication count does not change the magnitude of association with fall and fracture. Third, the association of fall and fracture and total medication count is largely attributable to the number of sedating medications and not to non-sedating medications.
METHODS
Study design
This is a secondary analysis of data collected as part of the Boston ARCH Cohort study; a longitudinal study of 250 PLWH with past-year substance dependence (DSM IV criteria assessed with Mini International Neuropsychiatric Interview Version 6.0 (MINI) (Sheehan et al., 1998) or ever injection drug use (IDU). Participants were recruited from the Center for Infectious Diseases at Boston Medical Center and the HIV Program at Boston Healthcare for the Homeless Program between December 2012 and November 2014. Inclusion criteria were: documentation of HIV infection in medical record, past-year substance dependence and/or ever IDU (hereafter collectively referred to as “substance dependence”), ability to speak English, age 18 or older, and willingness to provide contact information for one other person to assist with follow-up. Exclusion criteria were: pregnancy at time of enrollment, plans to leave the Boston area in the next year, or cognitive impairment such that the patient could not provide informed consent. Participants provided written informed consent and received compensation for each study assessment completed. The Institutional Review Board of Boston University Medical Campus approved the study. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) further protected participants with a Certificate of Confidentiality and the US Department of Health and Human Services approved the performance of follow-up assessments with incarcerated participants.
Data collection
Trained research associates administered standardized in-person interviews at study entry and at a 12-month follow-up. Medication data were extracted at study entry from the electronic medical record (EMR) at both recruitment sites. Duplicate medications were removed by cross-referencing the National Drug Code (NDC) number with the generic name as defined in the National Drug File (“Veterans Affairs (VA) National Formulary”). Because multiple NDC numbers exist for the same medication, unique counts of “generic name” were used as a proxy for total number of medications. Combination ARV medications were recoded into individual drug components (e.g., Atripla® was recoded into 3 distinct medications: tenofovir, emtricitabine, and efavirenz). Two of the study investigators (T.K. and A.W.), guided by the U.S. Department of Veterans Affairs Drug Classification System (“Veterans Affairs National Formulary”) classified medications as “systemically active,” sedating/non-sedating, opioid sedating/non-opioid sedating, opioids prescribed for pain/addiction (Fig. 1). Sedating medications were those widely known to be sedating. Medications not systemically active included emollients, irrigation solutions, vitamins (except for vitamin D), peritoneal solutions, eye drops, rectal or vaginally administered medications, complementary and alternative medications.
Figure 1.
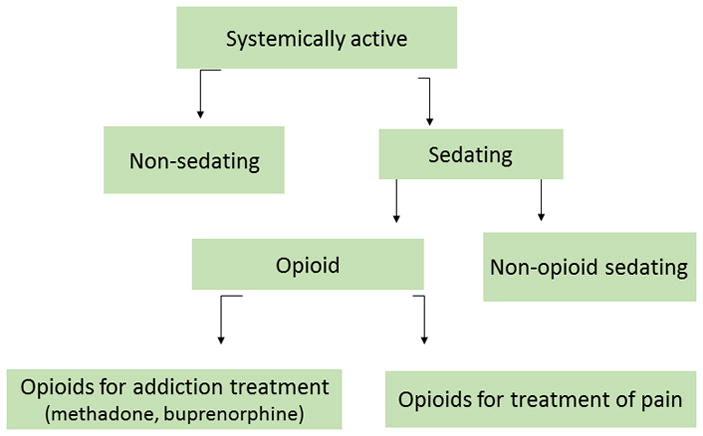
Summary of medication categories used in analyses
Measures
Outcomes
The primary outcomes were (1) any fall or accident requiring medical attention and (2) any fracture in the prior 12 months.
Main independent variables and covariates
The main independent variables were the number of prescribed 1) systemically active (“overall”) medications, 2) overall medications excluding ARV medications, 3) sedating medications, and 4) non-sedating medications. We also examined the effect of any opioid medication and any non-opioid sedating medication (Fig. 1).
Covariates included demographic data (age, sex, and race/ethnicity); medical comorbidity (Charlson Comorbidity Index Score [Quan et al., 2005]); physical functioning score (Kazis, Miller, and Clark, 2004), recent (past 30 day) use of the following: heavy alcohol (for men: > 14 drinks in a week or 5+ drinks in a day; for women: >7 drinks in a week or 4+ drinks in a day)(National Institutes of Health, 2016) (Timeline Follow Back [L.C. Sobell & M.B. Sobell, 1992]); cocaine; illicit opioids (includes prescription opioid misuse); and illicit sedatives. Drug use measures were assessed by Addiction Severity Index (McLellan, Luborsky, Woody, & O’Brien, 1980).
Statistical analysis
We used logistic regression to examine the association between the number of medications in each category (overall, non-ARV, sedating, and non-sedating) and each outcome assessed at baseline fitting separate models for each medication variable and each outcome. The analytic strategy is described below and summarized in Figure 1:
To assess whether the association of total medication count and falls and fracture is attributable to the number of sedating and/or non-sedating medications, we included the number of sedating and non-sedating medications in one model.
We assessed whether the association of sedating medications on falls or fractures is due to opioid and/or a non-opioid sedating medication by including the following in one logistic regression model: i) any prescribed opioid sedating medication (yes/no) and ii) any prescribed non-opioid sedating medication (yes/no). The latter 2 medication variables were examined as any vs. none because of the narrow range of the number of opioid and non-opioid sedating medications.
Given the possibility that the effect of opioid medications on falls or fractures is different for opioids prescribed for pain than for addiction treatment, we repeated this analysis replacing “any opioid medication” with two separate opioid medication variables (i.e. “any opioid prescribed for pain” (yes/no) and “any opioid for addiction” (yes/no). In this exploratory analysis, we defined opioids for addiction as buprenorphine or methadone, recognizing that they can be prescribed for pain.
To determine the best cutoff value for identifying the risk of each outcome, we used receiver operating characteristic (ROC) curve analyses to calculate the area under the curve for the number of medications, overall and sedating, without adjustment for covariates. We report the Youden Index (sensitivity + specificity − 1), a summary measure of the ROC curve, to determine the optimal cutoff point for significant associations. Given the number of falls (n=39) and fractures (n=21) in the sample, only a limited number of covariates could be included in a logistic regression model to control for confounding. Therefore, we performed a series of models for fall/accidents and fractures controlling separately for each covariate listed above.
We also performed confirmatory analyses adding fall and fracture data from the 12-month follow-up (fall, n=27; fracture, n=17) to the fall/fracture data at study entry. This analysis used two types of observations: i) medication data and past-year fall from the baseline assessment and ii) medication data at baseline and past-year fall from the 12-month follow-up. The same analytic strategy was used for the fracture analyses. We used separate generalized estimating equations (GEE) logistic regression models for each medication count and each outcome to account for non-independence of repeated measures.
RESULTS
Study participants
Of the 250 study participants (Table 1), most were male (62%) with a median age of 50 years (interquartile range [IQR] 44, 56) and an undetectable viral load (72%). In this cohort of patients with substance dependence, about half had both alcohol and drug dependence (51%). Although very few had alcohol dependence only (i.e., no drug dependence, 9%), about half of the sample reported recent heavy alcohol use. A substantial portion of the sample reported recent cocaine or illicit opioid use (30% and 23%, respectively). Only 9% reported illicit sedative use.
Table 1.
Baseline characteristics of study participants with HIV infection and substance dependence in previous year and/or lifetime injection drug use
| Characteristic | Total sample (n=250) | Fall a (n=39) | No fall (n=211) | Fracture a (n=21) | No fracture (n=229) |
|---|---|---|---|---|---|
| Age (median, IQR) | 50 (44, 56) | 50 (41, 57) | 50 (44, 56) | 49 (46, 57) | 50 (44, 56) |
|
| |||||
| Male | 157 (63%) | 20 (51%) | 137 (65%) | 13 (62%) | 144 (63%) |
|
| |||||
| Race/ethnicity | |||||
|
| |||||
| Hispanic | 62 (25%) | 7 (18%) | 55 (26%) | 3 (14%) | 59 (26%) |
|
| |||||
| Black | 125 (50%) | 18 (46%) | 107 (51%) | 12 (57%) | 112 (49%) |
|
| |||||
| White | 63 (25%) | 14 (36%) | 49 (23%) | 6 (29%) | 57 (25%) |
|
| |||||
| Employed | 40 (16%) | 5 (13%) | 35 (17%) | 0 (0%) | 40 (18%) |
|
| |||||
| Health insurance | 248 (99%) | 39 (100%) | 209 (99%) | 21 (100%) | 226 (99%) |
|
| |||||
| Currently taking antiretroviral medication | 220 (88%) | 32 (84%) | 188 (89%) | 16 (76%) | 204 (89%) |
|
| |||||
| HIV viral load < 200 | 178 (72%) | 23 (61%) | 155 (73%) | 12 (57%) | 166 (73%) |
|
| |||||
| Ever injection drug use | 143 (57%) | 26 (68%) | 117 (55%) | 12 (57%) | 131 (57%) |
|
| |||||
| Current tobacco | 196 (79%) | 32 (84%) | 164 (78%) | 17 (81%) | 179 (79%) |
|
| |||||
| Alcohol use, past month | |||||
|
| |||||
| Heavy alcohol use b | 127 (51%) | 23 (59%) | 104 (49%) | 14 (67%) | 113 (50%) |
|
| |||||
| Non-heavy alcohol Use | 40 (16%) | 3 (8%) | 37 (18%) | 3 (14%) | 37 (16%) |
|
| |||||
| No alcohol use | 83 (33%) | 13 (33%) | 70 (33%) | 4 (19%) | 78 (34%) |
|
| |||||
| DSM-IV substance dependence, c past year | |||||
|
| |||||
| Both alcohol and drug dependence | 127 (51%) | 20 (51%) | 107 (51%) | 14 (67%) | 113 (50%) |
|
| |||||
| Drug dependence Only | 53 (21%) | 8 (21%) | 45 (21%) | 0 (0%) | 52 (23%) |
|
| |||||
| Alcohol dependence Only | 23 (9%) | 3 (8%) | 20 (9%) | 2 (10%) | 21 (9%) |
|
| |||||
| No dependence (lifetime history of injection drug use) | 47 (19%) | 8 (21%) | 39 (18%) | 5 (24%) | 42 (18%) |
|
| |||||
| Drug use,d past month | |||||
|
| |||||
| Any illicit opioid use e | 58 (23%) | 10 (26%) | 48 (23%) | 8 (38%) | 50 (22%) |
|
| |||||
| Any illicit sedative use | 22 (9%) | 5 (13%) | 17 (8%) | 3 (14%) | 19 (8%) |
|
| |||||
| Any cocaine use | 76 (30%) | 15 (38%) | 61 (29%) | 8 (38%) | 68 (30%) |
|
| |||||
| Prescribed medications | |||||
|
| |||||
| Sedating medication, Any | 201 (80%) | 35 (90%) | 166 (79%) | 21 (100%) | 179 (79%) |
|
| |||||
| Opioid medication, Any | 124 (50%) | 23 (59%) | 101 (48%) | 12 (57%) | 111 (49%) |
|
| |||||
| Buprenorphine or methadone, any | 68 (27%) | 14 (36%) | 54 (26%) | 9 (43%) | 58 (25%) |
|
| |||||
| Non-opioid sedating medication, any | 179 (72%) | 34 (87%) | 145 (69%) | 21 (100%) | 157 (69%) |
|
| |||||
| Number of prescribed medications, median (IQR) f | |||||
|
| |||||
| Overall medications | 10 (7, 14) | 13 (9, 18) | 10 (6, 14) | 12 (9, 16) | 10 (6, 14) |
|
| |||||
| Non-antiretroviral Medications | 8 (5, 11) | 10 (7, 16) | 8 (4, 11) | 10 (7, 12) | 8 (5, 11) |
|
| |||||
| Sedating medications | 2 (1, 3) | 3 (2, 5) | 2 (1, 3) | 3 (2, 3) | 2 (1, 3) |
|
| |||||
| Non-sedating medications | 8 (5, 12) | 9 (6, 14) | 7 (5, 11) | 10 (6, 13) | 8 (5, 11) |
|
| |||||
| Past year fall/accident requiring medical attention | |||||
| Study entry | 39 (16%) | ---- | ---- | 15 (71%) | 23 (10%) |
| 12-month follow-up | 27 (12%) | 12 (36%) | 15 (8%) | 6 (38%) | 21 (10%) |
|
| |||||
| Past year fracture | |||||
| Study entry | 21 (8%) | 15 (39%) | 6 (3%) | ---- | ---- |
| 12-month follow-up | 17 (7%) | 4 (12%) | 13 (7%) | 2 (13%) | 15 (7%) |
At study entry
National Institute on Alcohol Abuse and Alcoholism criteria: for women: more than 7 drinks on average in a week or 4+ drinks in a day; and for men more than 14 drinks on average in a week or 5+ drinks in a day in the past 30 days
Mini International Neuropsychiatric Interview Version 6.0 DSM IV criteria
Assessed with Addiction Severity Index
Includes use of medications without a prescription or taken in amounts greater than prescribed
Includes systemically active medications. Medications determined by study investigators to not be systemically active (and therefore excluded) included emollients, irrigation solutions, vitamins (except for vitamin D), peritoneal solutions, eye drops, rectal or vaginally administered medications, complementary and alternative medications.
Participants were prescribed a median number of 10 (IQR 7,14) medications overall and 8 (IQR 5,11) non-ARV medications. Sedating medications were common: 80% were prescribed at least one sedating medication, half (50%) at least one opioid medication, and 72% a non-opioid sedating medication. The most frequent non-opioid sedating medications were (in descending order of frequency): gabapentin, mirtazapine, trazodone, hydroxyzine, diphenhydramine, amitriptyline, doxepin, zolpidem, quetiapine, and clonazepam.
At study entry, 16% (39/250) of the cohort had a fall/accident requiring medical attention in the previous year and 8% (21/250) had a fracture in the previous year.
Main findings
Falls/accidents
The odds of a fall/accident requiring medical attention were significantly greater with each additional medication overall (odds ratio [OR] 1.12; 95% Confidence Interval [CI]=1.05, 1.18) (Table 2). Excluding ARV medications from the overall number yielded similar results (OR 1.13; 95%CI=1.06, 1.20).
Table 2.
Results of unadjusted logistic regression analyses of the association of number of medications in each category and fall and fracture at study entry a
| Medication type | Fall OR (95%CI) |
Fracture OR (95%CI) |
|---|---|---|
| Each additional overall medication b | 1.12 (1.05, 1.18) | 1.05 (0.97, 1.13) |
| Each additional non-antiretroviral medication | 1.13 (1.06, 1.20) | 1.05 (0.97, 1.14) |
| Each additional sedating medication c | 1.36 (1.14, 1.62) | 1.11 (0.89, 1.38) |
| Each additional non-sedating medication c | 1.05 (0.98, 1.14) | 1.03 (0.94, 1.14) |
| Any opioid (sedating) medication d | 1.31 (0.64, 2.67) | 1.02 (0.37, 2.91) |
| Any non-opioid sedating medication d | 2.89 (1.06, 7.85) | 13.09 (2.77, --) e |
| Any opioid for addiction treatment f | 1.43 (0.66, 3.07) | 1.46 (0.49, 4.20) |
| Any opioid for pain f | 1.03 (0.45, 2.35) | 0.43 (0.07, 1.64) |
Separate unadjusted logistic regression models examining the association of the number of medications in each category and each outcome (fall or fracture) at study entry. Bolded numbers indicate statistically significant association (p<0.05). Results of adjusted models for were not different in magnitude, direction, or significance (see Appendix). Adjusted models included demographic data (age, sex, and race/ethnicity); medical comorbidity, physical functioning score, recent (past 30 day) use of the following: heavy alcohol (for men: > 14 drinks in a week or 5+ drinks in a day; for women: >7 drinks in a week or 4+ drinks in a day), cocaine, illicit opioids (includes prescription opioid misuse); and illicit sedatives. Drug use measures were assessed by Addiction Severity Index.
Includes only systemically active medications
Results of one model to examine whether the effect of the number of overall medications on the risk of fall or fracture is due to the prescription of sedating and/or non-sedating medications
Results of one model to examine whether the effect of the number of sedating medications on fall or fracture risk is due to prescription of an opioid and/or non-opioid sedating medication
The upper confidence interval could not be calculated due to the small number of events i.e. no participant with a past-year fracture was not prescribed at least one non-opioid sedating medication.
Results of one model to confirm that the effect of an opioid medication was not different based upon whether the opioid medication may have been prescribed for addiction treatment (buprenorphine or methadone) or for pain
The odds of a fall/accident were also significantly greater with each additional sedating medication (OR 1.36, 95%CI=1.14, 1.62), but not with each additional non-sedating medication (OR 1.05, 95%CI=0.98, 1.14). ROC curve analysis (Table 3) indicated that the optimal cutoffs for predicting falls were: ≥8 overall, ≥12 non-ARV medications, and ≥2 sedating medications.
Table 3.
Optimal cutoffs for number of prescribed medications predicting fall using receiver operating characteristic curve analysis a
| Medication type | Number of medications | Sensitivity (%) | Specificity (%) | Youden index |
|---|---|---|---|---|
| All | 8 | 90 | 37 | 0.26 |
| All non-antiretroviral medications | 12 | 44 | 79 | 0.23 |
| Sedating | 2 | 82 | 46 | 0.28 |
Obtained by calculating the Youden index: (sensitivity + specificity) −1 without adjustment for covariates using data from study entry. “All” indicates all systemically active medications
The odds of a fall/accident (Table 2) were significantly greater with prescription of a non-opioid sedating medication (OR 2.89, 95%CI=1.06, 7.85) but not with an opioid medication (OR 1.31, 95%CI =0.64, 2.67). Analyzing opioid medications as 2 separate variables (i.e., “opioid medication prescribed for addiction treatment” and “opioid medication for pain”) yielded similar results (OR 1.43, 95%CI=0.66, 3.07 and OR 1.03, 95%CI=0.45, 2.35, respectively). Results of adjusted models for these associations were not different in magnitude, direction, or significance (Appendix). Results of logistic regression models with data collected at study entry and 12 months yielded similar results (Table 4).
Table 4.
Results of unadjusted logistic regression analyses of number of medications and risk of fall and fracture with outcome data from study entry and 12-month follow-up a
| Medication type | Fall OR (95%CI) |
Fracture OR (95%CI) |
|---|---|---|
| Each additional overall medication b | 1.09 (1.04, 1.15) | 1.04 (0.99, 1.09) |
| Each additional non-antiretroviral medication | 1.11 (1.05, 1.17) | 1.04 (0.99, 1.09) |
| Each additional non-sedating medication c | 1.04 (0.98, 1.11) | 1.01 (0.95, 1.10) |
| Each additional sedating medication c | 1.30 (1.13, 1.50) | 1.11 (0.96, 1.27) |
| Any opioid (sedating) medication d | 0.98 (0.53, 1.81) | 1.55 (0.74, 3.24) |
| Any non-opioid sedating medication d | 2.52 (1.16, 5.48) | 2.56 (0.95, 6.87) |
| Any opioid for addiction treatment e | 1.04 (0.54, 2.01) | 1.78 (0.87, 3.65) |
| Any opioid for pain e | 0.93 (0.44, 1.98) | 0.81 (0.35, 1.85) |
Separate unadjusted GEE logistic regression models examined the association of the number of medications in each category (assessed at study entry) and fall and fracture (assessed at study entry and the 12 -month follow up study interview). The analysis used two types of observations: i) medication data and past year fall (or fracture) from the baseline assessment and ii) medication data at baseline and past year fall (or fracture) at the 12-month follow up. Bolded numbers indicate statistically significant association (p<0.05). Results of adjusted models were not different in magnitude, direction, or significance (see Appendix).
Includes only systemically active medications
Results of one model to examine whether the effect of the number of overall medications on the risk of fall or fracture is due to the prescription of sedating and/or non-sedating medications
Results of one model to examine whether the effect of the number of sedating medications on fall or fracture risk is due to prescription of an opioid and/or non-opioid sedating medication
Results of one model to confirm that the effect of an opioid medication was not different based upon whether the opioid medication may have been prescribed for addiction treatment (buprenorphine or methadone) or for pain
Fractures
While none of the odds ratios for fractures were statistically significant in any of the medication categories, the direction and magnitude followed a similar pattern of those for falls: overall medications (OR 1.05, 95%CI= 0.97, 1.13); non-ARV medications (OR 1.05, 95%CI= 0.97, 1.14); non-sedating medications (OR 1.03, 95%CI= 0.94, 1.14); sedating medications (OR 1.11, 95%CI=0.89, 1.38); any opioid medication (OR 1.02, 95%CI= 0.37, 2.91); and any non-opioid sedating medication (OR 13.09, 95%CI= 2.77, --) (Table 2). Analyzing opioid medications as 2 separate variables (i.e., “opioid medication prescribed for addiction treatment” and “opioid medication for pain”) yielded similar results (no significant increased odds) (OR 1.46, 95%CI=0.49, 4.20 and OR 0.43, 95%CI=0.07, 1.64, respectively). Results of adjusted models for these associations were not different in magnitude, direction, or significance (Appendix). The analyses using fracture data at study entry and the 12-month follow-up did not reveal any significant associations (Table 4).
DISCUSSION
In this sample of PLWH and substance dependence (or ever IDU), a higher number of systemically active medications listed in an EMR was associated with greater odds of having a fall requiring medical attention. Excluding the number of ARV medications from the total medication count did not change the direction or magnitude of the association. The odds of a fall were over 30% greater with each additional sedating medication. Any non-opioid sedating medication use was associated with substantially increased odds of a fall. Although there were no significant associations with number of medications and fracture, all odds ratios were in the hypothesized direction.
The association between number of sedating medications and increased odds of falls is consistent with a large body of work, primarily in elderly populations (Gnjidic et al., 2012; Munson et al., 2016). It is notable that 80% of the study sample was prescribed ≥1 sedating medication and half were prescribed ≥2 sedating medications. There is limited data on the risk of falls in PLWH. Erlandson et al., (2016) reported a higher risk of recurrent falls in PLWH with sedative, opioid, and antidepressant medications, although the definition of a fall was different than that used in this study (“unintentionally coming to a rest on the ground or lower level, not because of a major intrinsic event or external hazard”). Our definition of falls did not exclude those caused by an external hazard. Sedative medications may increase risk of falls due to both external causes and non-external causes.
The current study advances the literature on falls, fractures, and polypharmacy with its focus on PLWH and different medication classes: sedating, opioid, and non-opioid sedating. Both medication type and quantity warrant consideration when trying to predict and prevent falls. Muscle relaxants and antihistamine medications in elderly populations are associated with emergency department visits and hospitalizations for falls and fractures (Alvarez et al., 2015). Impaired hepatic or renal metabolism may contribute to the risk of multiple sedating medications (Edelman et al., 2013). Although we adjusted for illicit opioid and sedative use, participants may have taken higher doses of their prescribed sedating medications or used ones not prescribed to them (Vijayaraghavan et al., 2014). Medications such as gabapentin can have additive sedating and even dissociative effects when combined with opioids (Schifano, 2014). Minimizing the number of prescribed medications, especially sedating medications, may reduce the risk of falls that require medical attention in PLWH.
Among sedating medications, we found a particularly strong association with falls and fractures for non-opioid sedating medications, but not statistically significant for opioid sedating medications. In an exploratory analysis, we did not detect an association between more specific categories of opioids (i.e., prescribed for pain or addiction) with falls or fractures. To further investigate the relationship between more specific categories of sedating medications and falls and fractures, a larger data set is needed.
We also identified cutoffs: ≥2 sedating medications and ≥8 medications overall were associated with greater fall risk. While this may have clinical utility, it should be validated in other studies. Also, while the cutoffs were reasonably sensitive, specificity was poor.
Because almost all of the study participants were prescribed ≥5 medications (91%), this commonly used definition of polypharmacy was not useful in this cohort with a median age of fifty. This is higher than several other cohorts of similar age (Holtzman et al., 2013; Moore, Mao, & Oramasionwu, 2015; Zhou et al., 2014). We speculate that comorbid mental health and medical conditions as well as recruitment from clinical sites (rather than from the community) contributed to these differences.
Although most of the sample met criteria for substance dependence in the past year, nineteen percent had a history of IDU with no substance dependence. Inclusion of the latter group into the analyses is in line with the focus of the study to examine PLWH with a serious exposure to substances (either substance dependence in the past year or ever IDU). Those without past-year substance dependence may have been recently using unhealthy alcohol or drug use - not to the extent to meet criteria for a use disorder – but potentially risky use nonetheless. People with past-year substance dependence were not necessarily using substances recently (they simply meet dependence criteria in the past year). It is likely that HIV-infected individuals with a history of IDU but no current dependence have had a lifetime substance use disorder. It should be noted that analyses adjusted for recent substance use.
This study has several strengths. Examining falls that “required medical attention” allowed us to focus on clinically significant falls. Lack of medical insurance was not a barrier to care or receipt of prescribed medications, and therefore was likely not a significant confounding factor. The use of standardized, validated instruments to detect and assess alcohol and other drug use is also a study strength. Of note, while co-occurrence of heavy alcohol use and illicit drug use may have set the stage for polypharmacy risks, associations between polypharmacy and falls/accidents and fractures did not appear to be affected by alcohol or drug use.
The findings should be considered in the context of the study’s limitations. It is possible that the observed associations between polypharmacy and falls were due to “confounding by indication.” Participants who are prescribed more medications may be more likely to fall because of the medical conditions for which the medications are prescribed. However, a similar study failed to detect a significant association between the VACS Index, an indicator of physical function/comorbidity, and fall risk (Erlandson et al., 2012). Still, to mitigate this potential effect, models included adjustment with a comorbidity score and physical function scale. Also, models assessing the impact of sedating medications included the number of non-sedating medications, which itself is an indicator of medical comorbidity (Roberts, Green, & Kadam, 2014).
Another limitation is the relatively low number of fractures, compromising the power to detect differences and explore interactions between medication types. Although this study’s observation period was 12 months, future studies of polypharmacy and fracture risk should be examined in larger cohorts and/or over a longer observation period. Falls were assessed retrospectively without use of fall diary although one would expect that an inquiry about falls requiring medical attention would be less subject to recall errors. We did not assess frequency of falls or whether fractures were the result of a low-impact fall (i.e. “fragility fracture”). These are questions worthy of future examination. Assessment of medications listed in the EMR was not corroborated by study participants. Self-report has been shown to both underestimate and overestimate the number of prescribed medications (Garber, Nau, Erickson, Aikens, & Lawrence, 2004). Over-the-counter and complementary medications were not included in medication counts, which are likely conservative. Combination pills were counted as their component medications. While this might be seen as a limitation when studying adherence, we believe it was the correct approach for studying medication effects. Lastly, we can only draw conclusions regarding number of medications, not appropriateness or benefits derived from the medications. In some cases, increased risks might be balanced by benefits of the medication.
This study suggests that prescribers should be prudent about medications overall and should review medication lists regularly, eliminating medications that are no longer needed, particularly sedating medications. The benefits of life-saving ARV medications will be overshadowed if PLWH are suffering from morbidity, such as falls and functional impairment, related to over-prescribing of non-ARV medications. Whether de-prescribing is beneficial for prevention of falls, fracture, and ultimately frailty is worthy of examination in future studies.
Appendix: Polypharmacy and Falls and Fractures
Table 1a.
Results of adjusted logistic regression analyses of the associations between increasing number of medications and risk of fall a
| Systemically active medications OR (95%CI) |
Non-antiretroviral medications OR (95%CI) |
Sedating medications b OR (95%CI) |
Non-sedating medications b OR (95%CI) |
|
|---|---|---|---|---|
| Unadjusted | 1.12 (1.05, 1.18) | 1.13 (1.06, 1.20) | 1.36 (1.14, 1.62) | 1.05 (0.98, 1.14) |
|
| ||||
| Age | 0.98 (0.94, 1.02) | 0.98 (0.95, 1.02) | 1.00 (0.95, 1.04) | ---- |
|
| ||||
| Each additional medication | 1.12 (1.06, 1.19) | 1.13 (1.06, 1.21) | 1.36 (1.13, 1.63) | 1.06 (0.97, 1.15) |
|
| ||||
| Sex (female vs male) | 1.48 (0.73, 3.03) | 1.44 (0.70, 2.94) | 1.38 (0.67, 2.87) | --- |
|
| ||||
| Each additional medication | 1.11 (1.04, 1.18) | 1.12 (1.05, 1.19) | 1.35 (1.13, 1.61) | 1.05 (0.97, 1.14) |
|
| ||||
| Race | ||||
| Hispanic vs Black | 0.76 (0.29, 1.97) | 0.76 (0.29, 1.97) | 0.62 (0.23, 1.67) | --- |
| White vs Black | 1.65 (0.74, 3.68) | 1.55 (0.70, 3.46) | 1.20 (0.51, 2.83) | --- |
|
| ||||
| Each additional medication | 1.12 (1.05, 1.19) | 1.12 (1.05, 1.20) | 1.36 (1.13, 1.64) | 1.06 (0.98, 1.14) |
|
| ||||
| Charlson comorbidity Index | 0.90 (0.76, 1.06) | 0.90 (0.77, 1.07) | 0.93 (0.79, 1.10) | --- |
|
| ||||
| Each additional medication | 1.14 (1.06, 1.22) | 1.15 (1.07, 1.23) | 1.36 (1.14, 1.62) | 1.07 (0.98, 1.17) |
|
| ||||
| Alcohol use c | ||||
| Did not exceed daily/weekly limits vs no alcohol | 0.46 (0.12, 1.75) | 0.46 (0.12, 1.76) | 0.57 (0.15, 2.21) | --- |
| Exceeded daily/weekly limits vs no alcohol | 1.36 (0.63, 2.95) | 1.34 (0.62, 2.90) | 1.37 (0.63, 3.02) | --- |
|
| ||||
| Each additional medication | 1.12 (1.05, 1.19) | 1.13 (1.05, 1.20) | 1.34 (1.12, 1.61) | 1.06 (0.98, 1.15) |
|
| ||||
| Ilicit or misused prescription opioid, any d | 1.26 (0.56, 2.83) | 1.24 (0.55, 2.80) | 1.13 (0.49, 2.62) | --- |
| Each additional medication | 1.12 (1.05, 1.19) | 1.12 (1.06, 1.20) | 1.36 (1.14, 1.62) | 1.05 (0.98, 1.14) |
|
| ||||
| Non-prescribed sedative medication use, any d | 2.03 (0.67, 6.12) | 1.97 (0.65, 5.91) | 1.84 (0.60, 5.61) | --- |
|
| ||||
| Each additional medication | 1.12 (1.05, 1.19) | 1.13 (1.06, 1.20) | 1.36 (1.14, 1.62) | 1.06 (0.98, 1.14) |
|
| ||||
| Cocaine, any d | 1.50 (0.72, 3.13) | 1.46 (0.70, 3.03) | 1.44 (0.68, 3.03) | --- |
|
| ||||
| Each additional medication | 1.12 (1.05, 1.18) | 1.12 (1.05, 1.20) | 1.36 (1.14, 1.62) | 1.05 (0.98, 1.14) |
Results of separate logistic regression models examining the association of the number of medications in each category and fall/accident controlling for each covariate listed above. Each regression model included the number of medications and one covariate. Analyses used study entry data. The first odds ratio (95% CI) in each row is the parameter estimate for the covariate. The second OR is the parameter estimate for the number of medications in each category. Bolded numbers indicate statistically significant association (p<0.05)
Results of one model “number of sedating medications”,” number of non-sedating medications” and one covariate
NIAAA defined drinking limits (> 14 drinks in a week or 5+ drinks in a day, for men; or >7 drinks in a week or 4+ drinks in a day, for women)
Past 30 days
Table 2a.
Results of adjusted logistic regression analyses of the association between increasing number of medications and risk of fracturea
| Systemically active medications OR (95%CI) |
Non-antiretroviral medications OR (95%CI) |
Sedating medications b OR (95%CI) |
Non-sedating medications b OR (95%CI) |
|
|---|---|---|---|---|
| Unadjusted | 1.05 (0.97, 1.13) | 1.05 (0.97, 1.14) | 1.11 (0.89, 1.38) | 1.03 (0.94, 1.14) |
|
| ||||
| Age | 0.98 (0.93, 1.03) | 0.98 (0.94, 1.03) | 0.98 (0.93, 1.03) | ---- |
|
| ||||
| Each additional medication | 1.06 (0.98, 1.14) | 1.06 (0.97, 1.15) | 1.09 (0.87, 1.36) | 1.05 (0.94, 1.16) |
|
| ||||
| Sex (female vs male) | 1.48 (0.73, 3.03) | 0.95 (0.37, 2.45) | 0.94 (0.37, 2.42) | --- |
|
| ||||
| Each additional medication | 0.96 (0.38, 2.46) | 1.05 (0.97, 1.14) | 1.11 (0.89, 1.38) | 1.03 (0.94, 1.14) |
|
| ||||
| Race | ||||
| Hispanic vs Black | 0.48 (0.13, 1.77) | 0.48 (0.13, 1.77) | 0.45 (0.12, 1.69) | --- |
| White vs Black | 0.96 (0.34, 2.70) | 0.94 (0.33, 2.65) | 0.85 (0.28, 2.57) | --- |
|
| ||||
| Each additional medication | 1.05 (0.97, 1.13) | 1.05 (0.97, 1.13) | 1.13 (0.89, 1.42) | 1.03 (0.93, 1.14) |
|
| ||||
| Charlson comorbidity index | 1.03 (0.85, 1.25) | 1.04 (0.86, 1.25) | 1.04 (0.86, 1.26) | --- |
|
| ||||
| Each additional Medication | 1.04 (0.96, 1.14) | 1.04 (0.95, 1.14) | 1.11 (0.89, 1.38) | 1.02 (0.91, 1.14) |
|
| ||||
| Alcohol use c | ||||
| Did not exceed daily/weekly limits vs no alcohol | 1.64 (0.35, 7.73) | 1.64 (0.35, 7.75) | 0.57 (0.15, 2.21) | --- |
| Exceeded daily/weekly limits vs no alcohol | 2.58 (0.81, 8.20) | 2.55 (0.80, 8.09) | 1.37 (0.63, 3.02) | |
|
| ||||
| Each additional medication | 1.06 (0.98, 1.14) | 1.06 (0.97, 1.14) | 1.11 (0.89, 1.39) | 1.04 (0.94, 1.15) |
|
| ||||
| Illicit or misused prescription opioid, any d | 2.27 (0.89, 5.82) | 2.25 (0.88, 5.77) | 1.13 (0.49, 2.62) | --- |
|
| ||||
| Each additional medication | 1.05 (0.97, 1.14) | 1.05 (0.97, 1.14) | 1.09 (0.88, 1.35) | 1.04 (0.94, 1.15) |
|
| ||||
| Non-prescribed sedative medication use, any d | 1.98 (0.53, 7.41) | 1.94 (0.52, 7.25) | 1.84 (0.60, 5.61) | --- |
|
| ||||
| Each additional medication | 1.05 (0.97, 1.14) | 1.05 (0.97, 1.14) | 1.10 (0.88, 1.37) | 1.04 (0.94, 1.15) |
|
| ||||
| Cocaine, any d | 1.42 (0.56, 3.60) | 1.41 (0.56, 3.56) | 1.40 (0.55, 3.56) | --- |
|
| ||||
| Each additional medication | 1.05 (0.97, 1.13) | 1.05 (0.97, 1.14) | 1.10 (0.89, 1.37) | 1.03 (0.94, 1.14) |
Results of separate logistic regression models examining the association of the number of medications in each category and fall controlling for each covariate listed above. Each regression model included the number of medications and one covariate. The first odds ratio (95% CI) in each row is the parameter estimate for the covariate. The second OR is the parameter estimate for the number of medications in each category. Bolded numbers indicate statistically significant association (p<0.05). Analyses used data collected at study entry.
Results of one model “number of sedating medications”,” number of non-sedating medications” and one covariate
NIAAA defined drinking limits (> 14 drinks in a week, or 5+ drinks in a day, for men; or >7 drinks in a week, or 4+ drinks in a day, for women)
Past 30 days
Contributor Information
Theresa W. KIM, Clinical Addiction Research and Education (CARE) Unit, Section of General Internal Medicine, Boston Medical Center, Boston University School of Medicine.
Alexander Y. WALLEY, Clinical Addiction Research and Education (CARE) Unit, Section of General Internal Medicine, Boston Medical Center, Boston University School of Medicine.
Alicia S. VENTURA, Clinical Addiction Research and Education (CARE) Unit, Section of General Internal Medicine, Boston Medical Center, 801 Massachusetts Avenue, Boston 02118.
Gregory J. PATTS, Data Coordinating Center, Boston University School of Public Health.
Timothy C. HEEREN, Department of Biostatistics, Boston University School Public Health.
Gabriel B. LERNER, Boston University School of Medicine, Boston, MA 02118.
Nicholas MAURICIO, Boston University School of Medicine, Boston, MA 02118.
Richard SAITZ, Clinical Addiction Research and Education (CARE) Unit Section of General Internal Medicine, Boston Medical Center, Boston University School of Medicine. Department of Community Health Sciences, Boston University School of Public Health.
References
- Alvarez CA, Mortensen EM, Makris UE, Berlowitz DR, Copeland LA, Good CB, … Pugh MJV. Association of skeletal muscle relaxers and antihistamines on mortality, hospitalizations, and emergency department visits in elderly patients: a nationwide retrospective cohort study. BMC Geriatrics. 2015;15:2. doi: 10.1186/1471-2318-15-2. https://doi.org/10.1186/1471-2318-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, Rockwood K. Frailty in people aging with human immunodeficiency virus (HIV) infection. Journal of Infectious Diseases. 2014;210:1170–1179. doi: 10.1093/infdis/jiu258. https://doi.org/10.1093/infdis/jiu258. [DOI] [PubMed] [Google Scholar]
- Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, … Justice AC. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. https://doi.org/10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantudo-Cuenca MR, Jiménez-Galán R, Almeida-Gonzalez CV, Morillo-Verdugo R. Concurrent use of comedications reduces adherence to antiretroviral therapy among HIV-infected patients. Journal of Managed Care Specialty Pharmacy. 2014;20:844–850. doi: 10.18553/jmcp.2014.20.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Important Facts about Falls. [Accessed June 5, 2017];Home and Recreational Safety. 2015 Retrieved from http://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html.
- Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. https://doi.org/10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HV, Cortés YI, Shiau S, Yin MT. Osteoporosis and fractures in HIV/hepatitis C virus coinfection: a systematic review and meta-analysis. AIDS. 2014;28:2119–2131. doi: 10.1097/QAD.0000000000000363. https://doi.org/10.1097/QAD.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs & Aging. 2013;30:613–628. doi: 10.1007/s40266-013-0093-9. https://doi.org/10.1007/s40266-013-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, Duong S, MaWhinney S, Kohrt WM, Campbell TB. Risk factors for falls in HIV-infected persons. Journal of Acquired Immune Deficiency Syndromes. 2012;61:484–489. doi: 10.1097/QAI.0b013e3182716e38. https://doi.org/10.1097/QAI.0b013e3182716e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2013;63:209–215. doi: 10.1097/QAI.0b013e318289bb7e. https://doi.org/10.1097/QAI.0b013e318289bb7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, Plankey MW, Springer G, Cohen HS, Cox C, Hoffman HJ, … Brown TT. Fall frequency and associated factors among men and women with or at risk for HIV infection. HIV Medicine. 2016;17:740–48. doi: 10.1111/hiv.12378. https://doi.org/10.1111/hiv.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg MS, Chang CCH, Kuller LH, Skanderson M, Lowy E, Kraemer KL, … Justice AC. HIV Infection and the Risk of Acute Myocardial Infarction. Journal of American Medical Association Internal Medicine. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. https://doi.org/10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. Journal of American Geriatrics Society. 2014;62:2261–2272. doi: 10.1111/jgs.13153. https://doi.org/10.1111/jgs.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Medical Care. 2004;42:649–652. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- Gingo MR, Balasubramani GK, Rice TB, Kingsley L, Kleerup EC, Detels R, … Morris A. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulmonary Medicine. 2014;14:75. doi: 10.1186/1471-2466-14-75. https://doi.org/10.1186/1471-2466-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, … Le Couteur DG. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. Journal of Clinical Epidemiology. 2012;65:989–995. doi: 10.1016/j.jclinepi.2012.02.018. https://doi.org/10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Grant PM, Cotter AG. Tenofovir and bone health. Current Opinion in HIV and AIDS. 2016;11:326–232. doi: 10.1097/COH.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Kershaw T, Lin H, Heimer R, Goulet JL, Kraemer KL, … Justice AC. Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US Veteran cohort. Drug and Alcohol Dependence. 2010;110:208–220. doi: 10.1016/j.drugalcdep.2010.02.020. https://doi.org/10.1016/j.drugalcdep.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M, Justice AC, Lampiris HW, Valcour V. Management of Human Immunodeficiency Virus Infection in Advanced Age. Journal of American Medical Association. 2013;309:1397–14. doi: 10.1001/jama.2013.2963. https://doi.org/10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güerri-Fernandez R, Vestergaard P, Carbonell C, Knobel H, Avilés FF, Castro AS, … Diez-Perez A. HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population-based cohort study. Journal of Bone Mineral Research. 2013;28:1259–1263. doi: 10.1002/jbmr.1874. https://doi.org/10.1002/jbmr.1874. [DOI] [PubMed] [Google Scholar]
- Han BH, Moore AA, Sherman S, Keyes KM, Palamar JJ. Demographic trends in binge alcohol use and alcohol use disorders among older adults in the United States. Drug and Alcohol Dependence. 2017;170:198–207. doi: 10.1016/j.drugalcdep.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman C, Armon C, Tedaldi E, Chmiel JS, Buchacz K, … Wood K the HOPS Investigators. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. Journal of General Internal Medicine. 2013;28:1302–1310. doi: 10.1007/s11606-013-2449-6. https://doi.org/10.1007/s11606-013-2449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazis LE, Miller DR, Clark JA, Skinner KM, Lee A, Ren XS, … Ware JE. Improving the response choices on the Veterans SF-36 health survey role functioning scales: results from the Veterans Health Study. Journal of Ambulatory Care Management. 2004;27:263–280. doi: 10.1097/00004479-200407000-00010. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. Journal of Nervous Mental Diseases. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Miller TR, Lestina DC, Smith GS. Injury risk among medically identified alcohol and drug abusers. Alcoholism: Clinical and Experimental Research. 2001;25:54–59. [PubMed] [Google Scholar]
- Moore HN, Mao L, Oramasionwu CU. Factors associated with polypharmacy and the prescription of multiple medications among persons living with HIV (PLWH) compared to non-PLWH. AIDS Care. 2015;27:1443–1448. doi: 10.1080/09540121.2015.1109583. https://doi.org/10.1080/09540121.2015.1109583. [DOI] [PubMed] [Google Scholar]
- Munson JC, Bynum JP, Bell J-E, Cantu R, McDonough C, Wang Q, … Tosteson ANA. Patterns of Prescription Drug Use Before and After Fragility Fracture. JAMA Internal Medicine. 2016;176:1531–38. doi: 10.1001/jamainternmed.2016.4814. https://doi.org/10.1001/jamainternmed.2016.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. Retrieved from https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Nicholas PK, Kemppainen JK, Canaval GE, Corless IB, Sefcik EF, Nokes KM, … Gallagher DM. Symptom management and self-care peripheral neuropathy in HIV/AIDS. AIDS Care. 2007;19:179–89. doi: 10.1080/09540120600971083. [DOI] [PubMed] [Google Scholar]
- Pacek LR, Cioe PA. Tobacco use, tobacco use disorders, and smoking cessation interventions in persons living with HIV. Curr HIV/AIDS Rep. 2015;12:413–420. doi: 10.1007/s11904-015-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, … Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Roberts ER, Green D, Kadam UT. Chronic condition comorbidity and multidrug therapy in general practice populations: a cross-sectional linkage study. British Medical Journal Open. 2014;4:e005429. doi: 10.1136/bmjopen-2014-005429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs. 2014;2:491–496. doi: 10.1007/s40263-014-0164-4. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Humana Press; 1992. pp. 41–72. [Google Scholar]
- U.S. Department of Veterans Affairs National Formulary. VA Class Index Section. Retrieved from http://www.pbm.va.gov/nationalformulary.asp.
- Vijayaraghavan M, Freitas D, Bangsberg DR, Miaskowski C, Kushel MB. Non-medical use of non-opioid psychotherapeutic medications in a community-based cohort of HIV-infected indigent adults. Drug and Alcohol Dependence. 2014;143:263–267. doi: 10.1016/j.drugalcdep.2014.06.044. https://doi.org/10.1016/j.drugalcdep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Fitzgerald JF, Stump TE. The effect of hip fracture on mortality, hospitalization, and functional status: a prospective study. American Journal of Public Health. 1997;87:398–403. doi: 10.2105/ajph.87.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Martin K, Corbett A, Napravnik S, Eron J, Zhu Y, … Wohl DA. Total daily pill burden in HIV-infected patients in the southern United States. AIDS Patient Care and STDs. 2014;28:311–317. doi: 10.1089/apc.2014.0010. https://doi.org/10.1089/apc.2014.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]


