Graphical abstract
Keywords: Chitosan, Nanochitosan, Antimicrobial activity, Toxicity evaluation
Abstract
Chitosan, bio-polyaminosacharide, is derived from chitin. Two sources (shrimp wastes and fungus biomass) were used to produce chitosan. And then the chitosan was produced in the nano-form followed by characterization by transmission electron microscopy. The images obtained clearly showed that the size of nano-chitosan ranged between 7 and 13 and 3–6 nm with spherical shape for shrimp and fungal sources, respectively. The antimicrobial activities of the tested concentrations of chitosan and nano-chitosan were examined and found to have high activity against the tested pathogens. The evaluation of the toxicity of the tested concentrations of the produced chitosan and its nano-size were performed using brine shrimp and rat bioassay. Toxicity examination of chitosan and their nano derivatives is an essential procedure to assess the possibility of using these concentrations as food ingredient. Nine groups of rats were treated with either chitosan or nano-chitosan of both sources at 100 and 200 mg kg−1 bw. Adding chitosan in the diet of all groups showed no significant changes in both the blood biochemical and oxidative stress parameters when compared with control group. The histopathology of liver, kidney and stomach confirmed the results of the previous parameters. No signs of inflammation, fibrosis or cirrhosis were found in examined organs. It is concluded that chitosan and nano-chitosan of shrimp and Rhizopus stolonifer had high antimicrobial activity and are not toxic in the same time and it can be used as food ingredients.
1. Introduction
Chitosan, a natural linear bio-polyaminosacharide derived by the alkaline deacetylation of chitin. Skeleton of crustaceans like crab, shrimp and lobster is the main source of chitin. It also found in the skeleton of marine zooplankton spp. such as jellyfishes and coral [1]. Chitin is also a major component of the cell walls of yeast, mushrooms and some other fungi [2]. Usually, the zygomycetes has the highest chitin amounts in their cell walls when compared with other fungi classes as reported by Andrade et al. [3], Franco et al. [4] and Campos-Takaki [5]. The research for chitosan has increased during the last years due to its biocompatibility, biodegradability and safety. It is characterized by its antimicrobial activity, film forming ability, chelation and adsorption properties [[6], [7], [8]].
The uses of chitosan depend on its molecular weight and viscosity [9]. Shimojoh et al. [10] reported that high molecular weight chitosan was more effective as food additive than those with low molecular weight. Based on its polycationic properties, chitosan can be used as flocculating agent and act as chelating agent and heavy metal trapper [[11], [12]]). The waste of crustaceous industry is considered the most suitable way to get chitosan in a high amount and low cost. Filamentous fungi are considered an attractive source of both chitosan and chitin at industrial scale for it can be manufactured under controlled conditions [[13], [14]]). Comparing between the two sources of chitosan, the fungal chitosan distinguishes than another source depend on their properties as the degree of acetylation, molecular weight homogeneity, viscosity and charge distribution. Also, it does not have any contents of heavy metals especially nickel and copper [15]. Chitosan from fungal mycelia has medium-low molecular weight (1–12 × 104 Da), whereas that from crustaceans sources has high molecular weight (about 1.5 × 106 Da) [16]. Changing the basic structure of chitosan give an opportunity to obtain derivatives with a wide range of properties and consequently more application can be used [17]). One of the modern technologies is developing of chitosan micro-particles followed by nano-form, confirming the effective uses in many industries [18]. Although, many researches proofed the safety of chitosan for consumption either in food or drug sectors, there are no more available studies assess the toxicity of nano-chitosan particularly that derived from fungal source. So, the present study aimed to synthesize nano-chitosan from crustacean and fungal source and to assess its antimicrobial activity and toxicity using brine shrimp and rats in a preliminary evaluation to be used as food ingredient.
2. Materials and methods
2.1. Production of chitosan from shrimps exoskeletons
2.1.1. Extraction of chitin from shrimps wastes
The exoskeletons of shrimp were collected from shrimp local markets, air dried, washed several times by tab water, air dried and then crushed. These exoskeletons were conducted to deproteinization process as described by Lamarque et al. [19]. In brief, the crushed exoskeletons were placed in 1000 ml beakers and heated (∼80 °C) with 4% NaOH solution using hot plate in a portion of 1:4 (w/v). The mixture was left to cool for one hour, filtered in a normal sieve and washed three times by tab water. The exoskeletons were then dried and further grinded to pieces of 0.5–5.0 mm using a meat tenderizer.
2.1.2. Demineralization
The grinded exoskeleton was demineralized using 7% HCl in a portion of 1:4 (w/v). The samples were allowed to soak for 24 h to remove the minerals (mainly calcium carbonate). After draining, the remaining chitin was washed with deionized water [20].
2.1.3. Deacetylation
The deacetylation process was carried out on chitin by adding 40% NaOH solution onto chitin in a portion of 1:2 (w/v).The mixtures were boiled at 200 °C for 2 h and then cooled at room temperature. After that, it was washed continuously with deionized water. The chitosan was left uncovered and oven dried at 70 °C till getting a creamy-white form.
2.2. Production of fungal cell wall chitosan
2.2.1. Cultivation of fungal strain
Rhizopus stolonifer strain OSMR1 was activated on yeast peptone glucose agar (YPG) for 4 days at 28 ± 2 °C until sporulation. The spores were collected, suspended in sterile YPG broth, counted using a haemocytometer slide and diluted to 106 spores ml−1. One milliliter from fungal spore suspension was inoculated into sterilized 500 ml Erlenmeyer-flask containing 250 ml YPG broth. The flasks were placed in shaking incubator at 125 rpm and 28 ± 2 °C for 4 days. After incubation period, the mycelia were harvested by filtration using filter paper (Whatman No.1), washed many times with distilled water, dried in oven for overnight at 70 °C and then weighted.
2.2.2. Chitosan extraction from fungal biomass
Chitosan extraction was carried out by the method of Gharieb et al. [8]. Chitosan was extracted from dried mycelia according to the process involving: deproteinization with 2% sodium hydroxide solution in a portion of 1:4 w/v. After filtration, the alkali-insoluble material (AIM) was washed with distilled water till getting neutral pH and dried. One gram of dried AIM was added to 40 ml 20% acetic acid at 80 °C for 6 h. The suspension was centrifuged at 4000 rpm for 15 min, and the supernatant was collected. The pH of the supernatant was adjusted to pH 9.0 with 2 N NaOH solution and then centrifuged at 4000 rpm for 15 min. The precipitated chitosan was washed twice with distilled water and then with 95% ethanol (20:1 v/w). The washed chitosan was dried at 70 °C.
2.3. Purification of chitosan
The obtained crustaceans and fungal chitosan was purified to make it acceptable for application in the pharmaceutical and food industries. The chitosan at concentration of 1 mg ml−1 in acetic acid 1% (v/v) was prepared using magnetic stirrer to get homogenous solution. The insoluble particles were removed by filteration through Whatman filter paper. Chitosan was precipitated from solution by adding 1N NaOH until pH reached to 8. The obtained chitosan was washed several times with deionized water and centrifuged at 10,000 rpm.
2.4. Characterization of chitosan using Fourier transforms infrared spectroscopy (FTIR)
The degree of acetylation (DA) of produced chitosan (crustaceans or fungi) was determined according to Niamsa and Baimark (2009) based on their infrared spectra recorded on a FTIR instrument (Jasco, Model FTIR-6100, Japan) using the absorbance ratio (A1655/A3450). DA was calculated by the following equation:
| DA% = (A1655/A3450) × 100/1.33 |
The deacetylation was calculated using the following equation:
| Deacetylation% = 100 − % acetylation. |
2.5. Preparation of chitosan nanoparticles
The nanoparticles of crustaceans or fungal chitosan were prepared based on ionic gelation of tri-sodium polyphosphate (TPP) with chitosan [21]. Stock solutions of 1% chitosan (in 2.0% acetic acid) and 0.1% TPP (in distilled water) were prepared. The chitosan nanoparticles were obtained upon addition of 14 ml of TPP solution into 35 ml of chitosan solution under mild mechanical stirring (550 rpm) at room temperature. The chitosan nanoparticles were precipitated by centrifugation at 10,000 rpm for 10 min. The pellet was washed with distilled water following ethanol then air dried.
2.6. Characterization of chitosan nanoparticles using transmission electron microscopy (TEM)
The average particle size, size distribution and morphology of the chitosan nanoparticles were done using transmission electron microscopy (JEOL, JEM-2100 TEM). A drop of well dispersed nanoparticle was placed onto the amorphous carbon-coated 200 mesh carbon grid, followed by drying the sample at ambient temperature, before it was loaded into the microscope.
2.7. Antimicrobial evaluation of the produced chitosan and nano-chitosan
The antimicrobial activity of the produced crustaceans and fungi chitosan and nano-chitosan was evaluated on Gram positive bacteria (Staphylococcus aureus ATCC-47077, Bacillus cereus ATCC- 12228), Gram negative bacteria (Escherichia coli ATCC- 25922, Salmonella typhi ATCC 15566), Yeast (Candida albicans ATCC-10231) and Fungi (Aspergillus niger ATCC- 16888 and Fusarium oxysporum OS5). The pathogenic microbes were obtained from the American type culture collection (ATCC; Rockville, MD, USA) and National Research centre (NRC). The bacterial cells and fungal spore suspensions (106 CFU/ml) of each tested microbes were spreaded onto the nutrient agar plates for bacteria and potatoes dextrose agar plates for fungi. The wells (7 mm diameter) were dug on the inoculated plates [22], and 100 μl of 5 mg ml−1 chitosan or nano-chitosan at pH 6.5 were added to the wells. The plates were left 2 h at 4 °C to allow the diffusion. The plates were incubated at 37 °C for 24 h while fungi plates were incubated at 28 °C for 72 h, then, the inhibition zone diameter was measured expressed in millimeter and three replicates were averaged [23].
2.8. Toxicity determination of the produced chitosan and nano-chitosan by brine shrimp bioassay
Brine shrimp eggs were supplied by Avocet Artemin Inc., Utah, USA. Larvae were used within 24 h of hatching. Ten brine shrimp (Artemia salina leach) Larvae drown through a glass capillary and placed in a vial containing 5 ml of sea water. The chitosan from shrimp and fungal strain was added to 5 ml sea water to give four concentrations 5000, 10000, 15000 and 20000 ppm dry weight to detect any toxic activity on the brine shrimp [24]. The experimental maintained at room temperature for 24 h under light. The number of dead shrimps that was counted and percentage of mortality was calculated.
2.9. Toxicity evaluation of the produced chitosan and nano-chitosan using rat bioassay
2.9.1. Animals
Two-month old healthy male Sprague-Dawley rats (100 g) were purchased from the Animal House Colony, National Research Centre, Cairo, Egypt. The animals were kept in a well-ventilated room of 12 h light and 12 h darkness. All animals were fed with standard rat’s feed belts while water was provided ad libitum.
2.9.2. Kits
Alanineaminotransaminase (ALT), aspartateaminotransaminase (AST), urea, uric acid, Creatinine and cholesterol (Cho.) kits were purchased from Specrum Co. (Spain). Alkaline phosphatase (ALP), Triglysride (TriG), Malondialdehyde (MDA), Catalase (CAT), superoxide dismutase (SOD) and Total antioxidant capacity (TAC) kits were purchased from Biodiagnostics Co. (Egypt).
2.9.3. Experimental design and analysis
Chitosan and nano-chitosan produced from crustaceans or fungi at 2 concentrations (10000 and 20000 ppm) were prepared in 1% fresh acetic acid and the pH adjusted at 6.5. One centimeter of each concentration was administered to rat daily by oral gavage. Rats were divided into 9 groups (6 rats for each) based on concentration and type of the used chitosan (Table 1). Control group received diluted acetic acid (1% concentration pH 6.5) at 1.0 ml/rat. The prepared concentrations (1 and 2%) of each type of chitosan were administrated in the other 8 rats groups. Rat feeding was continued for 21 days and fasted for 12 h. Blood samples were collected on the day 22nd via the retro-orbital venous plexus from each animal under diethyl ether anaesthesia according to the method of [25] and allowed to clot. Serum was separated by centrifuging at 2500 rpm for 15 min and analyzed for various biochemical parameters such as ALT and AST [26], ALP [27], cholesterol [28], triglycerides [29], urea [30], creatinine [31] and uric acid [32]. After blood sample collection, the animals were sacrificed; the liver and kidneys from each animal were excised, rinsed in 0.25 M ice cold sucrose solution. Each tissue was homogenated (10% w/v) in 0.05 M phosphate buffer (pH 7) and centrifuged at 12,000 rpm for 60 min at 4 °C [33]. The supernatant was collected and stored at −20 °C until examination of oxidative stress parameters such CAT [34]), SOD Sun et al., 1998, TAC [35] and MDA as a result for lipid peroxidation [36]. Other Samples of the liver and kidney from all animals were fixed in 10% neutral formalin and paraffin embedded. Sections of each sample (5 μm thickness) were stained with hematoxylin and eosin (H&E) for the histological examination [37].
Table 1.
Experimental protocol of the bioassay.
| Experimental Groups | Dose | No. of animals | Times of treatment | Day of autopsy |
|---|---|---|---|---|
| Control | 6 | 21 | 22nd | |
| Fungal Chitosan (FC100) | 100 mg/kg bw | 6 | 21 | 22nd |
| Fungal Chitosan (FC200) | 200 mg/kg bw | 6 | 21 | 22nd |
| Nano- Fungal Chitosan (NFC100) | 100 mg/kg bw | 6 | 21 | 22nd |
| Nano- Fungal Chitosan (NFC200) | 200 mg/kg bw | 6 | 21 | 22nd |
| Shrimp chitosan (ShC100) | 100 mg/kg bw | 6 | 21 | 22nd |
| Shrimp chitosan (ShC200) | 200 mg/kg bw | 6 | 21 | 22nd |
| Nano-Shrimp chitosan (NShC100) | 100 mg/kg bw | 6 | 21 | 22nd |
| Nano-Shrimp chitosan (NShC200) | 200 mg/kg bw | 6 | 21 | 22nd |
bw = body weight.
2.10. Statistical analysis
Results were expressed as means ± standard deviation (SD). Differences between the groups were evaluated by using one-way ANOVA, followed by Duncan's test. All statistical analyses were performed using the statistical software SPSS 13.0 (SPSS Ltd., Surrey, UK). A P value of less than 0.05 was considered statistically significant.
3. Results and discussion
3.1. Production and characterization of chitosan nanoparticles
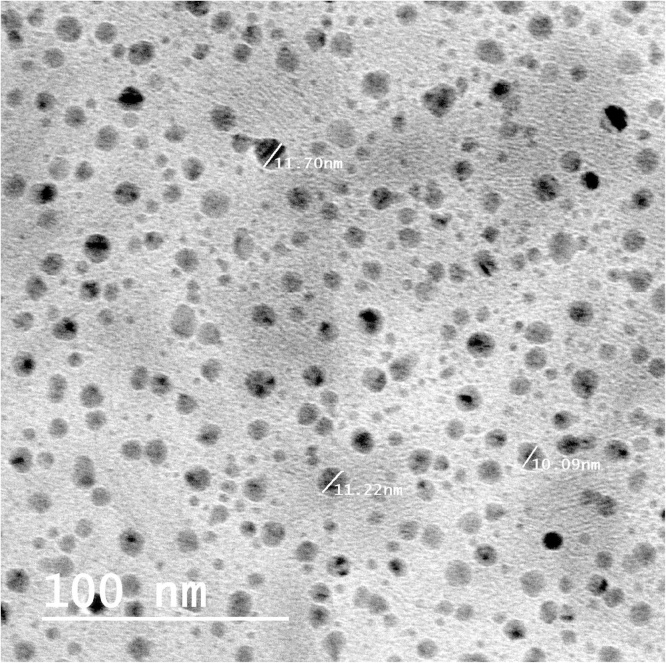
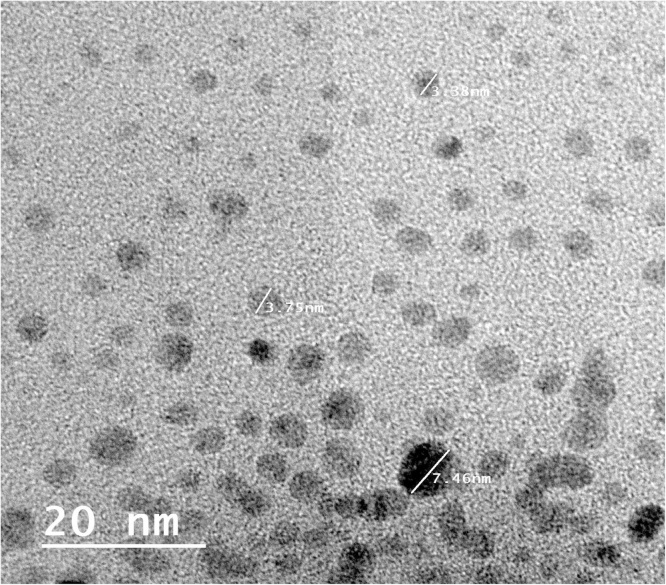
Chitosan is an amino polysaccharide, the second most abundant natural polymer after cellulose. Chitosan is biocompatible, biodegradable and nontoxic which made wide applicability in conventional pharmaceutics as a potential formulation excipient [38]). For that, we selected this biopolymer for examining its ability to be used as food ingredient in the present study. Two deferent sources (shrimp and fungi as the most global distributed sources of chitosan) were applied to produce chitosan followed by their nanostructures. Chitosan was produced at ratio of 100 and 400 g/kg dry biomass or shrimp waste for fungi and shrimp, respectively. Deacetylation degree (DD) of chitosan is one of the main parameters characterizing chitosan and considered about a percentage quantity of free amine groups in chitosan molecule [39]. In our study, we had determined the degree of chitosan deacetylation based on FTIR spectroscopy determinations. The DD obtained was 85.2 and 81.5% with fungi and shrimp chitosan, respectively. These degrees are good and found in the highest group in DD classifications based on properties [40]. We prepared nano-size molecules of shrimp and fungal chitosan using ionic gelation. Fig. 1, Fig. 2 illustrated the image produced by high resolution transmission electron microscopy for shrimp and fungal nano-chitosan. The size of produced chitosan nanoparticles was ranged between 7 and 13 nm with shrimp chitosan and ranged between 3 and 6 nm with fungal chitosan. On the other hand, the morphological and surface appearance of produced nano-chitosan was nearly spherical shape and smooth surface as represented in Fig. 1, Fig. 2.
Fig. 1.
High resolution transmission electron microscopy image of shrimp chitosan nanoparticles.
Fig. 2.
High resolution transmission electron microscopy image of fungal chitosan nanoparticles.
3.2. Antimicrobial examination of the produced chitosan and nano-chitosan
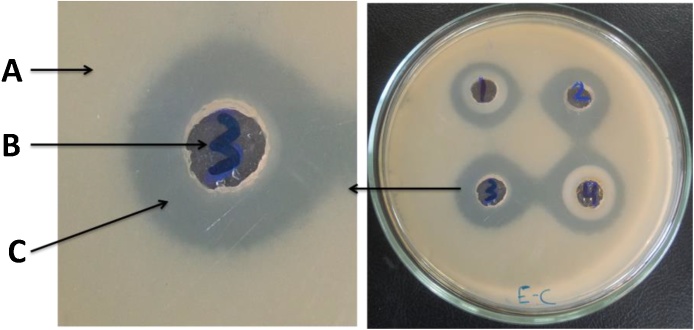
Antimicrobial activity of the produced chitosan (shrimp and fungal) and their nanoparticles was examined at concentration of 5 mg/ml of each. Well diffusion method was applied as the most accurate technique. After incubation period, the diameter of inhibition zone was recorded and tabulated in Table 2. The results indicated that high activity was obtained from the tested samples against all applied pathogens, and the diameter of inhibition zone reached 18 mm with fungal strains. However the diameter ranged between 19 and 30 mm with yeast and bacterial strains. Fig. 3 showed that 3 layers were produced resulting from the antimicrobial activity of chitosan and their nanoparticles against pathogenic microbes. The highest antibacterial activity was recorded with gram positive bacteria strains compared with gram negative bacteria. This may due to the fact that an outer membrane of gram positive bacteria containing peptidoglycan layers which is not enough to enable bacteria to survive in their different environments and it will be more sensitive toward antibacterial agents [41].
Table 2.
Inhibition zone diameter (mm) for antimicrobial activity of chitosan and nano-chitosan extracted from shrimp and fungi.
| Samples | Gram+ Bacteria |
Gram− Bacteria | Yeast |
Fungi |
|||
|---|---|---|---|---|---|---|---|
| B. cereus | St. aureus | E. Coli | S. typhi | C. albicans | A. niger | F. oxysporum | |
| Shrimp chitosan | 19 | 25 | 19 | 19 | 20 | 12 | 11 |
| Fungal chitosan | 21 | 26 | 21 | 21 | 22 | 15 | 13 |
| Shrimp nano-chitosan | 22 | 27 | 20 | 20 | 21 | 14 | 15 |
| Fungal nano-chitosan | 24 | 30 | 24 | 23 | 23 | 17 | 18 |
Fig. 3.
Three layers resulting from antimicrobial activity of chitosan and nano-chitosan against Escherichia coli strain. 1; shrimp chitosan, 2; fungal chitosan, 3; shrimp nano-chitosan, 4; fungal nano-chitosan, A; bacterial growth, B; well of sample, C; inhibition zone.
3.3. Toxicity determination of the produced chitosan and nano-chitosan by brine shrimp bioassay
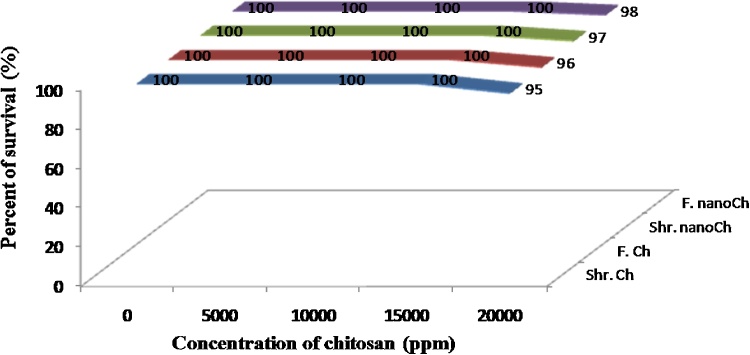
The use of aquatic organisms for biomonitoring is an important tool in aquatic ecotoxicology, allowing the detection and evaluation of the potential toxicity. Brine shrimp bioassay was used as an easy test to detect toxicity. Chitosan produced from shrimp wastes and fungal biomass (R. stolonifer) was tested for potential toxicity using brine shrimp bioassay technique. Fig. 4 showed that no toxic compounds could be detected in chitosan extracted from either shrimp or fungus strain sources or its nano-form at concentrations of 5000, 10000, 15000 ppm. While at concentration of 20000 ppm, the percentage of mortality was varied between 2 and 5%.
Fig. 4.
Survival of brine shrimp at 24 h after exposure to different concentrations of chitosan from shrimp and/or fungal strain and its nano-form. Shr.Ch; shrimp chitosan, F.Ch; fungal chitosan, Shr.nanoCh; shrimp nano-chitosan, F.nanoCh; fungal nano-chitosan.
3.4. Toxicity evaluation of the produced chitosan and nano-chitosan using rat bioassay
3.4.1. Blood parameters
The effects of the tested chitosan types and concentrations on liver and kidneys functions as well as on the histopathological changes in liver, kidneys and stomach tissues were studied. Table 3 exhibits the blood biochemical parameters of rats fed on different types of chitosan at 100 and 200 mg kg−1 bw. In general, there were no significant changes in the tested parameters due to chitosan treatments. However no changes were observed in ALT and AST values of chitosan treated rats revealing to normal liver functions. In this way, Abd El-Fattah et al. [42] found that Chitosan showed a significant decrease in normal liver function enzymes (ALT, AST and alkaline) of rats. In the present study, we reported that rats fed on chitosan significantly decreased the negative effect of the 2,3,7,8 tetrachlorodibenzo-p- dioxin (TCDD) when compared with those untreated with chitosan. Also serum ALP enzyme was noted in a normal range which considers a sensitive indicator of obstructive and space-occupying lesions of the liver. The only significant increases were observed in triglyceride values of rats treated with 100 mg kg−1 shrimp chitosan. The same result obtained by Karami et al. [43]. In these regard, Qujeq and Ataei [44] found that rats fed on chitosan at 5% level reduced triglyceride by 32.89%. The normality of urea, creatinine and uric acid levels in all treated groups when compared with the control rats revealed to the healthy kidney [45]. Furthermore, Jing et al. [46] used chitosan as a drug to improve renal function in patients with chronic renal failure. They found a significant reduction in urea and creatinine serum level in addition to improve the feeling of the physical strength, the appetite and the sleep of treated patients. Finally, these results clearly showed that the different types and concentrations of chitosan hadn’t any harmful influences on the hepatic and renal tissue.
Table 3.
Effects of tested types of chitosan on liver and kidneys functions of treated rats.
| Parameter | Control | FC (100 mg/kg BW) | FC (200 mg/kg BW) | NFC (100 mg/kg BW) | NFC (200 mg/kg BW) | ShC (100 mg/kg BW) | ShC (200 mg/kg BW) | NSh (100 mg/kg BW) | NSh (200 mg/kg BW) |
|---|---|---|---|---|---|---|---|---|---|
| ALT (IU/ml) | 28.3 ± 3.2a | 28.7 ± 0.33a | 30 ± 1.5a | 30.7 ± 0.9a | 34.6 ± 1.2a | 34.5 ± 1.1a | 34.3 ± 2.2a | 32.5 ± 1.8 a | 37.4 ± 1.4a |
| AST (IU/ml) | 35 ± 1.7a | 33.6 ± 2.5a | 34 ± 2a | 35.4 ± 1.5a | 33 ± 5.2a | 37.3 ± 2.5a | 33.8 ± 2.02a | 38 ± 2.6a | 38 ± 1.5a |
| ALP (IU/ml) | 54 ± 1.2a | 52.3 ± 1.4a | 53.7 ± 1.8a | 57.6 ± 1.45a | 54.6 ± 2.02a | 60.3 ± 1.4a | 63.4 ± 0.3a | 61.7 ± 1.2a | 63.4 ± 0.9a |
| TriG (mg/d) | 115.3 ± 2.9a | 116.6 ± 4.4a | 117.4 ± 1.4a | 116.3 ± 0.9a | 118.4 ± 1.6a | 130 ± 3.71b | 125 ± 2.9a | 127.7 ± 5.3a | 130 ± 2.9b |
| Urea (mg/d) | 38.7 ± 1.4a | 40 ± 1.5a | 36.3 ± 2.3a | 34.6 ± 2.03a | 39 ± 0.57a | 38.3 ± 2.8a | 39.3 ± 2a | 37.4 ± 1.6a | 39 ± 4.6a |
| Creatinine ((mg/d) | 0.5 ± 0.02a | 0.51 ± 0.05a | 0.52 ± 0.01a | 0.58 ± 0.03a | 0.48 ± 0.02a | 0.5 ± 1.7a | 0.6 ± 0.02a | 0.5 ± 0.07a | 0.48 ± 0.01a |
| Uric Acid (mg/d) | 4.1 ± 0.2a | 4.2 ± 0.2a | 4.3 ± 0.4a | 4.2 ± 0.2a | 4.4 ± 0.08a | 4.0 ± 0.05a | 4.1 ± 0.15a | 4.0 ± 0.08a | 4.1 ± 0.1a |
Within each row, means with superscript with different letters are significantly different at p ≤ 0.05; Control = Untreated Crop; FC = fungal chitosan; NFC = nano fungal chitosan; ShC = Shrimp chitosan; NShC = Nano shrimp chitosan; Bw = body weight.
In the same concern, changes in the oxidative stress parameters (SOD, CAT, TAC) and Malondialdehyde (MAD) of the treated rats with all type of chitosan at two mentioned doses were evaluated and the results tabulated in Table 4. No significant differences were observed in antioxidant enzymes and MDA when compared with control group. TAC gives more relevant data compared with measuring of each component individually as it is an accumulative consequence of an antioxidant compounds relative to plasma and body fluids. Otherwise, SOD has major role in removing of reactive oxygen species (ROS) resulting from the process of peroxidation in liver tissues [47]. SOD transfers superoxide to H2O2, then can be easily converted to water by cell catalase [48]. In the current study, the insignificancy changes in antioxidant enzyme level in MDA were confirmed with the histopathological investigation. The liver, Kidneys and stomach tissues were in normal architecture structure under all treatments compared with control groups. Wan et al. [49] reported that the serum malondialdehyde concentration of weaned pigs was decreased (P < 0.05) 26.59% by chitosan oligosaccharide ingestion.
Table 4.
Malondialdehyde and antioxidant enzymes of liver and kidneys homogenates of the treated rats.
| Parameter |
Control | FC |
FC |
NFC |
NFC |
ShC |
ShC |
NSh |
NSh |
|
|---|---|---|---|---|---|---|---|---|---|---|
| (100 mg/kg BW) | (200 mg/kg BW) | (100 mg/kg BW) | (200 mg/kg BW) | (100 mg/kg BW) | (200 mg/kg BW) | (100 mg/kg BW) | (200 mg/kg BW) | |||
| MDA | Liver | 43.8 ± 1.7a | 42.5 ± 2.2a | 44.8 ± 0.9a | 44.6 ± 1.7a | 43.6 ± 0.9a | 43.2 ± 1.2a | 43.2 ± 0.9a | 24.8 ± 0.4a | 42.9 ± 0.6a |
| Kidneys | 40.3 ± 0.8a | 39.2 ± 1.3a | 40.6 ± 1.8a | 38.06 ± 2.5a | 40.46 ± 1.6a | 43.7 ± 1.2a | 41.7 ± 1a | 42.1 ± 0.7a | 40.4 ± 2.1a | |
| SOD | Liver | 32.3 ± 1.5a | 34.1 ± 1.9a | 32.2 ± 1.7a | 35.3 ± 1.1a | 35.1 ± 2a | 32.3 ± 0.8a | 34.5 ± 1.2a | 33.8 ± 1.8a | 34.2 ± 1.1a |
| Kidneys | 38.1± 0.6a | 38.6 ± 1a | 37 ± 1.5a | 39.8 ± 0.7a | 41.03 ± 1.5a | 39.3 ± 0.4a | 38.2 ± 0.7a | 40.6 ± 0.9a | 39.02 ± 0.9a | |
| TAC Mol/ | Liver | 24 ± 0.5a | 23 ± 2a | 26.7 ± 1.2a | 28.4 ± 1.6a | 33.2 ± 2a | 27.7 ± 0.5a | 29 ± 0.8a | 26.4 ± 1.2a | 26.5 ± 0.7a |
| Kidneys | 23.7 ± 0.6a | 23.2 ± 1.2a | 24.3 ± 0.7a | 23.9 ± 0.2a | 24.5 ± 2a | 23.7 ± 1.2a | 24.5 ± 0.5a | 24.6 ± 1a | 24.1 ± 0.3a | |
| CAT | Liver | 42 ± 3.6a | 43 ± 1.7a | 45.1 ± 2a | 44.4 ± 1.5a | 47.3 ± 1.9a | 50.06 ± 0.7a | 47.2 ± 1.5a | 46.3 ± 0.5a | 45.7 ± 0.7a |
| Kidneys | 40.3 ± 0.8a | 39.2 ± 1.2a | 40.1 ± 1.7a | 38.06 ± 2.5a | 40.46 ± 1.6a | 43.7 ± 1.2a | 41.7 ± 1a | 42.1 ± 0.6a | 40.33 ± 2.1a | |
Within each row, means with superscript with different letters are significantly different at p ≤ 0.05; Control = Untreated Crop; FC = fungal chitosan; NFC = nano fungal chitosan; ShC = Shrimp chitosan; NShC = nano shrimp chitosan; Bw = body weight; SOD = superoxide dismutase; TAC = total antioxidant capacity; CAT = catalase; MDA = Malondialdehyde.
3.4.2. Histopathological study
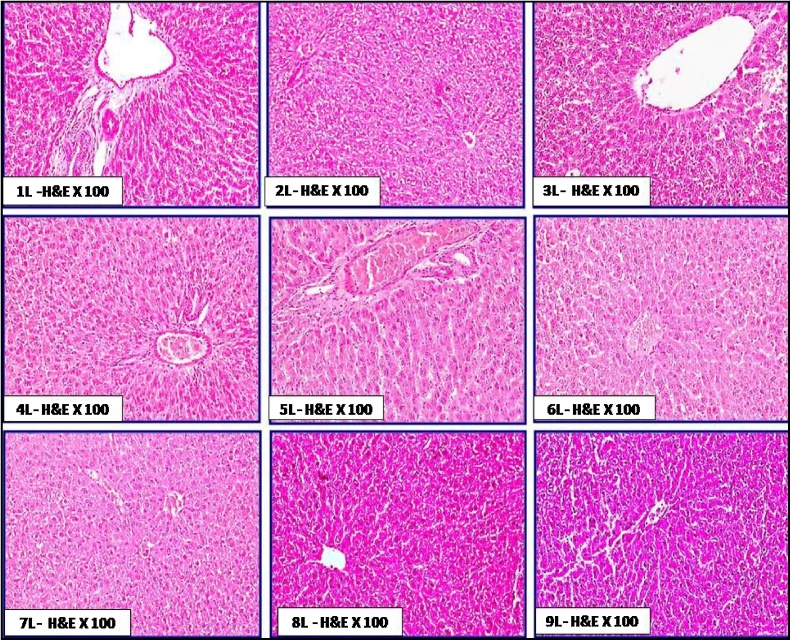
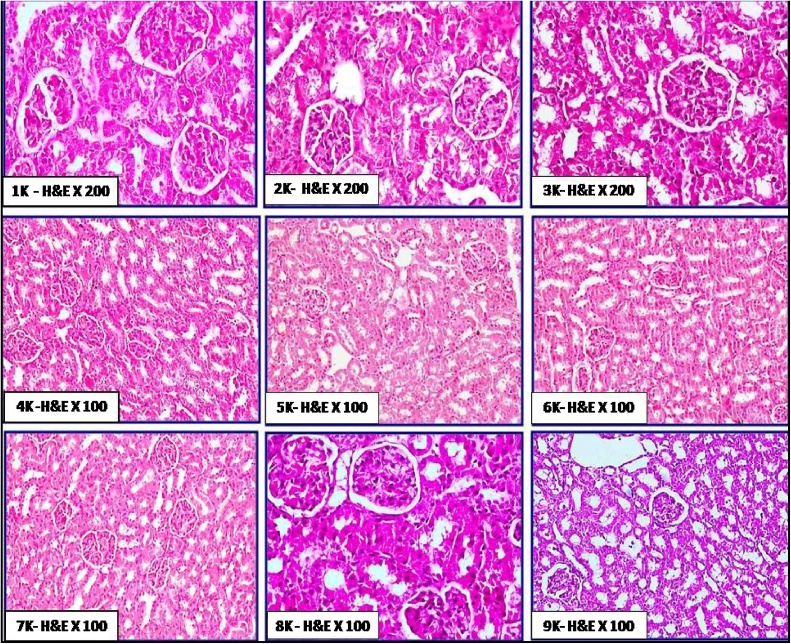
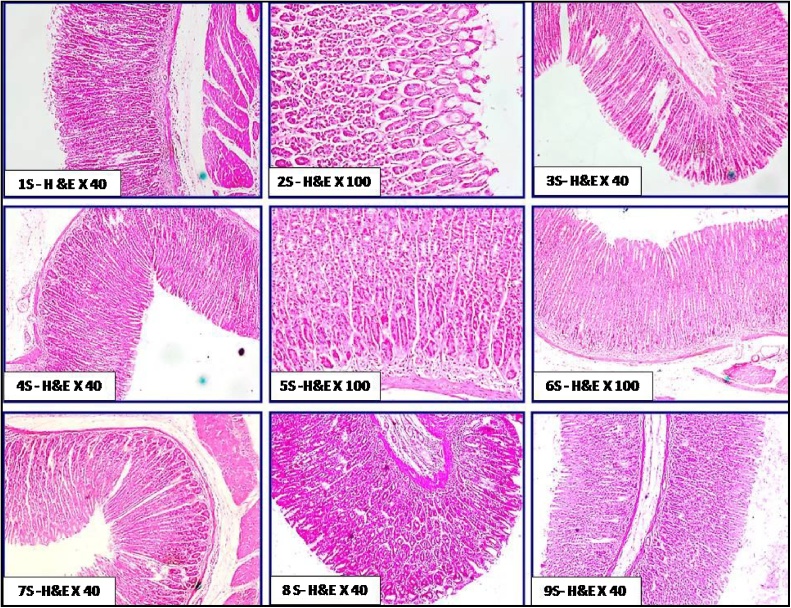
The histological features of liver in the different groups of rats orally administered with chitosan are shown in Fig. 5. No obvious histopathological changes in hepatic tissues were exhibited. These features included normal architecture of hepatic lobules, normal hepatic vein and sinusoids without congestion, normal bile ductules without dilatation; also hepatocytes fshowed hexagonal or polyhedral in out lines with no vacuolar degenerations. The cellular integrity and healthiness in hepatic tissue was more prominent in group 3L, 4L, 7L, 8L and 9L than groups 1L, 2L, 5L and 6L. Few studies evaluated the histological effect of chitosan on liver. Abd El-Fattah et al. [42] reported that no obvious histological changes were noticed in livers of rats fed on chitosan with ordinary hepatocytes surrounding central vein within normal architectural. Moreover, they found that feeding on 3.6 g (LMW) chitosan/kg diet enhanced the liver histology of treated rats with dioxins (5 μg dioxin/kg Bw) when compared with those treated with dioxin only. No available studies were examined the effect of nano-chitosan in diet on either kidney or stomach. In the same concern, The histological features of kidney in the treated groups (Fig. 6) showed no obvious histopathological changes in kidney tissue as; normal and active glomerular tuft. The proximal and distal convoluted tubules appeared normal with minimal degeneration in some areas. There are no any signs of toxicity as congestion, haemorrhages or tissue damage in renal tissue. Besides, the histological features of stomach (Fig. 7) in the different groups of rats revealed that the villi of gastric mucosa were nearly to be completely normal and intact without any tissue damage. There were no any signs of toxicity; as villus damages, ulcerations and sub-mucosal haemorrhages.
Fig. 5.
Plate of the histological status of hepatic tissue in rat treated orally with chitosan from fugal and shrimp sources; with two doses and also with normal and Nano particle sizes for 21days. 1L: control, 2L: FC100, 3L: FC200, 4L: NFC100, 5L: NFC200, 6L: ShC100, 7L: ShC200, 8L: NShC100, 9L: NShC200.
Fig. 6.
Plate of the histological status of kidney tissue in rat treated orally with chitosan from fungal and shrimp sources; with two doses and also with normal and Nano particle sizes for 21 days. 1 K: control, 2 K: FC100, 3 K: FC200, 4 K: NFC100, 5 K: NFC200, 6 K: ShC100, 7 K:ShC200, 8 K: NShC100, 9 K:NShC200.
Fig. 7.
Plate of the histological status of stomach tissue in rat treated orally with chitosan from fungal and shrimp sources; with two doses and also with normal and Nano particle sizes for 21 days. 1S: control, 2S: FC100, 3S: FC200, 4S: NFC100, 5S:NFC200, 6S:ShC100, 7S:ShC200, 8S: NShC100, 9S:NShC200.
4. Conclusion
Nano derivatives of both shrimp and fungal chitosan were well prepared in size ranging from 5 to 13 nm. Feeding on both normal and nano chitosan at 100 and 200 mg kg−1 bw rat had no effect on liver and kidney functions. Also, histopathology of these two organs in addition to stomach showed no obvious changes in their tissues. Finally, this subject needs more studies to confirm the obtained results and increase of chitosan applications instead of chemical food ingredients.
Conflict of interest
All authors have no conflicts of interest to disclose.
References
- 1.Shahidi F., Abuzaytoun R. Chitin, chitosan, and Co-products: chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005;49:93–135. doi: 10.1016/S1043-4526(05)49003-8. [DOI] [PubMed] [Google Scholar]
- 2.Tharanathan R.N., Kittur F.S. Chitin- the undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003;43:61–87. doi: 10.1080/10408690390826455. [DOI] [PubMed] [Google Scholar]
- 3.Andrade V.S., Neto B.B., Souza W., Campos-takaki G.M. A factorial designs analysis of chitin production by Cunninghamella elegans. Can. J. Microbiol. 2000;46:1042–1045. doi: 10.1139/w00-086. [DOI] [PubMed] [Google Scholar]
- 4.Franco L.O., Maia R.C.G., Porto A.L., Messias A.C., Fuskushima K., Campos-Takaki G.M. Heavy metal biosorption by chitin and chitosan isolated from Cunninghamella elegans (IFM 46109) Braz. J. Microbiol. 2004;35:243–247. [Google Scholar]
- 5.Campos-Takaki G.M. The fungal versatility on the copolymers chitin and chitosan production. In: Dutta P.K., editor. Chitin and Chitosan Opportunities and Challenges. SSM : Internat. P.; India: 2005. pp. 69–94. [Google Scholar]
- 6.Kumar M. A review of chitin and chitosan applications. React. Funct. Polym. 2000;46:1–27. [Google Scholar]
- 7.Chung Y.C., Chen C.Y. Antibacterial characteristics and activity of acid soluble chitosan. Biores. Technol. 2008;99:2806–2814. doi: 10.1016/j.biortech.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Gharieb M.M., El-Sabbagh S.M., Shalaby M.A., Darwesh O.M. Production of chitosan from different species of zygomycetes and its antimicrobial activity. Intern. J. Sci. Eng. Res. 2015;6:123–130. [Google Scholar]
- 9.No H.K., Lee M.Y. Isolation of chitin from crab shell waste. J. Korean Soc. Food Sci. Nutr. 1995;24:105–113. [Google Scholar]
- 10.Shimojoh M., Fukushima M., Kurita K. Low-molecular weight chitosans derived from beta-chitin preparation, molecular characteristics and aggregation activity. Carbohydr. Polym. 1998;35:223–231. [Google Scholar]
- 11.Kumar M.N., Muzzarelli R., Muzzarelli C. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 12.Matter I.A., Darwesh O.M., El-baz F.K. Using the natural polymer chitosan in harvesting scenedesmus species under different concentrations and cultural pH values. Intern. J. Pharm. Bio Sci. 2016;7(4)(B):254–260. [Google Scholar]
- 13.Pochanavanich P., Suntornsuk W. Fungal chitosan production and its characterization. Lett. Appl. Microbiol. 2002;35:17–21. doi: 10.1046/j.1472-765x.2002.01118.x. [DOI] [PubMed] [Google Scholar]
- 14.Nemtsev S.V., Zueva O.Y., Khismatullin M.R., Albulov A.I., Varlamov V.P. Isolation of chitin and chitosan from honeybees. Appl. Biochem. Microbiol. 2004;40:39–43. [Google Scholar]
- 15.Darwesh O.M., Shalaby M.A. LAP LAMBERT Academic Publishing, OmniScriptum GmbH & Co. KG; 2016. Production and Application of Fungal Chitosan and Chitosan Nanoparticles. ISBN: 978-3-659-83501-8, 170 p. [Google Scholar]
- 16.Nwe N., Stevens W.F. Production of fungal chitosan by solid substrate fermentation followed by enzymatic extraction. Biotechnol. Lett. 2002;24:131–134. [Google Scholar]
- 17.Bansal V., Sharma P.K., Sharma N., Pal O.P., Malviya R. Applications of chitosan and chitosan derivatives in drug delivery. Adv. Biol. Res. 2011;5:28–37. [Google Scholar]
- 18.Perera U.M., Rajapakse N. Chitosan nanoparticles: preparation, characterization, and applications. In: Kim S., editor. Seafood Processing By-Products. Springer; New York: 2014. pp. 371–387. Chapter 18. [Google Scholar]
- 19.Lamarque G., Lucas J., Viton C., Domard A. Physicochemical behavior of homogeneous series of acetylated chitosans in aqueous solution: role of various structural parameters. Biomacromolecules. 2005;6:131–142. doi: 10.1021/bm0496357. [DOI] [PubMed] [Google Scholar]
- 20.Huang M., Khor E., Lim L. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm. Res. 2004;21:344–353. doi: 10.1023/b:pham.0000016249.52831.a5. [DOI] [PubMed] [Google Scholar]
- 21.Liu H., Gao C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym. Adv. Technol. 2008;20:613–619. [Google Scholar]
- 22.Sultan Y.Y., Ali M.A., Darwesh O.M., Embaby M.A., Marrez D.A. Influence of nitrogen source in culture media on antimicrobial activity of Microcoleus lacustris and Oscillatoria rubescens. Res. J. Pharm. Biol. Chem. Sci. 2016;7(2):1444–1452. [Google Scholar]
- 23.Khalil A.M., Abdel-Monem R.A., Darwesh O.M., Hashim A.I., Nada A.A., Rabie S.T. Synthesis, characterization, and evaluation of antimicrobial activities of chitosan and carboxymethyl chitosan schiff-base/Silver nanoparticles. J. Chem. 2017:1–11. [Google Scholar]
- 24.Cocchetto D.M., Bjornsson T.D. Methods for vascular access and collection of body fluids from laboratory rat. J. Pharm. Sci. 1983;72:465–492. doi: 10.1002/jps.2600720503. [DOI] [PubMed] [Google Scholar]
- 25.Reitman S., Frankel S. Colorimetric method for aspartate and alanine tranferases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 26.Kind P.R., King E.J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino antipyrine. J. Clin. Pathol. 1954;7:322–326. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charles C.A., Richmond W. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 28.Fassati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- 29.Fawcett J.K., Scott J.E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartles H., Bohmer M., Heirli C. Serum creatinine determination without protein precipitation. Clin. Chim. Acta. 1972;37:193–197. doi: 10.1016/0009-8981(72)90432-9. [DOI] [PubMed] [Google Scholar]
- 31.Haisman P., Muller B. 1977. Glossary of Clinical Chemistry Terms Butterworth, London; p. 126. [Google Scholar]
- 32.Oyedemi S.O., Bradley G., Afolayan A.J. In −vitro and −vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. Afr. J. Pharm. Pharmacol. 2010;4:070–078. [Google Scholar]
- 33.Aebi E.H. 3rd ed. vol. III. Verlag Chemie; Deerfield Beach, FL: 1983. Catalase; pp. 273–282. (Bergmeyer Methods of Enzymatic Analysis). [Google Scholar]
- 34.Koracevic D., Koracevic G., Djordjevic V., Andrejevic S., Cosic V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yagi K. Lipid peroxides and human disease. Chem. Phys. Lipids. 1987;45:337–351. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- 36.Drury R.A., Wallington E.A., Cancerson R. 4th ed. Oxford University Press; Oxford, London, New York: 1980. Carlton’s Histopathological Techniques. [Google Scholar]
- 37.Puvvada Y.S., Vankayalapati S., Sukhavasi S. Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. Intern. Curr. Pharm. J. 2012;1(9):258–263. [Google Scholar]
- 38.Varan N. The use of titration technique and FTIR bands to determine the deacetylation degree of chitosan samples. J. Textile Sci. Eng. 2017;6:288–291. [Google Scholar]
- 39.Alvarenga E.S. In: Characterization and Properties of Chitosan, Biotechnology of Biopolymers. Elnashar Magdy., editor. InTech; 2011. ISBN: 978–953-307–179-4. [Google Scholar]
- 40.Mohamed A.A., Ali S.I., Darwesh O.M., El-Hallouty S.M., Sameeh M.Y. Chemical compositions, potential cytotoxic and antimicrobial activities of Nitraria retusa methanolic extract sub-fractions. Intern. J. Toxicol. Pharmacol. Res. 2015;7(4):204–212. [Google Scholar]
- 41.Abd El-Fattah H.M., Abdel- Kader Z.M., Hassnin E.A., Abd El-Rahman M.K., Hassan L.E. Chitosan as a hepato-protective agent against single oral dose of dioxin. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013;7(3):11–17. [Google Scholar]
- 42.Karami S., Ahmadi R., Siavashi M. Serum triglyceride, cholesterol and fasting blood sugar in male rats exposed to oil paint vapor. International Conference on Earth, Environment and Life Sciences (EELS-2014); Dec. 23–24, 2014 Dubai (UAE); 2014. [Google Scholar]
- 43.Qujeq D., Ataei G. Effects of dietary chitosan on serum lipid and lipoprotein concentrations in rats. Iran. Biomed. J. 2000;4:69–73. [Google Scholar]
- 44.Abdel Wahhab M.A., Nada S.A., Khalil F.A. Physiological and toxicological responses in rats fed aflatoxin contaminated diet with or without sorbent materials. Anim. Feed Sci. Technol. 2002;97:209–219. [Google Scholar]
- 45.Jing S.B., Li L., Ji D., Takiguchi Y., Yamaguchi T. Effect of chitosan on renal function in patients with chronic renal failure. J. Pharm. Pharmacol. 1997;49:721–723. doi: 10.1111/j.2042-7158.1997.tb06099.x. [DOI] [PubMed] [Google Scholar]
- 46.Packer J.E., Slater T.F., Willson R.L. Reactions of the carbon tetrachlonderelated peroxy free radical with amino acids: pulse radiolysis evidence. Life Sci. 1978;23:2617–2620. doi: 10.1016/0024-3205(78)90378-8. [DOI] [PubMed] [Google Scholar]
- 47.Abdel Wahhab M.A., Hassan N.S., El Kady A.A., Khadrawy Y.A., El Nekeety A.A., Mohamed S.R., Mannaa F.A. Red ginseng extract protects against aflatoxin B1 and Fumonisins induced hepatic precancerous lesions. Food Chem. Toxicol. 2010;48:733–742. doi: 10.1016/j.fct.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Wan J., Jiang F., Xu Q., Chen D., Yu B., Huang Z., Mao X., Yua J., He J. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv. 2017;7:9669–9679. [Google Scholar]
- 49.Edrington T.S., Sarr A.B., Kubena L.F., Harvey R.B., Phillips T.D. Hydrated sodium calcium aluminosilicate HSCAS, acidic HSCAS, and activated charcoal reduce urinary excretion of Aflatoxin M1 in turkey poults. Toxicol. Lett. 1996;89:115–122. doi: 10.1016/s0378-4274(96)03795-2. [DOI] [PubMed] [Google Scholar]