Highlights
-
•
Size and chemical identity of metallic nanoparticles Me-NPs are the main but interplaying determinants of their toxicity.
-
•
Organism’s responses to joint action of Me-NPs having different chemical compositions follow the patterns of combined toxicity common to different forms of metals.
-
•
Safe Me-NP occupational exposure levels should be substantiated, but principles of establishing them is still a problem for discussion.
-
•
It is possible to enhance significantly the organism’s resistance to the adverse health effects of Me-NP exposures.
Keywords: Metallic nanoparticles, Toxicity, Genotoxicity, Combined nano-impacts, Permissible exposure levels, Bioprotection
Abstract
During 2009–2017 we have studied nanoparticles of elemental silver or gold and of iron, copper, nickel, manganese, lead, zinc, aluminium and titanium oxides (Me-NPs) using, in most cases, a single low-dose intratracheal instillation 24 h before the bronchoalveolar lavage to obtain a fluid for cytological and biochemical assessment and, in all cases, repeated intraperitoneal injections in non-lethal doses to induce subchronic intoxications assessed by a lot of toxicodynamic and toxicokinetic features. We have also studied the same effects for a number of relevant combinations of these Me-NPs and have revealed some important patterns of their combined toxicity. Besides, we have carried out long-term inhalation experiments with Fe2O3, NiO and amorphous SiO2 nano-aerosols. We have demonstrated that Me-NPs are much more noxious as compared with their fine micrometric counterparts although the physiological mechanisms of their elimination from the lungs proved to be highly active. Even if water-insoluble, Me-NPs are significantly solubilized in some biological milieus in vitro and in vivo, which may explain some important peculiarities of their toxicity. At the same time, the in situ cytotoxicity, organ-systemic toxicity and in vivo genotoxicity of Me-NPs strongly depends on specific mechanisms characteristic of a particular metal. For some of the Me-NPs studied, we have proposed standards of presumably safe concentrations in workplace air. Along with this, we have proved that the adverse effects of Me-NPs could be significantly alleviated by background or preliminary administration of adequately composed combinations of some bioprotectors.
1. Introduction
Along with a rapid upsurge of technologies for manufacturing nanomaterials having unique physicochemical and hence biological properties and with widening the field of their scientific, technical and medical applications, has emerged and is actively developing a special branch of the toxicological science known as the nanotoxicology [78]. Beside many tens of works dedicated to toxicity of specific nanoparticles, several important mechanistic aspects of their biological activity were and still are being discussed [[26], [69]]
Starting from 2009, the authors of this synopsis, who constitute the core of what we tag as the Ekaterinburg nanotoxicology team,1 have focused their research on animal experiments with nanoparticles of metals and metalloids in elemental or oxidized state (Me-NPs). We maintain that this class of NPs is of special interest in the light of risks assessment and some other aspects of preventive toxicology because, along with purposely manufactured (engineered) Me-NPs, there usually is a substantial fraction of similar nanoscale (“ultrafine”) particles found within the particle size distribution of condensation aerosols generated by arc-welding, laser industrial processing, metallurgical and some chemical technologies and, thus, polluting both workplace and ambient air.
It is important to keep in mind, however, that such industrial aerosols (a) unlike engineered Me-NPs, do not have their nanoscale dimensions strictly preset; (b) typically also contain chemically similar or even identical micrometer particles (MPs), including submicron ones having dimensions >100 nm (see Fig. 1). That is why our priority questions to answer were: how does the Me-NP toxicity depend on particle size within the nanoscale range, and are Me-NPs really far more noxious for the exposed organism compared with their Me-MP chemical analogues? It is to be recalled that in the literature of the 2000 s the first question was virtually neglected while the second one was answered in the affirmative, though not unanimously [99].
Fig. 1.
(a) Particles sampled from copper smelter workplace air (SEM, magnification *25,080) (b) Percentage distribution of submicron particles by size obtained with programmed statistical processing of 500 measurements from the same SEM-image. N is the number of particles of a given diameter; No is the total number of particles. First published in [76].
To this end, we have carried out a series of animal experiments to compare the toxicity of different metallic particles in the nanometer and micrometer ranges.
The toxic effects of the Me-NP species listed in Section 2 (mostly as their commercially available engineered counterparts) have been studied by other researchers as well (e.g. iron oxides [[12], [56], [59], [60], [61], [68], [85], [88], [101], [106]], silver [[2], [3], [4], [8], [9], [13], [20], [22], [27], [28], [33], [35], [39], [50], [51], [53], [54], [55], [75], [76], [86], [89], [90], [95], [96]], gold [[10], [11], [18], [21], [24], [32], [52], [53], [67], [72], [82], [84], [86], [97], [105]], copper and copper oxide [[5], [6], [15], [17], [23], [33], [38], [58], [73], [91], [102]], nickel oxide [[16], [57], [58], [66], [103], [104]], manganese oxides [[14], [87]], zinc oxide [[1], [19], [30], [31], [36]], lead oxide [[7], [83]] but, unlike our experiments, theirs have never been comparative or involving combinations of those Me-NPs. It should also be stressed that up until very recently these studies have been conducted mostly in vitro on different immortalized or primary cell cultures and sometimes on small non-mammal organisms (e.g. [[71], [77], [81], [100]] and a lot of others), while ours have been oriented from the outset at in vivo exposure of laboratory rats.
We understood some well-known advantages of in vitro nano-toxicological experiments relating, in particular, to the analysis of primary toxicity mechanisms on cellular and sub-cellular levels. However, any extrapolation of the results of these experiments to the organ-systemic level is always associated with a number of uncertainties and assumptions, while some problems which are most important from the preventive toxicology point of view (in particular¸ organism level toxicokinetics, relationships between doses and systemic responses, the functioning and efficiency of self-regulatory and protective mechanisms, etc.) can generally be addressed only through experiments on the whole mammalian organism.
2. Materials and methods
All animal experiments were carried out on outbred white rats from our own breeding colony housed in conventional conditions, breathed unfiltered air, and fed standard balanced food. The experiments were planned and implemented in accordance with the – International guiding principles for biomedical research involving animals‖ developed by the Council for International Organizations of Medical Sciences (1985) and approved by the Ethics Committee of the Ekaterinburg Medical Research Center Medical for Prophylaxis and Health Protection in Industrial Workers We have used mostly two experimental models based either on a single low-dose intra-tracheal (IT) instillation of Me-NPs 24 h before the broncho-alveolar lavage, or on repeated intra-peritoneal (IP) injections of the same during 6–7 weeks in non-lethal single doses. (In all our experiments the dosage was chosen empirically as being too low to induce too heavy responses but high enough to induced measurable adverse shifts of relevant indices for the organism’s status.) All comparative assessments have been based on experiments conducted strictly in parallel.
We first studied iron oxide Fe3O4 (magnetite) NPs and MPs produced by a chemical technique [41], but later on other metallic nanoparticles were produced by laser ablation of respective 99.99% pure metals in deionized water [49]. All the particles excepting the rod-like ZnO-NPs were of spherical or near spherical shape. In some experiments, we compared particles of one and the same chemical composition having different diameters, while in others – equidimensional nanoparticles of different metals. The particles compared were those of: (a) gold – 3,9 nm, silver – 3,4 nm; (b) gold – 50 nm, silver – 49 nm and 1,1 mcm; (c) iron oxide Fe3O4 (magnetite) – 10 nm, 50 nm and 1000 nm (1 mcm); (d) copper oxide – 20 nm and 340 nm; (e) nickel (II) oxide – 20 nm, manganese (II, III) oxide – 19 nm; (f) lead oxide (47m), copper oxide (25 nm) and zinc oxide (83 × 30 nm); (g) aluminium (III) oxide (21 nm), titanium dioxide (27 nm), silicon dioxide (43 nm). Nanoparticles were produced as water suspensions (either sufficiently stable or, in some rare cases, needing ultrasonication before injection/instillation). No chemical stabilizer has ever been used in the suspensions. To avoid qualitative inequality of the tested Me-NP species beyond our control in any respect, we have never used any commercial batches of engineered Me-NPs.
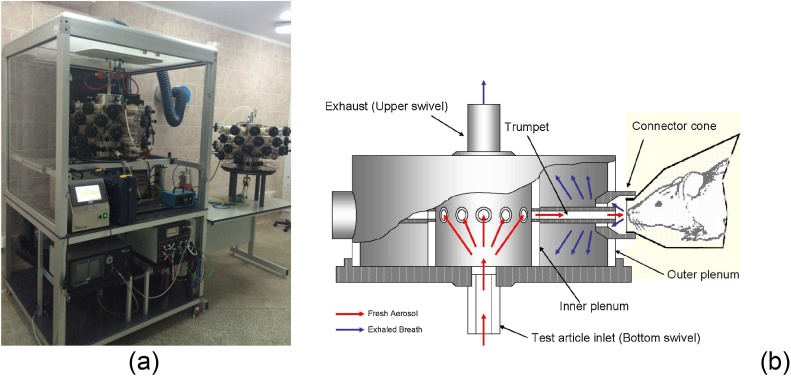
We have also carried out long-term inhalation exposures of rats in a “nose only” device (Fig. 2) to nano-aerosols of Fe2O3 [92], SiO2 in two different concentrations [93], and NiO [paper in preparation].
Fig. 2.
(a) General view of the inhalation device used in our experiments (photographed with the front chamber doors removed). Next to the exposure unit is a similar system setup for sham exposure of control rats. (b) Diagram of aerosol flows in this “nose only” inhalation exposure system (courtesy CH Technology, USA). First published in [93].
3. A brief overview of the most important inferences from our studies
3.1. Size and chemical identity of Me-NPs are the main determinants of their toxicity
Our research has demonstrated that metallic nanoparticles are much more noxious as compared with their fine micrometric or even submicron (above 100 nm) counterparts. At the same time, the cytotoxicity, organ-systemic toxicity and in vivo genotoxicity of nanoparticles having a given geometry is strongly dependent on their chemical nature and on properties associated with the latter (solubility included) as well as on specific mechanisms of action characteristic of a given metal in any chemical form [49].
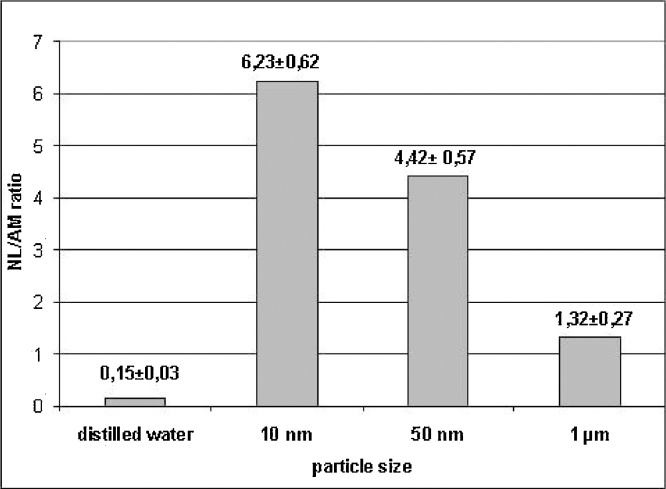
The examples given in Fig. 3, Fig. 4 illustrate these dependences based on the values of the neutrophil leukocytes to alveolar macrophages count ratio in a broncho-alveolar lavage fluid (BALF) obtained 24 h after an IT instillation of different Me-NP suspensions, which is a very informative index of the comparative cytotoxicity and pulmonary toxicity of particles deposited in the lower airways [44].
Fig. 3.
The ratio of the number of neutrophil leukocytes (NL) to the number of alveolar macrophages (AM) in the BALF of rats 24 h after the instillation of magnetite particles of different sizes at a dose of 2 mg in 1 ml of distilled water (х± s.e.m.).
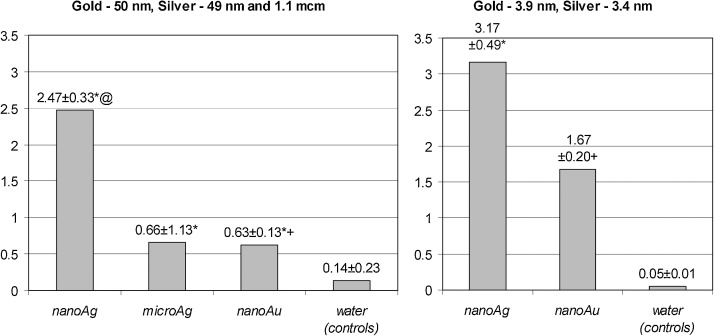
Fig. 4.
The same index for comparative cytotoxicity of silver and gold particles.
Note: * − statistically significant (P < 0.05) difference from controls;+- the same from nanoAg; @ − the same from microAg.
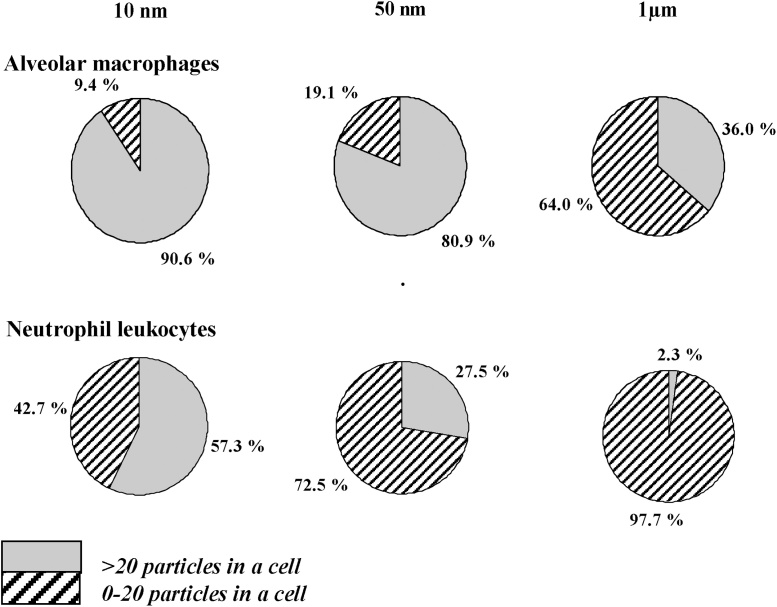
Meantime, the dependence of Me-NP organ-systemic subchronic toxicity on their dimensions within the conventional nanoscale range is not so simple. For instance, we found [42] that, although not only the in vivo cytotoxicity but also the subchronic organ-systemic toxicity of Fe3O4-NPs was higher compared to that of Fe3O4-MPs (even though the mean diameter of the latter was ca. 1 μm), the relationship between nanoscale size and systemic toxicity proved to be inverse for some adverse effects. Specifically, this paradoxical size dependence of Me-NP toxicity may be characteristic of target organs rich in RES cells and thus most capable of actively accumulating nanoparticles from the blood – such as the liver and the spleen. It should be taken into consideration that long-term accumulation of NPs in these and other organs depends, first of all, upon their more or less free penetration into the circulation by diffusion mechanisms through the biological barriers from the sites of their primary or secondary deposition, as a prerequisite to their being captured from blood by different tissues due to the same diffusion mechanism and to the specific function of resident macrophages. It stands to reason that this biphasic mechanism of particle translocation should be most effective for the smallest NPs because of both their higher penetrability and more avid engulfment by macrophages (exemplified by Fig. 5, Fig. 6).
Fig. 5.
Percentages of phagocytic cells with different particle burdens in rat BALF 24 h after an i.t. instillation of Fe3O4 particles having different diameters [49].
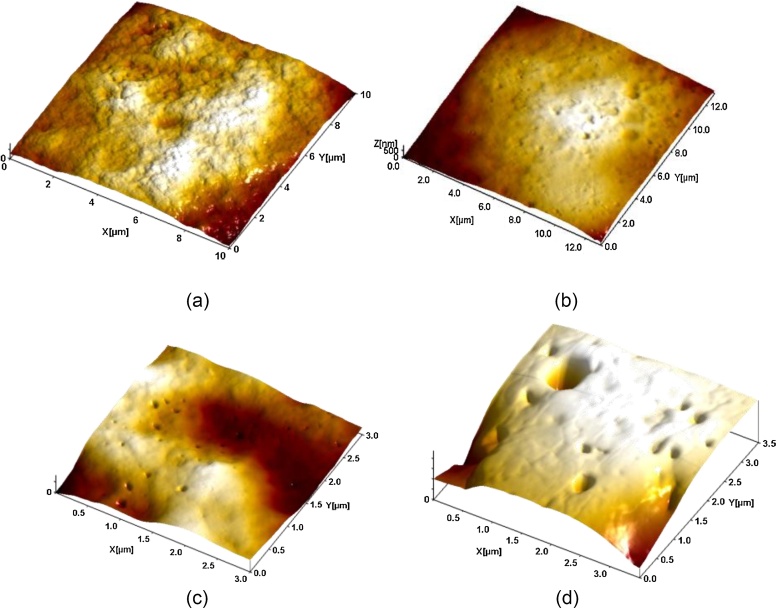
Fig. 6.
Alveolar macrophage surface topography measured by semi-contact AFM (a) control; (b) after instillation of 10 nm Fe3O4-NPs; (c) after instillation of 50 nm Fe3O4-NPs; (d) after instillation of 1 μm Fe3O4-MPs. First published and interpreted as associated with phagocytosis in [49].
However, the smaller a particle, the quicker it dissolves in these secondary depots due to its immense specific surface area. Besides, the smallest NPs are presumably more cytotoxic for any cells, resident macrophages included, and thus cause cellular death and destruction (with eventual release of NPs back into the bloodstream) more effectively. The balance between these oppositely acting mechanisms of toxicokinetics depends on many variables, but it is at least possible that for some larger Me-NPs the organ burden (and thus adverse effects on this organ) should be greater than for smaller Me-NPs of the same chemical nature, as we indeed found in experiments with Fe3O4-NPs [41].
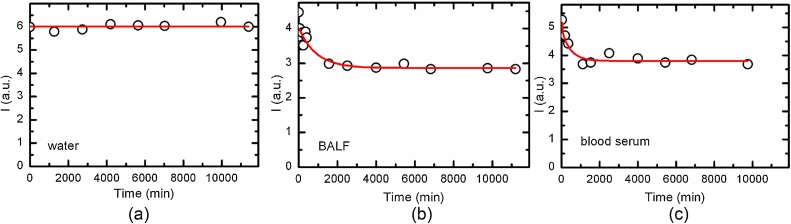
The data obtained in ex vivo experiments with another iron oxide Fe2O3-NP may illustrate the typical solubilization of water-insoluble Me-NPs when a biological solvent is present (Fig. 7).
Fig. 7.
Decay kinetics of the Fe+3 EPR signal from a filter on which Fe2O3-NPs deposited from the inhalation exposure chamber exhaust air (a) in de-ionized water, (b) in rat BALF supernatant, (c) in sterile bovine blood serum [49].
The kinetics of this ex or in vivo solubilization of Me-NPs is not only understandably associated with their dimension (being the higher, the smaller the particle’s size due, first of all, to its increased specific surface area) but depends on their chemical nature as well. Thus, for example, we demonstrated that Ag-NPs were more soluble than equidimensional Au-NPs [45] or Mn3O4-NPs as compared with equidimensional NiO-NPs [63]. In the meantime, the toxicological mechanistic significance of this property is not unique. On the one hand, the intracellular dissolution of an internalized Me-NP with release of toxic Me-ions close to ultrastructural and molecular targets of their impact (the so-called Trojan horse effect) is one of the widely recognized primary mechanisms explaining their especially high toxicity [27]. For instance, the key role of this mechanism was demonstrated in relation to ZnO-NP cytotoxicity [94]. Besides, it is quite probable (although not proved directly, as far as we know) that quicker dissolution of deposited Me-NPs with ion resorption into the bloodstream is a prerequisite to their higher systemic toxicity. On the other hand, however, our long-term experiments and their mechanistic multicompartmental modeling (Fig. 8) have proved that the same toxicokinetic process leads to a very low retention of inhaled Fe2O3-NPs [92] or inhaled SiO2-NPs [93] not only in the lungs and lung-associated lymph nodes but also in remote organs and thus to quite insignificant chronic adverse effects, both on local and systemic levels.
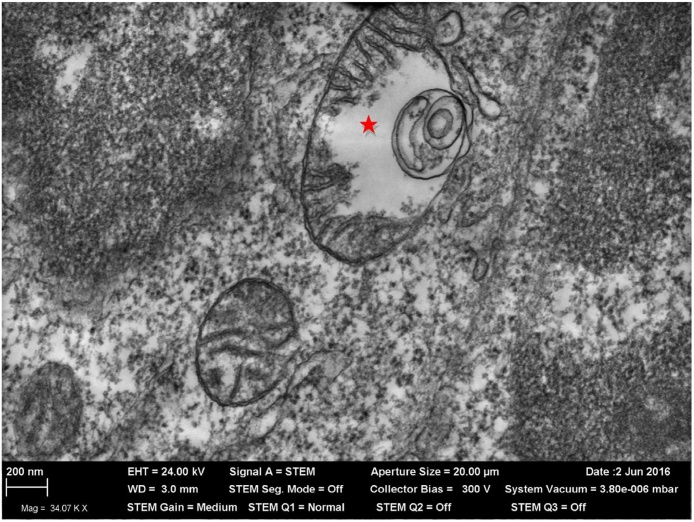
Fig. 8.
A partially destroyed mitochondrion (marked by asterisk) in a thymus cell of a rat exposed to PbO-NPs and ZnO-NPs. TEM, magnification *34,070. First published in [64].
We have found under both single-shot IT and repeated IP exposures that chemically different Me-NPs (e.g., equidimensional Ag-NP and Au-NP [45]) may differ not only in the degree of their cell-damaging action (cytotoxicity) but also in their intracellular distribution. However, the association between the latter and the prevalent intracellular loci of such damage is not always explicit. Thus, the ultrastructural abnormalities that we observed in cells of the liver, spleen, kidney, myocardium, brain, thymus and testicle tissues of rats exposed to subchronic intoxication with ZnO-NPs, CuO-NPs and Pb-NPs administered separately or in different combinations were visualized by qualitatively standard although quantitatively different TEM-pictures. The most frequent ones were vacuolization of the cytoplasm and concentric membranous inclusions in it and, especially, damage to mitochondria with partial or complete loss of cristae (Fig. 8), and demyelinization of nervous fibers in the brain. Paradoxically, the TEM picture of mitotoxicity was most frequent and most expressed in the testicles while explicit NP accumulation was the lowest just in this organ [49]
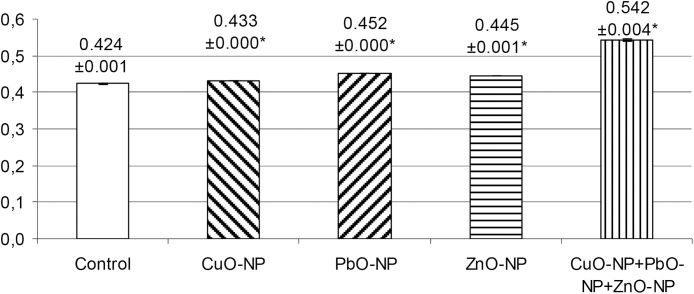
In all our subchronic experiments involving different Me-NPs, a RAPD-test was performed on cells of different tissues (on nucleated cells of circulating blood at the least). Of high importance is the fact that we have not yet found a Me-NP species that would not be thus genotoxic in vivo. Taking into account that the molecular mechanisms involved in DNA breakdown by such Me-NPs and Me-ions released in the course of their intracellular dissolution (either through direct action or by inducing ROS generation) are presumably common to all Me-NPs [29], we consider this ominous effect to be Me-independent and, in this sense, unspecific. As concerns its quantitative expression, the difference between the effects of chemically different particles was, as a rule, present but not always statistically significant and usually not very big (as exemplified by Fig. 9), although additive [49].
Fig. 9.
Genomic DNA fragmentation coefficients (based on the results of the RAPD-test) in rats exposed to subchronic administration of CuO-NPs, PbO-NPs and ZnO-NPs in equal doses separately or in combination (x ± s.e.m.). Asterisk * designates values that are statistically significantly different from the controls (p < 0.05 by Student’s t-test). First published in [64].
Thus, generally speaking, the roles played by Me-NP size and the chemical nature of a particular metal as determinants of their unspecific toxic effect level are closely intertwined and reciprocally interdependent, evidently linking size- and metal-depending differences to their in vivo solubilization. So the question which of these characteristics is more important seems rather vain. That is not the case, however, if we consider some adverse effects typical of toxicodynamics and/or toxicokinetics of this or that element in different chemical compounds and physical forms.
Thus, for example, Mn3O4-NPs, solubilized in vivo much more readily compared with NiO-NPs, would presumably be more toxic on the organ-systemic level and, especially, more nephrotoxic. We found [63], however, that damage to the tubular epithelium typical of all subchronic intoxications with Me-NPs was, on the contrary, higher under exposure to NiO-NPs alone or to a combination of (NiO-NPs + Mn3O4-NPs) than to Mn3O4-NPs alone. This can be explained by the fact that whereas the NiO-NP exposure drastically increased Ni renal excretion (and thus the impact of Ni-ions on the kidneys), the Mn3O4-NP exposure did not enhance the urinary excretion of Mn over the background level at all. The predominantly renal route of nickel excretion in contrast to a relatively low part of this route in manganese elimination under exposures to soluble salts of these metals is well known. Now we see that this fundamental difference in their toxicokinetic patterns is also present when the in vivo solubilization of Me-NPs takes place during the development of respective intoxications. Recall that in this experiment the two Me-NP species under comparison were virtually equidimensional, and the prevalent role of their chemistries becomes clearly obvious.
Let us consider as an example of the metal-determined specificity of adverse health effect patterns displayed by various nanoparticles, the much higher damage to the brain striatum and hippocampus caused by manganese oxide nanoparticles compared with nickel oxide ones [63] and very similar neurotoxicity effects observed in rats under exposure to CuO-NPs (illustrated by Fig. 10) or to submicron Cu/Cu2O-MPs [79].
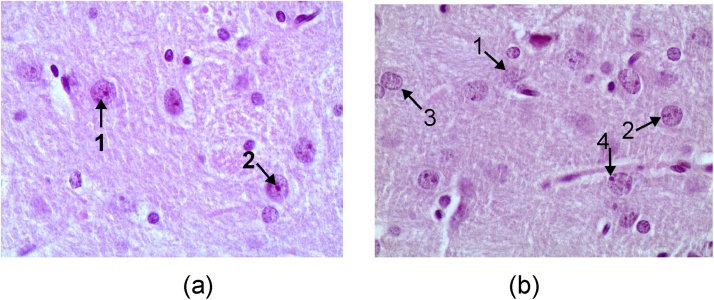
Fig. 10.
Rat brain in the nucleus caudatus area (hematoxylin-eosine stain, magnification *400). (a) A control rat. The neuron nuclei are predominantly spherical, with well-visible eosinophilic granulosity (arrow 1), and notable nucleoli in the center (arrow 2). (b) A rat exposed to subchronic i.p. intoxication with CuO-NPs. Arrows point to: poorly stained neuron nuclei, with an indistinct membrane (1), nucleoli that are pycnotic (2), often absent (3) or shifted towards the nuclear membrane (4). First published in [79].
Moreover, the subchronic toxicological syndrome caused by CuO-NPs involved the accumulation of Cu in the liver and brain, some decrease in the serum ceruloplasmin level, and anemia, thus bearing a resemblance to Wilson’s disease in humans associated with genetically determined disturbances of copper metabolism.
Yet another example of metal-specific systemic toxicity of Me-NPs was found by us [64] in a comparative subchronic experiment with CuO-, ZnO- and PbO-NPs. Not only did rats exposed to PbO-NPs have the highest urine concentration of coproporphyrins but in this group only was the urine concentration of the δ-aminolevulinic acid (δ-ALA) elevated compared with the control level: 17.4 ± 2.8 mg/L against 8.1 ± 2.7 mg/L (P < 0.05), compared with 5.3 ± 1.36 mg/L in CuO-NP exposed rats and 6.6 ± 1.8 mg/L in ZnO-NP exposed rats. As is well known, these two effects are typical of lead’s toxic action on porphyrin metabolism (and, thus, on hem synthesis). Along with this, one of the earliest hematological indices of lead intoxication, namely, increased proportion of reticulocytes, was again highest in the PbO-NP exposed rats: 24.7 ± 2.7‰ against 10.2 ± 1.4‰ in controls (P < 0.05), 19.3 ± 1.7‰ in ZnO-NP exposed rats and 11.6 ± 1.0‰ in CuO-NP exposed ones.
Furthermore, it is hardly by chance that among the 46 functional indices for the organism’s status used in that experiment, the only two that also testified to the higher toxicity of PbO-NPs compared with the other two Me-NP species were the indices characterizing exploratory activity inhibition. This effect may be tentatively interpreted as manifesting specific lead’s toxicity for the brain. In all other respects, the systemic toxicity of PbO-NPs was either the same or even lower than that of either one or both of the other Me-NPs. Moreover, the acute pulmonary toxicity of the same PbO-NPs instilled i.t., as judged by all cell counts and BALF biochemistry and their mitotoxicity for various organs, was significantly lower compared with ZnO-NPs although higher as opposed to CuO-NPs [64].
Summing up the main inferences from our experiments briefly overviewed above, we may conclude that:
-
(1)
Me-NP in vivo toxicity is much higher compared with that of their micrometric (even submicron) chemical counterparts, while within the nanoscale range it depends on both their size and chemical nature;
-
(2)
unequal solubilization in biological milieus (which, in turn, depends on both size and chemistry of a Me-NP) is one of the most probable but not necessarily the most important explanation of the quantitative difference between unspecific adverse outcomes of exposures to different Me-NPs;
-
(3)
along with this, these exposures may result in qualitatively different outcomes as manifestations of certain specific features pertinent to the toxicokinetics and toxicodynamics of the NP-forming metal;
-
(4)
in a real toxicological process, we observe a complicated interplay of these dependencies, which makes straightforward comparative assessment of health risks associated with different Me-NPs a rather difficult task.
Such definitive assessment, which is very much needed in the practice of predictive health risk analysis and regulatory toxicology, becomes even more difficult where one has to deal with the most frequent realistic situations in which industrial workers are being exposed to combined impacts of two or more Me-NP species differing in all the just mentioned intrinsic features.
3.2. Organism’s responses to a joint action of Me-NPs having different chemical compositions follow the complicated patterns of combined toxicity common to different forms of metals
As far as we know, our team was the first to start studying the combined toxicity of Me-NPs and still remains virtually the only one to be actively pursuing research into this topic [63]. Meanwhile, the extensive production and usage of nanomaterials in various industries, science and medicine make highly probable the multi-component impacts (either simultaneous or successive) of these materials on humans. This is even more true of spontaneously generated nanoparticles which the workers are exposed to in the industries mentioned in Section 1. For instance, the ultrafine condensation aerosols polluting workplace air in arc-welding and alloyed steel metallurgy usually have a complex chemical composition comprising oxides of iron, manganese, nickel, chrome, vanadium, silicon and other elements. Both the chemical identity of these NPs and the quantitative relationship between them vary depending on the specific technology or its phase, on the composition of the alloy being molten or welded and welding electrodes being used, on the metal melting temperature, etc. We believe it would be impracticable to investigate experimentally the toxic effects of each particular Me-NP mixture as well as the comparative toxicity of all these Me-NPs. What is really needed in this area is theoretically sound and experimentally corroborated conceptual and mathematical models of combined nano-toxicity.
The starting point of our journey in this direction was the proposal of a solution to this problem based on a summary of relevant literary data and the results of a series of special studies dealing with the combined action of soluble salts of manganese, lead, cadmium, nickel, chromium, fluoride [[46], [47], [62], [74], [98]]. In fact, we sought to find out whether the general patterns of Me-NP combined action were virtually the same. Generally speaking, we were able to answer this question in the affirmative without reservations. Since all of these works have already been overviewed and illustrated by us in this Journal [65], it would be inappropriate to do the same here again, and we may restrict ourselves to formulating just the main postulates inferred from our research and illustrate them with a few examples.
-
(1)
The widely assumed theoretical paradigms of effect additivity vs dose additivity as essentially different kinds of combined toxicity proved to be, in our experiments, virtually interchangeable and so might be regarded as different methods of combined toxicity modeling rather than as concepts reflecting fundamentally differing processes. The best approach to mathematical modeling uniting both paradigms is, in our experience, the Response Surface Methodology [64].
-
(2)
In one and the same case of intoxication resulting in different assessment outcomes (adverse health effects), the type of combined toxicity is not unique but is, first of all, outcome-dependent (as exemplified by Fig. 11).
-
(3)
In addition, there exist not mere three traditionally recognized types of combined unidirectional action (additivity, subadditivity and superadditivity), but also several complexes of these types and of oppositely directed action of one and the same pair of Me-NPs depending again on exactly which outcome is considered and, besides, on what its level is, as well as on dose levels and their ratios.
-
(4)
The type of binary combined toxicity may, in the presence of a third Me-NP species, transform into a more (class A) or, on the contrary, less dangerous one (class B), or remain virtually unchanged (class C). An example of three-factorial combined toxicity classified as B is given in Fig. 12. This classification was first proposed and developed in [47].
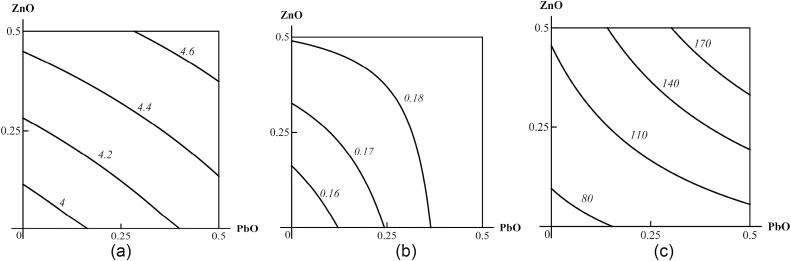
Fig. 11.
Examples of isoboles characterizing PbO-NP + ZnO-NP combined subchronic toxicity: (a) for de Ritis coefficient (additivity); (b) for follicle-stimulating hormone (FSH) (subadditivity); (c) for coproporphyrin in urine (superadditivity). The doses of CuO and ZnO are plotted on the axes in mg per rat. The numbers at the lines of the isoboles show the values of the effect Y. (FSH in IU/L, coproporphyrin in nMol/L). First published in [64].
Fig. 12.
The isobologram of a three-factorial toxicity case classified as “B”: the additivity of the [PbO-NP + CuO-NP] binary unidirectional action on the thrombocrit index in the absence of any other toxic exposure (left) transforms into a contra-directional (opposite) action of the same two Me-NPs against concomitant background exposure to ZnO-NPs (right). The doses of PbO and CuO are plotted on the axes in mg per rat. The numbers at the lines of the isoboles show the values of the effect. First published in [64].
3.3. Tentatively safe Me-NP occupational exposure levels should and can be substantiated, but how to do it is still a problem for discussion
We believe that the best protectively effective and in many, even if not in all cases, feasible approach to managing occupational health risks associated with Me-NP inhalation exposures would be to decrease the latter to virtually safe levels. A well-known alternative to this approach is the so-called precautionary paradigm according to which, until such safe levels have been substantiated to a certainty, the actual exposures should be either avoided completely or decreased to as low a level as is technically possible. However, what could be technically possible in a specific situation now or tomorrow is rather uncertain, to say nothing about the usefulness of a more concrete goal of prophylactic technical measures (especially, of enforceable ones) as an incentive for the industry to do anything at all. It is no wonder therefore that permissible workplace air concentrations of different nanoparticles, Me-NPs included, have been, at some point, adopted or at least recommended in some countries (whatever term a country or its various administrative agencies are using to denote such safe exposure standards).
In this respect, what may be called “regulatory nanotoxicology” would be based on a general presumption common to regulatory toxicology as a whole, namely that for some low level of any potentially dangerous exposure there may be a balance between its intrinsic noxiousness and the organism’s natural defenses preventing the development of any identifiable disease or condition. Is such a balance possible for nanoparticles (NPs) and, specifically, for Me-NPs? The question is not vain as we started our research into this field when the idea of an utterly low, if any at all, efficiency of the organism’s natural defenses against NPs was very popular among scientists of rather high repute − the idea which we criticized, first proceeding from evolutionary based doubts and then based on the very first experimental results of ours [48].
As stated in Section 3.1, we have confirmed another widespread belief according to which a substance, even if relatively innocuous in bulk or as a micro-scale particulate, may be markedly toxic in the form of nanoparticles. At the same time, our results entitled us to a strong opinion that the above-mentioned concept of the organism’s quasi-defenselessness against nanoparticles should be critically re-evaluated. We therefore argued [43] that: (a) safe levels of human exposure to nanoparticles are possible in principle; (b) for nanomaterials which are satisfactorily characterized by toxicological research comparing them with their micrometer counterparts, such levels can be proposed without delay, even if tentatively, based on a sufficiently conservative approach that involves decreasing by approximately one order of magnitude the exposure limits already established or recommended for respective micro-scale industrial aerosols. Based on these general premises, we then proposed for consideration a tentative reference value for engineered Fe3O4-NPs (nano-magnetite) in workroom air equal to 0.4 mg/m3 (as TWA) [92], being also sure that the same value might be suggested for the Fe2O3-NPs considering the toxicological similarity of all iron oxides.
However, now that the dual health risk-determining role of especially high (both size-dependently and chemistry-dependently) in vivo solubilization of Me-NPs has become clearer to us [92] (see Section 3.1), we are less confident that long-term inhalation of any and all Me-NPs is always more dangerous compared with their micrometric counterparts. We still believe though that the latter is a sound enough assumption to support the just characterized shortcut method for setting tentative standards with a sufficient safety margin (the precautionary principle again!) but we are inclined to think that time-consuming and expensive long-term inhalation experiments are needed to confirm or to reconsider these standards for establishing them on a permanent basis.
For instance, we have recently found that the NiO-NP, previously found to be highly toxic and genotoxic in a subchronic IP experiment [63], was very toxic in a long-term low-level inhalation experiment as well (paper in preparation). In such cases, the above-stated assumption that the permissible exposure level for a Me-NP should be set really very low can be relied upon.
On the other hand, we have demonstrated in similar long-term (of 3–10 months duration) experiments a very low pulmonary and systemic toxicity of ultrafine Fe2O3-NPs [92] and submicron (mostly nanoscale) amorphous SiO2 aerosol [93], explainable just by their low pulmonary and body burdens due to easy in vivo solubilization of respective particles. Nevertheless, we were hesitant about suggesting relatively high permissible exposure levels for these substances for two reasons.
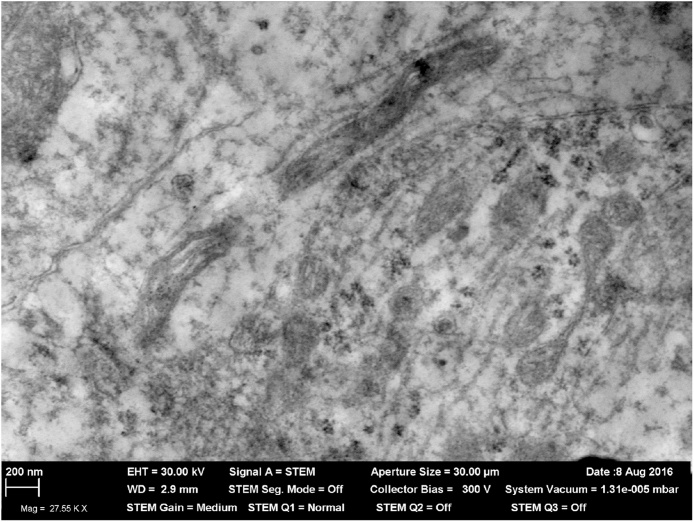
First, in both cases we detected a considerable number of nanoscale electron-dense round formations in some neurons of the brain olfactory area and in the myelin sheath of intra-cerebral nervous fibers (exemplified by Fig. 13, Fig. 14, respectively), which are most likely to be nanoparticles of the aerosols under study. Thus the ability of inhaled nanoparticles initially deposited in the nasal passages to penetrate along the olfactory nerve fibers into the brain demonstrated previous by other researchers [[25], [37], [70]] has been confirmed in our experiments as well.
Fig. 13.
Nanoparticles in the neuron body under inhalation exposure to nano-silica containing aerosol at a mean concentration of 2.6 mg/m3 during 3 months. STEM, magnification X 27550. First published in [93].
Fig. 14.
A longitudinal section of a nerve fiber in the olfactory brain region of a rat after 10-month inhalation exposure to Fe2O3-NP at a mean concentration of 1.14 mg/m3. Note focal damage to the myelin sheath (marked by an asterisk) associated with accumulation of the NPs pointed out by arrows (TEM, magnification _ 49,920). First published in [92].
In the experiment with Fe2O3-NP, the localization of particles within the myelin sheaths of the brain fibers was frequently associated with their ultrastructural damage (Fig. 14). Although we did not observe any clear signs of disturbance to either the ultrastructure of the particle-loaded neurons or to the not very sophisticated rat brain activity indices used by us,2 the potential danger of any cytotoxic Me-NP accumulation in the brain should not have been neglected.
Second, the genotoxic effect of inhalation exposure to Me-NPs (demonstrated by increased genomic DNA fragmentation in nucleated blood cells) called for even greater cautiousness.
As a result of all these pro et contra arguments, it was agreed that a reducing factor of ca. 3 should be applied to the experimental concentration of Fe2O3-NP and thus the labor-shift TWA value of 0.4 mg/m3 should be included into the Russian official list of Maximal Allowable Concentrations of noxious chemicals in workplace air. Respective decisions concerning the other Me-NPs that we have studied so far are pending.
3.4. It is possible to enhance the organism’s resistance to the adverse health effects of Me-NPs
As well as aspiring to keep Me-NP occupational exposures under the permissible and thus presumably safe (but not proven to a certainty as causing no harmful health effects) levels discussed above, and even more so where such levels have not been established and it could only be recommended that such exposures should be maintained as low as possible, we from the very beginning of our research into this field believed it to be both useful and possible to try and find ways of enhancing the organism’s resistance to these Me-NPs.
Based on the theoretical premises of beneficial interference with the toxicokinetics and toxicodynamics of any toxicants in different forms developed and reported by our research team quite a while ago [40] and on our studies of general and specific key mechanisms underlying the toxic action of different Me-NP species, we have proposed several bioprotective complexes (BPCs) comprising mainly pectin, some vitamins, glutamate, glycine, N-acetylcysteine, omega-3 PUFA, and various essential trace elements.
The results of our animal experiments with different Me-NPs described in a number of original papers [[45], [63], [64], [79]] and then summarized in [49] prove that oral background administration of or premedication with such a BPC can indeed help markedly attenuate the integral and specific toxicity of Me-NPs and even their genotoxicity.
We believe that this approach, which we term as biological prophylaxis, can be an efficient auxiliary tool of health risk management where both the protective effectiveness of a BPC and its own harmlessness have been reliably proved in animal experiments. Our previous positive experience of providing this kind of prophylaxis for alleviating the adverse health effects of many other toxicants, first in selective trials on volunteers and then as a large-scale preventive strategy [80], leads us to expect that it should be no less practicable and effective in the field of nanotoxicology as well. So we recommend the publications referred to, many of them in open access journals, to the attention of an interested reader while here we propose to give just a few examples of the above-mentioned experimental results.
Example 1
attenuation of Me-NP nephrotoxicity
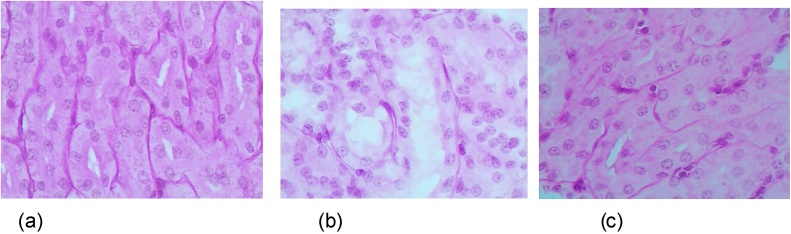
Not only those mentioned in Section 3.1, but virtually all Me-NPs studied by us up to now cause more or less significant damage to the epithelial cells of proximal convoluted renal tubules. As can be seen from the example in Fig. 15, the histological preparations of kidneys from rats exposed intraperitoneally to such nanoparticles during 6–7 weeks revealed marked degenerative and necrotic changes in these cells up to their disappearance with partial destruction of the brush border, while rats exposed to the same nanoparticles against background BPC administration demonstrated marked alleviation of such tubular damage. This renal-protective effect was confirmed by the morphometric indices given in Table 1.
Example 2
attenuation of Me-NP neurotoxicity
Fig. 15.
(a) Kidney of a control rat (proximal convoluted tubules with an intact brush border). (b) Kidney of a rat exposed to NiO-NPs + Mn3O4-NPs (marked degenerative and necrobiotic changes in tubular epithelial cells up to their disappearance; partial destruction of the brush border). (c) Kidney of a rat similarly exposed against background administration of a BPC comprising pectin, glutamate, glycine, N-acetylcysteine, vitamins A, C, E, selenium, iodide and omega-3 PUFA. Periodic Acid Schiff (PAS) stain, magnification × 400. First published in [63].
Table 1.
Some morphometric indices for tubular epithelium damage in the kidneys of rats after repeated intraperitoneal injections of some metallic oxide nanoparticles with or without background oral administration of BPCsa (x ± s.e.m.).
| Groups of rats given | Brush border loss (% lengthwise) | Epithelial desquamation (% lengthwise) |
|---|---|---|
| Subchronic IP exposure to NiO-NP + Mn3O4 − NP | ||
| Water (control) | 5.44 ± 0.90 | 0.00 ± 0.00 |
| Nanoparticles | 12.33 ± 2.30* | 2.43 ± 1.00* |
| Nanoparticles + BPC | 7.08 ± 1.70 | 0.00 ± 0.00+ |
| Subchronic IP exposure to CuO-NP | ||
| Water (control) | 5.39 ± 0.42 | 0.33 ± 0.13 |
| Nanoparticles | 8.36 ± 0.76* | 1.16 ± 0.38 * |
| Nanoparticles + BPC | 5.98 ± 0.46+ | 0.98 ± 0.35 |
Note: *Statistically significant difference from the control group; + from the group given nanoparticles without the BPC (p < 0.05 by Student’s t-test).
aThe main BPC components against NiO-NP + Mn3O4-NP are given in the caption to Fig. 15; those against CuO-NP are. in principle, the same plus vitamin B12 and biotic doses of iron, zinc, molybdenum and manganese.
As was shown in Section 3.1, a more specific adverse effect characterizing the toxicity of Mn3O4-NPs (acting either together with or without NiO-NPs) [63] and of CuO-NPs [64] consists in marked damage to some specialized structures of the brain (to the striatum and the hippocampus especially). In both cases, damage was also significantly attenuated by the respective BPCs. An example is given in Fig. 16
Example 3
attenuation of PbO-NP toxic action on the red blood.
Fig. 16.
Number of cells without a nucleolus per 100 Golgi cells in nucleus caudatus of rats exposed (A) to water (Control); (B) to water suspension of copper oxide nanoparticles; (C) to the same against background administration of the bioprotective complex (BPC), (D) to the BPC only (Average values with 95% CI). Differences are statistically significant between (B) and (A); (C) and (B) (p < 0.05 by Student’s t-test). First published in [64].
Still another metal-specific effect of subchronic Me-NP intoxications considered in Section 3.1 is an increased reticulocyte percentage under the impact of PbO-NPs (24.7 ± 2.7‰ against 10.2 ± 1.4‰ in control rats, P < 0.05). This effect was even more pronounced under a combined impact of PbO-NPs + CuO-NPs + ZnO-NPs (29.7 ± 3.2‰) but was significantly attenuated against background BPC administration (18.00 ± 1.6‰, P < 0.05). Similar attenuation (although statistically non-significant) was observed in relation to the decrease in the hemoglobin level (165.5 ± 10.7 g/L against 145.5 ± 4.0 g/L in the group exposed without bioprotectors and 158.8 ± 5.6 g/L in the control group) and to the increase in the δ-ALA urine concentration (11.1 ± 4.3, 15.2 ± 25 and 8.1 ± 2.7 mcg/mL, respectively)
Example 4
effectiveness of the BPCs tested against Me-NP genotoxicity.
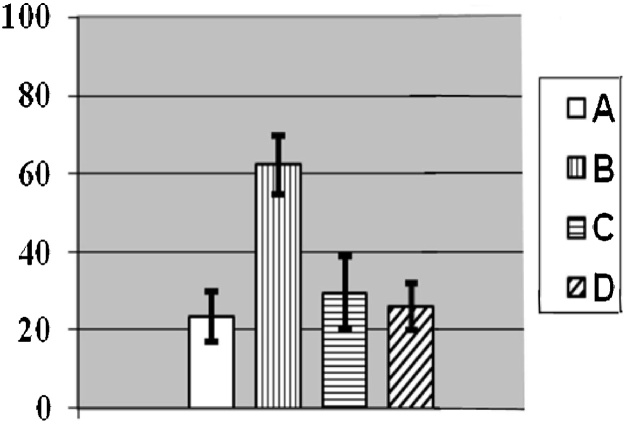
While all the Me-NPs that we have studied to date are genotoxic to various extents, this most worrying effect was significantly attenuated by background administration of any of the BPCs tested so far. Thus, in one of the experiments [45], the coefficient of genomic DNA fragmentation derived from the RAPD test (x ± s.e.m.) was equal to 0.40 ± 0.001 in the liver of control rats and to 0.46 ± 0.002* in the liver of rats exposed to subchronic intoxication with Ag-NP, whereas in similarly exposed rats with background BPC administration it was equal to 0.41 ± 0.011+. The respective mean group values were 0.39 ± 0.003, 0.46 ± 0.032*, 0.37 ± 0.003*+ in the bone marrow; 0.38 ± 0.002, 0.46 ± 0.001*, 0.42 ± 0.003*+ in the spleen; 0.39 ± 0.003, 0.42 ± 0.008*, 0.40 ± 0.006*+ in the kidneys; 0.38 ± 0.001, 0.41 ± 0.012*, 0.39 ± 0.007 in nucleated blood cells, respectively (in both exposed groups, the values differing statistically significantly from respective control ones are tagged with an asterisk; in the group exposed to NPs and administered the BPC, the values differing from respective ones in the group exposed without the BPC are marked with a cross). Similarly, the results of this test for nucleated blood cells in the experiment with NiO-NPs + Mn3O4-NPs [63] were: 0.42 ± 0.00, 0.50 ± 0.01*, 0.45 ± 0.01*+, respectively, and the results for spleen cells in the experiment with CuO-NPs [64] were: 0.37 ± 0.002, 0.46 ± 0.002*, 0.42 ± 0.002*+, respectively.
Let us give one more illustration of BPCs’ antigenotoxic effectiveness referring to yet unpublished data (Minigalieva et al., in preparation) obtained in an experiment of the same design with a combination of Al2O3-NP + TiO2-NP + SiO2-NP. In this case, the coefficient of DNA fragmentation in nucleated blood cells, equal to 0.40 ± 0.006 in control rats, was elevated up to 0.64 ± 0.019* in rats exposed to Me-NPs without protection and only to 0.47 ± 0.007*+ in rats injected with the same NPs and given a BPC. It is worth noticing that such BPC-induced decrease in the genotoxic action of these Me-NPs was commensurable with, and even somewhat more dramatic than the reduction achieved by halving their dosage (0.48 + 0.007*). Meantime, anybody who is familiar with the industrial realities would agree that attaining a 2 times decrease in occupational exposure to a hazard is not an easy task.
4. Conclusions
Nanoparticles of metals and of their oxides (Me-NPs) are not only purposely manufactured on a large scale but also constitute a substantial proportion in the typical particle size distribution of condensation aerosols generated by arc-welding, metallurgical processes, and some other technologies. Over the last 9 years we have been investigating the toxicity of mostly spherical Me-NPs using three complementary experimental models:
-
(1)
a single low-dose intra-tracheal (IT) instillation 24 h before the bronchoalveolar lavage performed to obtain a fluid (BALF) for cytological and biochemical study;
-
(2)
repeated intra-peritoneal (IP) injections during 6–7 weeks in non-lethal doses to assess the thus induced subchronic intoxication by a lot of functional and morphological indices and by the patterns of distribution and elimination of respective nanoparticles;
-
(3)
long-term (up to 10 months) inhalation exposure of rats in a nose-only device aimed at assessing the same indices of toxicokinetics and toxicodynamics.
In the first two types of experiment, we have tested the effects of Me-NPs not only where these acted alone but, for some of them, also in two-factorial and three-factorial combinations (for example, CuO-NP + PbO-NP; CuO-NP + ZnO-NP; PbO-NP + ZnO-NP; PbO-NP + CuO-NP + ZnO-NP). In several experiments, a special group of rats was being given per os a complex of innocuous bio-active substances along with IP exposure or during one month before IT exposure to nanoparticles.
Our experiments have demonstrated that Me-NPs are intrinsically much more noxious (especially on the cell level) as compared with their fine micrometric or even submicron counterparts, being generally the more toxic, the smaller their dimensions within the nano-scale range.
At the same time, the in situ cytotoxicity, organ-systemic toxicity and in vivo genotoxicity of Me-NPs of a given diameter strongly depend on their chemical nature and on the properties associated with the latter (in vivo solubility included) as well as on the specific mechanisms of action characteristic of a given metal in any chemical form. However, rapid solubilization of some Me-NPs in biological milieus may not only enhance their toxic action but also decrease their retention in both primary and secondary deposition sites and thus attenuate their subchronic and chronic toxicity on the organ − systemic − organism levels. The resulting vector of these contra-directional influences evidently depends on particle dimensions within the nanoscale range, their in vivo solubility and their metal-specific toxicity and so is not easy to predict. This fact makes it difficult to use the general principle of establishing safe Me-NP exposures at levels always much lower compared with those earlier established for respective micrometric particles. Nevertheless, for the time being this principle should be considered a common rule until proved wrong for any of the Me-NP species.
With these qualifications in mind, the most important postulate we consider proved is the very possibility to establish such permissible occupational exposures to airborne Me–NPs based on the demonstrated high activity of the pulmonary clearance mechanisms, both physiological and physico-chemical.
A mathematical analysis has shown that for the nanoparticles studied, as well as for the soluble salts of the respective metals, there exist not merely three traditionally acknowledged types of binary combined toxicity (additivity, subadditivity and superadditivity) but several variants of them depending on exactly which effect is considered, on its level, as well as on dose levels and their ratio. Where a 3rd component is present in a combination, these variants can change more or less significantly.
Last but not least, we have shown that the toxicity, and even genotoxicity of metallic nanoparticles could be markedly attenuated by background administration of, or premedication with adequately composed combinations of some bioactive agents in innocuous doses. We therefore believe that, along with decreasing exposure to nanoparticles, such bio-protectors can help enhance the organism’s resistance to their adverse effects and thus present an efficient auxiliary tool of health risk management in related occupations.
Conflicts of Interest
None.
Footnotes
It is a non-formal but stable group of researchers uniting not only toxicologists working together with the authors in the same Center but also physicists, histologists, and mathematicians of high competency employed in other scientific institutions. Their names and affiliations are always given in our original publications, briefly overviewed in the content of this paper (see References).
It should be noted, however, that in the experiment with SiO2-NP the relative brain mass towards the end of the 6-month exposure period was statistically significantly lower than the respective control value for both exposure levels used. Although rarely observed, this effect is nevertheless known to be associated with the impact of some neurotoxic substances [34].
References
- 1.Adamcakova-Dodd A., Stebounova L.V., Kim J.S., Vorrink S.U., Ault A.P., O’Shaughnessy P.T., Grassian V.H., Thorne P.S. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. J. Part. Fibre Toxicol. 2014;11:15. doi: 10.1186/1743-8977-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahamed M., Karns M., Goodson M., Rowe J., Hussain S.M., Schlager J.J., Hong Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. J. Toxicol. Appl. Pharmacol. 2008;233:404–410. doi: 10.1016/j.taap.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Ahamed M., AlSalhi M.S., Siddiqui M.K.J. Silver nanoparticles applications and human health. J. Clin. Chim. Acta. 2010;411:1841. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadi F., Kordestany A.H. Investigation on silver retention in different organs and oxidative stress enzymes in male broiler fed diet supplemented with powder of nano silver. Am-Euras J. Toxicol. Sci. 2011;3:28–35. [Google Scholar]
- 5.Akhtar M.J., Kumar S., Alhadlaq H.A., Alrokayan S.A., Abu-Salah K.M., Ahamed M. Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. J. Toxicol. Ind. Health. 2013;32(5):809–821. doi: 10.1177/0748233713511512. [DOI] [PubMed] [Google Scholar]
- 6.Alarifi S., Ali D., Verma A., Alakhtani S., Ali B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2013;32:296–307. doi: 10.1177/1091581813487563. [DOI] [PubMed] [Google Scholar]
- 7.Amiri A., Mohammadi M., Shabani M. Synthesis and Toxicity Evaluation of lead oxide (PbO) nanoparticles in rats. Electronic J. Biol. 2016;12:2. [Google Scholar]
- 8.Arora S., Jain J., Rajwade J.M., Paknikar K.M. Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. J. Toxicol. Appl. Pharmacol. 2009;236:310–318. doi: 10.1016/j.taap.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Asare N., Instanes C., Sandberg W.J., Refsnes M., Schwarze P., Kruszewski M., Brunborg G. Citotoxic and genotoxic effects of silver nanoparticles in testicular cell. J. Toxicol. 2012;291:65–72. doi: 10.1016/j.tox.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Bakri S.J., Pulido J.S., Mukerjee P., Marler R.J., Mukhopadhyay D. Absence of histologic retinal toxicity of intravitreal nanogold in a rabbit model. J. Retina. 2008;28:147–149. doi: 10.1097/IAE.0b013e3180dc9360. [DOI] [PubMed] [Google Scholar]
- 11.Balasurbamanian S.K., Jittiwat J., Manikandan J., Ong Ch.-N., Yu L.E., Ong W.-Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. J. Biomater. 2010;31:2034–2042. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 12.Barhoumi L., Dewez D. Toxicity of superparamagnetic iron oxide nanoparticles on green alga chlorella vulgaris. BioMed Res. Int. 2013;64797:4. doi: 10.1155/2013/647974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beer C., Foldbjerg R., Hayashi Y., Sutherland D.S., Autrup H. Toxicity of silver nanoparticles—nanoparticle or silver ion? J. Toxicol. Lett. 2012;208:286–292. doi: 10.1016/j.toxlet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Bellusci M., La Barbera A., Padella F., Mancuso M., Pasquo A., Grollino M.G., Leter G., Nardi E., Cremisini C., Giardullo P., Pacchierotti F. Biodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical process. Int. J. Nanomed. 2014;9:1919–1929. doi: 10.2147/IJN.S56394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bondarenko O., Ivask A., Käkinen A., Kahru A. Sub-toxic effects of CuO nanoparticles on bacteria: kinetics, role of Cu ions and possible mechanisms of action. J. Environ. Pollut. 2012;169:81–89. doi: 10.1016/j.envpol.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Capasso L., Camatini M., Gualtieri M. Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. J. Toxicol. Lett. 2014;226(1):28–34. doi: 10.1016/j.toxlet.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z., Meng H., Xing G., Chen C., Zhao Y., Jia G., Wang T., Yuan H., Ye C., Zhao F., Chai Z., Zhu C., Fang X., Ma B., Wan L. Acute toxicological effects of copper nanoparticles in vivo. J. Toxicol. Lett. 2006;25:109–120. doi: 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y-Sh., Hung Y-Ch., Huang G.S. Assessment of the in vivo toxicity of gold nanoparticles. J. Nanoscale Res. Lett. 2009;4:858–864. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho W.-S., Duffin R., Howie S., Scotton W.A.H., Wallace W.A.H., MacNee W., Bradley M., Megson I.L., Donaldson K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. J. Part. Fibre Toxicol. 2011;8:27. doi: 10.1186/1743-8977-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J.E., Kim S., Ahn J.H., Youn P., Kang J.S., Park K., Yi J., Ryu D.Y. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. J. Aquat. Toxicol. 2010;100:151–159. doi: 10.1016/j.aquatox.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Choi S.Y., Jeong S., Jang S.H., Park J., Park J.H., Ock K.S., Lee S.Y., Joo S.-W. In vitro toxicity protein-adsorbed citrate-reduced gold nanoparticles in human lung adenocarcinoma cells. J. Toxicol. In Vitro. 2012;26:229–237. doi: 10.1016/j.tiv.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Cronholm P., Karlsson H.L., Hedberg J., Lowe T.A., Winnberg L., Elihn K., Wallinder I.O., Möller L. Intracellular uptake and toxicity of Ag and CuO nanoparticles: a comparison between nanoparticles and their corresponding metal ions. J. Small. 2013;8:970–982. doi: 10.1002/smll.201201069. [DOI] [PubMed] [Google Scholar]
- 23.Cuillel M., Chevallet M., Charbonnier P., Fauquant C., Pignot-Paintrand I., Arnaud J., Cassio D., Michaud-Soret I., Mintz E. Interference of CuO nanoparticles with metal homeostasis in hepatocytes under sub-toxic conditions. J. Nanoscale. 2014;16:1707–1715. doi: 10.1039/c3nr05041f. [DOI] [PubMed] [Google Scholar]
- 24.Dykman L., Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. J. Chem. Soc. Rev. 2012;41:2256–2282. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 25.Elder A., Gelein R., Silva V., Feikert T., Opanashuk L., Carter J., Potter R., Maynard A., Ito Y., Finkelstein J., Oberdörster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006;114(8):1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engin A.B., Nikitovic D., Neagu M., Henrich-Noack P., Docea A.O., Shtilman M.I., Golokhvast K., Tsatsakis A.M. Mechanistic understanding of nanoparticles’ interactions with extracellular matrix: the cell and immune system. Part. Fibre Toxicol. 2017;14:22. doi: 10.1186/s12989-017-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flower N.A.L., Brabu B., Revathy M., Gopalakrishnan Ch., Raja S.V.K., Murugan S.S., Kumaravel T.S. Characterization of synthesized silver nanoparticles and assessment of its genotoxicity potentials using the alkaline comet assay. J. Mutat. Res. 2012;742:61–65. doi: 10.1016/j.mrgentox.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Foldbjerg R., Dang D.A., Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. J. Arch. Toxicol. 2011;85:743–750. doi: 10.1007/s00204-010-0545-5. [DOI] [PubMed] [Google Scholar]
- 29.Fröhlich E. Cellular targets and mechanisms in the cytotoxic action of non-biodegradable engineered nanoparticles. J. Curr. Drug Metab. 2013;14:976–988. doi: 10.2174/1389200211314090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao F., Ma N.J., Zhou H., Wang Q., Zhang H., Wang P., Hou H.L., Wen H., Li L.J. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. J. Toxicol. Lett. 2006;161(2):115–123. doi: 10.1016/j.toxlet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Gao F., Ma N.J., Zhou H., Wang Q., Zhang H., Wang P., Hou H.L., Wen H., Li L.J. Zinc oxide nanoparticles induced epigenetic change and G2/M arrest are associated with apoptosis in human epidermal keratinocytes. Int. J. Nanomed. 2016;11:3859–3874. doi: 10.2147/IJN.S107021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glazer E.S., Zhu C., Hamir A.N., Borne A., Thompson C.S., Curley S.A. Biodistribution and acute toxicity of naked gold nanoparticles in a rabbit hepatic tumor model. Nanotoxicology. 2011;5:459–468. doi: 10.3109/17435390.2010.516026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes T., Araújo O., Pereira R., Almeida A.C., Cravo A., Bebianno M.J. Genotoxicity of copper oxide and silver nanoparticles in the mussel Mytilus galloprovincialis. J. Mar. Environ. Res. 2013;84:51–59. doi: 10.1016/j.marenvres.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Grewal K.K., Sandhu G.S., Kaur R., Brar R.S., Sandhu H.S. Toxic impacts of cypermethrin on behavior and histology of certain tissues of albino rats. Toxicol. Int. 2010;17(2):94–98. doi: 10.4103/0971-6580.72679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hackenberg S., Scherzed A., Kessler M., Hummel S., Technau A., Froelich K., Ginzkey C., Koehler C., Hagen R., Kleinsasser N. Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cell. J. Toxicol. Lett. 2011;201:27–33. doi: 10.1016/j.toxlet.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen N.R., Stoeger T., van den Brule S., Saber A.T., Beyerle A., Vietti G., Mortensen A., Szarek J., Budtz H.C., Kermanizadeh A., Banerjee A., Ercal N., Vogel U., Wallin H., Møller P. Acute and subacute pulmonary toxicity and mortality in mice after intratracheal instillation of ZnO nanoparticles in three laboratories. Food Chem. Toxicol. 2015;85:84–95. doi: 10.1016/j.fct.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Kao Y.-Y., Cheng T.-J., Yang D.-M., Liu-Sh P. Demonstration of an olfactory bulb–brain translocation pathway for ZnO nanoparticles in rodent ells in vitro and in vivo. J. Mol. Neurosci. 2012;48(2):464–471. doi: 10.1007/s12031-012-9756-y. [DOI] [PubMed] [Google Scholar]
- 38.Karlsson H., Cronholm P., Gustafsson J., Möller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. J. Chem. Res. Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson H.L., Gliga A.R., Kohonen P., Wallbergb P., Fadeel B. Genotoxicity and epigenetic effects of silver nanoparticles. J. Toxicol. Lett. 2012;211:240. [Google Scholar]
- 40.Katsnelson B.A., Makeyev O.H., Kochneva N.I., Privalova L.I., Degtyareva T.D., Bukhantsev V.A., Minin V.V., Kostyukova S.V. Testing a set of bioprotectors against the genotoxic effect of a combination of ecotoxicants. Cent. Eur. J. Occup. Environ. Med. 2007;13:251–264. [Google Scholar]
- 41.Katsnelson B.A., Privalova L.I., Degtyareva T.D., Sutunkova M.P., Minigalieva I.A., Yeremenko O.S., Kireeva E.P., Khodos M.Y.a., Kozitsina A.N., Malakhova N.A., Glazyrina YuA, Shur V.Y.a., Nikolaeva E.V., Vazhenin V.A., Potapov A.P., Morozova M.V., Valamina I.E., Tulakina L.G., Pichugova S.V., Beikin J.B. Experimental estimates of the toxicity of iron oxide Fe3O4 (Magnetite) nanoparticles. Cent. Eur. J. Occup. Environ. Med. 2010;16(1–2):47–63. [Google Scholar]
- 42.Katsnelson B.A., Degtyareva T.D., Minigalieva I.A., Privalova L.I., Kuzmin S.V., Yeremenko O.S., Kireyeva E.P., Sutunkova M.P., Valamina I.I., Khodos M.Y., Kozitsina A.N., Shur V.Y., Vazhenin V.A., Potapov A.P., Morozova M.V. Sub-chronic systemic toxicity and bio-accumulation of Fe3O4 nano- and micro-particles following repeated intraperitoneal administration to rats. Int. J. Toxicol. 2011;30(1):60–67. doi: 10.1177/1091581810385149. [DOI] [PubMed] [Google Scholar]
- 43.Katsnelson B.A., Privalova L.I., Degtyaryova T.D., Kuzmin S.V., Gurvich V.B., Sutunkova M.P., Kireyeva Y.e.P., Minigaliyeva I.A., Yeryomenko O.S. About the validation of the tentative safe exposure level of the metalcontaining nanoparticles impact in occupational air. Toxicol. Rev. 2012;4:26–29. (in Russian) [Google Scholar]
- 44.Katsnelson B.A., Privalova L.I., Sutunkova M.P., Tulakina L.G., Pichugova S.V., Beykin J.B., Khodos M.J. Interaction of iron oxide Fe3O4 nanoparticles and alveolar macrophages in vivo. Bull. Exp. Biol. Med. 2012;152(5):627–631. doi: 10.1007/s10517-012-1593-z. [DOI] [PubMed] [Google Scholar]
- 45.Katsnelson B.A., Privalova L.I., Gurvich V.B., Makeyev O.H., Shur V.Y.a., Beikin Y.B., Sutunkova M.P., Kireyeva E.P., Minigalieva I.A., Loginova N.V., Vasilyeva M.S., Korotkov A.V., Shuman E.A., Vlasova L.A., Shishkina E.V., Tyurnina A.E., Kozin R.V., Valamina I.E., Pichugova S.V., Tulakina L.G. Comparative in vivo assessment of some adverse bioeffects of equidimensional gold and silver nanoparticles and the attenuation of nanosilver’s effects with a complex of innocuous bioprotectors. Int. J. Mol. Sci. 2013;14:2449–2483. doi: 10.3390/ijms14022449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katsnelson B.A., Privalova L.I., Gurvich V.B., Kuzmin S.V., Kireyeva E.P., Minigalieva I.A., Sutunkova M.P., Loginova N.V., Yarushin S.V., Soloboyeva J.I., Kochneva N.I. Enhancing population’s resistance to toxic exposures as an auxiliary tool of decreasing environmental and occupational health risks (a self-overview) J. Environ. Prot. 2014;5:1435–1449. [Google Scholar]
- 47.Katsnelson B.A., Panov V.G., Minigaliyeva I.A., Varaksin A.N., Privalova L.I., Slyshkina T.V., Grebenkina S.V. Further development of the theory and mathematical description of combined toxicity: an approach to classifying types of action of three factorial combinations (a case study of manganese-chromium-nickel subchronic intoxication) Toxicology. 2015;334:33–44. doi: 10.1016/j.tox.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Katsnelson B.A., Privalova L.I., Sutunkova M.P., Minigalieva I.A., Gurvich V.B., Shur V.Y., Makeyev O.H., Valamina I.E., Grigoryeva E.V. Is it possible to enhance the organism's resistance to toxic effects of metallic nanoparticles? Toxicol. 2015;337:79–82. doi: 10.1016/j.tox.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Katsnelson B.A., Privalova L.I., Sutunkova M.P., Minigalieva I.A., Gurvich V.B., VYa Shur, Shishkina E.V., Makeyev O.H., Valamina I.E., Varaksin A.N., Panov V.G. Experimental research into metallic and metal oxide nanoparticle toxicity in vivo. In: Yan B., Zhou H., Gardea-Torresdey J., editors. Bioactivity of Engineered Nanoparticles. Springer; 2017. pp. 259–319. (Chapter 11) [Google Scholar]
- 50.Kim H.R., Kim M.J., Lee S.Y., Oh S.M., Chung K.H. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cells. J. Mutat. Res. 2011;726:129–135. doi: 10.1016/j.mrgentox.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y.S., Song M.Y., Park J.D., Song K.S., Ryu H.R., Chung Y.H., Chang H.K., Lee J.H., Oh K.H., Kelman B.J., Hwang I.K., Yu I.J. Subchronic oral toxicity of silver nanoparticles. J. Part. Fibre Toxicol. 2010;7:20. doi: 10.1186/1743-8977-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J.J., Lo S.L., Ng C.T., Gurung R.L., Hartono D., Hande M.P., Ong C.N., Bay B.H., Yung L.Y. Genomic instability of gold nanoparticle treated human lung fibroblast cells. J. Biomater. 2011;32:5515–5523. doi: 10.1016/j.biomaterials.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 53.Li T., Albee B., Alemayehu M., Diaz R., Ingham L., Kamal S., Rodriguez M., Bishnoi S.W. Comparative toxicity study of Ag, Au, Ag-Au bimetallic nanoparticles on Daphnia magna. J. Anal. Bioanal. Chem. 2010;398:689–700. doi: 10.1007/s00216-010-3915-1. [DOI] [PubMed] [Google Scholar]
- 54.Li Y., Chen D.H., Yan J., Chen Y., Mittelstaedt R.A., Zhang Y., Biris A.S., Heflich R.H., Chen T. Genotoxicity of silver nanoparticles evaluated using the Ames test and in vitro micronucleus assay. J. Mutat. Res. 2012;745:4–10. doi: 10.1016/j.mrgentox.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Lim D.-H., Jang J., Kim S., Kang T., Lee K., Choi I.H. The effects of sub-lethal concentrations of silver nanoparticles on inflammatory and stress in human macrophages using cDNA microarray analysis. J. Biomater. 2012;33:4690–4699. doi: 10.1016/j.biomaterials.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Liu G., Gao J., Ai H., Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. J. Small. 2013;9(9–10):1533–1545. doi: 10.1002/smll.201201531. [DOI] [PubMed] [Google Scholar]
- 57.Magaye R., Zhao J. Recent progress in studies of metallic nickel and nickel-based nanoparticles' genotoxicity and carcinogenicity. Environ. Toxicol. Pharmacol. 2012;34(3):644–650. doi: 10.1016/j.etap.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Magaye R., Zhao J., Bowman L., Ding M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. J. Exp. Ther. Med. 2012;4:551–561. doi: 10.3892/etm.2012.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahmoudi M., Simchi A., Milani A.S., Stroeved P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2009;336(2):510–518. doi: 10.1016/j.jcis.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 60.Mahmoudi M., Laurent S., Shokrgozar M.A., Hosseinkhani M. Toxicity evaluations of superparamagnetic iron oxide nanoparticles: cell vision versus physicochemical properties of nanoparticles. J. ACS Nano. 2011;5(9):7263–7276. doi: 10.1021/nn2021088. [DOI] [PubMed] [Google Scholar]
- 61.Markides H., Rotherham M., El Haj A.J. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012;61409:4. [Google Scholar]
- 62.Minigaliyeva I.A., Katsnelson B.A., Privalova L.I., Gurvich V.B., Panov V.G., Varaksin A.N., Makeyev O.H., Sutunkova M.P., Loginova N.V., Kireyeva E.P., Grigoryeva E.V., Slyshkina T.V., Ganebnykh E.V., Grebenkina S.V. Toxicodynamic and toxicokinetic descriptors of combined chromium (VI) and nickel toxicity. Int. J. Toxicol. 2014;33(6):498–505. doi: 10.1177/1091581814555915. [DOI] [PubMed] [Google Scholar]
- 63.Minigalieva I.A., Katsnelson B.A., Privalova L.I., Sutunkova M.P., Gurvich V.B., Shur V.Y., Shishkina E.V., Valamina I.E., Makeyev O.H., Panov V.G., Varaksin A.N., Grigoryeva E.V., Meshtcheryakova E.Y. Attenuation of combined nickel (II) oxide and manganese (II, III) oxide nanoparticles’ adverse effects with a complex of bioprotectors. Int. J. Mol. Sci. 2015;16(9):22555–22583. doi: 10.3390/ijms160922555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minigalieva I.A., Katsnelson B.A., Panov V.G., Privalova L.I., Varaksin A.N., Gurvich V.B., Sutunkova M.P., Shur V.Y.a., Shishkina E.V., Valamina I.E., Zubarev I.V., Makeyev O.H., Meshtcheryakova E.Y., Klinova S.V. In vivo toxicity of copper oxide, lead oxide and zinc oxide nanoparticles acting in different combinations and its attenuation with a complex of innocuous bio-protectors. Toxicol. 2017;380(2017):72–93. doi: 10.1016/j.tox.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Minigalieva I.A., Katsnelson B.A., Panov V.G., Varaksin A.N., Gurvich V.B., Privalova L.I., Sutunkova M.P., Klinova S.V. Experimental study and mathematical modeling of toxic metals combined action as a scientific foundation for occupational and environmental health risk assessment (a synthesis of results obtained by the Ekaterinburg research team, Russia) Toxicol. Rep. 2017;4C:194–201. doi: 10.1016/j.toxrep.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morimoto Y., Hirohashi M., Ogami A., Oyabu T., Myojo T., Hashiba M., Mizuguchi Y., Kambara T., Lee B.W., Kuroda E., Tanaka I. Pulmonary toxicity following an intratracheal instillation of nickel oxide nanoparticle agglomerates. J. Occup. Health. 2011;53(4):293–295. doi: 10.1539/joh.11-0034-br. [DOI] [PubMed] [Google Scholar]
- 67.Mustafa T., Watanabe F., Monroe W., Mahmood M., Xu Y., Saeed L.M., Karmakar A., Casciano D., All S., Biris A.S. Impact of gold nanoparticle concentration on their cellular uptake by MC3T3-E1 mouse osteoblastic cells as analyzed by transmission electron microscopy. J. Nanomed. Nanotechnol. 2011;2:1–8. [Google Scholar]
- 68.Naqvi S., Samim M., Abdin M.Z., Ahmed F.J., Maitra A., Prashant C., Dinda A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010;5:983–989. doi: 10.2147/IJN.S13244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Neagu M., Piperigkou Z., Karamanou K., Engin A.B., Docea A.O., Constantin C., Negrei C., Nikitovic D., Tsatsakis A. Protein bio-corona: critical issue in immune nanotoxicology. Arch. Toxicol. 2017;91(3):1031–1048. doi: 10.1007/s00204-016-1797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: an emerging discipline evolving from studied of ultrafine particles. J. Environ. Health Persp. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onodera A., Nishiumi F., Kakiguchi K., Tanaka A., Tanabe N., Honma A., Yayama K., Yoshioka Y., Nakahira K., Yonemura S., Yanagihara I., Tsutsumi Y., Kawai Y. Short-term changes in intracellular ROS localisation after the silver nanoparticles exposure depending on particle size. Toxicol. Rep. 2015;2:574–579. doi: 10.1016/j.toxrep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan Y., Leifert A., Ruau D., Neuss S., Bornemann J., Schmid G., Brandau W., Simon U., Jahnen-Dechent W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. J. Small. 2009;5:2067–2076. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 73.Pang C., Selck H., Misra S.K., Berhanu D., Dybowska A., Valsami-Jones E., Forbes V.E. Effects of sediment-associated copper to the deposit-feeding snail, Potamopyrgus antipodarum: a comparison of Cu added in aqueous form or as nano- and micro-CuO particles. J. Aquat. Toxicol. 2012;15:114–122. doi: 10.1016/j.aquatox.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 74.Panov V.G., Katsnelson B.A., Varaksin A.N., Privalova L.I., Kireyeva E.P., Sutunkova M.P., Valamina I.E., Beresneva O.Y.u. Further development of mathematical description for combined (a case study of lead–fluoride combination) Toxicol. Rep. 2015;2:297–307. doi: 10.1016/j.toxrep.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park E.-J., Bae E., Yi Y., Kim Y., Choi K., Lee S.H., Yoon J., Lee B.C., Park K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nano-particles. J. Environ. Toxicol. Pharmacol. 2010;30:162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Park M.V., Neigh A.M., Vermeulen J.P., de la Fonteyne L.J., Verharen H.W., Briedé J.J., van Loveren H., de Jong W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. J. Biomater. 2011;32:9810–9817. doi: 10.1016/j.biomaterials.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 77.Pauksch L., Rohnke M., Schnettler R., Lips K.S. Silver nanoparticles do not alter human osteoclastogenesis but induce cellular uptake. Toxicol. Rep. 2014;1:900–908. doi: 10.1016/j.toxrep.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piperigkou Z., Karamanou K., Engin A.B., Gialeli C., Docea A.O., Vynios D.H., Pavão M.S., Golokhvast K.S., Shtilman M.I., Argiris A., Shishatskaya E., Tsatsakis A.M. Emerging aspects of nanotoxicology in health and disease: from agriculture and food sector to cancer therapeutics. Food Chem. Toxicol. 2016;91:42–57. doi: 10.1016/j.fct.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Privalova L.I., Katsnelson B.A., Loginova N.V., Gurvich V.B., Shur V.Y., Valamina I.E., Makeyev O.H., Sutunkova M.P., Minigalieva I.A., Kireyeva E.P., Rusakov V.O., Tyurnina A.E., Kozin R.V., Meshtcheryakova E.Y., Korotkov A.V., Shuman E.A., Zvereva A.E., Kostykova S.V. Subchronic toxicity of copper oxide nanoparticles and its attenuation with the help of a combination of bioprotectors. Int. J. Mol. Sci. 2014;15:12379–12406. doi: 10.3390/ijms150712379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Privalova L.I., Katsnelson B.A., Sutunkova M.P., Minigalieva I.A., Gurvich V.B., Makeyev O.H., Shur V.Y.a., Valamina I.E., Klinova S.V., Shishkina E.V., Zubarev I.V. Looking for biological protectors against adverse health effects of some nanoparticles that can pollute workplace and ambient air (a summary of authors’ experimental results) J. Environ. Protect. 2017;8:844–866. [Google Scholar]
- 81.Rajanahalli P., Stucke C.J., Hong Y. The effects of silver nanoparticles on mouse embryonic stem cell self-renewal and proliferation. Toxicol. Rep. 2015;2:758–764. doi: 10.1016/j.toxrep.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rudolf R., Friedrich B., Stopic S., Anzel I., Tomic S., C’Olic M. Cytotoxicity of gold nanoparticles prepared by ultrasonic spray pyrolysis. J. Biomater. Appl. 2012;26:595–612. doi: 10.1177/0885328210377536. [DOI] [PubMed] [Google Scholar]
- 83.Shaikh S.M., Shyama S.K., Desai P.V. Absorption, LD50 and effects of CoO, MgO and PbO nanoparticles on mice mus musculus. IOSR-JESTFT. 2015;9(2):32–38. [Google Scholar]
- 84.Shulz M., Ma-Hock L., Brill S., Strauss V., Treumann S., Gröters S., van Ravenzwaay B., Landsiedel R. Investigation on the genotoxicity of different sizes of gold nanoparticles administered to the lungs of rats. J. Mutat. Res. 2012;745:51–57. doi: 10.1016/j.mrgentox.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 85.Singh N., Jenkins G.J.S., Asadi R., Doak S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION) J. Nano Rev. 2010;1:5358. doi: 10.3402/nano.v1i0.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh S., D’Britto V., Prabhune A.A., Ramana C.V., Dhawan A., Prasad B.L.V. Cytotoxic and genotoxic assessment of glycolipid-reduced and -capped gold and silver nanoparticles. New J. Chem. 2011;34(2):294–2301. [Google Scholar]
- 87.Singh S.P., Kumari M., Kumari S.I., Rahman M.F., Mahboob M., Grover P. Toxicity assessment of manganese oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral exposure. J. Appl. Toxicol. 2013;33(10):1165–1179. doi: 10.1002/jat.2887. [DOI] [PubMed] [Google Scholar]
- 88.Soenen S.J., De Cuyper M., De Smedt S.C., Braeckmans K. Investigating the toxic effects of iron oxide nanoparticles. J. Methods Enzymol. 2012;509:195–224. doi: 10.1016/B978-0-12-391858-1.00011-3. [DOI] [PubMed] [Google Scholar]
- 89.Srivastava M., Singh S., Self W.T. Exposure to silver nanoparticles inhibits selenoprotein synthesis and the activity of thioredoxin reductase. J. Environ. Health Perspect. 2011;120:56–61. doi: 10.1289/ehp.1103928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stebounova L.V., Adamcakova-Dodd A., Kim J.S. Nanosilver induces minimal lung toxicity or inflammation in a subacute murine inhalation model. J. Part. Fibre Toxicol. 2011;8:5. doi: 10.1186/1743-8977-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Studer A.M., Limbach L.K., Van Duc L., Krumeich F., Athanassiou E.K., Gerber L.C., Moch H., Stark W.J. Nanoparticle cytotoxicity depends on intracellular solubility: comparison of stabilized copper metal and degradable copper oxide nanoparticles. J. Toxicol. Lett. 2010;1:169–174. doi: 10.1016/j.toxlet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 92.Sutunkova M.P., Katsnelson B.A., Privalova L.I., Gurvich V.B., Konysheva L.K., Shur V.Y.a., Shishkina E.V., Minigalieva I.A., Solovjeva S.N., Grebenkina S.V., Zubarev I.V. On the contribution of the phagocytosis and the solubilization to the iron oxide nanoparticles retention in and elimination from lungs under long-term inhalation exposure. Toxicol. 2016;363–364(1):19–28. doi: 10.1016/j.tox.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Sutunkova M.P., Solovyeva S.N., Katsnelson B.A., Gurvich V.B., Privalova L.I., Minigalieva I.A., Slyshkina T.V., Valamina I.E., Shur V.Y.a., Zubarev I.V., Kuznetsov D.K., Shishkina E.V. A paradoxical rat organism’s response to a long-term inhalation of silica-containing submicron (predominantly, nanoscale) particles of an actual industrial aerosol at realistic exposure levels. Toxicol. 2017;384:59–68. doi: 10.1016/j.tox.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 94.Tada-Oikawa S., Ichihara G., Suzuki Y., Izuoka K., Wu W., Yamada Y., Mishima T., Ichihara S. Zn (II) released from zinc oxide nano/micro particles suppresses vasculogenesis in human endothelial colony-forming cells. Toxicol. Rep. 2015;2:692–701. doi: 10.1016/j.toxrep.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tavares P., Balbino F., de Oliveira H. Martins, Fagundes G.E., Venâncio M., Ronconi J.V.V., Merlini A., Streck E.L., da Silva Paula M. Marques, de Andrade V. Moraes. Evaluation of genotoxic effect of silver nanoparticles (Ag-NPs) in vitro and in vivo. J. Nanopart. Res. 2012;14:791. [Google Scholar]
- 96.Trickler W.J., Lantz S.M., Murdock R.C., Schrand A.M., Robinson B.L., Newport G.D., Schlager J.J., Oldenburg S.J., Paule M.G., Slikker W., Jr., Hussain S.M., Ali S.F. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain micro vessel endothelial cells. J. Toxicol. Sci. 2010;118:160–170. doi: 10.1093/toxsci/kfq244. [DOI] [PubMed] [Google Scholar]
- 97.Trickler W.J., Lantz S.M., Murdock R.C., Schrand A.M., Robinson B.L., Newport G.D., Schlager J.J., Oldenburg S.J., Paule M.G., Slikker W., Jr., Hussain S.M., Ali S.F. Brain microvessel endothelial cells responses to gold nanoparticles: In vitro pro-inflammatory mediators and permeability. J. Nanotoxicol. 2011;5:479–492. doi: 10.3109/17435390.2010.540356. [DOI] [PubMed] [Google Scholar]
- 98.Varaksin A.N., Katsnelson B.A., Panov V.G., Privalova L.I., Kireyeva E.P., Valamina I.E., Beresneva O.Y. Some considerations concerning the theory of combined toxicity: a case study of subchronic experimental intoxication with cadmium and lead. Food Chem. Toxicol. 2014;64:144–156. doi: 10.1016/j.fct.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 99.Warheit D.B., Reed K.L., Sayes C.M. A role fore surface reactivity in TiO2 and quartz-related nanoparticle pulmonary toxicity. J. Nanotoxicol. 2009;3:181–187. [Google Scholar]
- 100.Wehmas L.C., Anders C., Chess J., Punnoose A., Pereira C.B., Greenwood J.A., Tanguay R.L. Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicol. Rep. 2015;2:702–715. doi: 10.1016/j.toxrep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu X., Tan Y., Mao H., Zhang M. Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int. J. Nanomed. 2010;5:385–399. doi: 10.2147/ijn.s10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu J., Li Z., Xu P., Xiao L., Yang Z. Nanosized copper oxide induces apoptosis through oxidative stress in podocytes. J. Arch. Toxicol. 2013;87:1067–1073. doi: 10.1007/s00204-012-0925-0. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Q., Yukinori K., Sato K., Nakakuki K., Koyahama N., Donaldson K. Differences in the extent of inflammation caused by intratracheal exposure to three ultrafine metals: role of free radicals. J. Toxicol. Environ. Health. 1998;53:423–438. doi: 10.1080/009841098159169. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Q., Yukinori K., Zhu X. Comparative toxicity of standard nickel and ultrafine nickel after intratracheal instillation. J. Occup. Health. 2003;45:23–30. doi: 10.1539/joh.45.23. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Q., Hitchins V.M., Schrand A.M., Hussain S.M., Goering P.L. Uptake of gold nanoparticles in murine macrophage cells without cytotoxicity or production of proinflammatory mediators. J. Nanotoxicol. 2010;5:284–295. doi: 10.3109/17435390.2010.512401. [DOI] [PubMed] [Google Scholar]
- 106.Zhu M.T., Feng W.Y., Wang B., Wang T.C., Gu Y.Q., Wang M., Wang Y., Ouyang H., Zhao Y.L., Chai Z.F. Comparative study of pulmonary responses to nano- and submicron ferric oxide in rats. J. Toxicol. 2008;247:102–111. doi: 10.1016/j.tox.2008.02.011. [DOI] [PubMed] [Google Scholar]