Abstract
Purpose:
To report the endovascular reconstruction of the superior vena cava (SVC), innominate and internal jugular veins following stenosis due to mediastinal fibrosis.
Case Report:
A 36-year-old female with mediastinal fibrosis was referred for symptomatic SVC syndrome (SVCS). A covered stent was inserted in the SVC with 2 kissing stents in the innominate and jugular veins via anterograde right femoral vein access with sandwich technique. She exhibited near-immediate relief of debilitating symptoms. Computed tomographic scan demonstrated patent vessels at 1 year.
Conclusions:
Extensive endovascular venous reconstruction is an effective treatment for SVCS due to mediastinal fibrosis.
Keywords: Superior vena cava syndrome, innominate vein, superior vena cava bifurcation, mediastinal fibrosis, endovascular repair, percutaneous procedure, stenosis, stent, kissing, sandwich technique
Introduction
First described in 1757, superior vena cava syndrome (SVCS) is caused by malignancy in 80% to 90% of cases.1,2 However, the incidence of non-malignant SVCS is increasing (10%-40%) due to a growing use of indwelling central venous catheters and implanted cardiac devices.2,3 Mediastinal fibrosis (MF) is a frequent cause of benign SVCS.1,3 The symptoms of SVCS depend on the site, degree, and speed of onset of obstruction, but can be devastating. Severe symptoms occur in patients with rapid obstruction of the superior vena cava (SVC), with slower onset allowing the development of collateral circulation. Contrast-enhanced computed tomographic (CT) scans and venography are important procedures in the diagnosis and management of SVCS.1 According to Stanford et al, 4 types of SVCS have been described depending on the collateral venous return and serve to identify patients at risk of cerebral or airway compromise.1–8
Historically, open debulking of fibrosis and venous reconstruction was the only therapeutic option. However, this palliative procedure carried significant risk and frequent recurrence. We describe a rare case of endovascular recanalization and SVCS stenting to reconstruct the head and neck veins following MF.
Case Report
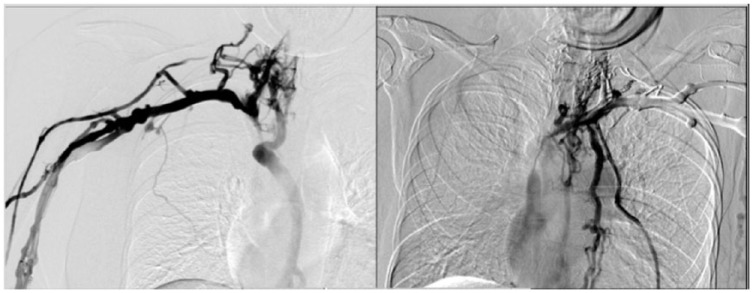
A 36-year-old female from Tunisia presented with slow-onset, moderate SVCS. She was diagnosed with nodal and oesophageal tuberculosis in 2012 and treated with a 6-month course of anti-tubercular treatment. One year prior she had a mediastinal mass that regressed with corticoids. She presented with symptoms including cough, weakness, weight loss (15 kg), thoracic pain, distended collateral venous circulation of the thorax, and significant oedema for 2 years (moderate SVCS and Kishi score = 5).1–8 She had no history of central venous device placement or thrombophilic risk. Lymphoma or recurrence of tuberculosis was suspected. Bronchoscopy with biopsy, analysis of bronchoalveolar fluid, positron emission tomography (PET) CT, and 2 endobronchial ultrasounds ruled out infection or malignant cause. Pathology demonstrated inflammatory tissue without granuloma. Streptococcus parasanguinis was found on bacterial culture of bronchoalveolar fluid and was treated by antibiotics. A contrast-enhanced CT scan with 3-dimensional reconstruction (Figure 1) revealed significant superior vena cava compression secondary to MF. Intraoperative venograms performed via the right and left arms confirmed pre-occlusive stenosis of the right internal jugular (IJ) and innominate veins and SVC with extensive collateral veins and type 4 SVCS according to the Standford Classification (Figure 2).1–8 We decided not to perform mediastinoscopy because of prior negative biopsies on endobronchial ultrasounds.
Figure 1.
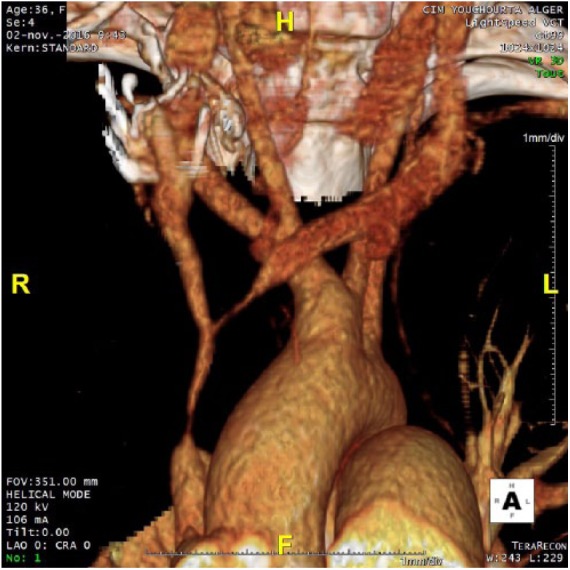
Contrast-enhanced computed tomographic (CT) scan with 3-dimensional reconstruction showing the stenosis.
Figure 2.
Intraoperative venograms via the right and left arm, respectively.
Primary endovascular repair was discussed and planned by a multidisciplinary team including a thoracic surgeon, interventional radiologist, pulmonologist, and oncologist. The ethical committee approved the informed patient consent, procedure, and report.
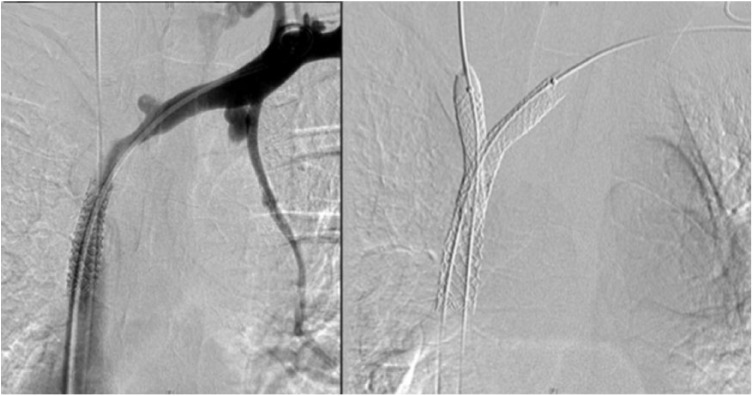
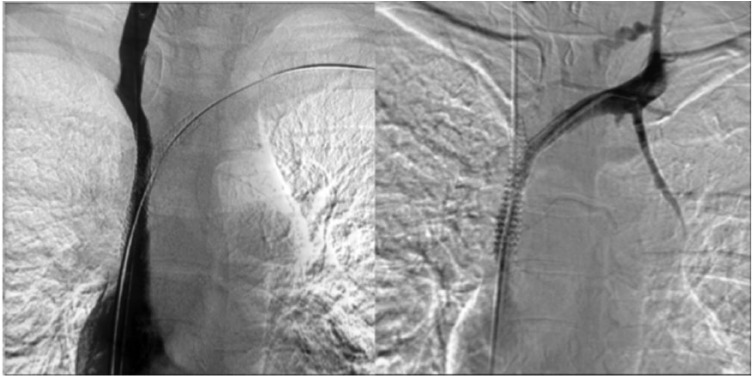
Under local anaesthesia, a percutaneous venous access was achieved through the right common femoral vein. A 12F sheath was inserted, and two 0.035-in hydrophilic guidewires (Glidewire Terumo, Somerset, NJ, USA) were manoeuvred in parallel past the venous obstruction (Figure 3). Pre-dilation was performed using a 6-mm Armada balloon (Abbott Vascular, Lakeside Drive, Santa Clara, CA, USA) inflated in the confluence of SVC, right IJ and innominate veins. A 10 mm × 37 mm balloon-expandable covered stent (Begraft Bentley InnoMed GmbH, Hechingen, Germany) was deployed at the beginning of the SVC. Complementary simultaneous kissing stents were deployed in the SVC bifurcation, with a 8 mm × 29 mm stent in the right IJ vein and a 8 mm × 39 mm balloon-expandable bare metal stent (Omnilink Elite; Abbott Vascular) in the innominate vein. The parallel stents extended beyond the initial part of the stent in the SVC (Figure 4). A venogram demonstrated complete coverage of the SVC bifurcation and patent right IJ and innominate veins and SVC without any extravasation (Figure 5). The pressure gradient across the occlusions before angioplasty was 20 mm Hg and it decreased to 8 mm Hg on both sides. There were no procedural complications. There was a marked improvement in symptoms. Dual anticoagulation therapy with acetylsalicylic acid (160 mg once daily) and therapeutic low-molecular-weight heparin was started immediately after the intervention. The patient was discharged on the fourth day post-surgery. Her drug regimen at discharge included long-term anticoagulation (vitamin K inhibitors) and antiplatelet therapy. A CT scan confirmed stent patency at discharge and 1 year (Figure 6).
Figure 3.
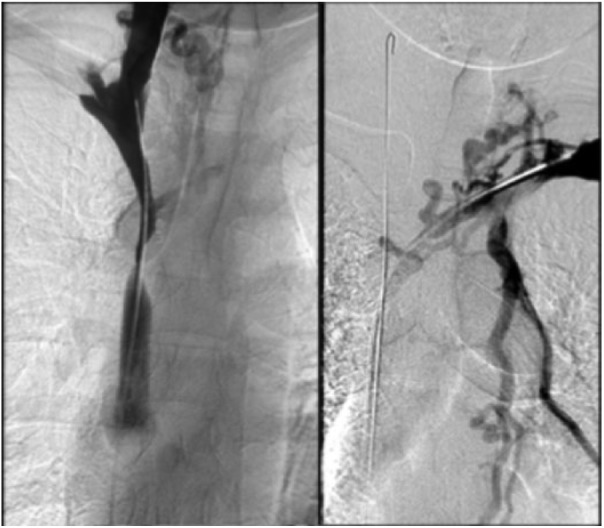
Recanalization of right IJ and innominate veins.
Figure 4.
Stenting of the superior vena cava and kissing stents in right IJ and innominate veins.
Figure 5.
Venograms showing complete patency after stenting.
Figure 6.
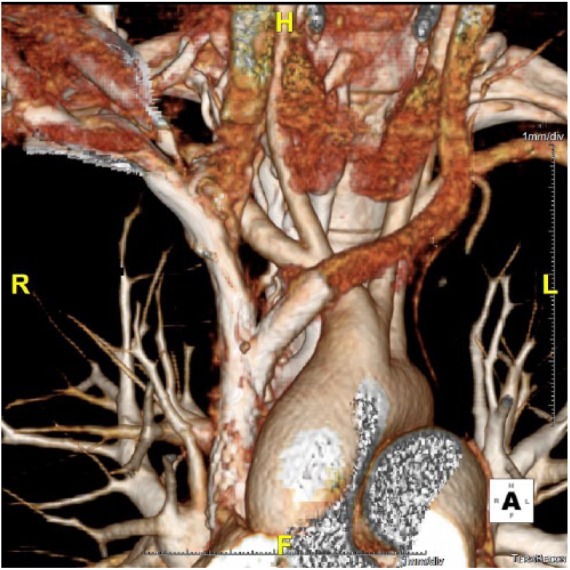
Computed tomographic scan with 3-dimensional reconstruction before patient discharge.
Discussion
MF is a rare and benign disorder affecting preferentially young women.9 The most common cause is histoplasmosis, but it has also been described in association with tuberculosis, fungal or bacterial infections, trauma, radiation therapy, Hodgkin disease, sarcoidosis and autoimmune disease, drugs, and idiopathic fibrosing disease.9 MF involves the mediastinal structures, especially the SVC, leading to SVCS in 23% to 59% of cases.9 MF was previously the most common cause of benign SVCS but has now been overtaken by catheter-related thrombosis.1,3–5,8 CT scans confirm the diagnosis of MF and reveal the vein compression in cases of SVCS.9 A bilateral brachial phlebogram is recommended in cases of SVC obstruction.9 Further testing should include upper gastrointestinal and airway fibroscopy, diagnostic tests for infection, mediastinoscopy with tissue biopsy, and PET scans to confirm diagnosis and rule out neoplasia.9 After reconstructive surgery for SVCS or endovascular treatment, long-term anticoagulation therapy is recommended with an antiplatelet agent.7
Surgery for SVCS depends on urgency, cause, and location of the obstruction.1–8 Open surgical repair is an option if endovascular repair is not appropriate or fails.3–5,7,8 Emergency treatment is required in cases of airway obstruction, cerebral oedema, or pericardial effusion.1 If hemodynamic, respiratory, or cerebral symptoms are well tolerated and there is no immediate emergency, diagnostic procedures such as biopsy are recommended.1 Patients with benign disease have a normal life expectancy. The goal of treatment is to provide prompt and long-term symptomatic relief.2,5,10 In case of MF, an extra-anatomical reconstruction can be performed for SVC or inominate thrombosis using polytetrafluoroethylene graft.4,5,7,8 Primary patency ranges from 71% to 100%, with a mortality rate of 0% to 12% and 5-year survival of 11% to 77%.3,7 Regular follow-up and reintervention are required to maintain patency and long-term clinical success.3,7
SVC stenting was first described in 19862,6,7 and is now a well-established treatment in benign cases of SVCS2–5,8,11 related to MF.8,10 The advantages of stenting include the rapid relief of symptoms, low morbidity, long-term patency, and a minimally invasive approach that can be performed under local anaesthesia or conscious sedation.1,3,4,6–8 It can be combined with other therapeutic approaches, local fibrinolysis and allows easy central venous access.1,6,7,11 Either balloon-expandable or self-expanding stents can be used.6 Kishi et al proposed a score to indicate endovascular repair if >4.1–4,6–8 In benign SVCS, primary patency ranges from 40% to 95% and secondary patency from 80% to 100%.1,3,6,7 Endovascular repair is more effective and has reduced reinterventions compared with percutaneous balloon angioplasty.3–5,8,12 Y-stenting has already been proposed in malignant SVCS and in MF with good resolution of symptoms.10,13 Karuppasamy et al14 proposed a train-track technique for reconstruction of SVC bifurcation in chronic SVCS due to central venous catheters. A recent report of 27 SVCS stenting procedures showed 100% technical success, 19.7% first recurrences at 1 year, and 2 major complications with a mean follow-up of 1275 days.2 Regular follow-up and reinterventions are required to maintain patency and long-term clinical success.3
Morbidity and mortality rates of 29% and 4%, respectively, have been reported following SVCS stenting.6,7 Malpositioning and stent migration decrease with surgical experience and stent improvement.6,8 Tamponade due to intrapericardial SVC rupture is a dreaded complication along with cardiac injury, haemothorax, and rupture of the ascending aorta.2,6 Pulmonary embolism may occur in the absence of anticoagulation.6 Thrombosis has been described following long and complex procedures.6,7 SVCS recurrence is due to different mechanisms (in-stent thrombosis, intimal hyperplasia, shortening of the prosthesis, disease progression) resulting in in-stent restenosis requiring reintervention.2,6,7 Volume overload can precipitate complications in patients with pre-existing heart disease.6,7 Infection of the device is a rare but serious complication which can be prevented with antibiotic prophylaxis.6
Conclusions
Endovascular treatment of SVCS for MF is a safe procedure with excellent results. Complex endovascular venous reconstruction using kissing stents can provide symptomatic relief with stent patency beyond 1 year.
Footnotes
Declaration Of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: JB wrote the first draft of the manuscript. JB, DF, CW contributed to the writing of the manuscript. DF, CW, PB, EF made critical revisions and approved final version. All authors reviewed and approved of the final manuscript.
Ethical Approval: Ethical committee was sought, Department of Thoracic and Vascular Surgery and Heart-Lung Transplantation, Marie-Lannelongue Hospital, Paris-Sud University, Le Plessis-Robinson, France.
References
- 1. Straka C, Ying J, Kong FM, et al. Review of evolving etiologies, implications and treatment strategies for the superior vena cava syndrome. Springerplus. 2016;5:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Breault S, Doenz F, Jouannic AM, Qanadli SD. Percutaneous endovascular management of chronic superior vena cava syndrome of benign causes: long-term follow-up. Eur Radiol. 2017;27:97–104. [DOI] [PubMed] [Google Scholar]
- 3. Sfyroeras GS, Antonopoulos CN, Mantas G, et al. A review of open and endovascular treatment of superior vena cava syndrome of benign aetiology. Eur J Vasc Endovasc Surg. 2017;53:238–254. [DOI] [PubMed] [Google Scholar]
- 4. Bagan P, De Dominicis F, Berna P. Syndrome cave supérieur. EMC – Cardiol. 2015;10:1–7. [Google Scholar]
- 5. Rizvi AZ, Kalra M, Bjarnason H, Bower TC, Schleck C, Gloviczki P. Benign superior vena cava syndrome: stenting is now the first line of treatment. J Vasc Surg. 2008;47:372–380. [DOI] [PubMed] [Google Scholar]
- 6. El Hajjam M, Lagrange C, Desperramons J, et al. Imagerie diagnostique et thérapeutique de la veine cave supérieure. EMC – Radiologie et Imagerie Médicale – Cardiovasculaire – Thoracique – Cervicale. 2010;32-225-F-20. [Google Scholar]
- 7. Solovei L, Marty-Ané CH, Alric P, et al. Chirurgie de la veine cave supérieure. EMC – Tech Chir – Thorax. 2016;11:1–22. [Google Scholar]
- 8. Johansen M, Hoyer M, Kleiman M. Transcatheter treatment of SVC syndrome from histoplasmosis-related mediastinal fibrosis in a 9-year old male. Catheter Cardiovasc Interv. 2013;82:E708–111. [DOI] [PubMed] [Google Scholar]
- 9. Urschel HC, Patel AN, Razzuk MA, et al. Chronic mediastinitis. In: Patterson GA, Cooper JD, Deslauriers J, et al., eds. Pearson’s Thoracic and Esophageal Surgery (Vol 1). 3rd ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2008:1532–1536. [Google Scholar]
- 10. Amin P, Sharafuddin MJ, Laurich C, et al. Anatomic bifurcated reconstruction of chronic bilateral innominate-superior vena cava occlusion using the Y-stenting technique. Ann Vasc Surg. 2012;26:276.e5–276.e9. [DOI] [PubMed] [Google Scholar]
- 11. O’Sullivan GJ, Mhuircheartaigh JN, Ferguson D, Delappe E, O’Riordan C, Browne AM. Isolated pharmacomechanical thrombolysis plus primary stenting in a single procedure to treat acute thrombotic superior vena cava syndrome. J Endovasc Ther. 2010;17:115–123. [DOI] [PubMed] [Google Scholar]
- 12. Seckeler MD, Villa C, Hirsch R. Percutaneous recanalization of occluded brachiocephalic vein-superior vena cava connection after resection of mediastinal mass. JACC Cardiovasc Interv. 2014;7:e69–70. [DOI] [PubMed] [Google Scholar]
- 13. Cordial R, Moussavian MR, Corvalan J, Görtz H, Teßarek J. Percutaneous endovascular Y-stenting of a malignant superior vena cava and innominate vein obstruction. Vasc Endovascular Surg. 2014;48:77–79. [DOI] [PubMed] [Google Scholar]
- 14. Karuppasamy K, Al-Natour M, Gurajala RK. A “train-track” technique in anatomic reconstruction of SVC bifurcation complicated by cardiac tamponade: an introspection. Cardiovasc Intervent Radiol. 2017;40:629–633. [DOI] [PubMed] [Google Scholar]