Abstract
Background
There is little research done on non-mass cancers (NMCs) on breast ultrasound (US).
Purpose
To evaluate large-sectional histopathology findings of NMCs on breast US.
Material and Methods
The mammographic and histopathology features of biopsy proven 36 breast cancers which showed pure non-mass lesions on US were retrospectively reviewed.
Results
The most common mammographic finding was microcalcification (23/35, 65.7%); fine pleomorphic microcalcification was predominant (18/23, 78.3%). The main tumor type was pure ductal carcinoma in situ (DCIS) (14/36, 38.9%) and DCIS with micro- or minimal invasion (11/36, 30.6%). Among the 25 DCIS, histologic grade was high in 15 (60.0%) and intermediate in nine (36%); comedo necrosis was seen in 17 (68%). Immunohistochemical analysis was available in 27 lesions and showed HER2-overexpression in 12 (44.4%) and triple-negative in two (7.4%).
Conclusion
According to our limited patient sample, NMCs on breast US were mainly associated with high-grade DCIS.
Keywords: Breast cancer, ductal carcinoma in situ, DCIS, non-mass lesion, ultrasound, histopathology findings
Introduction
A non-mass lesion (NML) on breast ultrasound (US) is defined as a lesion that occupies space in two different sonographic planes but that cannot be characterized as a mass due to lack of a conspicuous margin or shape. Since Uematsu et al. (1) first described sonographic NMLs in their review article, few studies (2–5) have noted that the NMLs were related with malignancies such as ductal carcinoma in situ (DCIS). Most recently, Lee et al. (4) reported that the likelihood of malignancy in NMLs on breast screening US was >2%, and they could be classified as BI-RADS category 4A lesions. However, knowledge and understanding about non-mass cancers (NMCs) on breast US are still scarce; moreover, to date, there has been no study on their biologic features related with prognosis. Therefore, the purpose of this brief clinical study was to evaluate large-section histological findings of breast cancers solely presented as NMLs on US.
Material and Methods
The institutional review board approved this study and informed consent was waived.
Study cohort
From our radiologic records of all breast US examinations from January 2011 to December 2015, we found 151 lesions in 146 patients described as the NMLs based on the reports; we excluded eight (5.3%) of the 151 lesions because additional data were unavailable. Among the remaining 143 lesions, 139 (97.2%) were pathologically confirmed and 42 (30.2%) of the 139 were diagnosed as malignant. Among the 42 lesions, 36 (85.7%) were pure NMLs and the remaining six (14.3%) were combined with mass-forming cancers. Finally, 36 lesions in 36 patients were enrolled in the study.
Mammography
Two radiologists with 6–11 years of experience in breast imaging retrospectively reviewed the mammographic findings together blinded to the US findings, in consensus. The findings were categorized as negative, mass, calcifications, asymmetry, architectural distortion, or combined calcifications and asymmetry.
Ultrasound
Whole-breast US examinations were performed for all 36 patients by one of our dedicated breast-specific radiologists with 11–14 years of experience in breast imaging. The radiologist used Aixploler (Supersonic Imagine, Aix-en-Provence, France), IU 22 (Philips Medical Systems, Bothell, WA, USA), or HDI 5000 (Philips Advanced Technology Laboratories, Bothell, WA, USA) US systems equipped with linear 8- to 13-MHz transducers. The radiologist had been informed of the mammographic and clinical findings before the US examinations. With real-time scanning, if a lesion was visible on two orthogonal planes but could not be characterized as a distinct mass because of lack of a conspicuous margin or shape, the radiologist categorized it as a NML (Fig. 1). For each lesion, we, the two experienced radiologists, retrospectively reviewed the sonographic features together and reached consensus of that it was a NML.
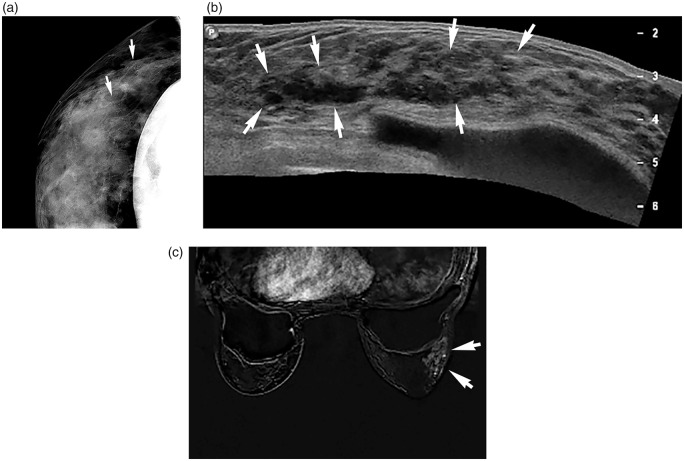
Fig. 1.
A 42-year-old woman with 6.3-cm ductal carcinoma in situ. (a) Magnification mammography shows segmentally distributed pleomorphic microcalcifications (arrows) at the right upper outer breast. (b) Sonography panoramic scan shows a linear or segmentally distributed non-mass lesion with duct ectasia and calcifications (arrows) correlating with the mammographic abnormality in the right breast. (c) Axial first contrast-enhanced subtraction MR image shows heterogenous, segmental non-mass enhancement (arrows) in the corresponding area in the right breast. US-guided core biopsy and surgery confirmed the lesion to be DCIS (high nuclear grade, comedo type) with ER-negative/PR-negative/HER2-positive subtype.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) was performed using a 3.0-T unit (Signa HDxt; GE Medical Systems, Milwaukee, WI, USA) with a dedicated surface breast coil (eight channels) and we retrospectively analyzed the MRI findings for amount of fibroglandular tissue, degree of background parenchymal enhancement, lesion size (longest diameter), and lesion type (mass or non-mass enhancement). We also interpreted each lesion according to shape, margin, and internal enhancement patterns for mass and distribution and internal enhancement patterns for non-mass enhancement.
Histopathology
After we reviewed the available histopathological data for each lesion, we recorded tumor type, tumor size, surgery type, lymph node status, histologic grade, nuclear grade, the presence of necrosis for DCIS, and hormone receptor status: estrogen receptors (ER); progesterone receptors (PR); and human epidermal growth factor receptor 2 (HER2) overexpression. When DCIS and invasive cancers were combined, the combined maximum tumor size was used. A re-cut of the corresponding paraffin block was immunostained with commercially available antibodies to ER, PR, and HER-2. The cut-off point for ER- and PR-positive expression was 10%. HER2 status was graded as 0, 1+, 2+, and 3+; 3+ was deemed to be positive. If HER2 status was 2+ and it was amplified HER2 expression on fluorescence in situ hybridization, it was also regarded to be positive.
Results
The mean age of the patients was 49 years (age range = 33–69 years). Twelve (33.3%) patients had no symptoms. The most common symptom was palpability (20/36, 55.6%), followed by nipple discharge (3/36, 8.3%) and pain (1/36, 2.8%).
Mammographic findings
Mammography was available within six months of the US examinations in 35 patients (97.2%) and the most common findings were combined calcifications and asymmetry (13/35, 37.1%), followed by calcifications (10/35, 28.6%) (Fig. 1), asymmetry (5/35, 14.3%), negative (5/35, 14.3%), and architectural distortion (2/35, 5.7%). Microcalcification was visualized in 23 lesions (65.7%), with fine pleomorphic calcifications (Fig. 1) being the most frequently seen (18/23, 78.3%), followed by coarse heterogenous calcifications (3/23, 13.0%), fine linear calcifications (2/23, 8.7%), and amorphous calcifications (2/23, 8.7%); two lesions showed combined microcalcifications.
MRI findings
MRI was available for 15 of the 36 lesions; the histological types of the 15 lesions were as follows: DCIS (n = 6, 40%); minimally invasive DCIS (n = 5, 33.3%); invasive ductal carcinoma (IDC) with DCIS (n = 3, 20%); IDC (n = 1, 6.7%). The lesions presented as non-mass (n = 13, 87%) or mass (n = 2, 13%) enhancement. Among the 13 non-mass enhancement lesions, the distribution was mostly linear or segmental (9/13, 69%) (Fig. 1) and regional (3/13, 23%). The two mass-enhancing lesions were micro- and minimally invasive DCIS (0.1 cm single focus of IDC and 1.5 cm DCIS) and IDC.
Histopathology findings
The 36 lesions were pathologically confirmed by US-guided 14-gauge core needle biopsy (n = 9, 25%) and surgical excision (n = 27, 75%); Table 1 summarizes the detailed histopathology findings. DCIS or DCIS with micro- or minimal invasion (25/36, 69.4%) (Fig. 1) was the most predominant histologic type. Three (75%) of four microinvasive DCIS had multifocal microinvasion. Among 31 lesions including DCIS, 14 DCIS (45.2%) were extensive. Invasive or minimally invasive component was identified in 16 lesions with being high histologic grade in seven (43.8%), intermediate in six (37.5%), and low in three (18.8%). Tumor size was measured in 27 surgically excised lesions (18 DCIS or DICS with micro- or minimal invasion and nine invasive cancers). Tumor size of all invasive cancers combined with DCIS was determined by DCIS because all the lesions had larger DCIS component than invasive component. Nuclear grade, lymph node status, and biologic markers (ER, PR, and HER2) were available in the 27 surgically excised lesions. Lymph node status was negative in all of 18 pure DCIS or DCIS with micro- or minimal invasion, and in three (30%) of nine invasive cancers. The most common tumor subtype was ER- or PR-positive/HER2-negative (13/27, 48.1%). Triple-negative subtype was the least (2/27, 7.4%).
Table 1.
Large section histology findings of non-mass breast cancers.
| Histologic findings (n) | n (%) |
|---|---|
| Tumor type (n = 36) | |
| DCIS | 14 (38.9) |
| Micro- or minimally invasive DCIS | 11 (30.6) |
| IDC with DCIS | 6 (16.7) |
| IDC only | 2 (5.6) |
| Others | 3 (8.3) |
| Tumor size (mm) (n = 27*) | |
| <20 | 5 (20) |
| <50 | 10 (37) |
| ≥50 m | 12 (44.4) |
| Lymph node status (n = 27*) | |
| Negative | 21 (77.8) |
| Positive | 6 (22.2) |
| DCIS grade (n = 28†) | |
| High | 18 (64.3) |
| Intermediate | 9 (32.1) |
| Low | 1 (5.6) |
| Type of DCIS (n = 28†) | |
| Comedo | 14 (50) |
| Non-comedo | 10 (35.7) |
| Mixed | 4 (14.3) |
| Tumor subtype (n = 27*) | |
| ER(+) PR (+) HER2 (−) | 12 (44.4) |
| ER(+) PR (−) HER2 (−) | 1 (3.7) |
| ER(+) PR (+) HER2(+) | 4 (14.8) |
| ER(+) PR(−) HER2(+) | 2 (7.4) |
| ER(−) PR(−) HER2(+) | 6 (22.2) |
| ER(−) PR(−) HER2 (−) | 2 (7.4) |
Pathologic tumor size, lymph node status, and tumor subtype analysis were available in 27 surgically excised lesions.
14 DCIS, 11 micro- or minimally invasive DCIS, and three extensive DCIS with IDC.
DCIS, ductal carcinoma in situ; IDC, invasive ductal cancer; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor.
Discussion
According to our results, DCIS or DCIS with micro- or minimal invasion comprised the main histopathologic type (25/36, 69.5%). Additionally, six (66.7%) of nine IDCs were accompanied with extensive DCIS. DCIS with microinvasion is an uncommon pathological entity representing <1% of all breast cancers (6), but there were four cases (11.1%) in the current study. These results corresponded to the results of previous studies (2–5). Therefore, DCIS was a major element for deciding the sonographic features of the NMCs in this study, and mammography and MRI features were also compatible with DCIS mostly, showing calcifications or asymmetry (29/35, 82.9%) on mammography and non-mass enhancement (13/15, 86.7%) on MRI. On mammography, we noted suspicious microcalcification in 23 (74.2%) of 31 lesions with DCIS, and most microcalcifications were either fine pleomorphic or fine linear microcalcifications which were significantly associated with the presence of high-grade DCIS and necrosis (7).
Histopathologically, high-grade DCIS (18/28, 64.3%) was more identified than low-grade DCIS (1/28, 5.6%) in this study. These results are consistent with those of previous studies, where non-mass parenchymal changes with microcalcifications were more common sonographic findings in high-grade DCIS (11/31, 35.5%) and masses in non-high-grade DCIS (13/26, 50.0%) (8–9). Low- and high-grade DCIS are fundamentally different diseases and each is genetically related to its invasive counterpart (10). Therefore, knowledge of these characteristic US features will be helpful in management as well as diagnosis of DCIS.
It is well-known that breast cancer subtypes approximated by ER, PR, and HER2 are important prognostic factors. These subtypes are similar to the subtypes based on mRNA gene expression profiling alone. Each molecular subtype has shown varying risk for progression, response to treatment, and survival outcomes. Research on imaging phenotype of breast cancers has revealed that non-calcified, circumscribed masses are common in the basal-like subtype (triple-negative), spiculated masses with posterior acoustic shadowing in the luminal subtype (ER-positive), and microcalcifications in the HER2-enriched subtype (ER-negative/HER2-positive) (11–14). Moreover, low- and high-grade DCIS shows different molecular profile; HER2 is rarely expression in low-grade DCIS and is detected in about 70% of intermediate- and high-grade DCIS (10). In this study, considering general distribution of breast cancer subtypes (luminal subtype, 71.3%; HER2-enriched subtype, 11.0%; basal-like subtype, 17.7%) (15), luminal type cancers (70.3%) were comparable, HER2-enriched cancers (22.2%) were more depicted, and triple negative cancers were less detected (7.4%). These results relatively were correlated with those of previous studies (11–14) and suggest that HER2-enriched type might be more related with NMCs than mass-forming cancers on breast US. Further extended studies are needed to refine knowledge on molecular subtypes of NMCs on breast US.
There are some limitations to our study. First, it was a retrospective study with a small population of only 36 NMCs; to better represent the characteristics of NMCs, a prospective study with a larger population is needed. Second, even though experienced breast imaging subspecialists reached consensus regarding selecting cases and regarding imaging findings, the decisions on imaging features could have been subjective; additional studies with inter-observer variability are needed. Third, this descriptive study had no statistical power, thus our results should be validated by further studies using statistical analysis.
In conclusion, according to our limited patient sample, NMCs on breast US were mainly associated with high-grade DCIS.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Uematsu T. Non-mass-like lesions on breast ultrasonography: a systematic review. Breast cancer 2012; 19: 295–301. [DOI] [PubMed] [Google Scholar]
- 2.Kim SJ, Park YM, Jung HK. Nonmasslike lesions on breast sonography: comparison between benign and malignant lesions. J Ultrasound Med 2014; 33: 421–430. [DOI] [PubMed] [Google Scholar]
- 3.Ko KH, Hsu HH, Yu JC, et al. Non-mass-like breast lesions at ultrasonography: feature analysis and BI-RADS assessment. Eur J Radiol 2015; 84: 77–85. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Lee JH, Baik S, et al. Non-mass lesions on screening breast ultrasound. Med Ultrason 2016; 18: 446–451. [DOI] [PubMed] [Google Scholar]
- 5.Park JW, Ko KH, Kim EK, et al. Non-mass breast lesions on ultrasound: final outcomes and predictors of malignancy. Acta Radiol 2017; 58: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 6.Intra M, Zurrida S, Maffini F, et al. Sentinel lymph node metastasis in microinvasive breast cancer. Ann Surg Oncol 2003; 10: 1160–1165. [DOI] [PubMed] [Google Scholar]
- 7.Barreau B, de Mascarel I, Feuga C, et al. Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlations. Eur J Radiol 2005; 54: 55–61. [DOI] [PubMed] [Google Scholar]
- 8.Park JS, Park YM, Kim EK, et al. Sonographic findings of high-grade and non-high-grade ductal carcinoma in situ of the breast. J Ultrasound Med 2010; 29: 1687–1697. [DOI] [PubMed] [Google Scholar]
- 9.Lee MH, Ko EY, Han BK, et al. Sonographic findings of pure ductal carcinoma in situ. J Clin Ultrasound 2013; 41: 465–471. [DOI] [PubMed] [Google Scholar]
- 10.Balleine RL, Webster LR, Davis S, et al. Molecular grading of ductal carcinoma in situ of the breast. Clin Cancer Res 2008; 14: 8244–8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamini RM, Baer HJ, Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res 2008; 10: R67–R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology 2009; 250: 638–647. [DOI] [PubMed] [Google Scholar]
- 13.Ko ES, Lee BH, Kim HA, et al. Triple-negative breast cancer: correlation between imaging and pathological findings. European radiology 2010; 20: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Ikeda DM, Narasimhan B, et al. Estrogen receptor-negative invasive breast cancer: imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression. Radiology 2008; 246: 367–375. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Kang E, Lee AS, et al. Outcomes and recurrence patterns according to breast cancer subtypes in Korean women. Breast Cancer Res Treat 2015; 151: 183–190. [DOI] [PubMed] [Google Scholar]