Abstract
Mast cells (MCs) can influence the maturation of collagen fibers. This study evaluated the relationship between the distribution and degranulation of MCs and collagen maturation in human gingival tissue in chronic periodontitis. A total of 16 specimens of patients clinically diagnosed as periodontitis and 18 controls clinically diagnosed as healthy or gingivitis were included. Immunohistochemistry and Picrosirius staining were performed to identify MCs and assess collagen fibers, respectively. Chi-square, t test, and Pearson’s correlation test (p<0.05) were used. In control specimens, there was a positive association between MCs in the connective tissue and the presence of immature collagen (p=0.001); in periodontitis samples, this association was not confirmed (p≥0.12). There was no significant relationship between periodontal diagnosis and collagen maturation or MC degranulation (p≥0.35). MC density was significantly higher (p=0.04) in periodontitis tissue (339.01 ± 188.94 MCs/mm2) than in control tissue (211.14 ± 131.13 MCs/mm2) in the area of connective tissue containing inflammatory infiltrate. There was a correlation between the number of MCs and probing depth (r = 0.34, p=0.04). MCs are involved in the pathogenesis of periodontal diseases and might be associated with collagen maturation in periodontal tissue during the early stages of periodontal disease pathogenesis.
Keywords: collagen, human, inflammation, mast cells, periodontitis
Introduction
Periodontitis is a chronic inflammatory disease that is initiated by an inflammatory response to dental plaque bacteria. Disease progression is due to a combination of factors, including host inflammatory response to periodontopathic bacteria, which leads to the destruction of periodontal tissues.1 Significant changes occur in specific cell populations, including fibroblasts and mast cells, during the pathogenesis of periodontitis.2
Mast cells (MCs) have been determined to be crucial effectors in chronic inflammatory diseases such as rheumatoid arthritis,3 atherosclerosis,4 and periodontitis.5 In periodontal tissues, they are present within the gingival epithelium and connective tissue,6 and are important initiators and effectors of innate immunity. MCs are activated during the innate immune response to pathogens, and can secrete products, including tryptase, chymase, cathepsin G,7 histamine, heparin, serotonin,8 acid hydrolases,9 metalloproteinases,10–12 tumor necrosis factor-α,13 and other interleukins.14 Furthermore, these cells have a role in the cellular immune response, facilitating the development, amplifying the magnitude, or regulating the kinetics of adaptive immune responses.15 Furthermore, MCs are able to capture, process, and present antigens.5
MCs are found in both healthy and diseased gingival tissues, and their density in periodontal diseases is a topic of much speculation. Some studies have reported that in inflamed and healing gingival tissues, the number of MCs is elevated,16–19 whereas other studies have reported decreased numbers.20–22
The activation of MCs is a feature of chronic inflammation, and this can lead to tissue fibrosis as a result of increased collagen synthesis by fibroblasts.23 However, MCs can also stimulate fibroblasts to secrete collagenases, suggesting that these cells might modulate the function of connective tissue cells24 and influence the maturation and degradation of collagen fibers in periodontal tissue. Interactions between MCs and collagen degradation is crucial for the development of several conditions such as keloid formation, chronic graft versus host disease, and wound repair,25,26 and may also be important in the periodontal diseases. However, their interaction in the periodontal tissues is unknown. We hypothesized that there is an association between MCs and collagen maturation during chronic periodontitis. Therefore, the present study was designed to investigate the relationship between the distribution and degranulation of MCs and collagen maturation in periodontal tissues and during chronic periodontitis.
Materials and Methods
This study was approved by the Ethics Committee of Research (06-306). A written explanation of the study purpose was provided, and signed consent, according to the Helsinki Declaration, was obtained from each participant.
The individuals included in the present study were males and females, 18 to 62 years of age, with no history of systemic disease; subjects did not use antibiotics or anti-inflammatory medications 6 months before this study and were not smokers or former smokers. A total of 34 gingival tissue specimens were obtained from 16 subjects with chronic periodontitis and 18 individuals demonstrating periodontal health (n=8) or gingivitis (n=10) (control group).
All permanent, fully erupted teeth were examined. Probing depth (PD) and distance between the cement-enamel junction and the free gingival margin (CEJ-GM) were measured at six sites per tooth (mesiobuccal, midbuccal, distobuccal, distolingual, midlingual, and mesiolingual). Measurements were made in millimeters and were rounded to the nearest whole number. Clinical attachment level (CAL) was calculated as the sum of PD and CEJ-GM measurements. The diagnosis and classification of periodontal diseases was performed for each patient.27 In the biopsy site, chronic periodontitis was defined as the loss of clinical attachment (level ≥4 mm), which was associated with bleeding upon probing.28 Sites that did not show any of the above symptoms in individuals with periodontal health or gingivitis were included in the control group. One single trained periodontist performed periodontal examinations, using a manual periodontal probe (PCP-UNC 15; Hu-Friedy, Chicago, IL) and biopsies.
Histological Staining and Grading
Immunohistochemistry was performed on paraffin sections (3-µm thick). The tissue sections were routinely deparaffinized and rehydrated. Endogenous peroxidase activity was blocked using hydrogen peroxide. A polyclonal anti-tryptase antibody (Clone AA1; Dako Corporation, Carpinteria, CA; dilution 1:50) was used with the EnVision System (Dako). The sections were incubated with MC tryptase (Dako), at a dilution of 1:50 for 12 hr at room temperature. The immunohistochemical reactions were developed with diaminobenzidine (Dako) as the chromogenic peroxidase substrate, and the slides were counterstained with Harris’s hematoxylin. The positive control was a squamous cell carcinoma sample.
MCs were counted manually by two previously trained examiners, using one entire tissue section from each sample. The oral epithelium (OE), junctional epithelium (JE), and connective tissue (CT) containing inflammatory cell infiltrates (CTI), and sites adjacent to inflammatory cell infiltrates (CTWI) were evaluated separately. The images were digitized by a camera (Zeiss; Germany, 2008) and the corresponding OE, JE, and CT areas were measured using Image J software (Image J 1.48; National Institutes of Health, Bethesda, MD). Subsequently, the density of MCs was calculated by determining the number of MCs per unit area (cell number/mm2). For degranulation evaluating, any cluster of MC granules with a dark-brown appearance that was clearly distinct from the adjacent cell membrane was considered degranulated.19 The degranulated MCs were semiquantitatively categorized as ≥50% or <50% degranulated for each specimen.
To assess collagen maturation, 5-µm thick sections were stained with Picrosirius. The sections were classified according to the following scores: ≥50%, regular mature collagen fibers (intense red color and thick fibers), or ≥50%, immature fibers (light red color, fine fibers, and interleaved). Red color in normal oral mucosa was used as control.
All sections were analyzed by light microscopy (Axio Lab; Zeiss, Germany, 2008) at a 400× magnification at the Laboratory of Surgical Pathology of the School of Dentistry, Federal University of Bahia.
Statistical Analysis
The mean MC density in OE, JE, and CT were compared between periodontitis and control specimens by performing a Student’s t-test. A Mann–Whitney test was performed when homogeneity of variances was not detected.
A chi-square test was used to analyze the relationship between MC degranulation and collagen maturation, as well as the relationship between periodontal status and MC degranulation, and between periodontal status and collagen maturation.
A Pearson’s correlation was used to analyze the relationship between MC density and periodontal clinical parameters (PD, gingival recession [GR], and CAL).
Data analysis was performed using a statistical software program (SPSS version 13.0; SPSS Inc., Chicago, IL), and the significance level was set to p<0.05.
Results
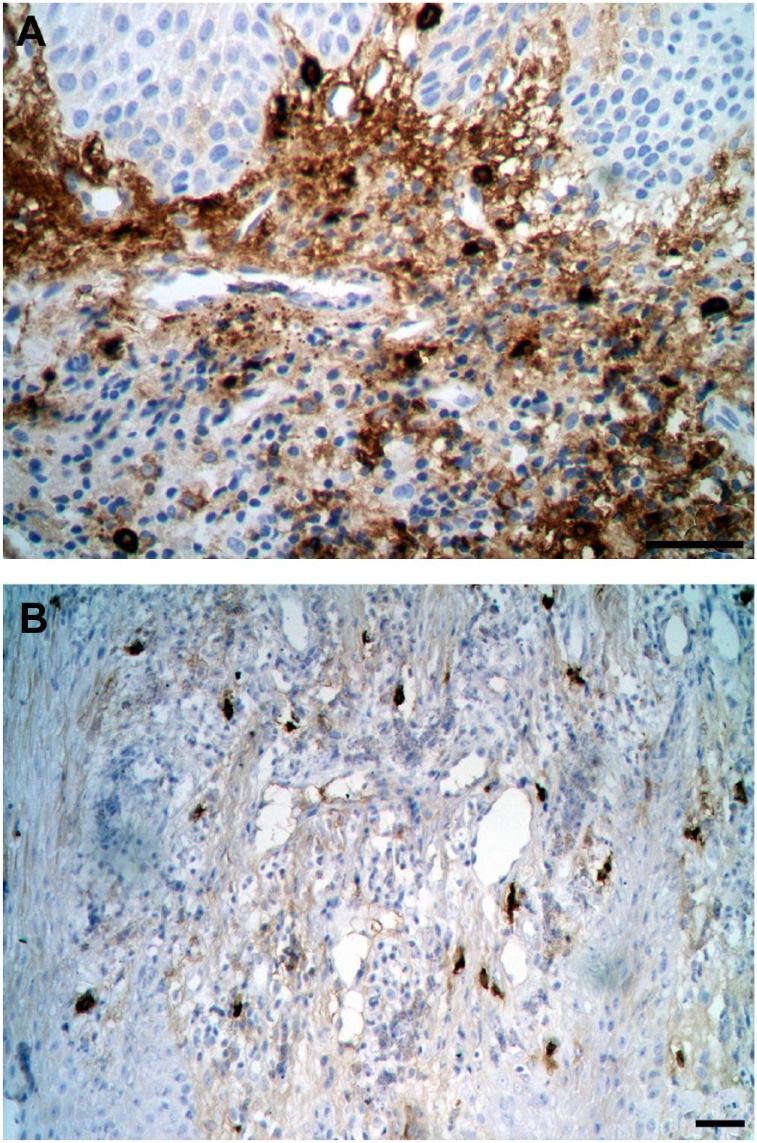
MC density was significantly higher in periodontitis tissue (mean ± SD: 339.01 ± 188.94) than in controls (mean ± SD: 211.14 ± 131.13) in CTI (p=0.04). There were no differences between periodontitis and control specimens in terms of MC density in OE, JE, and CTWI (p≥0.19). See Table 1 and Fig. 1.
Table 1.
MC Density (Mean ± SD) by Histological Region in Periodontitis and Control Tissues.
| Area | Mast Cell Density (Mean ± SD) | p Value | |
|---|---|---|---|
| Periodontitis (n=16) | Control (n=18) | ||
| JE | 12.95 ± 14.78 | 29.21 ± 46.77 | 0.19a |
| OE | 13.01 ± 16.57 | 12.73 ± 12.74 | 0.96a |
| CTI | 339.01 ± 188.94 | 211.14 ± 131.13 | 0.04b |
| CTWI | 196.42 ± 122.22 | 183.32 ± 236.85 | 0.84a |
Abbreviations: MC, mast cell; JE, junctional epithelium; OE, oral epithelium; CTI, connective tissue with inflammatory cells; CTWI, connective tissue without inflammatory cells.
Student’s t-test.
Mann–Whitney test.
Figure 1.
Immunohistochemistry with mast cell tryptase antibody showing mast cell density. (A) Periodontitis sample (scale bar = 50 µm); (B) Control sample (scale bar = 20 µm).
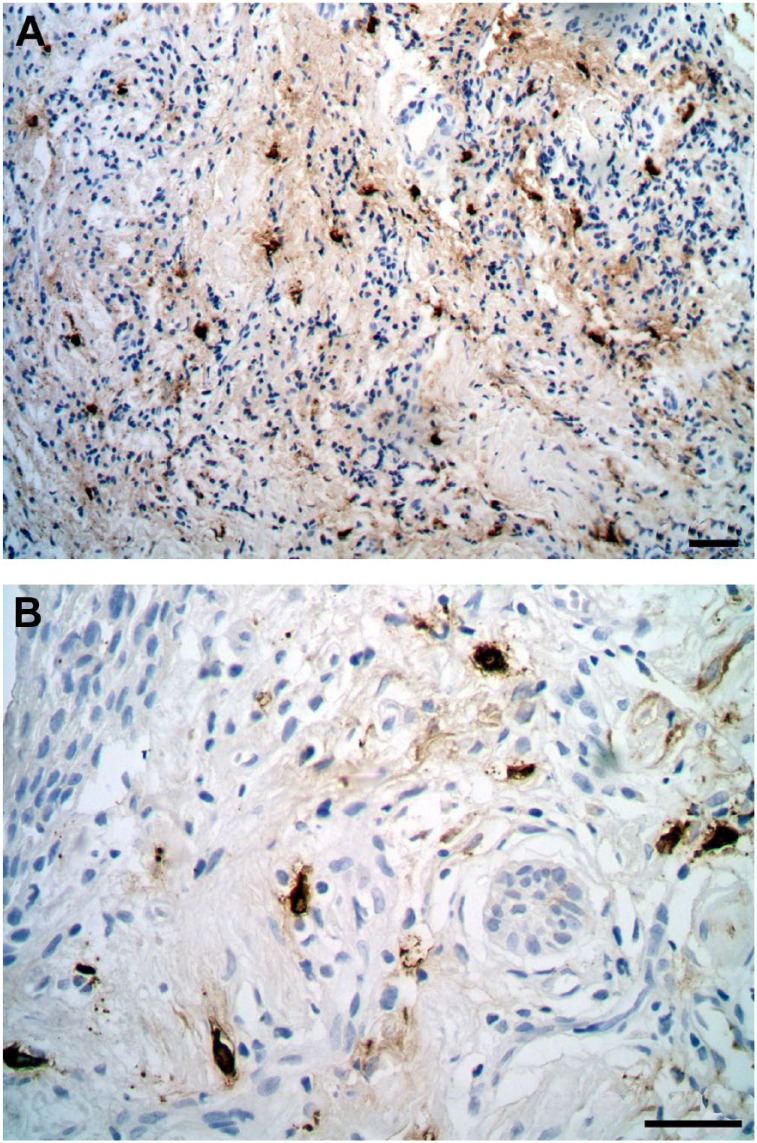
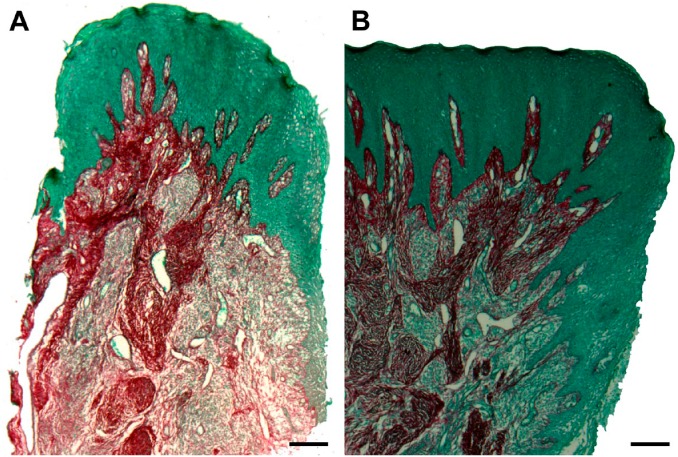
Only in the control group, there was a statistically significant association between an enhancement of immature collagen and increased MC density in the CTWI (p=0.001; Table 2). No statistically significant association between collagen maturation and MC degranulation was found (p≥0.30; Table 3). There was no significant association between periodontal status and either MC degranulation (Table 4 and Fig. 2) or collagen fiber maturation (Table 5 and Fig. 3).
Table 2.
MC Density (Mean ± SD) by Histological Region According to Immature Collagen Fibers, in Control (n=18) and Periodontitis (n=16) Tissues.
| Control | MC Density Immature Collagen Fibers |
p Valuea | |
|---|---|---|---|
| Yes (n=15) | No (n=3) | ||
| CTWI | |||
| JE | 05.02 ± 8.69 | 34.05 ± 49.95 | 0.34 |
| OE | 12.88 ± 08.43 | 12.70 ± 13.70 | 0.98 |
| CTI | 265.30 ± 109.67 | 200.30 ± 135.66 | 0.45 |
| CTWI | 549.28 ± 444.27 | 110.13 ± 74.05 | 0.001 |
| CTI | |||
| JE | 46.33 ± 80.62 | 24.32 ± 35.29 | 0.42 |
| OE | 03.10 ± 03.95 | 15.48 ± 13.12 | 0.09 |
| CTI | 195.74 ± 225.40 | 215.54 ± 103.27 | 0.80 |
| CTWI | 181.47 ± 110.84 | 183.85 ± 265.56 | 0.99 |
| Periodontitis | Yes (n=13) | No (n=3) | |
| CTWI | |||
| JE | 09.34 ± 13.21 | 13.47 ± 15.37 | 0.73 |
| OE | 15.16 ± 21.44 | 12.70 ± 16.75 | 0.85 |
| CTI | 364.45 ± 251.21 | 335.37 ± 190.33 | 0.85 |
| CTWI | 295.38 ± 83.15 | 182.28 ± 122.41 | 0.23 |
| CTI | |||
| JE | 16.77 ± 14.54 | 12.07 ± 15.27 | 0.64 |
| OE | 16.16 ± 27.98 | 12.27 ± 14.47 | 0.73 |
| CTI | 183.92 ± 108.95 | 374.80 ± 187.74 | 0.12 |
| CTWI | 111.69 ± 24.66 | 215.97 ± 127.92 | 0.19 |
Abbreviations: MC, mast cell; CTWI, connective tissue without inflammatory cells; JE, junctional epithelium; OE, oral epithelium; CTI, connective tissue with inflammatory cells.
Chi-square test.
Table 3.
Relationship Between Collagen Maturation in CTI and CTWI and MCs’ Degranulation in Periodontitis (n=16) and Control (n=18) Tissues.
| ≥50% of Immature Collagen Fibers | MCs’ Degranulation | Odds Ratio (95% CI) |
p Valuea | |
|---|---|---|---|---|
| CTWI | ||||
| Control | Yes—n (%) | No—n (%) | 2.00 [0.13, 30.16] | 0.55 |
| No | 3 (75.0) | 12 (85.7) | ||
| Yes | 1 (25.0) | 2 (14.3) | ||
| Periodontitis | ||||
| No | 4 (66.7) | 9 (90) | 4.50 [0.31, 65.23] | 0.30 |
| Yes | 2 (33.3) | 1 (10) | ||
| CTI | ||||
| Control | Yes—N (%) | No—N (%) | 1.00 [0.11, 9.23] | 0.71 |
| No | 7 (77.8) | 7 (77.8) | ||
| Yes | 2 (22.2) | 2 (22.2) | ||
| Periodontitis | ||||
| No | 3 (100) | 11 (84.6) | 0.79 [0.60, 1.03] | 0.65 |
| Yes | 0 (0) | 2 (15.4) | ||
Abbreviations: CTI, connective tissue with inflammatory cells; CTWI, connective tissue without inflammatory cells; MC, mast cell; CI, confidence interval.
Chi-square test.
Table 4.
MC Degranulation in CTI and CTWI Infiltrate in Periodontitis (n=16) and Control (n=18) Tissues.
| ≥50% of Degranulated MCs | N (%) | Odds Ratio (95% CI) |
p Valuea | |
|---|---|---|---|---|
| Periodontitis | Control | |||
| CTI | ||||
| Yes | 6 (37.5) | 9 (50.0) | 0.60 [0.15, 2.36] | 0.35 |
| No | 10 (62.5) | 9 (50.0) | ||
| CTWI | ||||
| Yes | 3 (42.9) | 4 (57.1) | 0.81 [0.15, 4.32] | 0.57 |
| No | 13 (48.1) | 14 (51.9) | ||
Abbreviations: MC, mast cell; CTI, connective tissue with inflammatory cells; CTWI, connective tissue without inflammatory cells; CI, confidence interval.
Chi-square test.
Figure 2.
Immunohistochemistry with mast cell tryptase antibody showing mast cell degranulation. (A) Periodontitis sample (scale bar = 20 µm); (B) Control sample (scale bar = 20 µm).
Table 5.
Immature Collagen in CTI and CTWI in Periodontitis (n=16) and Control (n=18) Tissues.
| ≥50% of Immature Collagen Fibers | Periodontitis | Control | Odds Ratio (95% CI) |
p Valuea |
|---|---|---|---|---|
| CTI | ||||
| Yes | 3 (18.7) | 4 (22.2) | 0.81 [0.15, 3.31] | 0.57 |
| No | 13 (81.3) | 14 (77.8) | ||
| CTWI | ||||
| Yes | 2 (12.5) | 3 (16.7) | 0.71 [0.10, 4.93] | 0.56 |
| No | 14 (87.5) | 15 (83.3) | ||
Abbreviations: CTI, connective tissue with inflammatory cells; CTWI, connective tissue without inflammatory cells; CI, confidence interval.
Chi-square test.
Figure 3.
Picrosirius coloring of collagen fibers (an intense red color represents mature and thick collagen fibers; a lighter red color represents immature and thin collagen fibers). (A) Periodontitis sample (scale bar = 200 µm); (B) Control sample (scale bar = 200 µm).
A Pearson’s correlation test indicated a significant correlation between MC number and probing depth (r = 0.34; p=0.04; Table 6). No further associations were found between other clinical parameters and MC density (p≥0.12).
Table 6.
Correlations Between MC Density Based on Region and PD, CAL, and CEJ-GM (N=34).
| Clinical Parameters | Histological Area | ||||
|---|---|---|---|---|---|
| JE | OE | CTI | CTWI | CTT | |
| PD |
r = −0.21 p=0.24 |
r = 0.09 p=0.62 |
r = 0.34 p=0.04 |
r = 0.01 p=0.94 |
r = 0.24 p=0.18 |
| CAL |
r = −0.22 p=0.20 |
r = 0.09 p=0.59 |
r = 0.27 p=0.12 |
r = 0.09 p=0.60 |
r = 0.25 p=0.16 |
| CEJ-GM |
r = −0.19 p=0.28 |
r = 0.003 p=0.99 |
r = 0.23 p=0.20 |
r = 0.09 p=0.59 |
r = 0.22 p=0.21 |
A Pearson Correlation test was performed to assess statistically significant differences. Abbreviations: MC, mast cell; PD, probing depth; CAL, clinical attachment level; CEJ-GM, cement-enamel junction to gingival margin distance; JE, junctional epithelium; OE, oral epithelium; CTI, connective tissue with inflammatory cells; CTWI, connective tissue without inflammatory cells; CTT, connective tissue total.
Discussion
This study revealed that in health and gingivitis (control) specimens, there was an association between higher MC density and more immature collagen. Furthermore, MC density was higher in periodontitis tissues than in control tissues in areas of CT with inflammatory infiltrate, and there was a positive correlation between MC density and PD. No significant relationship was observed between periodontal diagnosis and collagen maturation or MC degranulation.
It was hypothesized that there is an interaction between MCs and collagen maturation during chronic periodontitis. As in the control specimens, there was a significant association between increased immature collagen and increased MC density, and it was not observed in the periodontitis group; therefore, the hypothesis was confirmed. It may be suggested that this interaction is disturbed in periodontitis. The immature collagen may represent newly formed collagen fibers due to collagen degradation.
In contrast, no significant relationship between degranulated MCs and periodontal diagnosis was found. No significant relationship between degranulated MCs and periodontal diagnosis was found. This result contrasts those of previous studies, which found an increased population of degranulated MCs in periodontitis19,29,30 and observed weak evidence of MC degranulation in healthy gingival tissues. This suggests that MC products might not be the primary driver of gingival destruction, but rather are contributing or secondary factors involved in the evolution of periodontal disease.
A higher MC density was observed in periodontitis tissue in the area surrounding the CT with inflammatory infiltrate and a positive correlation between MC density and PD was also found. Whereas many authors have shown increased MC density with periodontitis,17–19,30 others have shown a decreased number with periodontal inflammation.20–22 These contrasting results can be explained by the fact that periodontal disease is an inflammatory disease with different stages of development and activity.31 In addition, specimens exhibiting different inflammation statuses might have been analyzed. Independent of MC density in periodontitis, it is clear that these cells participate in host defense and inflammation in gingival tissues.
MCs have important effector functions against pathogens.17,18,32–35 Once released into the circulation, undifferentiated precursors complete maturation and differentiation after being recruited to specific tissues, a process that is influenced by environmental factors, cellular origin, bacterial products (lipopolysaccharides), and cytokines.5 MCs are distributed throughout the tissue, and are typically located adjacent to blood vessels and lymph vessels and near the epithelium.5 In this study, it was shown that MCs are distributed in the OE, JE, and CT in both clinically healthy and periodontitis gingival tissue.
Sample size can be considered a limitation of this study. Studies with a larger sample size should be subsequently performed. Future studies should also attempt to better characterize the stage of inflammation for the included samples. Furthermore, the age of the patients should be taken into account in future studies, as age-related changes in rates of collagen synthetic and degradative processes have been described.36
In conclusion, MCs are involved in periodontal disease pathogenesis, independent of disease stage, and might be associated with collagen fiber maturation in periodontal tissue during the early stages of periodontal disease. The present results improve the understanding of periodontal disease pathogenesis and might help in the development of new therapies based on host response.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: LSFR developed all the research. JNS carried out the photos and collaborated in the analysis of the images. CAGR collaborated to make the blades. PRC was responsible for research and statistical analysis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Cury is supported by the National Council for Scientific and Technological Development (303003/2015-4).
Contributor Information
Lívia S.F. e Ribeiro, Master’s degree student at the Postgraduate Program in Health and Dentistry, School of Dentistry, Federal University of Bahia, Salvador, Brazil
Jean N. dos Santos, Department of Oral Pathology, School of Dentistry, Federal University of Bahia, Salvador, Brazil
Clarissa A.G. Rocha, Department of Oral Pathology, School of Dentistry, Federal University of Bahia, Salvador, Brazil
Patricia R. Cury, Department of Periodontics, School of Dentistry, Federal University of Bahia, Salvador, Brazil.
Literature Cited
- 1. Gemmell E, Seymour GJ. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol 2000. 2004;35:21–41. [DOI] [PubMed] [Google Scholar]
- 2. Cho M, Garant PR. Development and general structure of the periodontium. Periodontol 2000. 2000;24:9–27. [DOI] [PubMed] [Google Scholar]
- 3. Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 2002. 297(5587):1689–92. [DOI] [PubMed] [Google Scholar]
- 4. Hansson GK. Inflammation and immune response in atherosclerosis. Curr Atheroscler Rep. 1999;1:150–5. [DOI] [PubMed] [Google Scholar]
- 5. Steinvoll S, Helgeland K, Schenck K. Mast cells—a role in periodontal diseases? J Clin Periodontol. 2004;31(6):413–9. [DOI] [PubMed] [Google Scholar]
- 6. Steinsvoll S, Halstensen TS, Schenck K. Extensive expression of TGF-b1 in chronically-inflamed periodontal tissue. J Clin Periodontol. 1999;26(6):366–73. [DOI] [PubMed] [Google Scholar]
- 7. Irani AA, Schechter NM, Craig SS, Deblois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986;83(12):4464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soter N, Austen KF. The diversity of mast cell-derived mediators: implications for acute, subacute, and chronic cutaneous inflammatory disorders. J Invest Dermatol. 1976;67(3):313–9. [DOI] [PubMed] [Google Scholar]
- 9. Stevens RL, Austen KF. Recent advances in the cellular and molecular biology of mast cells. Immunol Today. 1989;10(11):381–6. [DOI] [PubMed] [Google Scholar]
- 10. Befus AD, Dyck N, Goodacre R, Bienenstock J. Mast cells from the human intestinal lamina propria. Isolation, histochemical subtypes, and functional characterization. J Immunol. 1987;138(8):2604–10. [PubMed] [Google Scholar]
- 11. Befus AD, Mowat C, Gilchrist M, Hu J, Solomon S, Bateman A. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol. 1999;163(2):947–53. [PubMed] [Google Scholar]
- 12. Naesse EP, Schereurs O, Helgeland K, Schenck K, Steinvoll S. Matrix metalloproteinases and their inhibitors in gingival mast cells in persons with and without human immunodeficiency virus infection. J Periodontal Res. 2003;38(6):575–82. [DOI] [PubMed] [Google Scholar]
- 13. Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule-1. Proc Natl Acad Sci U S A. 1991;88(10):4220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328(4):257–65. [DOI] [PubMed] [Google Scholar]
- 15. Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–42. [DOI] [PubMed] [Google Scholar]
- 16. Shapiro S, Ulmansky M, Scheuer M. Mast cell population in gingiva affected by chronic destructive periodontal disease. J Periodontol. 1969;40(5):276–8. [DOI] [PubMed] [Google Scholar]
- 17. Kennett CN, Cox SW, Eley BM, Osman IA. Comparative histochemical and biochemical studies of mast cell tryptase in human gingiva. J Periodontol. 1993;64(9):870–7. [DOI] [PubMed] [Google Scholar]
- 18. Batista A, Rodini CO, Lara VS. Quantification of mast cells in different stages of human periodontal disease. Oral Dis. 2005;11(4):249–54. [DOI] [PubMed] [Google Scholar]
- 19. Huang S, Lu F, Chen Y, Huang B, Liu M. Mast cell degranulation in human periodontitis. J Periodontol. 2013;84(2):248–55. [DOI] [PubMed] [Google Scholar]
- 20. Shelton LE, Hall WB. Human gingival mast cells. Effects of chronic inflammation. J Periodontol Res. 1968;3(3):214–2. [DOI] [PubMed] [Google Scholar]
- 21. Robinson LP, De Marco TJ. Alteration of mast cell densities in experimentally inflamed human gingiva. J Periodontol. 1972;43(10):614–22. [DOI] [PubMed] [Google Scholar]
- 22. Gemmell E, Carter CL, Seymour GJ. Mast cells in human periodontal disease. J Dent Res. 2004;83(5):384–7. [DOI] [PubMed] [Google Scholar]
- 23. Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest. 1997;15;99(6):1313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atkins FM, Friedman MM, Pillarisetti V, Subba Rao PV, Metcalfe D. Interactions between mast cells, fibroblasts and connective tissue components. Int Arch Allergy Appl Immunol. 1985;77(1–2):96–102. [DOI] [PubMed] [Google Scholar]
- 25. Atkins FM. Mast cells and fibrosis. Arch Dermatol. 1987;123(2):191–3. [PubMed] [Google Scholar]
- 26. De Castro IC, Rocha CA, Gomes Henriques AC, Cavalcanti de, Sousa AP, Lisboa MV, Sotero Dda R, Pinheiro AL, Cury PR, Santos JN. Do laser and LED phototherapies influence mast cells and myofibroblasts to produce collagen? Lasers Med Sci. 2014;29(4):1405–10. [DOI] [PubMed] [Google Scholar]
- 27. Baelum V, López R. Defining a periodontitis case: analysis of a never-treated adult population. J Clin Periodontol. 2012;39(1):10–9. [DOI] [PubMed] [Google Scholar]
- 28. Huang S, Lu F, Li J, Lan T, Huang B, Yin X, Jin H. Quantification of tryptase-TIM-3 double-positive mast cells in human chronic periodontitis. Arch Oral Biol. 2014;59(6):654–61. [DOI] [PubMed] [Google Scholar]
- 29. Golijanin R, Kujundzic B, Milisavljevic Z, Milovanovic DR, Andjelkovic Z, Obrenovic M, Nikolic R. Morphometric analysis of collagen and inflammatory cells in periodontal disease. Vojnosanit Pregl. 2015;72(3):219–24. [DOI] [PubMed] [Google Scholar]
- 30. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387–99. [DOI] [PubMed] [Google Scholar]
- 31. Angelopoulos AP. Studies of mast cells in the human gingiva. J Periodontal Res. 1973;8(5):28–36. [DOI] [PubMed] [Google Scholar]
- 32. Barnett MJ. The fine structure of human connective tissue mast cells in periodontal disease. J Periodontal Res. 1973;9(2):84–91. [DOI] [PubMed] [Google Scholar]
- 33. Walsh LJ, Davis MF, Xu LJ, Savage NW. Relationship between mast cell degranulation and inflammation in the oral cavity. J Oral Pathol Med. 1995;24(6):266–72. [DOI] [PubMed] [Google Scholar]
- 34. Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med. 2003;14(3):188–98. [DOI] [PubMed] [Google Scholar]
- 35. Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. [DOI] [PubMed] [Google Scholar]
- 36. Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J. 1991;276(2):307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]