Abstract
La protein is an abundant 47-kDa phosphoprotein found mostly in the nucleus of eukaryotic cells with a small fraction present in the cytoplasm. Nascent RNA transcripts synthesized by RNA polymerase III are known to be associated with La protein. This binding has been shown to occur to the 3′ end of RNA via RNA recognition motifs and to the 5′ triphosphate via the Walker A motif of the La protein. In this study, we developed an in vitro immunoprecipitation assay to quantitate the 5′ ppp-dependent binding of small RNAs to the human La protein. Using this assay, we found that oligonucleotides five bases or longer bind to the human La protein in a 5′ ppp-dependent manner; pppG did not bind to La protein in this assay. In addition, CH3pppN cap structure present on the 5′ ends of U6 and B2 small RNAs reduced the ability of these RNAs to bind the human La protein. These data show that Walker motif in the human La protein can bind to short RNAs containing 5′ ppp and removal of 5′ ppp from RNAs, or modification of 5′ pppN to CH3pppN or m7GpppN, significantly reduces the ability of small RNAs to bind the human La protein. These data suggest that one of the functions of methylphosphate cap structure in U6 snRNA and B2 RNAs is possibly to reduce the affinity of these RNAs to La protein.
Keywords: La protein, Methylphosphate cap structure, Walker motif
RNA polymerase III (pol III) is responsible for the synthesis of many different RNA species in eukaryotic cells. These RNAs include all tRNAs, 5S rRNA, U6, 7SL (SRP), MRP, RNaseP, 7SK, Ro, Alu, and many other small RNAs [reviewed in (31,33,47)]. Immediately after synthesis, these nascent RNA molecules transcribed by pol III are found to be associated with La protein, which was shown to bind and stabilize these transcripts (12,24,29,35,36). Association of the La protein with RNA occurs through the 3′-oligouridylate stretch common to most pol III transcripts (32,36,42). La protein was initially discovered as a human autoantigen (27) and its homologues have been identified in many species from yeast to mammals (3,20,21,38,44,48). In humans, the La protein is an abundant 47-kDa phosphoprotein that is present mostly in the nucleus (7,15), although some La protein can be found in the cytoplasm (28).
In addition to pol III RNA transcripts, human La (hLa) has also been shown to associate with several viral RNAs including leader transcripts of different viral RNAs (1,18,20,30) and some pol II transcripts like U1 snRNA (17,23). The binding of La protein to the viral mRNAs occurs at the 5′ untranslated region (UTR). This La protein–viral 5′ UTR interaction is required for internal ribosome entry site (IRES)-mediated translation (2,6,43). In addition to binding small RNAs, studies from different groups have indicated that hLa protein plays an active role in transcription termination, transcript release, and reinitiation of transcription from pol III promoters. Results suggest that La protein may be associated with the pol III machinery and bind to the 3′-UUUU-OH of the nascent RNA immediately after transcription (9,11,12,24,25). However, other studies suggest that efficiency of transcription initiation and termination do not depend on La protein (22,46).
Studies on the role of La protein in the maturation process of tRNAs has shown that the carboxy-terminal domain (CTD) of La protein plays an important role in protecting the 5′ termini of pre-tRNAs and may play an important role in posttranscriptional modification of nascent tRNAs (10,16). Previous studies from our laboratory demonstrated that translation of mRNA molecules having γ-monomethyl-phosphate cap structure at the 5′ end was severely inhibited (5). The mRNAs tested contained IRES sequences for cap-independent translation initiation. As La protein binding to the 5′ UTR in viral RNAs had been shown to play a significant role in IRES-mediated translation (2,6), it was suggested that changes in the 5′ end of the mRNA were responsible for reduction in the La protein binding, resulting in reduced levels of translation (5). These data, as well as the data showing the involvement of the Walker A motif of La protein in interaction to the 5′ end of pre-tRNA (16), led us to investigate the role of the 5′ terminal triphosphate in binding with La protein. In this study, we report that La protein specifically interacts with the 5′ triphosphate moiety of small RNAs including U6, 5S, and B2 RNAs. Modifications in the 5′ ppp structure caused a significant decrease in La protein binding to these small RNAs. We also demonstrate that a minimal length of RNA is required for this ppp-dependent specific interaction of RNAs with the La protein.
MATERIALS AND METHODS
Chemicals and Isotopes
Fine chemicals and unlabeled nucleotides were obtained from Sigma. [α-32P]GTP and [γ-32P]GTP were obtained from ICN. Unlabeled 5′ cap analog m7G(5′)-ppp(5′)G was obtained from Ambion. α-32P-labeled and unlabeled γ-monomethyl GTP was prepared as described previously (13). Restriction enzymes were obtained from Invitrogen and New England Biolabs. T7 RNA polymerase and RNase-free DNase were obtained from New England Biolabs. All recombinant plasmids were propagated in E. coli DH5α and plasmid DNAs were prepared according to standard protocols (37).
In Vitro Transcription
Different RNA transcripts were synthesized in vitro using pUC19 plasmid DNA containing the genes for either mouse B2 or human U6 RNAs under the T7 promoter (39). Plasmid DNA carrying mouse B2 DNA was linearized by different restriction enzymes to obtain templates for synthesizing either full-length or shorter RNA transcripts. The enzymes used and the sizes of transcripts obtained were: DraI, 180 nt; BglII, 104 nt; AvaII, 50 nt; BsiHKAI, 30 nt; BlpI, 15 nt. Full-length human U6 RNA (106 nt) was transcribed using plasmid DNA containing the U6 gene, which was linearized with DraI restriction enzyme.
Linearized DNA (1 μg) was incubated with 0.5 mM each of ATP, CTP, UTP, 0.05 mM GTP, 50 units of T7 RNA polymerase, 2 μl 10 × polymerase buffer (New England Biolabs), 20 units RNase inhibitor (Invitrogen), and 50 μCi [α-32P]GTP in a total volume of 20 μl for 1 h at 37°C. The reaction mixture was then treated with 2 units of RNase-free DNase (New England Biolabs) for 30 min at 37°C. Labeled RNAs were fractionated on 10% (for longer RNAs) or 20% (for 5–15-nucleotide-long transcripts) denaturing polyacrylamide gel, subjected to autoradiography, and the portions of the gel corresponding to the RNAs of interest were excised. The RNAs were eluted by incubating the excised gel pieces in extraction buffer containing 0.03% SDS, 100 mM NaCl, and 50 mM sodium acetate (pH 5.2) at 37°C overnight. After extraction with phenol/chloroform/iso-amyl alcohol (25:24:1) and chloroform, the RNAs were precipitated with ethanol using 10 μg yeast tRNA as carrier.
Transcripts having 5′ 32P label were synthesized in the presence of [γ-32P]GTP using similar reaction conditions. Incorporated radioactivity in all RNAs was quantitated by liquid scintillation counting.
Synthesis of RNAs With Modified 5′ Ends
RNAs capped at the 5′ end were synthesized using a 100-fold molar excess of the modified GTP analogs m7G(5′)ppp(5′)G or γ-monomethyl GTP compared with GTP in the in vitro reaction mixtures described above. RNA substrates without triphosphate at the 5′ end were synthesized as follows: RNAs were transcribed in the absence of modified GTP analogs as described above and fractionated on denaturing poly-acryamide gels. After purification, the labeled RNAs were treated with 1 unit of calf intestinal alkaline phosphatase (Invitrogen) for 1 h at 37°C. RNA molecules without the 5′ triphosphate were purified by phenol/chloroform extraction, followed by ethanol precipitation.
Preparation of Cell Extracts
S100 extracts were prepared from HeLa cells grown in suspension culture by the procedure of Dignam et al. (8). The final protein concentration of the extract was 2.5 mg/ml. Yeast whole cell extract was prepared as described by Chen and Moore (4) and the protein concentration of the extract was 10 mg/ml.
Recombinant La Proteins and Antibodies
C-terminal histidine-tagged, full-length or mutated La proteins (recombinant) expressed from cDNA clones in E. coli and anti-La antibodies were as described in Goodier et al. (11). The proteins were used at a concentration of 50 μg/ml.
Gu Protein and Antibodies
The purified recombinant RNA helicase II/Gu protein and anti-Gu antibodies (45) were kind gifts from Ben Valdez (Baylor College of Medicine) and used as controls in some experiments.
RNA Binding and Immunoprecipitation
Immunoprecipitation reactions were done essentially as described in Lerner and Steitz (19). Labeled RNA was incubated with 50 ng of recombinant La protein and 20 μl yeast extract in binding buffer (10 mM Tris-HCl, pH 7.5, 80 mM KCl, 5 mM MgCl2, 0.1% Triton X-100, 1 mM DTT, 1 mM EDTA, 100 ng poly rGTP, and 5% glycerol) in a total volume of 50 μl. The mixture was incubated first on ice for 5 min and then at 30°C for 15 min. Equal volume of cold 2× immunoprecipitation (IPP) buffer (200 mM NaCl, 2 mM MgCl2, and 20 mM Tris-HCl, pH 7.5) and 4 μl of anti-La antibody was added to the reaction and incubated on ice for 1 h. Pansorbin (Calbio-chem) (75 μl) was added to the mixture and further incubated on ice for 30 min. The mixture was centrifuged at 3500 × g for 5 min at 4°C and the supernatant removed and stored. The pellet was washed thoroughly with cold NET2 buffer (150 mM NaCl, 2 mM EDTA, 50 mM Tris-HCl, pH 7.4, and 0.05% Nonidet P-40) three times to remove unbound materials and was used for scintillation counting. The stored supernatant was counted to estimate the unbound RNA. For competition immunoprecipitation assays, multiple RNA substrates were mixed together before addition of La protein or HeLa cell S100 fraction. In immunoprecipitation reactions using the HeLa cell S100 fraction, 30 μl of the extract was used instead of recombinant La protein and yeast extract was not added to the reaction. All other steps were the same as before.
To analyze the immunoprecipitated RNA on a gel, the pellet containing the labeled RNA was treated successively with phenol and phenol/chloroform. RNA was precipitated with ethanol using 10 μg of yeast tRNA as carrier. The precipitated RNAs were electrophoresed on denaturing 10% polyacrylamide gel followed by autoradiography. Starting material (5%) of each reaction was loaded as control and used for comparative analyses. Intensity of the bands was quantitated by ImageQuant.
RESULTS
The 5′ Triphosphate Is an Important Factor in Binding of La Protein to Several Small RNAs
To ascertain the importance of the 5′ triphosphate in different poll III transcripts in binding to La protein, an immunoprecipitation assay was developed. Recombinant human La protein was incubated with in vitro-transcribed α-32P-labeled U6 or B2 RNAs (two representative pol III RNA transcripts), which either had the 5′ triphosphate or had been treated with alkaline phosphatase to remove 5′ ppp. Anti-La antibodies were used to immunoprecipitate the La protein-labeled RNA complexes in the reaction mixtures. La protein bound significantly better to both U6 and B2 RNAs containing triphosphate on their 5′ ends (Fig. 1A, lanes 1 and 3) compared with the RNAs lacking the triphosphate moieties (Fig. 1A, lanes 2 and 4). Quantitation of the immunoprecipitated RNAs and comparison with the starting material showed that La protein has a 3.5- to 5-fold higher affinity for RNA molecules having the 5′ triphosphate compared to the same RNA without the 5′ ppp (Fig. 1B).
Figure 1.
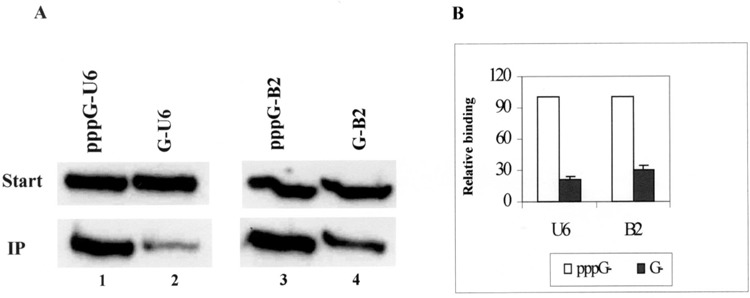
Immunoprecipitation assay for 5′ triphosphate-mediated La protein–RNA binding. (A) Recombinant human La protein was incubated with either α-32P-labeled 5′ ppp-containing (lanes 1 and 3) or phosphatase-treated (lanes 2 and 4) RNAs in the presence of RNA binding buffer. The La protein–RNA complexes were immunoprecipitated using anti-La antibodies and the bound RNAs were isolated and purified. These RNAs from the immunoprecipitates and 5% of the starting material for each reaction were electrophoresed on 10% polyacrylamide/7 M urea gels and subjected to autoradiography. The labeling of the RNAs and conditions of immunoprecipitation are described in detail in Materials and Methods. Start: starting material. IP: immunoprecipitated RNAs. (B) The intensity of the bands obtained in the autoradiograms were quantitated using ImageQuant and the percentage of RNAs bound to the La protein was calculated. Taking the percentage of binding by the RNAs having intact 5′ triphosphate as 100%, the relative binding of the substrate pppG and G RNAs were plotted. The open columns correspond to RNAs having pppG at the 5′ end and the filled columns to those with 5′ G. The results are from an average of three independent experiments.
Similar results were obtained when labeled ribosomal 5S RNA substrates, with or without the 5′ ppp, were used for immunoprecipitation (data not shown). To measure the extent of RNAs bound to the La protein, the radioactivity in both the immunoprecipitates and the supernatants was measured. Several independent experiments showed that the extent of La protein binding to RNAs varied between 4% and 9% of the total input RNA in the case of intact RNA molecules containing 5′ ppp and between 1% and 2% for RNAs without the 5′ ppp moiety (data not shown). Similar results were obtained when HeLa cell extract was used as source of the La protein instead of the recombinant La protein. Though the binding efficiency of La protein with the RNA substrates was only about 6% on an average, there was a significant reduction in binding in the absence of the 5′ triphosphate in each case. These results suggest that the 5′ triphosphate plays an important role and is one of the significant factors determining the extent of the La protein-RNA interaction. These data are consistent with and are similar to results reported by Maraia’s lab using tRNAs either with or without the 5′ triphosphate (10).
Modifications in the 5′ Triphosphate Reduces Binding of RNAs to the La Protein
The experiments described above show that removal of the 5′ triphosphate leads to a reduction in the binding of the RNA with La protein. However, in vivo, the 5′ ends of the pol III RNA transcripts are not only processed where the 5′ ppp is no longer present but are also modified to yield 5′ γ-monomethylated or trimethylguanosine-capped structures (34,41). Because our earlier studies showed a reduction in the La protein binding due to the presence of 5′ cap structures (5), the effect of the 5′ cap structures in U6 and B2 RNAs on La protein-RNA interaction was examined.
The α-32P-labeled B2 and U6 RNAs were synthesized in the presence of GTP analogs as described in Materials and Methods to obtain 5′ m7GpppG or methyl-pppG-capped RNAs. Immunoprecipitation assays were performed using the capped, alkaline phosphatase-treated and control unmodified B2 and U6 RNAs. Modification of the 5′ ppp by introduction of a CH3 group or m G on the gamma phosphate of the RNAs reduced the efficiency of the binding of La protein with U6 and B2 RNAs (Fig. 2A). The RNA substrates having intact 5′ ppp (pppG-U6 and pppG-B2) were more efficiently immunoprecipitated with La protein (Fig. 2A, lanes 1 and 5) compared with corresponding RNAs that contained modified 5′ ends such as G, CH3pppG or m7GpppG (Fig. 2A, lanes 2–4 and 6–8). Quantitation of the radioactivity in the immunoprecipitates and comparison with the respective starting materials showed that modifications in the 5′ triphosphate resulted in an approximately fivefold decrease in binding of the RNAs with La protein (Fig. 2B).
Figure 2.
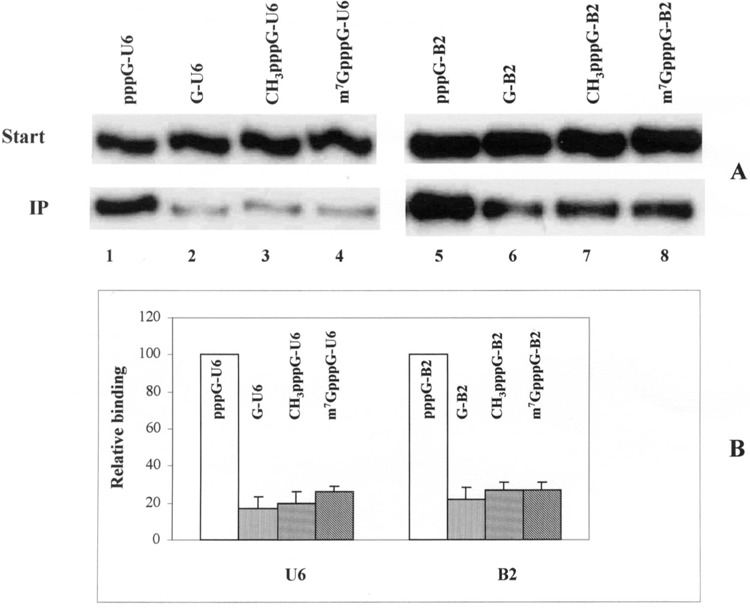
Effect of 5′ end modification in RNA on binding to the La protein. (A) Recombinant human La protein was incubated with different U6 and B2 RNA substrates labeled by [α-32P]GTP having either pppG, G, CH3pppG, or m7GpppG at their 5′ ends. The immunoprecipitated RNAs were extracted, purified, and fractionated on 10% polyacrylamide/7 M urea gels and subjected to autoradiography. For each of the RNAs, 5% of the starting material was loaded as control. (B) The percentage of La protein-bound RNAs was calculated by measuring the intensities of the bands using the ImageQuant software and comparing with their respective starting materials. Relative binding efficiencies of different RNAs were plotted taking the binding efficiency of the unmodified RNA (5′ pppG) as 100%.
Previous studies have shown that La protein–RNA (pol III transcript) binding was influenced by the 3′ terminal uridylic acid residues (42). However, in the experiments described above, the reduction in binding of the La protein to the RNAs was virtually due to the absence or modification of the 5′ triphosphate. The full-length RNA transcripts of both U6 and B2 from DraI-linearized plasmid DNA templates had three uridylic acid residues at their 3′ ends (40). These residues were present in both U6 and B2 RNAs, either with 5′ pppG, 5′ G, or 5′ cap-G, and hence the difference in binding with La protein can only be due to the removal or modification of the 5′ triphosphate. These results also explain observations made by Stefano (42), where the complexes of La protein with substrates giving optimal binding (i.e., 5′-p-tRNA-UUUU-OH) were less stable than those with natural tRNA precursors. Reduced affinity of phosphatase-treated tRNAs with the La protein has also been described in Fan et al. (10). This lower affinity can be attributed to the absence of 5′ ppp, which plays an important role in La protein–RNA interaction. The results described above show that not only removal of the 5′ ppp, but other modifications at the 5′ end of RNAs also lead to lower affinity between the RNA and La protein.
Mononucleotide (pppG) Is Not Sufficient to Bind to the La Protein
Pol III RNA transcripts invariably initiate with a purine and, in a majority of cases, the initiation nucleotide is a G residue. As available data suggest a possible interaction between the WAM/PBS motif of La protein and the 5′ triphosphate of the RNA, we wanted to test whether pppG itself would have higher binding affinity to La protein compared with modified pppG analog. Labeled pppG and CH3pppG were used to test this possibility using the immunoprecipitation assay. In several attempts we did not find any detectable binding of labeled pppG or CH3pppG to La protein (data not shown) using either recombinant La protein or S100 extracts. These results suggest that in addition to the triphosphate, a minimum length of RNA appears to be required for La protein–RNA interaction mediated through the 5′ ppp. Because this 5′ ppp-dependent RNA binding was observed in RNAs having different sequences, the size of the RNA molecules rather than a specific sequence appears to be the determining factor in the interaction with the La protein. Therefore, experiments were done to test the possibility that a minimum size of RNA is required for ppp-dependent binding of RNA to La protein.
Determination of Minimum RNA Length Required for La Protein Binding
To determine the minimum length of the RNA required for binding to La protein, B2 and U6 RNAs were transcribed in vitro in the absence of ATP to produce small abortive transcripts of different sizes (oligonucleotides of 1–15 in length). These small RNA fragments were purified from a 20% denaturing polyacrylamide gel and used in immunoprecipitation assays with either recombinant La protein or with HeLa cell extracts. Oligonucleotides having a length of 5 or more bound to La protein in the case of B2 (Fig. 3, lanes 2 and 3) and U6 (Fig. 3, lanes 4 and 5), while there was no detectable binding with shorter oligonucleotides or with pppG (Fig. 3). Most efficient binding was observed for transcripts of 12 nucleotides in this assay (Fig. 3, lanes 2–5). The binding was more efficient as the length of the RNA increased. For example, compared with the starting material (see lane 6), there was very little radioactivity corresponding to 1, 4, 5, or 6 nucleotides, whereas there was significant radioactivity corresponding to 7- to 12-nucleotide-long RNAs. These data strongly indicate that though RNA molecules can bind La protein in a triphosphate-dependent manner in vitro, a minimum size of about 5 nucleotides or longer is required for this type of interaction. These additional nucleotides following pppG perhaps are required to stabilize the interaction between the triphosphate and the WAM/PBS motif of the La protein.
Figure 3.
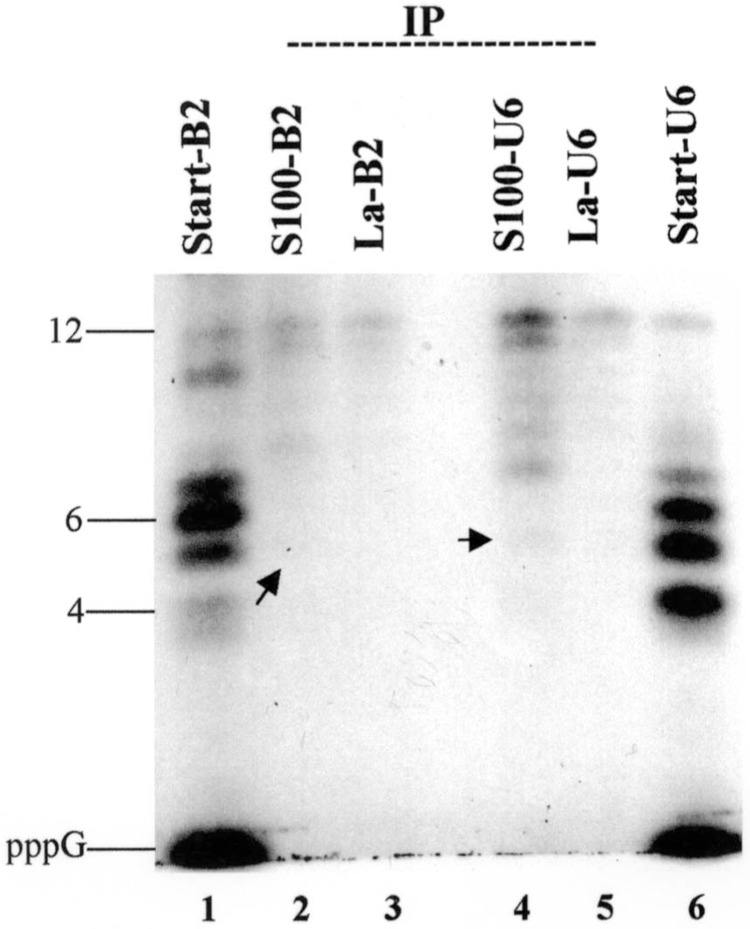
Determination of minimal size requirement for RNA–La protein interaction. U6 and B2 RNAs were transcribed from plasmid DNA templates in the absence of ATP to generate abortive transcripts. Immunoprecipitation of these short oligonucleotides containing 5′ ppp was performed using Hela cell extracts or recombinant La protein, as described in Materials and Methods. Bound RNAs for B2 (lanes 2–3) and U6 (lanes 4–5) were fractionated along with the starting material and visualized by autoradiography.
La Protein Has Higher Affinity to Partial RNAs Compared With the Full-Length RNAs
Plasmid DNA containing B2 DNA was digested by different restriction enzymes to yield templates for full-length B2 RNA as well as truncated B2 RNA transcripts. RNAs of different lengths were synthesized in vitro in the presence of [γ-32P]GTP to obtain labeled RNAs containing 5′ ppp. Equal amounts of radioactivity corresponding to each transcript were used in immunoprecipitation assays and the bound and unbound RNAs were quantitated by scintillation counting. Surprisingly, determination of the percentage of binding showed that the smaller transcripts (15, 30, and 50 nucleotides long) had better binding than the longer (104 nucleotides) and full-length (180 nucleotides) B2 RNA transcripts (Fig. 4A). In several independent experiments using B2 RNAs (full-length or smaller), the 50-nucleotide-long transcript showed the highest affinity for La protein binding, with about 25% of the total RNA forming complex with La protein under conditions of assay used in this study. The shorter transcripts also showed higher affinity compared with the full-length B2 RNA, with an average of 9% and 16% binding for 15- and 30-nucleotide-long RNAs, respectively (Fig. 4A). In contrast, approximately 5% of the 104- and 180-nucleotide-long B2 RNAs could be immunoprecipitated in association with La protein (Fig. 4A).
Figure 4.
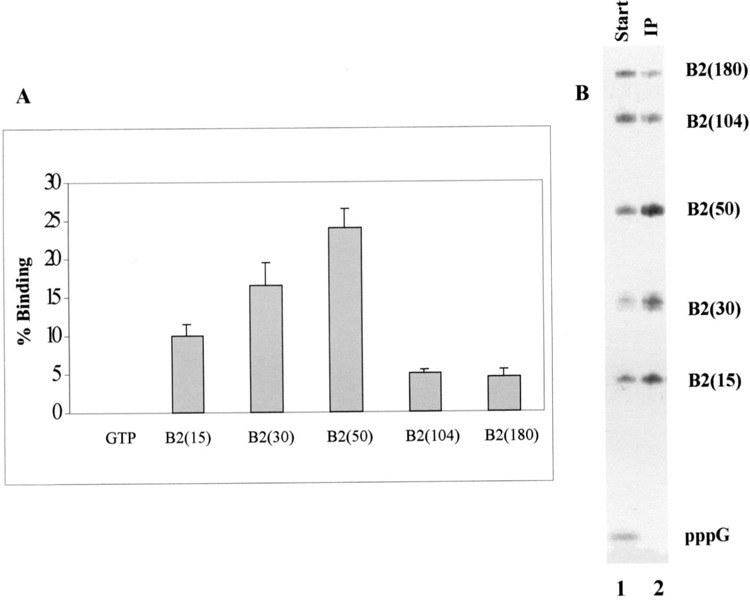
Binding of La protein to B2 RNA transcripts of different sizes. Plasmid DNA containing B2 DNA was digested with different enzymes (see text) and transcribed in vitro to produce run-off transcripts of different sizes. (A) These RNAs and GTP were used in immunoprecipitation reactions with the La protein. The bound RNAs were measured by scintillation counting and percentage of the total RNA precipitated was plotted for each transcript of different size. The plots are an average of three independent experiments. (B) Equal counts of the different transcripts and pppG were mixed together. This mixture was immunoprecipitated using the La protein and bound RNAs were purified. These purified RNAs were fractionated on a 10% denaturing polyacrylamide gel (lane 2) along with 5% of the starting RNA mixture (lane 1) and subjected to autoradiography.
To get a better understanding of the in vivo situation where different sized RNAs can compete for binding to the La protein, competition immunoprecipitation experiments were performed. Approximately equal number of 5′ γ-32P-labeled RNA molecules (estimated by scintillation counts) of various sizes were mixed together and used in La protein binding experiments. Comparison of the immunoprecipitated RNAs with the starting material showed that the La protein has highest affinity for the 50-nucleotide-long B2 RNA (Fig. 4B, lane 2). Other B2 RNA fragments bound La protein with lesser affinity, while there was no binding with the mononucleotide pppG, which was consistent with previous results (see Fig. 3). The percentage of binding obtained by densitometric scanning of the signals (data not shown) was also very similar to immunoprecipitation results shown in Figure 4A.
The 5′ ppp Interacts With the WAM of the La Protein
Accumulating evidence suggested that the Walker A motif in La protein recognizes the 5′ pppG of nascent pol III transcripts (10,16). This WAM has been shown to bind to NTPs in other proteins [for references see (44)]. Though La protein could not be shown to bind to pppG alone, all the experiments described above indicate that the 5′ triphosphate-mediated RNA–La protein interactions are presumably due to recognition and binding to the ppp moiety by the WAM of the La protein. Wild-type and a mutant La protein (hLa GXK>DE), where amino acids 328 to 344 were replaced with alternating aspartate and glutamate residues leading to a disruption of the consensus (GXXXXGKX) NTP binding site (11), were used in immunoprecipitation assays with labeled B2 and U6 RNA as binding substrates. Compared with the wild-type La protein (Fig. 5A, lane 2), mutant La protein was very inefficient in binding to B2 RNA (Fig. 5A, lane 1). Quantitative analyses from multiple experiments showed approximately a 10-fold difference in RNA binding (Fig. 5B) by these two proteins. These data show that with all other experimental conditions being similar, the recognition of the 5′ tri-phosphate by the Walker A domain of the La protein is critical for RNA–protein interaction.
Figure 5.
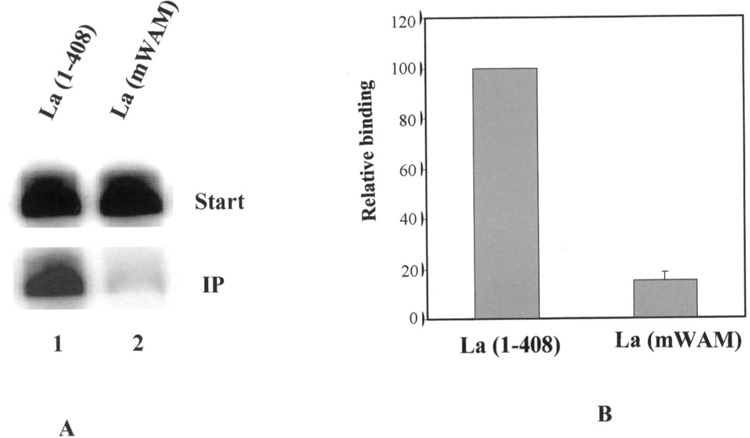
Effect of Walker A motif mutation in RNA–La protein interaction. (A) Recombinant wild-type La protein (hLa 1–408) (lane 1) and a mutant of La protein (hLa GXK>DE) (11) (lane 2), which has mutations in the Walker A motif, were used in immunoprecipitation reactions using 32P-labeled B2 RNA as substrate. La protein-bound RNAs were purified and fractionated on 10% denaturing polyacrylamide gel. Starting material (10%) was also loaded to determine the extent of RNA binding by the wild-type and mutant La proteins. (B) Immunoprecipitation reactions similar to above were performed. The RNA in the pellets and supernatants were measured by liquid scintillation. The RNA binding efficiency of the proteins was plotted, taking the binding of full-length protein to be 100%. The plots are an average of three independent experiments.
The 5′ Triphosphate-Dependent RNA Recognition by WAM Is Specific for the La Protein
Many RNA binding proteins have been predicted to have Walker A motif-containing regions. Therefore, it is possible that the interaction studied so far is not specific to La protein and any protein with WAM may bind pol III RNA transcripts in a ppp-dependent manner. If this is true, then there would be little biological significance in the triphosphate-mediated RNA–La protein binding. Immunoprecipitation reactions were done using intact or phospha-tase-treated internally labeled B2 RNA with either La or Gu proteins, both of which are RNA binding proteins and predicted to have Walker A motif (44,45). There was only 1–2% binding of the input RNA to the Gu protein and this binding of Gu protein to B2 RNA was found to be independent of the presence or absence of the 5′ triphosphate. RNAs with both ppp-G or G at the 5′ end were immunoprecipitated equally (Fig. 6B). However, La protein showed preferential binding to RNA having 5′ ppp (Fig. 6A) as observed in earlier experiments. These results show that 5′ ppp-mediated binding is specific for La protein, and proteins like Gu, with potential Walker A motifs, do not bind small RNAs in a 5′ ppp-dependent manner. The data suggest that Walker A motif alone is probably not sufficient to bind RNAs in a ppp-dependent manner and the context in which Walker A motif is present in La protein is important for this binding.
Figure 6.
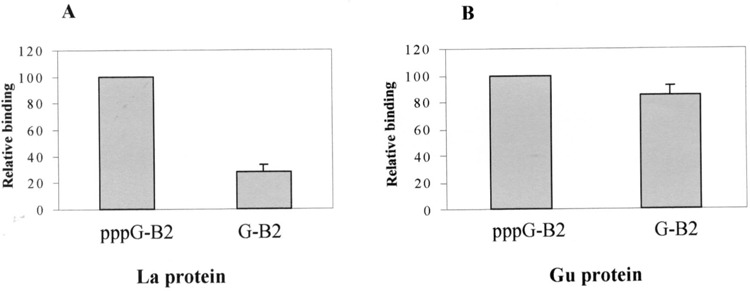
Binding of B2 RNA with La and RNA helicase Gu protein. Recombinant La and Gu proteins, containing Walker motifs, were used in binding assays with labeled B2 RNA having either 5′ pppG or 5′ G. The RNA–La or RNA–Gu protein complexes were immunoprecipitated with either anti-La or anti-Gu antibodies, respectively. Free and bound RNAs were quantitated in a scintillation counter and binding efficiency calculated. Comparative binding of La and Gu proteins with the two substrate RNAs are plotted in (A) and (B), respectively. The plots are an average of three independent experiments.
DISCUSSION
The main observation made in this study is that the γ-monomethyl phosphate cap structure present on the 5′ end of U6 and B2 small RNAs reduces the affinity of these RNAs to La protein. Studies from Maraia’s lab have shown that the triphosphate moiety of tRNA binds to the Walker motif of the La protein and phosphorylation of the serine 366 of human La protein reduces the affinity of La protein to the 5′ ppp of the tRNA (10). The results obtained in this study indicate that there are alternate ways to reduce the affinity of RNA to La protein. These include removal of one or more phosphate residues from the 5′ end of the RNA or methylation of the γ-phosphate.
In the case of some RNAs such as U6, 7SK, and B2, the 5′ end contains a γ-monomethyl phosphate cap structure. While trying to understand the function of γ-monomethyl phosphate cap structure, which is present on the 5′ end of these small RNAs, we showed that this cap structure increases the stability of these RNAs in the Xenopus oocyte system (39). In addition to increasing stability of these RNAs, it is possible that methylation of the γ-phosphate in B2 and U6 RNAs results in reduction of affinity to La protein. This may help in the release of these RNA transcripts from La protein.
The structure of La protein shows that the carboxy-terminus domain (CTD) contains a potential Walker A motif (WAM). Analysis of La protein also indicated the presence of a potential phosphate binding site (PBS) downstream of the WAM (26). These motifs are possibly involved in the recognition and binding to 5′ ppp structure of interacting tRNAs, because mutant La proteins having deletions in these regions are unable to protect precursor tRNAs against 5′ processing (16). Our studies also indicate that the Walker A motif interacts with the 5′ triphosphate present in a variety of other RNAs, including U6 and B2 RNAs. These RNA molecules have no sequence homology to each other. So, it may be assumed that the 5′ end and not any particular sequence or structural aspect determines the initial interaction between La protein and the nascent RNA, and this interaction probably occurs before the 3′ uridylic residues are synthesized.
The experimental evidence presented in this study shows that La protein has a higher affinity for shorter RNA molecules rather than full-length RNAs. This is very interesting considering the fact that the shorter versions of RNA do not have 3′ uridylic residues. However, we do not have an explanation how this occurs. Previous studies with the vaccinia virus transcription showed that the 5′ end of the RNA was capped when nascent chains were 31 nt or longer (14). Though there are no reports describing the minimum length of RNA pol III transcripts when 5′ capping occurs, it may be assumed that 5′ capping occurs when the nascent RNA is 50 nt or shorter. As shown in this study, the transcripts that bind with highest affinity to La protein are also of similar length. It is possible that La protein initially binds the nascent transcript when it is 5 nt or longer and protects the 5′ end from degradation, but as soon as the RNA is capped at the 5′ end the La protein–RNA interaction gets weaker. This may facilitate the release of the La protein from the RNA transcripts, which may be utilized in further rounds of transcription.
ACKNOWLEDGMENT
We acknowledge Dr. Ben Valdez for the recombinant Gu protein, anti-Gu antibodies, and also for critically reviewing the manuscript.
REFERENCES
- 1. Ali N.; Siddiqui A. The La antigen binds 5′ noncoding region of the hepatitis C virus RNA in the context of the initiator AUG codon and stimulates internal ribosome entry site-mediated translation. Proc. Natl. Acad. Sci. USA 94:2249–2254; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali N.; Pruijn G. J.; Kenan D. J.; Keene J. D.; Siddiqui A. Human La antigen is required for the hepatitis C virus internal ribosome entry site-mediated translation. J. Biol. Chem. 275:27531–27540; 2000. [DOI] [PubMed] [Google Scholar]
- 3. Bai C.; Li Z.; Tolias P. P. Developmental characterization of a Drosophila RNA-binding protein homologous to the human systemic lupus erythematosus-associated La/SS-B autoantigen. Mol. Cell. Biol. 14:5123–5129; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J.; Moore C. Separation of factors required for cleavage and polyadenylation of yeast pre-mRNA. Mol. Cell. Biol. 12:3470–3481; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Y.; Perumal K.; Reddy R. Inhibition of translation of mRNAs containing gamma-monomethylphosphate cap structure in frog oocytes and in mammalian cells. Gene Expr. 9:133–143; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Das S.; Kenan D. J.; Bocskai D.; Keene J. D.; Dasgupta A. Sequences within a small yeast RNA required for inhibition of internal initiation of translation: Interaction with La and other cellular proteins influences its inhibitory activity. J. Virol. 70:1624–1632; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deng J. S.; Takasaki Y.; Tan E. M. Nonhistone nuclear antigens reactive with autoantibodies. Immunofluorescence studies on distribution in synchronized cells. J. Cell Biol. 91:654–660; 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dignam J. D.; Lebovitz R. M.; Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475–1489; 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fan H.; Sakulich A. L.; Goodier J. L.; Zhang X.; Qin J.; Maraia R. J. Phosphorylation of the human La antigen on serine 366 can regulate recycling of RNA polymerase III transcription complexes. Cell 88:707–715; 1997. [DOI] [PubMed] [Google Scholar]
- 10. Fan H.; Goodier J. L.; Chamberlain J. R.; Engelke D. R.; Maraia R. J. 5′ processing of tRNA precursors can be modulated by the human La antigen phosphoprotein. Mol. Cell. Biol. 18:3201–3211; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodier J. L.; Fan H.; Maraia R. J. A carboxy-terminal basic region controls RNA polymerase III transcription factor activity of human La protein. Mol. Cell. Biol. 17:5823–5832; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gottlieb E.; Steitz J. A. The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J. 8:841–850; 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta S.; Busch R. K.; Singh R.; Reddy R. Characterization of U6 small nuclear RNA cap-specific antibodies. Identification of gamma-monomethyl-GTP cap structure in 7SK and several other human small RNAs. J. Biol. Chem. 265:19137–19142; 1990. [PubMed] [Google Scholar]
- 14. Hagler J.; Shuman S. A freeze-frame view of eukaryotic transcription during elongation and capping of nascent mRNA. Science 255:983–986; 1992. [DOI] [PubMed] [Google Scholar]
- 15. Hendrick J. P.; Wolin S. L.; Rinke J.; Lerner M. R.; Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: Further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol. Cell. Biol. 1:1138–1149; 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Intine R. V.; Sakulich A. L.; Koduru S. B.; Huang Y.; Pierstorff E.; Goodier J. L.; Phan L.; Maraia R. J. Control of transfer RNA maturation by phosphorylation of the human La antigen on serine 366. Mol. Cell 6:339–348; 2000. [DOI] [PubMed] [Google Scholar]
- 17. Kufel J.; Allmang C.; Chanfreau G.; Petfalski E.; Lafontaine D. L.; Tollervey D. Precursors to the U3 small nucleolar RNA lack small nucleolar RNP proteins but are stabilized by La binding. Mol. Cell. Biol. 20:5415–5424; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurilla M. G.; Keene J. D. The leader RNA of vesicular stomatitis virus is bound by a cellular protein reactive with anti-La lupus antibodies. Cell 34:837–845; 1983. [DOI] [PubMed] [Google Scholar]
- 19. Lerner M. R.; Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 76:5495–5499; 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lerner M, R.; Boyle J. A.; Hardin J. A.; Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science 211:400–402; 1981. [DOI] [PubMed] [Google Scholar]
- 21. Lin-Marq N.; Clarkson S. G. A yeast RNA binding protein that resembles the human autoantigen La. J. Mol. Biol. 245:81–85; 1995. [DOI] [PubMed] [Google Scholar]
- 22. Lin-Marq N.; Clarkson S. G. Efficient synthesis, termination and release of RNA polymerase III transcripts in Xenopus extracts depleted of La protein. EMBO J. 17:2033–2041; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madore S. J.; Wieben E. D.; Pederson T. Eukaryotic small ribonucleoproteins. Anti-La human autoantibodies react with U1 RNA–protein complexes. J. Biol. Chem. 259:1929–1933; 1984. [PubMed] [Google Scholar]
- 24. Maraia R. J.; Kenan D. J.; Keene J. D. Eukaryotic transcription termination factor La mediates transcript release and facilitates reinitiation by RNA polymerase III. Mol. Cell. Biol. 14:2147–2158; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maraia R. J. Transcription termination factor La is also an initiation factor for RNA polymerase III. Proc. Natl. Acad. Sci. USA 93:3383–3387; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maraia R. J.; Intine R. V. Recognition of nascent RNA by the human La antigen: Conserved and divergent features of structure and function. Mol. Cell. Biol. 21:367–379; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattioli M.; Reichlin M. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum. 17:421–429; 1974. [DOI] [PubMed] [Google Scholar]
- 28. Meerovitch K.; Svitkin Y. V.; Lee H. S.; Lejbkowicz F.; Kenan D. J.; Chan E. K.; Agol V. I.; Keene J. D.; Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 67:3798–3807; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pannone B. K.; Xue D.; Wolin S. L. A role for the yeast La protein in U6 snRNP assembly: Evidence that the La protein is a molecular chaperone for RNA polymerase III transcripts. EMBO J. 17:7442–7453; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park Y. W.; Katze M. G. Translational control by influenza virus. Identification of cis-acting sequences and trans-acting factors which may regulate selective viral mRNA translation. J. Biol. Chem. 270:28433–28439; 1995. [DOI] [PubMed] [Google Scholar]
- 31. Paule M. R.; White R. J. Survey and summary: Transcription by RNA polymerases I and III. Nucleic Acids Res. 28:1283–1298; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reddy R.; Henning D.; Tan E.; Busch H. Identification of a La protein binding site in a RNA polymerase III transcript (4.5 I RNA). J. Biol. Chem. 258:8352–8356; 1983. [PubMed] [Google Scholar]
- 33. Reddy R.; Singh R. Synthesis of small nuclear RNAs. In: Jeanteur P.; Kuchino Y.; Muller W. E. G.; Paine P. L., eds. Progress in molecular and subcellular biology. Berlin: Springer Verlag; 1991. [Google Scholar]
- 34. Reddy R.; Singh R.; Shimba S. Methylated cap structures in eukaryotic RNAs: Structure, synthesis and functions. Pharmacol. Ther. 54:249–267; 1992. [DOI] [PubMed] [Google Scholar]
- 35. Rinke J.; Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell 29:149–159; 1982. [DOI] [PubMed] [Google Scholar]
- 36. Rinke J.; Steitz J. A. Association of the lupus antigen La with a subset of U6 snRNA molecules. Nucleic Acids Res. 13:2617–2629; 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sambrook J.; Fritsh E. F.; Maniatis T. Molecular cloning: A laboratory manual, 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38. Scherly D.; Stutz F.; Lin-Marq N.; Clarkson S. G. La proteins from Xenopus laevis. cDNA cloning and developmental expression. J. Mol. Biol. 231:196–204; 1993. [DOI] [PubMed] [Google Scholar]
- 39. Shumyatsky G.; Wright D.; Reddy R. Methylphosphate cap structure increases the stability of 7SK, B2 and U6 small RNAs in Xenopus oocytes. Nucleic Acids Res. 21:4756–476; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shumyatsky G.; Shimba S.; Reddy R. Capping signals correspond to the 5′ end in four eukaryotic small RNAs containing gamma-monomethylphosphate cap structure. Gene Expr. 4:29–41; 1994. [PMC free article] [PubMed] [Google Scholar]
- 41. Singh R.; Reddy R. Gamma-monomethyl phosphate: A cap structure in spliceosomal U6 small nuclear RNA. Proc. Natl. Acad. Sci. USA 86:8280–8283; 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3′ termini of RNA polymerase III transcripts. Cell 36:145–154; 1984. [DOI] [PubMed] [Google Scholar]
- 43. Svitkin Y. V.; Pause A.; Sonenberg N. La autoantigen alleviates translational repression by the 5′ leader sequence of the human immunodeficiency virus type 1 mRNA. J. Virol. 68:7001–7007; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Topfer F.; Gordon T.; McCluskey J. Characterization of the mouse autoantigen La (SS-B). Identification of conserved RNA-binding motifs, a putative ATP binding site and reactivity of recombinant protein with poly(U) and human autoantibodies. J. Immunol. 150:3091–3100; 1993. [PubMed] [Google Scholar]
- 45. Valdez B. C.; Henning D.; Busch R. K.; Woods K.; Flores-Rozas H.; Hurwitz J.; Perlaky L.; Busch H. A nucleolar RNA helicase recognized by autoimmune antibodies from a patient with watermelon stomach disease. Nucleic Acids Res. 24:1220–1224; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weser S.; Bachmann M.; Seifart K. H.; Meibetaner W. Transcription efficiency of human polymerase III genes in vitro does not depend on the RNP-forming autoantigen La. Nucleic Acids Res. 28:3935–3942; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Willis I. M. RNA polymerase III. Genes, factors and transcriptional specificity. Eur. J. Biochem. 212:1–11; 1993. [DOI] [PubMed] [Google Scholar]
- 48. Yoo C. J.; Wolin S. L. La proteins from Drosophila melanogaster and Saccharomyces cerevisiae: A yeast homolog of the La autoantigen is dispensable for growth. Mol. Cell. Biol. 14:5412–5424: 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


