Abstract
Trunk neural crest cells delaminate from the dorsal neural tube and migrate on two distinct pathways: a dorsolateral route, between the ectoderm and somites, and a ventromedial route, through the somitic mesoderm. Neural crest cells that migrate ventromedially travel in a segmental manner through rostral half-somites, avoiding caudal halves. Recent studies demonstrate that various molecular cues guide the migration of neural crest cells, primarily by serving as inhibitors to premature pathway entry or by preventing neural crest from entering inappropriate territories. Trajectories of migrating trunk neural crest are well organized and generally linear in nature, suggesting that positive, migration-promoting factors may be responsible for this organized cell behavior. However, the identity of these factors and their function are not well understood. Here we examine the expression of members of the EphA subclass of receptor tyrosine kinases and ephrins using RT-PCR and immunocytochemistry. Neural crest cells express ephrins and EphA4 at distinct stages during their migration. In functional analyses, addition of ephrin-A2-, ephrin-A5-, and EphA4-Fc disrupted the segmental organization of trunk neural crest migration in explants: neural crest cells entered rostral and caudal halves of somites. Finally, to test the specific effects of these factors on cell behavior, neural crest cells were exposed in vitro to substrate-bound EphA and ephrin-As. Surprisingly, neural crest cells avoided ephrin-A2 or ephrin-A5 substrates; this avoidance was abolished by the addition of EphA4. Together, these data suggest that ephrin-As and EphA4 cooperate to positively promote the migration of neural crest cells through rostral half somites in vivo.
Keywords: Migration, Guidance, Chicken, Segmentation, Ephrin, EphA4
NEURAL crest cells emanate from the dorsal neural tube and migrate extensively to their final destinations where they give rise to a wide range of derivatives, including sensory and sympathetic ganglia, bones of the face, and pigment cells (21). At trunk levels, neural crest cells migrate on two distinct pathways: a ventromedial route through the somites and a dorsolateral pathway, between the ectoderm and somites (22,25). Neural crest cells that migrate ventromedially move in a segmental manner through the somites, entering the rostral but not caudal somitic sclerotome (13).
A large variety of molecular cues are thought to control the segmental movement of neural crest cells through the somites (15). The predominant notion that has emerged over the past few years is that repulsive or inhibitory cues, localized to the caudal half sclerotome, control neural crest migration (3,16,17,20,24,26). However, neural crest migration through the rostral half sclerotome is well organized and coordinated, suggesting that cues present in the rostral half sclerotome, and perhaps associated with neural crest themselves, influence neural crest movement in a positive manner (4,8,16,23,27).
Members of the Eph family are strong candidate guidance factors for migrating neural crest, due to their known involvement in cell migrations and boundary formation during embryonic development (2,15,33). Previously, we examined the function of members of the EphB subclass of receptor tyrosine kinases (RTKs) and their ephrin-B ligands during trunk neural crest migration [(17); see also (29,32)]. Disruption of EphB–ephrin-B1 interactions perturbed the normal segmental migration of trunk neural crest through the somites: neural crest cells entered both rostral and caudal half somites. In vitro, neural crest cells specifically avoided ephrin-B1 substrates. These data suggested that EphB–ephrin-B1 interactions controlled the segmental migration of neural crest by serving to inhibit cell entry into the caudal half sclerotome. However, this analysis also revealed that neural crest trajectories through rostral half somites of treated embryos were linear and organized, similar to controls. These results and others have implicated other positive, migration-promoting factors in the control of trunk neural crest migration.
Here, the spatiotemporal distribution of EphA subclass members during early stages of trunk neural crest migration was examined using RT-PCR and avian-specific antibodies. To determine the role of EphA/ephrin-A interactions, avian trunk explants were treated with EphA4-, EphA7-, ephrin-A2-, or ephrin-A5-Fc fusion proteins and the effects on trunk neural crest migration assayed using cell-specific markers. Finally, to examine the specific effects of these factors, trunk neural crest cells were exposed to striped substrates containing ephrins or Eph RTKs in vitro. The results of our functional experiments to date and expression analysis suggest that neural crest migration through the rostral half somite is promoted in a positive manner by interactions between ephrin-As and EphA4.
MATERIALS AND METHODS
Embryos
Fertilized White Leghorn chicken eggs and Japanese quail eggs (Coturnix coturnix japonica) were acquired from suppliers (Hy-Line International, Spencer, IA; Bear Bayou Quail Farm, Channelview, TX) and incubated at 38°C in a humidified incubator until the appropriate developmental stage (8). Embryos were collected in Ringer’s solution in preparation for vibratome sectioning or explant cultures. Embryos to undergo vibratome sectioning were fixed for 2 h to overnight in 4% paraformaldehyde.
RT-PCR
Chicken neural tube/neural crest cultures were prepared as previously described (19). After 12 h in vitro, neural tubes were removed from culture plates using a tungsten needle and the remaining neural crest cells were washed with fresh PBS. The identity of the remaining cells as neural crest was verified by HNK-1 antibody staining and morphological characteristics. The Boehringer Mannehim High Pure RNA Isolation Kit was used to extract total RNA from neural crest cells. RT-PCR was then performed using primers for ephrin-A2, ephrin-A5, ephrin-A6, EphA4, Wnt-3a (a marker for nonmigratory, neural tube cells) and β-actin (controls). Primers used for ephrin-A2 detection were 5′-CCGCAGCAACCCCAGGTTCCAC-3′ (forward) and 5′-CCGCGGCACTAAGAGCCCAGCA-3′ (reverse) resulting in a 504-bp DNA product. Primers used for ephrin-A5 detection were 5′-TGGCCGACCGCTACGCCGTCTA-3′ (forward) and 5′-CCACGGGATGGCTCGGCTGACT-3′ (reverse), resulting in a 520-bp DNA product. Primers used for ephrin-A6 detection were 5′-CGTTCGTCCCCGTTCGGTTCTCC-3′ (forward) and 5′-CCCCCACGTTTTGGGGGTCCAT-3′ (reverse), resulting in a 506-bp DNA product. Primers used for EphA4 detection were 5′-CATGTGCCAAATGCCCGCCTCA-3′ (forward) and 5′-TTCCAGCCAGGCCAAGGCAACG-3′ (reverse), resulting in a 524-bp fragment. Primers used for Wnt-3a detection were 5′-TCGCCGATGCCCGAGAGAAC-3′ (forward) and 5′-TCCTGGCAGCGGACGTAGCA-3′ (reverse), resulting in a 517-bp DNA product. Primers used for β-actin detection were 5′-CGGTTTCGCCGGGGACGATG-3′ (forward) and 5′-CGTCAGGTCACGGCCAGCCAGA-3′ (reverse), resulting in a 502-bp DNA product. Touchdown PCR was carried out under the following conditions: 95°C for 2 min, then 94°C for 1 min, annealing temperature decreased 1°C every two cycles from 76°C to 62°C for 1 min, 72°C for 1.5 min (30 cycles), then 94°C for 1 min, 50°C for 1 min, 72°C for 1.5 min (15 cycles). RT-PCR products were then visualized by electrophoresis in 1% agarose in 1× TAE buffer with ethidium bromide.
Antibodies/Immunocytochemistry
Avian-specific antibodies to EphA4, ephrin-A2, ephrin-A5, and ephrin-A6 were applied to 100-μm vibratome sections, as previously described (5). Most sections were also stained with HNK-1 antibody, a marker for avian neural crest, except for ephrin-A2 antibody-labeled sections that were labeled with DiI, as previously described (17). Appropriate Alexa Fluor secondary antibodies were applied to detect primary antibody binding (5).
Trunk Explant Cultures
Trunk explants were prepared as previously described (17). Trunk regions were preincubated in a solution of 10 μg/ml of unclustered ligand (ephrin-A2, ephrin-A5) or receptor-Fcs (EphA4, EphA7) or Fc alone (controls) in culture medium for 4 h at 37°C in 5% CO2. Explants were placed on Millicell inserts (Millipore) and grown at 37°C in a 5% CO2 incubator for 24 h. All explants were fixed in 4% paraformaldehyde, rinsed extensively with PBS, and double-stained with HNK-1 antibody, to mark neural crest, and ephrin-B1 antibody, to label the caudal half sclerotome. In some cases, the nuclear marker To-Pro-3 (Molecular Probes, t-3605) was applied. HNK-1-positive/To-Pro-3-positive cells in the rostral and caudal half somites in midregions of trunk explants were counted in experimental (n = 5 per treatment condition) and control explants (n = 5) using a Bio-Rad Radiance 2000 laser scanning confocal microscope (Molecular Cytology Core, University of Missouri-Columbia). Percentages of neural crest cells entering the caudal half somite under each condition were then determined.
Neural Crest Cultures/Stripe Assays
Primary neural crest cell cultures were prepared from Japenese quail embryos at stages 12–14, using standard procedures (19). Striped substrates were prepared as previously described (17,31).
Confocal Imaging
Optical sections at 2-μm intervals were collected from vibratome sections or trunk explants previously stained with antibodies using a BioRad Radiance 2000 laser scanning confocal microscope. Z series stacks of 10 μm were compiled. Each image in a Z series was viewed and analyzed individually to assure that antibody labeling was assigned to the correct cell type. Images were processed in Metamorph and compiled into figures using Adobe Photoshop 6.0.
RESULTS
Trunk Neural Crest Cells Express Ephrin-A2, Ephrin-A5, Ephrin-A6, and EphA4 RTK at Distinct Stages
Previously, we examined the distribution and function of EphB subclass members during trunk neural crest migration through the somitic mesoderm (17). Our recent analysis of the expression of EphA subclass members during motor axon guidance showed that cells in the dorsal root ganglia express ephrin-A2 and ephrin-A5 (5). Therefore, we examined the distribution of EphA4 RTK and ephrin-As during early stages of neural crest migration, to determine if these factors were in position to influence neural crest movements in a positive manner.
First, we performed semiquantitative RT-PCR with EphA4-, ephrin-A2-, ephrin-A6-, ephrin-A6-specific primers. The results indicated that migratory neural crest cells expressed ephrin-A2, ephrin-A5, ephrin-A6, and EphA4, albeit at lower levels, but not Wnt 3a, a marker for nonmigratory dorsal neural tube cells (Fig. 1). We next examined the spatiotemporal pattern expression of these factors in the trunk regions of chicken embryos at stages 12–18, when neural crest are migrating into somites. Ephrin-A2 protein localizes to the neural tube prior to neural crest emigration and is found on the earliest migrating neural crest cells (Fig. 2). As neural crest migration through the somites progresses, ephrin-A2 remains associated with migrating neural crest cells. Ephrin-A5 is also associated with newly emigrated neural crest cells (Fig. 3). As neural crest migration proceeds, ephrin-A5 is apparent on most neural crest cells but more strongly on ventrally positioned neural crest that are loosely associated. In the neural tube, ephrin-A5 protein is most prominent in its dorsal half and eventually demarcates an intermediate region. Ephrin-A6 is present on newly emigrated neural crest cells at early stages of migration, prior to somite entry (Fig. 4). However, ephrin-A6 appears to be downregulated in neural crest cells that migrate in the somitic mesoderm.
Figure 1.
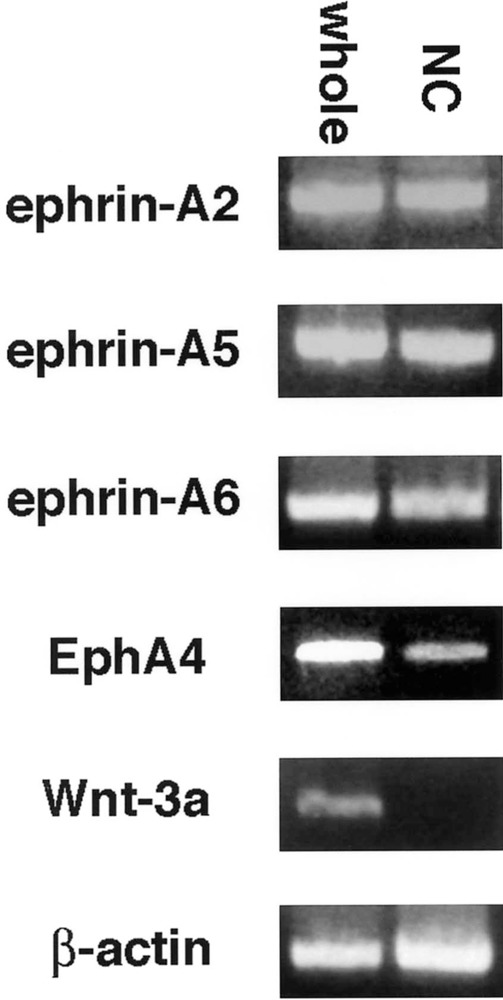
Ephrin-As and EphA4 are expressed by neural crest cells, using RT-PCR. Ephrin-A2, ephrin-A5, ephrin-A6, and EphA4 transcripts were all detected in RNA samples from stage 15 whole embryos (whole) and samples from isolated migratory neural crest cells (NC). Wnt-3a, a marker for dorsal neural tube, was used as a control for neural tube contamination of neural crest RNA. β-Actin was used as an internal control.
Figure 2.
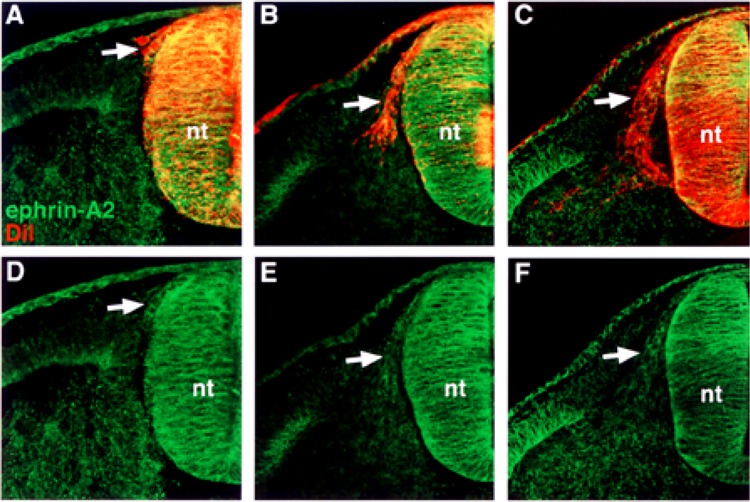
Ephrin-A2 is expressed by recently delaminated and migratory neural crest cells in the somite. (A, B, C) Cross sections through stage 14–18 embryos stained with ephrin-A2 antibody (green) and DiI (red), by prelabeling neural tubes prior to neural crest migration. (D, E, F) Ephrin-A2 antibody labeling. Ephrin-A2 is associated with the surfaces of neural crest cells that have recently delaminated (A, D), initiated entry into the somites (B, E), and migrated into the rostral half sclerotome (C, F). nt, neural tube.
Figure 3.
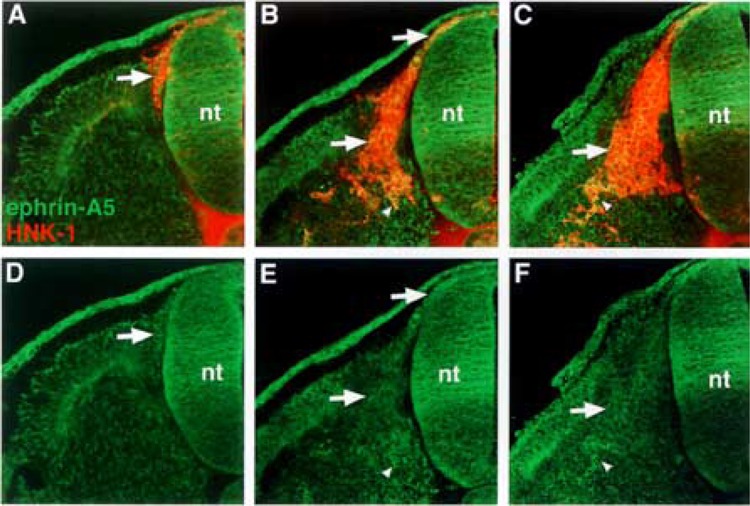
Ephrin-A5 is most strongly expressed by neural crest cells that have delaminated and migrated ventrally in the sclerotome. (A, B, C) Cross sections through stages 14–18 embryos stained with ephrin-A5 antibody (green) and HNK-1 antibody (red), a marker for avian neural crest. (D, E, F) Ephrin-A5 antibody labeling. Ephrin-A5 localizes predominantly to recently delaminated neural crest prior to somite entry (A–F: arrows), to migratory neural crest (B, E: arrows), and to neural crest at ventral locations in the somite (B, C, E, F: arrowheads).
Figure 4.
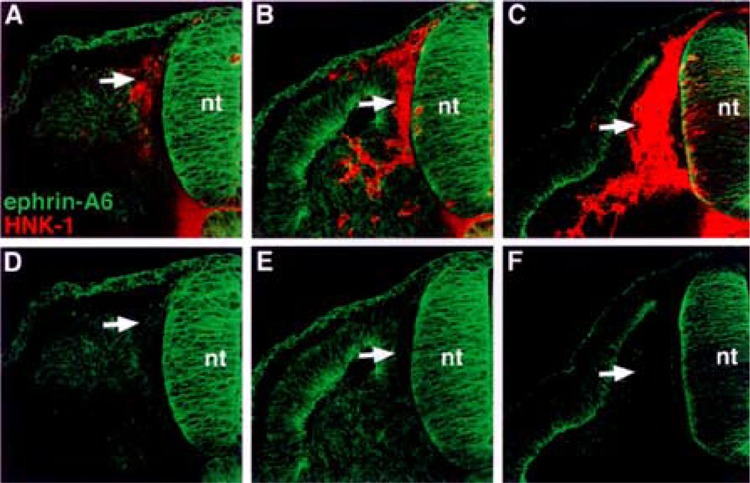
Ephrin-A6 protein associates with neural crest cells prior to their entry into the somite. (A, B, C) Cross sections through stages 14–18 embryos stained with ephrin-A6 antibody (green) and HNK-1 antibody (red). (D, E, F) Ephrin-A6 antibody labeling. Ephrin-A6 protein is evident on recently delaminated neural crest that lie between the neural tube and somite (A, D: arrows). As neural crest cells enter the somite, ephrin-A6 protein is not detectable (B–F: arrows).
We examined the distribution of EphA4, a potential receptor for ephrin-A2, ephrin-A5, and ephrin-A6, using antibody labeling. EphA4 protein is expressed at very low levels by neural crest cells (Fig. 5). This weak expression of EphA4 appears associated with neural crest cells that have recently delaminated from the neural tube as well as those migrating in the sclerotome. The expression of EphA7 during the process of neural crest migration was not characterized, as it was previously reported localized to the caudal half sclerotome (1).
Figure 5.
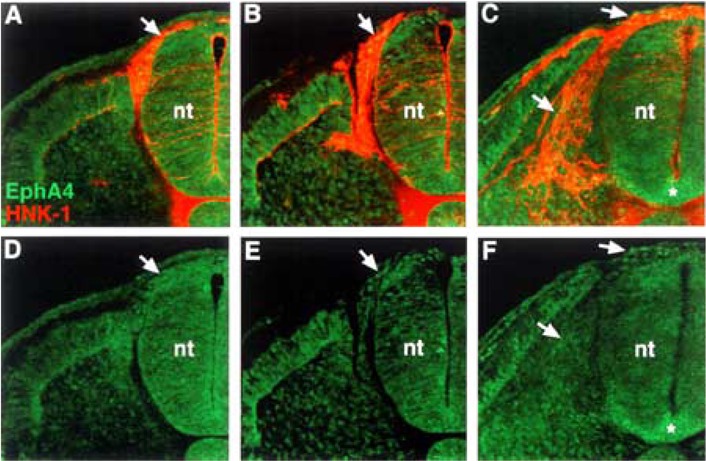
EphA4 protein is present at very low levels on neural crest cells. (A, B, C) Cross sections through stage 18 embryos, at different trunk axial levels, stained with EphA4 antibody (green) and HNK-1 antibody (red). (D, E, F) EphA4 antibody labeling. EphA4 protein is apparent at very low levels on neural crest that have exited the neural tube and are migrating in the somites (arrows). Asterisk (*) marks EphA4 expression in the floor plate.
The Segmental Migration of Trunk Neural Crest in the Somitic Mesoderm Is Disrupted in the Presence of Ephrin-A2-, Ephrin-A5-, or EphA4-Fcs
To test the function of EphA4–ephrin interactions in trunk neural crest migration, we prepared trunk explants and applied EphA- and ephrin-A-Fc fusion proteins (17). Neural crest cells migrated in the rostral and caudal half somites in the presence of ephrin-A2-Fc (n = 38), ephrin-A5-Fc (n = 47), and EphA4-Fc (n = 39) (Fig. 6A, C, D). However, in the presence of EphA7-Fc (n = 35) and Fc (n = 41; controls), neural crest typically migrated through the rostral half somites, as observed normally in vivo, supporting the specificity of our effects (Fig. 6B, E). We quantified the numbers of neural crest cells in rostral and caudal half somites in treated explants and found that 33.1%, 35.2%, and 36.9% of HNK-1-positive neural crest cells were found in caudal somite halves in explants treated with ephrin-A2-, ephrin-A5-, and EphA4-Fcs, respectively. In contrast, 4.1% and 9.6% of neural crest cells were found in caudal somite halves in Fc- and EphA7-Fc-treated explants, respectively. These data suggest that interactions between EphA4, ephrin-A2, and ephrin-A5 control the segmental migration of neural crest cells through the somitic mesoderm. Eph–ephrin interactions could establish the rostrocaudal polarity of the somites by forming borders between these cellular compartments. To determine if somite polarity was altered, treated explants were labeled with ephrin-B1 antibody, a marker for caudal half somites. No alterations in the rostrocaudal polarity of the somites were evident under any treatment condition (Fig. 6).
Figure 6.
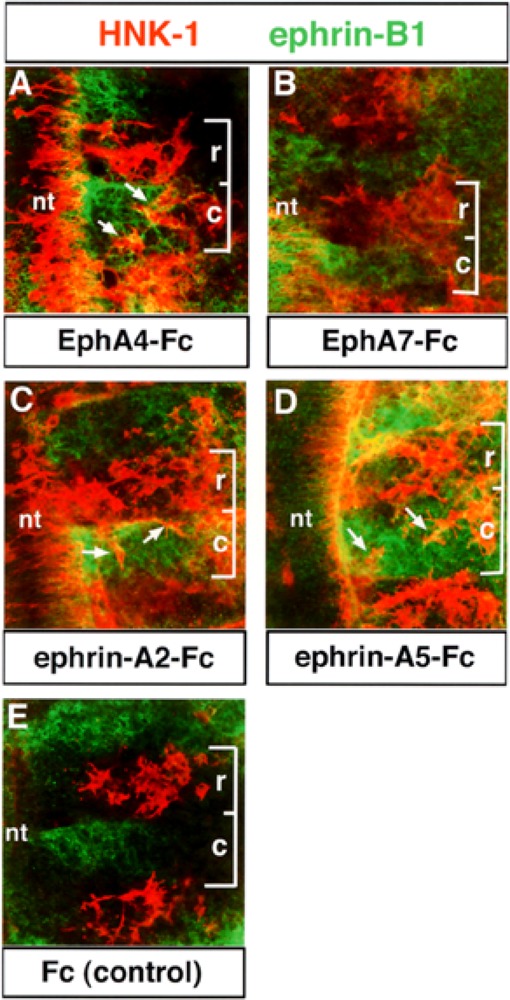
The segmental migration of neural crest cells is disrupted in explants treated with EphA4- or ephrinA-Fc. Trunk explants containing premigratory neural crest cells were isolated, as previously described (17). Explants were treated with 10 μg/ml of EphA4-Fc (A), EphA7-Fc (B), ephrin-A2-Fc (C), ephrin-A5-Fc (D), or Fc (E, controls). The disposition of neural crest cells was then examined using HNK-1 antibody (red) and ephrin-B1 antibody (green) was used as a marker for the caudal half somite. Brackets mark the extent of a single somite: r, rostral; c, caudal. Neural crest cells invade the caudal half somites in EphA4-Fc-, ephrin-A2-Fc-, and ephrin-A5-Fc-treated explants (A, C, D: arrows). In EphA7-Fc and Fc controls, neural crest cells migrate in the rostral half somite, typical of normal embryos (B, E).
Neural Crest Cells Migrate on Substrates Composed of Ephrin-A2 or Ephrin-A5 Combined With EphA4
Because ephrin-As and EphA4 RTK are expressed by neural crest cells, we reasoned that the disruption of segmental migration observed in our treated ex-plants might be due to the perturbation of positive signals that guide neural crest cells in the rostral half somites. Thus, we examined the effects of substrate-bound EphA4 and ephrins on migrating neural crest cells in vitro, using stripe assays (17,31). Surprisingly, neural crest cells avoided migrating on ephrin-A5 (n = 13; Fig. 7A) or ephrin-A2 (n = 9; data not shown) in culture. When ephrin-A2 and ephrin-A5 were applied together, neural crest cells seemed to exhibit a stronger avoidance of these substrate-bound factors (n = 7; Fig. 7C). These results were unexpected in light of the expression of these ligands by migratory neural crest cells. Uniform migration across all lanes occurred when neural crest cells were confronted with EphA4 (n = 14) or EphA7 (n = 14), suggesting these proteins do not act as repulsive agents for neural crest (Fig. 7B, E). To determine if the addition of EphA4 to ephrin substrates would allow or promote neural crest cell migration on ephrin stripes, we constructed stripes composed of EphA4/ephrin-A2 or ephrin-A5/fibronectin versus fibronectin. Neural crest cells migrated on EphA4/ephrin-A2 (n = 11) or EphA4/ephrin-A5 stripes (n = 12) whereas they had previously avoided ephrins as simple substrates (Fig. 7D). In control dishes treated with Fc, neural crest migrated uniformly across all lanes, as expected (n = 16; Fig. 7F). These data, combined with our expression analysis and explant results, suggest that EphA4, ephrin-A2, and ephrin-A5 interact together to promote trunk neural crest migration through the somites.
Figure 7.
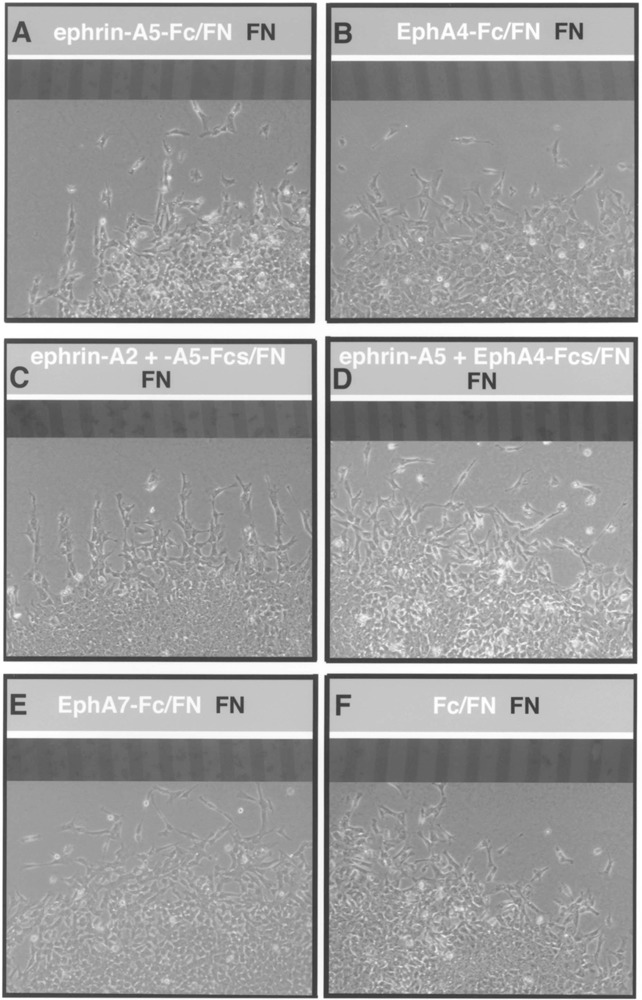
Neural crest cells migrate uniformly on substrates containing ephrin-A2 or ephrin-A5 plus EphA4. Neural tubes were placed perpendicular to striped substrates composed of ephrins or Eph RTKs/fibronectin (light lanes) versus fibronectin (dark lanes) and neural crest were allowed to migrate for 24 h. Digital images were collected of the same field containing the lanes using fluorescence optics and migratory neural crest cells, using phase optics. (A) Neural crest cells avoid ephrin-A5/fibronectin lanes and migrate on fibronectin-alone lanes. This behavior was also observed in the presence of ephrin-A2 substrates (data not shown). (B) Neural crest cells migrate uniformly on lanes containing EphA4/fibronectin vs. fibronectin. (C) Neural crest cells distinctly avoid lanes containing ephrin-A2 and ephrin-A5/ fibronectin. (D) Neural crest migrate uniformly on lanes of ephrin-A5 and EphA4/fibronectin vs. fibronectin. This behavior was also observed with ephrin-A2/EphA4/fibronectin substrates (data not shown). (E, F) Neural crest cells migrate across all lanes in dishes containing EphA7 or Fc (controls) lanes.
DISCUSSION
One common theme in development is that inhibitory factors sculpt the nervous system by preventing the entry of neural cells into particular territories. A diverse collection of inhibitory molecules is thought to prohibit the entry of trunk neural crest cells into the caudal half sclerotome, generating the segmental organization of the peripheral nervous system (15). Relatively little is understood about the positive factors that permit or promote neural crest migration in the rostral half sclerotome. A small number of guidance factors including fibronectin, laminin, integrins, and tenascin localize to migrating neural crest cells or the rostral sclerotome, and results from in vitro analyses support positive roles for these molecules in neural crest guidance (4,18,23,27,30).
Members of the Eph family interact to prohibit neural crest cells from entering the caudal half sclerotome (17) and participate in neural crest pathway choices in the trunk (28). Our analysis here demonstrates that EphA subclass members localize to neural crest cells during their migration. Ephrin-A2, ephrin-A5, and ephrin-A6 are associated with neural crest cells at distinct stages in their migration from the neural tube into the somitic mesoderm. EphA4 is coexpressed but weakly by neural crest cells during their delamination from the neural tube and early entry into the somites. We examined the functions of EphA4 and its cognate ephrins by disrupting Eph–ephrin signaling in trunk explants and examining the subsequent disposition of migrating trunk neural crest. Our results show that neural crest cells inappropriately enter the caudal half somite when explants are treated with ephrin-A-Fcs or EphA4-Fc. These data, combined with our expression analysis, led us to hypothesize that EphA–ephrin-A interactions promoted neural crest migration in the rostral half sclerotome normally. The results of our in vitro analyses also support this hypothesis: neural crest cells migrate on ephrin-A2 or ephrin-A5 substrates in the presence of EphA4, a situation remarkably similar to the in vivo environment.
Interactions between Eph RTKs and ephrins are known to exert inhibitory effects, keeping cells out of inappropriate regions or forming boundaries between cellular compartments that prevent cell mixing. However, recent studies support more positive, growth-promoting functions of ephrin-As. Ephrin-A2 and ephrin-A5 promote neurite outgrowth by sympathetic neurons, and the growth and branching of hippocampal neurons in vitro, suggesting that these ephrins exhibit bifunctional properties (6,7). Evidence for a positive role for Eph–ephrin signaling in vivo comes from studies in the vomeronasal system, where axons with high concentrations of receptors project into regions of high ligand concentration (14). In the somitic mesoderm, activation of ephrin-A2 or ephrin-A5 may result in a β1 integrin-dependent increased adhesion of neural crest cells to laminin, as previously described (11).
If ephrins display bifunctional properties, what is the mechanism by which cells respond differently to ephrins? One possible explanation that would account for our findings in vitro is that coexpression of EphA4 reduces the typical repulsive effects of ephrin-As on neural crest cells. This scenario would be similar to that described in the retinotectal system, where ephrin coexpression with Eph receptors in retinal neurons decreases the inhibitory effects of ephrins in the tectum on retinal growth cones (10). Alternatively, downstream components could be differentially activated in Eph–ephrin interactions that are positive or inhibitory. In cells that are inhibited by ephrins, Rac1 could be activated, resulting in endocytosis of the plasma membrane and the collapse of the leading edge of migration, as recently demonstrated for axonal growth cones (12). In another example, the metal-loprotease kuzbanian apparently cleaves ephrin-A2, converting Eph–ephrin adhesion to repulsion (9). Whether kuzbanian or other proteases are involved in EphA–ephrin-A signaling in neural crest cells in vivo awaits further analysis.
The results of these studies suggest that neural crest cells utilize ephrin-As and EphA4 cooperatively to navigate in the rostral half sclerotome. Loss-of-function studies, combined with time-lapse imaging, are in progress in vivo to test the requirement for EphA4–ephrin-A interactions in shaping the migratory trajectories of neural crest.
ACKNOWLEDGMENTS
We thank Elena Pasquale for avian-specific antibodies to EphA4, ephrin-A5, and ephrin-A6, and Hideaki Tanaka for anti-ephrin-A2 antibody. Thanks to members of the Krull laboratory for helpful discussions and to the Molecular Cytology Core, University of Missouri-Columbia. Support provided by NIH/NIMH and the Muscular Dystrophy Association (C.E.K.).
REFERENCES
- 1. Araujo M.; Nieto M. A. The expression of chick EphA7 during segmentation of the central and peripheral nervous system. Mech. Dev. 68:173–177; 1997. [DOI] [PubMed] [Google Scholar]
- 2. Davis S.; Gale N.; Aldrich T. H.; Maisonpierre P. C.; Lhotak V.; Pawson T.; Goldfarb M.; Yancopoulos G. D. Ligands for Eph-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science 266:816–819; 1994. [DOI] [PubMed] [Google Scholar]
- 3. Debby-Brafman A.; Burstyn-Cohen T.; Klar A.; Kalcheim C. F-spondin, expressed in somite regions avoided by neural crest cells, mediates inhibition of distinct somite domains to neural crest migration. Neuron 22:475–488; 1999. [DOI] [PubMed] [Google Scholar]
- 4. Duband J. L.; Dufour S.; Yamada S. S.; Yamada K. M.; Thiery J. P. Neural crest cell locomotion induced by antibodies to beta 1 integrins. A tool for studying the roles of substratum molecular avidity and density in migration. J. Cell Sci. 98:517–532; 1991. [DOI] [PubMed] [Google Scholar]
- 5. Eberhart J.; Swartz M.; Koblar S. A.; Pasquale E. B.; Tanaka H.; Krull C. E. Expression of EphA4, ephrin-A2 and ephrin-A5 during axon outgrowth to the hind-limb indicates potential roles in pathfinding. Dev. Neurosci. 22:237–250; 2000. [DOI] [PubMed] [Google Scholar]
- 6. Gao P. P.; Yue Y.; Cerretti D. P.; Dreyfus C.; Zhou R. Ephrin-dependent growth and pruning of hippocampal axons. Proc. Natl. Acad. Sci. USA 96:4073–4077; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao P. P.; Sun C. H.; Zhou X. F.; DiCicco-Bloom E.; Zhou R. Ephrins stimulate or inhibit neurite outgrowth and survival as a function of neuronal cell type. J. Neurosci. Res. 60:427–436; 2000. [DOI] [PubMed] [Google Scholar]
- 8. Hamburger V.; Hamilton H. L. A series of normal stages in the development of the chick embryo. J. Morphol. 88:49–92; 1951. [PubMed] [Google Scholar]
- 9. Hattori M.; Osterfield M.; Flanagan J. G. Regulated cleavage of a contact-mediated axon repellent. Science 289:1360–1365; 2000. [DOI] [PubMed] [Google Scholar]
- 10. Hornberger M. R.; Dutting D.; Ciossek T.; Yamada T.; Handwerker C.; Lang S.; Weth F.; Huf J.; Wessel R.; Logan C.; Tanaka H.; Drescher U. Modulation of EphA receptor function by co-expressed ephrinA ligands on retinal ganglion cell axons. Neuron 22:731–742; 1999. [DOI] [PubMed] [Google Scholar]
- 11. Huai J.; Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J. Biol. Chem. 276:6689–6694; 2001. [DOI] [PubMed] [Google Scholar]
- 12. Jurney W. M.; Gallo G.; Letourneau P. C.; McLoon S. C. Rac1-mediated endocytosis during ephrin-A2- and semaphoring-3A-induced growth cone collapse. J. Neurosci. 22:6019–6028; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keynes R. J.; Stern C. D. Mechanisms of vertebrate segmentation. Development 103:413–429; 1988. [DOI] [PubMed] [Google Scholar]
- 14. Knoll B.; Zarbalis K.; Wurst W.; Drescher U. A role for the EphA family in the topographic targeting of vomeronasal axons. Development 128:895–906; 2001. [DOI] [PubMed] [Google Scholar]
- 15. Krull C. E. Segmental organization of neural crest migration. Mech. Dev. 105:37–45; 2001. [DOI] [PubMed] [Google Scholar]
- 16. Krull C. E.; Collazo A.; Fraser S. E.; Bronner-Fraser M. Segmental migration of trunk neural crest: Time-lapse analysis reveals a role for PNA-binding molecules. Development 121:3733–3743; 1995. [DOI] [PubMed] [Google Scholar]
- 17. Krull C. E.; Lansford R.; Gale N. W.; Collazo A.; Marcelle C.; Yancoupoulos G. D.; Fraser S. E.; Bronner-Fraser M. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr. Biol. 7:571–580; 1997. [DOI] [PubMed] [Google Scholar]
- 18. Lallier T. E.; Deutzmann R.; Perris R.; Bronner-Fraser M. Neural crest cell interactions with laminin: Structural requirements and localization of the binding site for alpha 1 beta 1 integrin. Dev. Biol. 162:451–464; 1994. [DOI] [PubMed] [Google Scholar]
- 19. Lallier T. E.; Bronner-Fraser M. Avian neural crest cell adhesion to laminin: Involvement of divalent cation dependent and independent integrins. Development 113:1069–1081; 1991. [DOI] [PubMed] [Google Scholar]
- 20. Landolt R. M.; Vaughan L.; Winterhalter K. H.; Zimmermann D. R. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest migration and axon outgrowth. Development 121:2303–2312; 1995. [DOI] [PubMed] [Google Scholar]
- 21. Le Douarin N. M.; Kalcheim C. The neural crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 22. Loring J. F.; Erickson C. A. Neural crest cell migratory pathways in the trunk of the chick embryo. Dev. Biol. 121:220–236; 1987. [DOI] [PubMed] [Google Scholar]
- 23. Perris R.; Krotoski D.; Bronner-Fraser M. Collagens in avian neural crest development: Distribution in vivo and migration-promoting properties in vitro. Development 113:969–984; 1991. [DOI] [PubMed] [Google Scholar]
- 24. Ranscht B.; Bronner-Fraser M. T-cadherin expression alternates with migrating neural crest cells in the trunk of the avian embryo. Development 111:15–22; 1991. [DOI] [PubMed] [Google Scholar]
- 25. Rickmann M.; Fawcett J. W.; Keynes R. J. The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J. Embryol. Exp. Morphol. 90:437–455; 1985. [PubMed] [Google Scholar]
- 26. Ring C.; Hassell H.; Halfter W. Expression pattern of collagen IX and potential role in segmentation of the peripheral nervous system. Dev. Biol. 180:41–53; 1996. [DOI] [PubMed] [Google Scholar]
- 27. Rovasio R. A.; Delouvee A.; Yamada K. M.; Timpl R.; Thiery J. P. Neural crest cell migration: Requirements for exogenous fibronectin and high cell density. J. Cell Biol. 96:462–473; 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santiago A.; Erickson C. A. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development 129:3621–3632; 2002. [DOI] [PubMed] [Google Scholar]
- 29. Smith A.; Robinson V.; Patel K.; Wilkinson D. G. Eph-related receptor tyrosine kinases EphA4 and EphB1 and the ligand ephrin-B2 regulate the targeted migration of branchial neural crest cells. Curr. Biol. 7:561–570; 1997. [DOI] [PubMed] [Google Scholar]
- 30. Tucker R. P.; McKay S. E. The expression of tenascin by neural crest cells and glia. Development 112:1031–1039; 1991. [DOI] [PubMed] [Google Scholar]
- 31. Vielmetter J.; Stolze B.; Bonhoeffer F.; Stuermer C. A. In vitro assay to test differential substrate affinities of growing axons and migratory cells. Exp. Brain Res. 81:283–287; 1990. [DOI] [PubMed] [Google Scholar]
- 32. Wang H. U.; Anderson D. J. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron 18:383–396; 1997. [DOI] [PubMed] [Google Scholar]
- 33. Wilkinson D. G. Multiple roles of Eph receptors and ephrins in neural development. Nat. Rev. Neurosci. 2:155–164; 2001. [DOI] [PubMed] [Google Scholar]


