Abstract
Splicing of nuclear precursor messenger RNAs is an important and ubiquitous type of gene regulation in metazoans. Splicing joins the coding sequences called exons by removing the intervening noncoding sequences, introns, from primary transcripts. Alternative splicing generates an enormous repertoire of functional diversity by producing multiple RNAs and proteins from a single gene. In fact, recent genome sequences from several organisms suggest that splicing regulation is likely to provide an important source of functional diversity in more complex organisms. Because splice sites are short sequences at the ends of introns, the functional splice sites have to be distinguished from an excessively large number of sequences in the primary transcripts that resemble a splice site. Furthermore, alternative splice sites have to be correctly chosen at appropriate times. Thus, selection of proper splice sites remains a daunting biological problem. This review focuses on a few examples in which the molecular and biochemical basis for splice site selection is better understood.
Keywords: RNA binding proteins, Splicing factors, Enhancers, Silencers, Small nuclear ribonucleoprotein, Alternative splicing, Exon definition, Polypyrimidine tract, Combinatorial control
RECENT genome sequences of vertebrates and invertebrates have unexpectedly revealed that in more complex organisms there may be fewer genes than expected from the DNA content or the complexity of an organism. For example, humans (and mice) may have only three times (∼26,000–38,000) as many gene as fruit flies (∼13,600), twice as many as nematodes (19,000), and six times as many as yeast (∼6000) (1,20,21,121). Thus, it appears that complexity arises to a large extent by increasing the network of interactions between proteins rather than by merely increasing the number of genes. Moreover, an increase in size and number of the noncoding sequences has significantly contributed to the increase in the DNA content in more complex organisms. The coding portion of the human genome, for example, constitutes less than 1.5% of the entire genome. One form of the noncoding sequences, introns, which interrupt the coding sequences within genes, constitutes 24% of the genome. Approximately 85–95% of the genes in fruit flies, mammals, nematodes, and plants contain introns. Precursor messenger RNAs are spliced to remove introns and join exons together before translation into protein. Interestingly, it is estimated that at least 30% of the human genes are alternatively spliced to generate multiple forms of mRNA, and therefore protein, from a single gene (34). Thus, alternative splicing is likely to represent a major source of functional diversity and complexity in humans. Because abnormality in splice site selection can profoundly affect gene function, alternative splicing must be rigorously regulated. In fact, approximately 15% of the mutations in mammalian genes that have been implicated in various diseases affect RNA splicing signals, linking abnormal splicing to cellular transformation, Duchenne muscular dystrophy, and tumor metastasis (22,54).
SPLICING
The splicing machinery recognizes specific sequences around the intron boundaries: the 5′ splice site, the branchpoint, the polypyrimidine tract, and the 3′ splice site. The architectures of known introns are schematized in Figure 1A. Proper recognition of these splicing signals is critical for accurate splicing. Splicing of nuclear pre-mRNAs involves a stepwise assembly of five (U1, U2, U4, U5, and U6) small nuclear ribonucleoproteins (snRNPs) and several other proteins onto a pre-mRNA to form a large complex called the spliceosome (10,91,108). The assembly begins with the binding of the U1 snRNP to the 5′ splice site and the U2 snRNP to the branch site. Subsequently, the U4/U5/U6 snRNPs join to form the spliceosome. The ordered assembly of the spliceosome involves several rearrangements, including RNA–RNA, protein–protein, and RNA–protein interactions. Several of these rearrangements require members of the SR family of proteins, ATPases, RNA binding proteins, kinases, and RNA helicases or unwindases.
Figure 1.
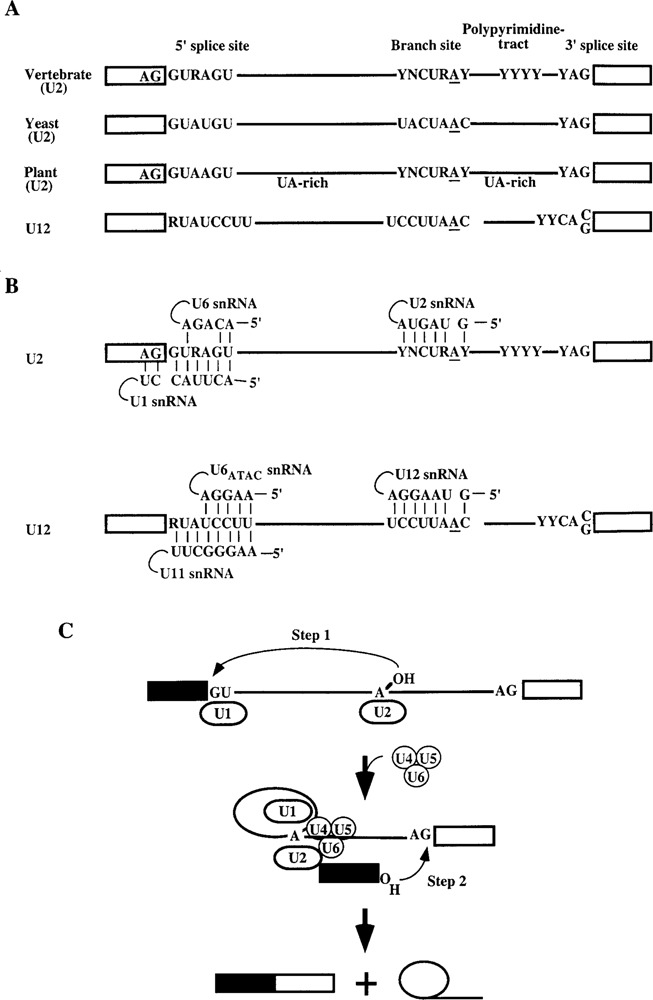
Intron splicing signals and their recognition. (A) The 5′ splice site, the branch site, the polypyrimidine tract, and the 3′ splice site consensus sequences are shown for both the U2- and U12-dependent introns. (B) Base pairing interactions between the splicing signals and the U snRNAs for the U2- and U12-dependent introns are shown. (C) Two transesterification reactions. Arrows show step 1 and step 2 of splicing. For clarity, only U snRNPs are shown. A, adenine; G, guanine; C, cytosine; U, uracil; T, thymine; R, adenine or guanine; Y, cytosine or uracil.
In addition, the recently discovered minor class of introns is spliced by the U12-dependent spliceosome, which includes U11, U12, U4ATAC, and U6ATAC snRNPs; the U5 snRNP is common to both spliceosomes (Fig. 1B) (10,131). The U2 and U12 spliceosomes use both common and distinct proteins (126).
Following the assembly of the spliceosome, the splicing reaction involves two transesterification reactions within the catalytic center of the spliceosome (76). During the first step, the 2′-hydroxyl group of the branchpoint adenosine attacks the phosphodiester linkage at the 5′ splice site, leaving a 3′-hydroxyl at the end of the first exon. During the second step, this 3′-hydroxyl group attacks the phosphodiester linkage at the 3′ splice site, leading to the joining of the two exons and release of the lariat intron (Fig. 1C). Both steps of splicing involve a direct SN2 type in-line nucleophilic attack. For example, an Sp phosphorothioate (in which one of the nonbridging oxygens is replaced by sulfur) at either splice junction undergoes inversion of configuration to Rp isomer (76). It has been shown that the spliceosome, like the self-splicing group II ribozyme, is a metalloenzyme (104,105,130). This conclusion is based on metal specificity switch experiments in which replacement of an oxygen by a sulfur interferes with splicing in the presence of Mg2+ only, but addition of Mn2+ rescues splicing. This is because Mn2+ coordinates sulfur well but Mg2+ does not (13,29). Divalent metal ions play important roles by promoting RNA folding, active site chemistry, or both (13,29). The fact that splicing by the spliceosome and the self-splicing group II introns, which do not need proteins, are mechanistically similar (76,86,105,125) led to the proposal that the spliceosome is an RNA-based and not a protein-based enzyme, although this issue remains unresolved (19,83). Regardless, proteins play an important role in regulating splice site selection in pre-mRNA splicing.
ALTERNATIVE SPLICING
Alternative splicing generates multiple RNA and protein isoforms from a single gene. It involves use of alternative 5′ or 3′ splice sites, introns, and exons within a single pre-mRNA. Alternative splicing is widely used in many important biological processes, including cell fate determination, programmed cell death, learning and memory, and sex determination (116). For example, a subset of over 500 alternatively spliced transcripts of the Slowpoke (slo) gene are expressed in a gradient along the basilar papilla in the inner ear of birds. These isoforms allow response to various sound frequencies (82,93). Alternative splicing can potentially generate more than 38,000 isoforms of the Drosophila homologue of human Down syndrome cell adhesion molecule (DSCAM), which functions as an axon guidance receptor. Finally, alternative promoter use and splicing can generate over 1000 isoforms of neuroxin mRNA from three different genes that are important for synaptogenesis (111). The multitude of these isoforms should provide an underlying mechanism for the specificity of neural connectivity.
Sex determination in Drosophila is one of the best understood examples of splicing regulation. In Drosophila melanogaster, the key sex determining genes, Sex-lethal (Sxl), transformer (tra), and double-sex (dsx), are transcribed in an identical manner in both sexes. However, their sex-specific alternative splicing controls the sex of fruit flies (18,99) (Fig. 2). For additional details on splicing, the reader is directed to several excellent review articles (5,6,37,60,103).
Figure 2.
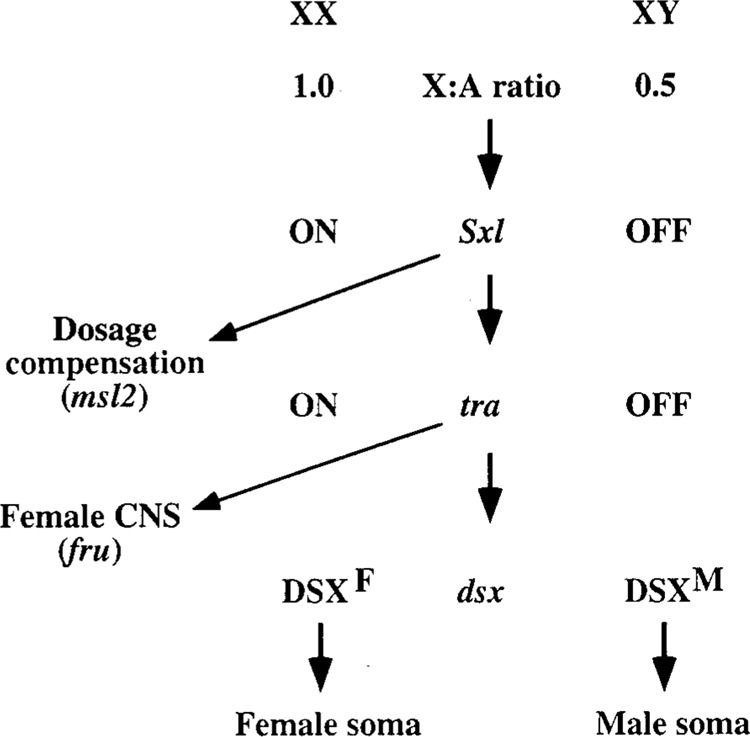
Sex determination pathway in Drosophila melanogaster. Only the genes that are known to be relevant for alternative splicing are shown. DSXM and DSXF are the male- and female-specific forms of the DSX protein. Sex-lethal, Sxl; transformer, tra; doublesex, dsx; male-specific lethal 2, msl2; and fruitless, fru.
MECHANISMS OF ALTERNATIVE SPLICING
In a general sense, splicing regulation involves activation of weak splice sites and repression of strong splice sites. This involves regulatory sequences within RNA through which trans-acting factors modulate splice site selection. From the known examples of alternative splicing, it is becoming increasingly evident that the majority of even simple decisions are orchestrated by the combined action of multiple factors. These include cell type-specific factors as well as those that are widely expressed. The issue of combinatorial control has been extensively discussed in a recent review (103). The examples discussed below demonstrate how multiple factors collaborate to confer cell type specificity and compensate for the paucity of information in splicing signals, which are short consensus sequences.
Exon Exclusion by cis-Acting Sequence
The Drosophila gene Sxl is transcribed from two promoters. The establishment promoter (Pe) responds to the X chromosome to autosome ratio early during development and is active for a short period only in females (18). These early transcripts skip by default the mate-specific exon, which contains a translation termination codon (Fig. 3A). The skipping most likely involves the masking of the splice sites by RNA secondary structures (44,136). Later during development, transcription switches to the maintenance promoter (Pm), which is active in both sexes. However, the late transcripts include the male-specific exon by default. The mechanism by which this exon is excluded from the late transcripts in females is discussed later.
Figure 3.
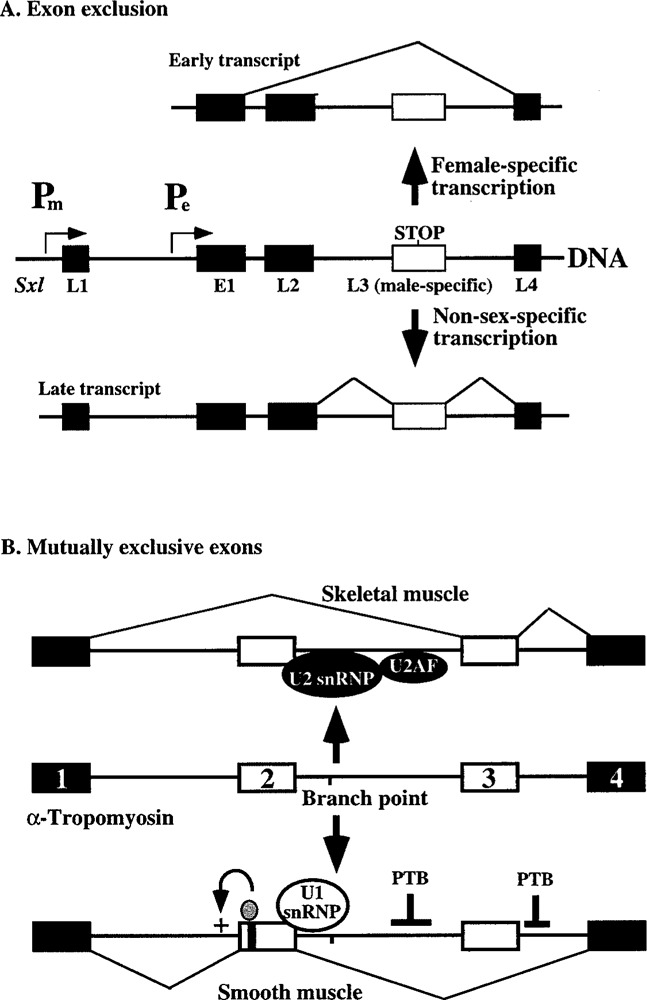
Role of RNA cis elements in alternative splicing. (A) The early transcripts of the Sxl gene exclude the male-specific exon by default (top). However, late transcripts include this exon by default (bottom). (B) Mutually exclusive exons. Recruitment of U2AF and U2 snRNP blocks the assembly of the spliceosome at the 5′ splice site of exon 2 (top). However, blockage of the 5′ and 3′ splice sites of exon 3 and activation of the 3′ splice site of exon 2 facilitate inclusion of exon 2 (bottom).
Mutually Exclusive Exons
Exons 2 and 3 of the α-tropomyosin pre-mRNA are mutually exclusive: either exon 2 or 3 but not both can be included in the processed mRNA. Nearly all cell types select exon 3, but the smooth muscle cells include exon 2 (78). The basis for this regulation is that the branch site associated with exon 3 is unconventionally located very close to the 5′ splice site of exon 2 (Fig. 3B). The recognition of polypyrimidine tract by the general splicing factor U2AF is one of the earliest steps in spliceosome assembly and the polypyrimidine tract of exon 3 is stronger than that of exon 2. Therefore, recruitment by U2AF of the U2 snRNP to the branch site of exon 3 hinders spliceosome assembly at the adjacent 5′ splice site, leading to the splicing of exons 1 and 3 in most cell types. The polypyrimidine tract binding protein (PTB), which binds to the negative regulatory sequences flanking exon 3, blocks the splicing of exon 3 (35,58,102). In addition, the activation of exon 2 also requires positive regulators that bind to enhancer sequences in this exon (28). Thus, differences in the strengths of the two polypyrimidine tracts, involvement of negative and positive regulators, and an unusual positioning of the branchpoint control the mutually exclusive splicing of exons 2 and 3 of α-tropomyosin in a cell-specific manner.
The role of PTB as a splicing repressor has been shown for several other pre-mRNAs: β-tropomyosin (77), c-src (14,17,69), FRGR (12,47), glycine receptor alpha 2 (88), MAP tau (124), GABA(A) receptor gamma 2 (2), and α-actinin (106). PTB plays a dual role in the inclusion of exon 4 of the human calcitonin/calcitonin gene-related peptide (CT/CGRP) mRNA by positively regulating polyadenylation and negatively regulating splicing (62,63). A neuronal isoform of PTB also plays an important role in the inclusion of the neuron-specific exons that are present in the GABA(A) receptor gamma 2, clathrin light chain B, N-methyl-D-aspartate receptor NR1, and c-src pre-mRNAs (69,133). Moreover, PTB has also been implicated in translation regulation (114).
5′ Splice Site Switching
The SV-40 pre-mRNA uses alternative 5′ splice sites for the synthesis of the large T antigen or the small t antigen. The alternative splicing factor (ASF), which was also independently purified as a splicing factor (SF2), activates the proximal 5′ splice site. It likely facilitates the recruitment of the U1 snRNP to both 5′ splice sites, but the downstream splice site is favored because of its proximity to the 3′ splice site (32,53). In contrast, the heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) promotes the use of the distal 5′ splice site and exon skipping (7,11,40,71,128) (Fig. 4A). The exact mechanism of hnRNP A1 action, however, remains poorly understood.
Figure 4.
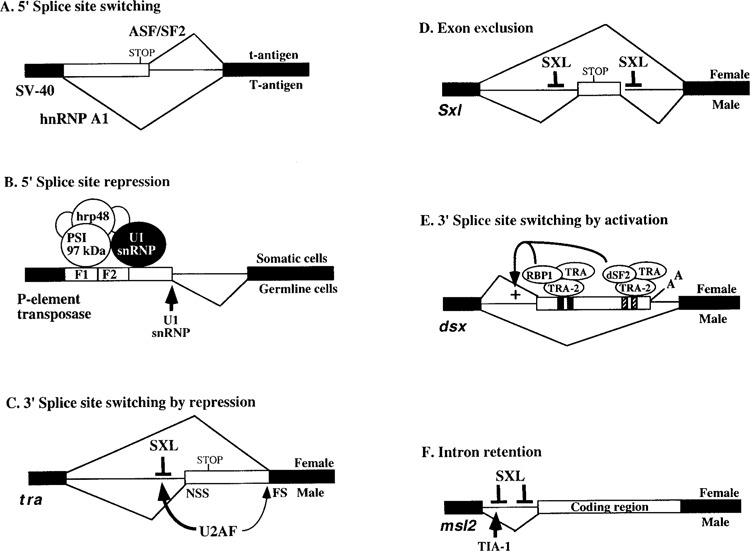
Role of trans-acting factors in alternative splicing. (A) Alternative 5′ splice site selection of the SV-40 pre-mRNA by ASF/SF2 and hnRNP A1. (B) 5′ splice site repression of the Drosophila P element intron by a large complex assembled onto the pseudo 5′ splice sites (F1 and F2) in somatic cells. (C) 3′ splice site switching of the tra pre-mRNA. SXL directly competes with U2AF for the NSS polypyrimidine-tract/3′ splice and mediates 3′ splice site switching in females. (D) Exon exclusion by SXL during splicing autoregulation. The binding of SXL to uridine-rich sequences in the flanking introns mediates skipping of the male-specific exon in its own pre-mRNA. (E) 3′ splice site activation of the dsx pre-mRNA by TRA, TRA2, RBP1, and dSF2 proteins, which assemble on the exonic splicing enhancers. The site of polyadenylation for female-specific transcripts is also shown. (F) Intron retention in msl2 by SXL, which is a negative regulator. Removal of this intron in males requires TIA-1, which is a positive regulator. Alternative splicing patterns are shown by thin lines below and above the transcripts. The names of the genes are indicated below the transcripts on the left. Boxes, exons; horizontal lines, introns; STOP, translation termination codon.
The osmotic shock or the ultraviolet irradiation activates the signaling cascade that phosphorylates and relocalizes the hnRNP A1 protein from the nucleus to the cytoplasm. The relocalization of hnRNP A1 activates proximal splice sites (119), which is consistent with the role of hnRNP A1 in the activation of distal splice sites.
One of the few known examples of alternative splicing in Saccharomyces cerevisiae is the MER2 protein, which is necessary for meiotic recombination. Synthesis of functional MER2 occurs only during meiosis and involves removal of an intron. This process requires a meiosis-specific protein called MER1. The 5′ splice site of the MER2 intron differs from the consensus sequence. Mutation of this 5′ splice site to the consensus or extension of the base pairing between the 5′ splice site and the U1 snRNA relieves dependence on the MER1 protein, suggesting that MER1 facilitates an otherwise weak base pairing interaction between the U1 snRNA and the 5′ splice site (80,81). The MER1 protein is also required for the splicing regulation of MER3 (79) and SP070 (24). However, the nature of the MER1 responsive element in these target mRNAs remained undefined. Recently, Ares and coworkers have identified an intronic splicing enhancer (AYACCCUY, where Y is pyrimidine) downstream of the 5′ splice site, which mediates MER1 response (107).
5′ Splice Site Repression
The Drosophila transposon, P element, transposes in the germline cells but not in the somatic cells. This is because intron 3 of the transposase pre-mRNA is not removed in somatic cells, which prevents the synthesis of transposase. Several factors, including the P element somatic inhibitor (PSI) and hrp48 (a Drosophila homologue of hnRNP A1), form a large complex on nearby pseudo 5′ splice sites (Fig. 4B). This complex prevents the accessibility of the U1 snRNP to the correct 5′ splice site in somatic cells (38,100). Failure to form this nonproductive complex allows removal of this intron and synthesis of transposase in germline cells.
3′ Splice Site Switching by Repression
SXL controls the 3′ splice site choice of the tra pre-mRNA. This allows the synthesis of functional TRA protein only in females, by skipping an in-frame translation stop codon. The general splicing factor U2AF65 has a higher affinity for the non-sex-specific (NSS) polypyrimidine tract/3′ splice site rather than the alternative, female-specific (FS) polypyrimidine tract/3′ splice site. In addition, the proximity of the NSS polypyrimidine tract/3′ splice site to the 5′ splice site also contributes to the default splicing pattern in males. In females, the SXL protein mediates 3′ splice site switching by specifically competing with the binding of U2AF65 to the NSS polypyrimidine tract/3′ splice, thereby diverting U2AF65 to the lower affinity FS polypyrimidine tract/3′ splice site in vitro (Fig. 4C) (117). The regulation of tra by SXL in vivo may require additional interactions (129). The SXL binding site of tra is a uridine-rich sequence, which has been extensively characterized by using multiple approaches [(101), and references therein].
Exon Exclusion by trans-Acting Factors
Once SXL is synthesized from the early transcripts in females, which was discussed earlier, it maintains its synthesis by feedback regulation. SXL binds to uridine-rich sequences present in the introns flanking its own male-specific exon, which has a translation stop codon. The binding of SXL to these sequences allows exon skipping and thus continued synthesis of functional SXL protein from the late transcripts (Fig. 4D) (36,45,98). SXL regulation requires cofactors that are encoded by snf, fl(2)d, and vir genes (99); the SNF protein is a component of both the U1 and the U2 snRNPs and may be a direct target of SXL (26). The amino-terminal domain of SXL is required for the regulation of Sxl but not tra (36,122). Deletion of the amino-terminal region of SXL can uncouple the splicing and translation regulation functions of SXL (129).
Another example of this type of regulation is found in the c-src gene in which a short exon (N1) of 18 nucleotides is excluded in nonneuronal cells and included in neuronal cells. The PTB binding sites in the introns flanking the N1 exon favor its exclusion in nonneuronal cells. Several proteins, including a neuronal isoform of PTB (nPTB), KSRP, hnRNP F and H, collaborate for the inclusion of N1 in neuronal cells (15–17,69,73–75).
3′ Splice Site Switching by Activation
In the absence of TRA, the Drosophila female-specific exon of dsx is skipped because it has a weak polypyrimidine tract. However, in females TRA, along with other proteins (TRA2, RBP1, and dSF2), binds to the exonic splicing enhancers (six 13-nucleo-tide repeats and a purine-rich sequence) in the female-specific exon and activates an otherwise weak 3′ splice site (Fig. 4E). Improving the strength of this weak polypyrimidine tract or shortening the distance between the 3′ splice site and the enhancer alleviates the requirement of TRA (42,66). Moreover, TRA also activates an alternative 5′ splice site in the fruitless pre-mRNA, which controls courtship behavior in fruit flies (97).
Intron Retention
Dosage compensation is a process by which essentially all heterogametic organisms equalize the expression of their X-chromosome-linked genes. In Drosophila, it involves hypertranscription of genes on the single X-chromosome in males by several essential factors, including the MSL2 protein (31,109). SXL prevents the removal of the intron in the 5′ non-coding region of the msl2 transcripts by specifically binding to uridine-rich sequences adjacent to both splice sites (3,33,52,134). The spacing between the 3′ splice site AG dinucleotide and the polypyrimidine tract, which is one of the SXL binding sites, is also crucial for this regulation (30). Furthermore, binding of SXL to the uridine-rich sequences in the retained intron blocks translation in females. Thus, SXL also controls msl2 expression at the level of translation by a nonsplicing mechanism.
The splicing of msl2 is also under positive regulation by the apoptosis promoting factor TIA-1, which prefers uridine-rich sequences (25). TIA-1 binds to the uridine-rich sequences downstream of the 5′ splice site to which SXL also binds. TIA-1 is required for the activation of otherwise weak 5′ splice sites of msl2 as well as the human apoptotic gene Fas. TIA-1 shows structural and functional similarities with the Saccharomyces cerevisiae splicing factor Nam8, suggesting conservation of splicing mechanisms in diverse processes such as meiosis, dosage compensation, and programmed cell death (30).
ADDITIONAL WAYS TO REGULATE SPLICING
There are also others ways to alter splice site choice.
Posttranslational Modification
The adenovirus encoded protein ORF4 promotes dephosphorylation and thus inactivation of the HeLa SR protein, which controls the temporal shift in adenoviral alternative splicing (50). Similarly, phosphorylation of the hnRNP A1 protein promotes its relocalization from the nucleus to the cytoplasm, which activates proximal splice sites (119).
Protein Sequestration
Expansion of the CUG repeat in the 3′ untranslated region of the myotonic dystrophy, DM, gene sequesters the conserved heterogeneous nuclear ribonucleoprotein, CUG-binding protein (CUG-BP), which disrupts splicing and likely contributes to myotonic dystrophy pathogenesis (87).
Transcriptional Control
Transcription pausing can facilitate inclusion of an exon that is normally skipped (92), and a promoter-specific RNA polymerase complex can modulate alternative splicing by recruiting specific splicing factors (ASF/SF2) to nascent transcripts (23).
RNA Editing
The double-stranded RNA-specific adenosine deaminase (ADAR2) autoregulates its own synthesis by mRNA editing. The conversion of an intronic adenosine to inosine (AA to AI) generates a novel 3′ splice site because an inosine mimics the guanosine of the 3′ splice site AG (96).
EXON DEFINITION
The mammalian exons are usually short (<300 nucleotides) and introns can be rather long, up to 100,000 nucleotides. Thus, identification of exons or splice sites can be very challenging for the splicing machinery. Normally, splicing factors bound at the 5′ and the 3′ ends of an intron interact with each other as a part of spliceosome assembly (interaction “b” in Fig. 5). However, Berget and coworkers observed that a downstream 5′ splice site led to the activation of a weak 3′ splice site upstream of an exon. This observation led to the proposal for “exon definition model” in which molecular interactions across an exon rather than across an intron can help initially define splice sites (interaction “a” in Fig. 5) (4), although once splice sites are selected, the spliceosome assembly would proceed normally. The serine-arginine-rich (SR) family of proteins plays an important role in these cross-exon interactions (8,67,103,115).
Figure 5.
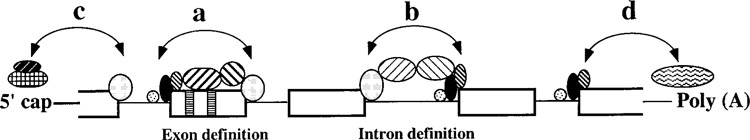
Exon definition. For internal exons, initial recognition of the splice sites may be facilitated by interactions among splicing factors either (a) across exons or (b) across intron. For terminal exons, initial recognition of the splice sites may involve interactions with either (c) the cap binding complex at the 5′ end or (d) the polyadenylation machinery at the 3′ end. The vertical bars within box are splicing enhancers. Circles and ovals are snRNPs, splicing factors/activators, cap binding complex, or polyadenylation machinery.
Unlike internal exons, which have both splicing partners, the terminal introns lack one of the partners, either the 5′ or the 3′ splice site. For the terminal exons, interactions of the splicing machinery with the nuclear cap binding complex at the 5′ end of mRNA (interaction “c” in Fig. 5) (46,57) or with the polyadenylation machinery at the 3′ end (interaction “d” in Fig. 5) (64,84,85,112,123) could define the first and the last splice sites, respectively. Although plant introns are generally short, splicing of several Arabi-dopsis introns is also thought to involve exon definition (61). In summary, several factors are expected to define whether such initial interactions would occur across exons or introns; for example, the strength of splicing signals, length of introns, and location and number of binding sites for various regulators around the splice sites.
RECOGNITION OF THE POLYPYRIMIDINE TRACT
In metazoans, the polypyrimidine tract present adjacent to the 3′ splice site is an important signal for both constitutive and regulated pre-mRNA splicing. Structure–function analysis has revealed important insights into the molecular functions of two of the polypyrimidine tract binding proteins, U2AF and SXL.
Function of the Large Subunit of U2AF65
The large subunit of U2AF is essential for splicing in vitro and viability in vivo (49,89,132,138). It recognizes a diverse array of polypyrimidine tracts and has two functional domains: a carboxyl-terminal RNA binding domain and an amino-terminal activation domain, which includes a region rich in arginine and serine residues (RS domain) (132). The RS domain of U2AF65 functions at least in part by promoting an otherwise weak base pairing between the pre-mRNA branch site and U2 snRNA, which is an essential step in splicing (113). Fusion of the activation domain of U2AF65 to the RNA binding domain of SXL, a repressor, converted it into an activator (117). U2AF also interacts with other splicing factors, UAP-56, U2AF35, BBP/mBBP, and SAP-155 (91). All of these activities of U2AF likely contribute to the recruitment of the U2 snRNP to pre-mRNA. Interestingly, the RS domain on either the large (dU2AF50) or the small (dU2AF38) subunit of the Drosophila U2AF is sufficient for function (94,95), suggesting a redundant role for the RS domain. The RS (or SR) domain, which is present in several splicing factors, is also known to mediate protein–protein interactions (67,115).
Function of the Small Subunit of U2AF35
For several years, the function of the small subunit of U2AF (U2AF35) remained a mystery because the large subunit alone was found sufficient for U2AF function in vitro. Recently, three independent groups showed that the small subunit of U2AF contacts the 3′ splice site AG. First, both subunits of U2AF are required for binding to 3′ splice sites in C. elegans, which comprise a short polypyrimidine tract followed by an AG dinucleotide (UUUUCAG) (137). Second, the AG dinucleotide and the polypyrimidine tract of msl2 are spaced apart, which is crucial for regulation. The spacing reduces the affinity for U2AF, because of the missing contacts between the small subunit of U2AF and the 3′ splice site AG dinculeotide. Thus, SXL effectively competes with U2AF for intron retention (72). Third, an iterative selection–amplification experiment revealed that the U2AF heterodimer selected a uridine-rich sequence followed by an AG dinucleotide from a random pool of RNA (127), but the large subunit alone selected only the uridine-rich sequence (102). Thus, U2AF35 appears to facilitate the recognition of the 3′ splice site AG dinucleotide and is likely relevant for the splicing of regulated 3′ splice sites and the AG-dependent introns, which are characterized by weak polypyrimidine tracts (91).
Polypyrimidine Tract Recognition
Both SXL and U2AF65 contain ribonucleoprotein-consensus RNA binding motifs (RNP-CS) (9,70). This motif appears to be a versatile RNA binding fold and recognizes various kinds of RNA targets—structured and unstructured (120). Whereas U2AF65 recognizes a wide variety of polypyrimidine tracts, SXL recognizes specific polypyrimidine tracts. U2AF65 and SXL appear to interact with the polypyrimidine tract differently, which may provide at least in part the basis for the different RNA binding specificities of U2AF65 and SXL (101). The X-ray structure of SXL with its target RNA (39) has offered the basis for polypyrimidine tract recognition, which is an unstructured RNA sequence. In particular, extensive intra- and intermolecular interactions with backbone sugars and phosphates and with the N3 and O4 positions of several uracil residues provide at least in part the structural basis for the preferential binding of SXL to uridine-rich sequences (39,48,101).
COUPLING BETWEEN SPLICING AND OTHER PROCESSES
Over the years, many of the aspects of RNA biogenesis/processing have been studied as individual processes. Now there is ample evidence that several of these cellular processes are, in fact, integrated. For example, the splicing machinery communicates with the transcription, capping, polyadenylation, and RNA export machineries (43,90,135). Moreover, the splicing process has been linked to nonsense-mediated mRNA decay (NMD), which is a surveillance system for discarding defective mRNAs (41,68). NMD targets the mRNAs that have an in-frame translation stop codon located upstream of the last exon–exon junction, suggesting a link to translation. Identification of several proteins—the splicing-associated factors SRm160, DEK, and RNPS1, the mRNA shuttling protein Y14, and the mRNA export factor REF—that are deposited at the site of splicing or the exon–exon junctions and the human Upf proteins could provide the link between splicing and sensing of premature termination codons (51,55,56,65). In addition, nonsense mutations were linked to exon skipping or nonsense-mediated altered splicing (NAS) (27,118), suggesting a nuclear mechanism for stop codon recognition. However, Krainer and coworkers demonstrated that the NAS phenomenon for the BRCA1 gene results from the disruption of a splicing enhancer in the exon, which argues against a nuclear reading-frame scanning mechanism (59). These examples provide an overwhelming case for the integration of various cellular processes at the molecular level.
FUTURE CHALLENGES
Availability of the entire genome sequences from a number of organisms and of the powerful DNA microarray technology provides an excellent opportunity and a challenge towards understanding the basis for splice site choice on a global rather than a single gene basis. This is particularly important because regulation in more complex organisms involves a network of interactions among multiple factors. Thus, combinatorial control is likely to provide an important source of versatility, specificity, and complexity of gene regulation in more complex organisms.
ACKNOWLEDGMENTS
I thank Tin Tin Su, Massimo Buvoli, William Davis, Andrew Rahn, and Greg Odorizzi for critical comments on the manuscript. I apologize to those colleagues whose work could not be cited more directly because of space limitations. This work was supported by grants from the National Institutes of Health and the Basil O’Connor Starter Scholar Research Award from the March of Dimes.
REFERENCES
- 1. Adams M. D.; Celniker S. E.; Holt R. A.; et al. The genome sequence of Drosophila melanogaster . Science 287:2185–2195; 2000. [DOI] [PubMed] [Google Scholar]
- 2. Ashiya M.; Grabowski P. J. A neuron-specific splicing switch mediated by an array of pre-mRNA repressor sites: Evidence of a regulatory role for the polypyrimidine tract binding protein and a brain-specific PTB counterpart. RNA 3:996–1015; 1997. [PMC free article] [PubMed] [Google Scholar]
- 3. Bashaw G. J.; Baker B. S. The regulation of the Drosophila msi-2 gene reveals a function for Sex-lethal in translational control. Cell 89:789–798; 1997. [DOI] [PubMed] [Google Scholar]
- 4. Berget S. M. Exon recognition in vertebrate splicing. J. Biol. Chem. 270:2411–2414; 1995. [DOI] [PubMed] [Google Scholar]
- 5. Black D. L. Protein diversity from alternative splicing: A challenge for bioinformatics and post-genome biology. Cell 103:367–370; 2000. [DOI] [PubMed] [Google Scholar]
- 6. Black D. L. Splicing in the inner ear: A familiar tune, but what are the instruments? Neuron 20:165–168; 1998. [DOI] [PubMed] [Google Scholar]
- 7. Blanchette M.; Chabot B. Modulation of exon skipping by high-affinity hnRNP A1-binding sites and by intron elements that repress splice site utilization. EMBO J. 18:1939–1952; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blencowe B. J. Exonic splicing enhancers: Mechanism of action, diversity and role in human genetic diseases. Trends Biochem. Sci. 25:106–110; 2000. [DOI] [PubMed] [Google Scholar]
- 9. Burd C. G.; Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science 265:615–621; 1994. [DOI] [PubMed] [Google Scholar]
- 10. Burge C. B.; Tuschl T.; Sharp P. A. Splicing of precursors to mRNAs by the spliceosomes, In: Gesteland R. F.; Cech T. R.; Atkins J. F. , eds. The RNA world, 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1999:525–560. [Google Scholar]
- 11. Caceres J. F.; Stamm S.; Helfman D. M.; Krainer A. R. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science 265:1706–1709; 1994. [DOI] [PubMed] [Google Scholar]
- 12. Carstens R. P.; Wagner E. J.; Garcia-Blanco M. A. An intronic splicing silencer causes skipping of the IIIb exon of fibroblast growth factor receptor 2 through involvement of polypyrimidine tract binding protein. Mol. Cell. Biol. 20:7388–7400; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cech T. R.; Golden B. L. Building a catalytic active site using only RNA. In: Gesteland R. F.; Cech T. R.; Atkins J. F., eds. The RNA world, 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1999:321–349. [Google Scholar]
- 14. Chan R. C.; Black D. L. Conserved intron elements repress splicing of a neuron-specific c-src exon in vitro. Mol. Cell. Biol. 15:6377–6385; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan R. C.; Black D. L. The polypyrimidine tract binding protein binds upstream of neural cell-specific c-src exon N1 to repress the splicing of the intron downstream. Mol. Cell. Biol. 17:4667–4676; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chou M. Y.; Rooke N.; Turek C. W.; Black D. L. hnRNP H is a component of a splicing enhancer complex that activates a c-src alternative exon in neuronal cells. Mol. Cell. Biol. 19:69–77; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chou M. Y.; Underwood J. G.; Nikolic J.; Luu M. H.; Black D. L. Multisite RNA binding and release of polypyrimidine tract binding protein during the regulation of c-src neural-specific splicing. Mol. Cell 5:949–957; 2000. [DOI] [PubMed] [Google Scholar]
- 18. Cline T. W.; Meyer B. J. Vive la difference: Males vs females in flies vs worms. Annu. Rev. Genet. 30:637–702; 1996. [DOI] [PubMed] [Google Scholar]
- 19. Collins C. A.; Guthrie C. The question remains: Is the spliceosome a ribozyme? Nat. Struct. Biol. 7:850–854; 2000. [DOI] [PubMed] [Google Scholar]
- 20. The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 282:2012–2018; 1998. [DOI] [PubMed] [Google Scholar]
- 21. International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature 409:860–921; 2001. [DOI] [PubMed] [Google Scholar]
- 22. Cooper T. A.; Mattox W. The regulation of splice-site selection, and its role in human disease. Am. J. Hum. Genet. 61:259–266; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cramer P.; Caceres J. F.; Cazalla D.; Kadener S.; Muro A. F.; Baralle F. E.; Komblihtt A. R. Coupling of transcription with alternative splicing: RNA pol II promoters modulate SF2/ASF and 9G8 effects on an exonic splicing enhancer. Mol. Cell 4:251–258; 1999. [DOI] [PubMed] [Google Scholar]
- 24. Davis C. A.; Grate L.; Spingola M.; Ares M. Jr. Test of intron predictions reveals novel splice sites, alternatively spliced mRNAs and new introns in meiotically regulated genes of yeast. Nucleic Acids Res. 28:1700–1706; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dember L. M.; Kim N. D.; Liu K. Q.; Anderson P. Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J. Biol. Chem. 271:2783–2788; 1996. [DOI] [PubMed] [Google Scholar]
- 26. Deshpande G.; Samuels M. E.; Schedi P. D. Sex-lethal interacts with splicing factors in vitro and in vivo. Mol. Cell. Biol. 16:5036–5047; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dietz H. C. Nonsense mutations and altered splice-site selection. Am. J. Hum. Genet. 60:729–730; 1997. [PMC free article] [PubMed] [Google Scholar]
- 28. Dye B. T.; Buvoli M.; Mayer S. A.; Lin C. H.; Patton J. G. Enhancer elements activate the weak 3′ splice site of alpha-tropomyosin exon 2. RNA 4:1523–1536; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feig A. L.; Uhlenbeck O. C. The role of metal ions in RNA biochemistry. In: Gesteland R. F.; Cech T. R.; Atkins J. F., eds. The RNA world, 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1999:287–319. [Google Scholar]
- 30. Forch P.; Puig O.; Kedersha N.; Martinez C.; Granneman S.; Seraphin B.; Anderson P.; Valcarcel J. The apoptosis-promoting factor TIA-1 is a regulator of alternative pre-mRNA splicing. Mol. Cell 6:1089–1098; 2000. [DOI] [PubMed] [Google Scholar]
- 31. Franke A.; Baker B. S. Dosage compensation rox! Curr. Opin. Cell. Biol. 12:351–354; 2000. [DOI] [PubMed] [Google Scholar]
- 32. Ge H.; Manley J. L. A protein factor, ASF, controls cell-specific alternative splicing of SV40 early pre-mRNA in vitro. Cell 62:25–34; 1990. [DOI] [PubMed] [Google Scholar]
- 33. Gebauer F.; Merendino L.; Hentze M. W.; Valcarcel J. The Drosophila splicing regulator sex-lethal directly inhibits translation of male-specific-lethal 2 mRNA. RNA 4:142–150; 1998. [PMC free article] [PubMed] [Google Scholar]
- 34. Gelfand M. S.; Dubchak I.; Dralyuk I.; Zorn M. ASDB: Database of alternatively spliced genes. Nucleic Acids Res. 27:301–302; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gooding C.; Roberts G. C.; Smith C. W. Role of an inhibitory pyrimidine element and polypyrimidine tract binding protein in repression of a regulated alpha-tropomyosin exon. RNA 4:85–100; 1998. [PMC free article] [PubMed] [Google Scholar]
- 36. Granadino B.; Penalva L. O. F.; Green M. R.; Valcarcel J.; Sanchez L. Distinct mechanisms of splicing regulation in vivo by the Drosophila protein Sex-lethal. Proc. Natl. Acad. Sci. USA 94:7343–7348; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Graveley B. R. Alternative splicing: Increasing diversity in the proteomic world. Trends Genet. 17:100–107; 2001. [DOI] [PubMed] [Google Scholar]
- 38. Hammond L. E.; Rudner D. Z.; Kanaar R.; Rio D. C. Mutations in the hrp48 gene, which encodes a Drosophila heterogeneous nuclear ribonucleoprotein particle protein, cause lethality and developmental defects and affect P-element third-intron splicing in vivo. Mol. Cell. Biol. 17:7260–7267; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Handa N.; Nureki O.; Kurimoto K.; Kim I.; Sakamoto H.; Shimura Y.; Muto Y.; Yokoyama S. Structural basis for recognition of the tra mRNA precursor by the Sex-lethal protein. Nature 398:579–585; 1999. [DOI] [PubMed] [Google Scholar]
- 40. Harper J. E.; Manley J. L. A novel protein factor is required for use of distal alternative 5′ splice sites in vitro. Mol. Cell. Biol. 11:5945–5953.; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hentze M. W.; Kulozik A. E. A perfect message: RNA surveillance and nonsense-mediated decay. Cell 96:307–310; 1999. [DOI] [PubMed] [Google Scholar]
- 42. Hertel K. J.; Lynch K. W.; Maniatis T. Common themes in the function of transcription and splicing enhancers. Curr. Opin. Cell. Biol. 9:350–357; 1997. [DOI] [PubMed] [Google Scholar]
- 43. Hirose Y.; Manley J. L. RNA polymerase II and the integration of nuclear events. Genes Dev. 14:1415–1429; 2000. [PubMed] [Google Scholar]
- 44. Horabin J. I.; Bopp D.; Waterbury J.; Schedl P. Selection and maintenance of sexual identity in the Drosophila germline. Genetics 141:1521–1535; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horabin J. I.; Schedl P. Sex-lethal autoregulation requires multiple cis-acting elements upstream and downstream of the male exon and appears to depend largely on controlling the use of the male exon 5′ splice site. Mol. Cell. Biol. 13:7734–7746; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Izaurraide E.; Lewis J.; McGuigan C.; Jankowska M.; Darzynkiewicz E.; Mattaj 1. W. A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell 78:657–668; 1994. [DOI] [PubMed] [Google Scholar]
- 47. Jin W.; McCutcheon I. E.; Fuller G. N.; Huang E. S.; Cote G. J. Fibroblast growth factor receptors alpha-exon exclusion and polypyrimidine tract-binding protein in glioblastoma multiforme tumors. Cancer Res. 60:1221–1224; 2000. [PubMed] [Google Scholar]
- 48. Kanaar R.; Lee A. L.; Rudner D. Z.; Wemmer D. E.; Rio D. C. Interaction of the sex-lethal RNA binding domains with RNA. EMBO J. 14:4530–4539; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kanaar R.; Roche S. E.; Beall E. L.; Green M. R.; Rio D. C. The conserved pre-mRNA splicing factor U2AF from Drosophila: Requirement for viability. Science 262:569–573; 1993. [DOI] [PubMed] [Google Scholar]
- 50. Kanopka A.; Muhlemann O.; Petersen-Mahrt S.; Estmer C.; Ohrmalm C.; Akusjarvi G. Regulation of adenovirus alternative RNA splicing by dephosphorylation of SR proteins. Nature 393:185–187; 1998. [DOI] [PubMed] [Google Scholar]
- 51. Kataoka N.; Yong J.; Kim V. N.; Velazquez F.; Perkinson R. A.; Wang F.; Dreyfuss G. Pre-mRNA splicing imprints MRNA in the nucleus with a novel RNA-binding protein that persists in the cytoplasm. Mol. Cell 6:673–682; 2000. [DOI] [PubMed] [Google Scholar]
- 52. Kelley R. L.; Wang J.; Bell L.; Kuroda M. I. Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature 387:195–199; 1997. [DOI] [PubMed] [Google Scholar]
- 53. Krainer A. R.; Conway G. C.; Kozak D. The essential pre-mRNA splicing factor SF2 influences 5′ splice site selection by activating proximal sites. Cell 62:35–42; 1990. [DOI] [PubMed] [Google Scholar]
- 54. Krawczak M.; Reiss J.; Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: Causes and consequences. Hum. Genet. 90:41–54; 1992. [DOI] [PubMed] [Google Scholar]
- 55. Le Hir H.; Izaurraide E.; Maquat L. E.; Moore M. J. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon–exon junctions. EMBO J. 19:6860–6869; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Le Hir H.; Moore M. J.; Maquat L. E. Pre-mRNA splicing alters mRNP composition: Evidence for stable association of proteins at exon–exon junctions. Genes Dev. 14:1098–1108; 2000. [PMC free article] [PubMed] [Google Scholar]
- 57. Lewis J. D.; Izaurralde E.; Jarmolowski A.; McGuigan C.; Mattaj I. W. A nuclear cap-binding complex facilitates association of U1 snRNP with the cap-proximal 5′ splice site. Genes Dev. 10:1683–1698; 1996. [DOI] [PubMed] [Google Scholar]
- 58. Lin C. H.; Patton J. G. Regulation of alternative 3′ splice site selection by constitutive splicing factors. RNA 1:234–245; 1995. [PMC free article] [PubMed] [Google Scholar]
- 59. Liu H. X.; Cartegni L.; Zhang M. Q.; Krainer A. R. A mechanism for exon skipping caused by nonsense or missense mutations in BRCA1 and other genes. Nat. Genet. 27:55–58; 2001. [DOI] [PubMed] [Google Scholar]
- 60. Lopez A. J. Alternative splicing of pre-mRNA: Developmental consequences and mechanisms of regulation. Annu. Rev. Genet. 32:279–305; 1998. [DOI] [PubMed] [Google Scholar]
- 61. Lorkovic Z. J.; Wieczorek Kirk D. A.; Lambermon M. H.; Filipowicz W. Pre-mRNA splicing in higher plants. Trends Plant Sci. 5:160–167; 2000. [DOI] [PubMed] [Google Scholar]
- 62. Lou H.; Gagel R. F.; Berget S. M. An intron enhancer recognized by splicing factors activates polyadenylation. Genes Dev. 10:208–219; 1996. [DOI] [PubMed] [Google Scholar]
- 63. Lou H.; Helfman D. M.; Gagel R. F.; Berget S. M. Polypyrimidine tract-binding protein positively regulates inclusion of an alternative 3′-terminal exon. Mol. Cell. Biol. 19:78–85; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lutz C. S.; Alwine J. C. Direct interaction of the U1 snRNP-A protein with the upstream efficiency element of the SV40 late polyadenylation signal. Genes Dev. 8:576–586; 1994. [DOI] [PubMed] [Google Scholar]
- 65. Lykke-Andersen J.; Shu M.; Steitz J. A. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell 103:1121–1131; 2000. [DOI] [PubMed] [Google Scholar]
- 66. Lynch K. W.; Maniatis T. Assembly of specific SR protein complexes on distinct regulatory elements of the Drosophila doublesex splicing enhancer. Genes Dev. 10:2089–2101; 1996. [DOI] [PubMed] [Google Scholar]
- 67. Manley J. L.; Tacke R. SR proteins and splicing control. Genes Dev. 10:1569–1579; 1996. [DOI] [PubMed] [Google Scholar]
- 68. Maquat L. E. Defects in RNA splicing and the consequence of shortened translational reading frames. Am. J. Hum. Genet. 59:279–286; 1996. [PMC free article] [PubMed] [Google Scholar]
- 69. Markovtsov V.; Nikolic J. M.; Goldman J. A.; Turck C. W.; Chou M. Y.; Black D. L. Cooperative assembly of an hnRNP complex induced by a tissue-specific homolog of polypyrimidine tract binding protein. Mol. Cell. Biol. 20:7463–7479; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mattaj I. W. RNA recognition: A family matter? Cell 73:837–840; 1993. [DOI] [PubMed] [Google Scholar]
- 71. Mayeda A.; Krainer A. R. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell 68:365–375; 1992. [DOI] [PubMed] [Google Scholar]
- 72. Merendino L.; Guth S.; Bilbao D.; Martinez C.; Valearcel J. Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature 402:838–841; 1999. [DOI] [PubMed] [Google Scholar]
- 73. Min H.; Chan R. C.; Black D. L. The generally expressed hnRNP F is involved in a neural-specific pre-mRNA splicing event. Genes Dev. 9:2659–2671; 1995. [DOI] [PubMed] [Google Scholar]
- 74. Min H.; Turck C. W.; Nikolic J. M.; Black D. L. A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev. 11:1023–1036; 1997. [DOI] [PubMed] [Google Scholar]
- 75. Modafferi E. F.; Black D. L. Combinatorial control of a neuron-specific exon. RNA 5:687–706; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Moore M. J.; Sharp P. A. Evidence for two active sites in the spliceosome provided by stereochemistry of pre-mRNA splicing. Nature 365:364–368; 1993. [DOI] [PubMed] [Google Scholar]
- 77. Mulligan G. J.; Guo W.; Wormsley S.; Helfman D. M. Polypyrimidine tract binding protein interacts with sequences involved in alternative splicing of beta-tropomyosin pre-mRNA. J. Biol. Chem. 267:25480–25487; 1992. [PubMed] [Google Scholar]
- 78. Nadal-Ginard B.; Smith C. W.; Patton J. G.; Breitbart R. E. Alternative splicing is an efficient mechanism for the generation of protein diversity: Contractile protein genes as a model system. Adv. Enzyme Regul. 31:261–286; 1991. [DOI] [PubMed] [Google Scholar]
- 79. Nakagawa T.; Ogawa H. The Saccharomyces cere-visiae MER3 gene, encoding a novel helicase-like protein, is required for crossover control in meiosis. EMBO J. 18:5714–5723; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nandabalan K.; Price L.; Roeder G. S. Mutations in U1 snRNA bypass the requirement for a cell type-specific RNA splicing factor. Cell 73:407–415; 1993. [DOI] [PubMed] [Google Scholar]
- 81. Nandabalan K.; Roeder G. S. Binding of a cell-type-specific RNA splicing factor to its target regulatory sequence. Mol. Cell. Biol. 15:1953–1960; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Navaratnam D. S.; Bell T. J.; Tu T. D.; Cohen E. L.; Oberholtzer J. C. Differential distribution of Ca2+-activated K+ channel splice variants among hair cells along the tonotopic axis of the chick cochlea. Neuron 19:1077–1085; 1997. [DOI] [PubMed] [Google Scholar]
- 83. Nilsen T. W. The case for an RNA enzyme. Nature 408:782–783; 2000. [DOI] [PubMed] [Google Scholar]
- 84. Niwa M.; Berget S. M. Mutation of the AAUAAA polyadenylation signal depresses in vitro splicing of proximal but not distal introns. Genes Dev. 5:2086–2095; 1991. [DOI] [PubMed] [Google Scholar]
- 85. Niwa M.; Rose S. D.; Berget S. M. In vitro polyadenylation is stimulated by the presence of an upstream intron. Genes Dev. 4:1552–1559; 1990. [DOI] [PubMed] [Google Scholar]
- 86. Padgett R. A.; Podar M.; Boulanger S. C.; Perlman P. S. The stereochemical course of group II intron self-splicing. Science 266:1685–1688; 1994. [DOI] [PubMed] [Google Scholar]
- 87. Philips A. V.; Timchenko L. T.; Cooper T. A. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280:737–741; 1998. [DOI] [PubMed] [Google Scholar]
- 88. Polydorides A. D.; Okano H. J.; Yang Y. Y.; Stefani G.; Darnell R. B. A brain-enriched polypyrimidine tract-binding protein antagonizes the ability of Nova to regulate neuron-specific alternative splicing. Proc. Natl. Acad. Sci. USA 97:6350–6355; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Potashkin J.; Naik K.; Wentz-Hunter K. U2AF homolog required for splicing in vivo. Science 262:573–575; 1993. [DOI] [PubMed] [Google Scholar]
- 90. Proudfoot N. Connecting transcription to messenger RNA processing. Trends Biochem. Sci. 25:290–293; 2000. [DOI] [PubMed] [Google Scholar]
- 91. Reed R. Mechanisms of fidelity in pre-mRNA splicing. Curr. Opin. Cell. Biol. 12:340–345; 2000. [DOI] [PubMed] [Google Scholar]
- 92. Roberts G. C.; Gooding C.; Mak H. Y.; Proudfoot N. J.; Smith C. W. Co-transcriptional commitment to alternative splice site selection. Nucleic Acids Res. 26:5568–5572; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rosenblatt K. P.; Sun Z. P.; Heller S.; Hudspeth A. J. Distribution of Ca2+-activated K+ channel isoforms along the tonotopic gradient of the chicken’s cochlea. Neuron 19:1061–1075; 1997. [DOI] [PubMed] [Google Scholar]
- 94. Rudner D. Z.; Breger K. S.; Kanaar R.; Adams M. D.; Rio D. C. RNA binding activity of heterodimeric splicing factor U2AF: At least one RS domain is required for high-affinity binding. Mol. Cell. Biol. 18:4004–4011; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rudner D. Z.; Breger K. S.; Rio D. C. Molecular genetic analysis of the heterodimeric splicing factor U2AF: The RS domain on either the large or small Drosophila subunit is dispensable in vivo. Genes Dev. 12:1010–1021; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rueter S. M.; Dawson T. R.; Emeson R. B. Regulation of alternative splicing by RNA editing. Nature 399:75–80; 1999. [DOI] [PubMed] [Google Scholar]
- 97. Ryner L. C.; Goodwin S. F.; Castrillon D. H.; Anand A.; Villella A.; Baker B. S.; Hall J. C.; Taylor B. J.; Wasserman S. A. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87:1079–1089; 1996. [DOI] [PubMed] [Google Scholar]
- 98. Sakamoto H.; Inoue K.; Higuchi I.; Ono Y.; Shimura Y. Control of Drosophila Sex-lethal pre-mRNA splicing by its own female-specific product. Nucleic Acids Res. 20:5533–5540; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schutt C.; Nothiger R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development 127:667–677; 2000. [DOI] [PubMed] [Google Scholar]
- 100. Siebel C. W.; Admon A.; Rio D. C. Soma-specific expression and cloning of PSI, a negative regulator of P element pre-mRNA splicing. Genes Dev. 9:269–283; 1995. [DOI] [PubMed] [Google Scholar]
- 101. Singh R.; Banerjee H.; Green M. R. Differential recognition of the polypyrimidine-tract by the general splicing factor U2AF65 and the splicing repressor sex-lethal. RNA 6:901–911; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Singh R.; Valcarcel J.; Green M. R. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science 268:1173–1176; 1995. [DOI] [PubMed] [Google Scholar]
- 103. Smith C. W.; Valcarcel J. Alternative pre-mRNA splicing: The logic of combinatorial control. Trends Biochem. Sci. 25:381–388; 2000. [DOI] [PubMed] [Google Scholar]
- 104. Sontheimer E. J.; Gordon P. M.; Piccirilli J. A. Metal ion catalysis during group II intron self-splicing: Parallels with the spliceosome. Genes Dev. 13:1729–1741; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sontheimer E. J.; Sun S.; Piccirilli J. A. Metal ion catalysis during splicing of premessenger RNA. Nature 388:801–805; 1997. [DOI] [PubMed] [Google Scholar]
- 106. Southby J.; Gooding C.; Smith C. W. Polypyrimidine tract binding protein functions as a repressor to regulate alternative splicing of alpha-actinin mutally exclusive exons. Mol. Cell. Biol. 19:2699–2711; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Spingola M.; Ares M. Jr., A yeast intronic splicing enhancer and Nam8p are required for Mer1p-activated splicing. Mol. Cell 6:329–338; 2000. [DOI] [PubMed] [Google Scholar]
- 108. Staley J. P.; Guthrie C. Mechanical devices of the spliceosome: Motors, clocks, springs, and things. Cell 92:315–326; 1998. [DOI] [PubMed] [Google Scholar]
- 109. Stuckenholz C.; Kageyama Y.; Kuroda M. I. Guilt by association: Non-coding RNAs, chromosome-specific proteins and dosage compensation in Drosophila . Trends Genet. 15:454–458; 1999. [DOI] [PubMed] [Google Scholar]
- 110. Tacke R.; Manley J. L. Determinants of SR protein specificity. Curr. Opin. Cell. Biol. 11:358–362; 1999. [DOI] [PubMed] [Google Scholar]
- 111. Llitich B.; Ushkaryov Y. A.; Sudhof T. C. Cartography of neurexins: More than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 14:497–507; 1995. [DOI] [PubMed] [Google Scholar]
- 112. Vagner S.; Vagner C.; Mattaj I. W. The carboxyl terminus of vertebrate poly(A) polymerase interacts with U2AF 65 to couple 3′-end processing and splicing. Genes Dev. 14:403–413; 2000. [PMC free article] [PubMed] [Google Scholar]
- 113. Valcarcel J.; Gaur R. K.; Singh R.; Green M. R. Interaction of U2AF65 RS region with pre-mRNA branch point and promotion of base pairing with U2 snRNA. Science 273:1706–1709; 1996. [DOI] [PubMed] [Google Scholar]
- 114. Valcarcel J.; Gebauer F. Post-transcriptional regulation: The dawn of PTB. Curr. Biol. 7:R705–R708; 1997. [DOI] [PubMed] [Google Scholar]
- 115. Valcarcel J.; Green M. R. The SR protein family: Pleiotropic functions in pre-mRNA splicing. Trends Biochem. Sci. 21:296–301; 1996. [PubMed] [Google Scholar]
- 116. Valcarcel J.; Singh R.; Green M. R. Mechanisms of regulated pre-mRNA splicing. Austin, TX: The R. G. Landes Company, Biomedical Publisher: 1995:97–112. [Google Scholar]
- 117. Valcarcel J.; Singh R.; Zamore P. D.; Green M. R. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature 362:171–175; 1993. [DOI] [PubMed] [Google Scholar]
- 118. Valentine C. R. The association of nonsense codons with exon skipping. Mutat. Res. 411:87–117; 1998. [DOI] [PubMed] [Google Scholar]
- 119. van der Houven van Oordt W.; Diaz-Meco M. T.; Lozano J.; Krainer A. R.; Moscat J.; Caceres J. F. The MKK(3/6)-p38-signaling cascade alters the sub-cellular distribution of hnRNP A1 and modulates alternative splicing regulation. J. Cell Biol. 149:307–316; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Varani G.; Nagai K. RNA recognition by RNP proteins during RNA processing. Annu. Rev. Biophys. Biomol. Struct. 27:407–445; 1998. [DOI] [PubMed] [Google Scholar]
- 121. Venter J. C.; Adams M. D.; Myers E. W.; et al. The sequence of the human genome. Science 291:1304–1351; 2001. [DOI] [PubMed] [Google Scholar]
- 122. Wang J.; Bell L. R. The Sex-lethal amino terminus mediates cooperative interactions in RNA binding and is essential for splicing regulation. Genes Dev. 8:2072–2085; 1994. [DOI] [PubMed] [Google Scholar]
- 123. Wassarman K. M.; Steitz J. A. Association with terminal exons in pre-mRNAs: A new role for the U1 snRNP? Genes Dev. 7:647–659; 1993. [DOI] [PubMed] [Google Scholar]
- 124. Wei M.; Memmott J.; Screaton G.; Andreadis A. The splicing determinants of a regulated exon in the axonal MAP tau reside within the exon and in its upstream intron. Mol. Brain Res. 80:207–218; 2000. [DOI] [PubMed] [Google Scholar]
- 125. Weiner A. M. mRNA splicing and autocatalytic introns: Distant cousins or the products of chemical determinism? Cell 72:161–164; 1993. [DOI] [PubMed] [Google Scholar]
- 126. Will C. L.; Schneider C.; Reed R.; Luhrmann R. Identification of both shared and distinct proteins in the major and minor spliceosomes. Science 284:2003–2005; 1999. [DOI] [PubMed] [Google Scholar]
- 127. Wu S.; Romfo C. M.; Nilsen T. W.; Green M. R. Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature 402:832–835; 1999. [DOI] [PubMed] [Google Scholar]
- 128. Yang X.; Bani M. R.; Lu S. J.; Rowan S.; Ben-David Y.; Chabot B. The A1 and A1B proteins of heterogeneous nuclear ribonucleoparticles modulate 5′ splice site selection in vivo. Proc. Natl. Acad. Sci. USA 91:6924–6928; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yanowitz J. L.; Deshpande G.; Calhoun G.; Schedl P. D. An N-terminal truncation uncouples the sex-transforming and dosage compensation functions of sex-lethal. Mol. Cell. Biol. 19:3018–3028; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yean S. L.; Wuenschell G.; Termini J.; Lin R. J. Metal-ion coordination by U6 small nuclear RNA contributes to catalysis in the spliceosome. Nature 408:881–884; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yu Y.; Scharl E. C.; Smith C. M.; Steitz J. A. The growing world of small nuclear ribonucleoproteins. In: Gesteland R. F.; Cech T. R.; Atkins J. F., eds. The RNA world, 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1999:487–524. [Google Scholar]
- 132. Zamore P. D.; Patton J. G.; Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature 355:609–614; 1992. [DOI] [PubMed] [Google Scholar]
- 133. Zhang L.; Liu W.; Grabowski P. J. Coordinate repression of a trio of neuron-specific splicing events by the splicing regulator PTB. RNA 5:117–130; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhou S.; Yang Y.; Scott M. J.; et al. Male-specific lethal 2, a dosage compensation gene of Drosophila, undergoes sex-specific regulation and encodes a protein with a RING finger and a metallothionein-like cysteine cluster. EMBO J. 14:2884–2895; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhou Z.; Luo M. J.; Straesser K.; Katahira J.; Hurt E.; Reed R. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 407:401–405; 2000. [DOI] [PubMed] [Google Scholar]
- 136. Zhu C.; Urano J.; Bell L. R. The Sex-lethal early splicing pattern uses a default mechanism dependent on the alternative 5′ splice sites. Mol. Cell. Biol. 17:1674–1681; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zorio D. A.; Blumenthal T. Both subunits of U2AF recognize the 3′ splice site in Caenorhabditis elegans . Nature 402:835–838; 1999. [DOI] [PubMed] [Google Scholar]
- 138. Zorio D. A.; Blumenthal T. U2AF35 is encoded by an essential gene clustered in an operon with RRM/cyclophilin in Caenorhabditis elegans . RNA 5:487–494; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


