Graphical abstract
Keywords: Fitnox, Sports nutritional powder, Oral toxicity, Moringa leaf, Black ginger, Pomegranate peel
Highlights
-
•
Fitnox is a newly developed dietary ingredient for physical endurance.
-
•
Fitnox is a unique blend of M. oleifera, P. granatum and K. parviflora extracts.
-
•
Fitnox has no toxic potential even at oral dose of 1000 mg/kg in rats.
-
•
No significant changes in body weight, biochemistry and hematological parameters.
-
•
NOAEL is considered to be 1000 mg/kg body weight in both the sexes rats.
Abstract
Fitnox is a newly developed dietary ingredient for physical endurance composed of the extracts of Moinga oleifera leaf (45–50%), Kaempferia parviflora (black ginger) root (15–20%) and Punica granatum peel (25–30%). The aim of this study was to assess the subchronic oral toxicity of Fitnox (test substance) - in Wistar albino rats. Forty rats equally divided into 4 groups (control male, control female, treatment male and treatment female) administrated the test substance at 1000 mg/kg per rat daily for 90 days. All the animals were observed for body weight, mortality and clinical observations during the entire study. Results revealed no significant changes between the control and Fitnox treated groups. Based on the results, it was concluded that orally administered Fitnox to rats (dose of 1000 mg/kg per rat, orally-90 days) is safe with no drug-related toxicity was observed during the study period. Thus, the no-observed adverse effect level (NOAEL) for the present study is evaluated to be 1000 mg/kg body weight in both the sexes.
1. Introduction
Subchronic studies evaluate the toxicity results of the natural products on animals like rodents and its adverse effects. These studies also provide the data of the target organs in which the product adversely affects and helps to assess the no observed adverse effect level (NOAEL). The information obtained from the subchronic studies could be used for the dose protocols for the long-term examinations. Hence, in this study we tried to evaluate the subchronic toxicity of Fitnox which contains extracts of moringa leaf, Kaempferia parviflora (black ginger) root and pomegranate peel in rats at doses of 1000 mg/kg/day for 90 days.
Kaempferia parviflora (K. parviflora) is an herbal plant that has been used by athletes to delay fatigue and improve physical fitness [1]. In addition, Sudwan et al. tested the toxicity of black ginger ethanolic extract at doses of 60, 120 and 240 mg/kg extract body weight of male rats for sixty days and the impact on the sexual activity. The findings revealed that all male rats groups had remarkably higher mating activity while the toxicological analysis showed no significant changes in hemoglobin, white blood cells (WBC) or differential cell count. No negative effects on renal and hepatic functions were observed at any doses, as assessed by typical values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine and blood urea nitrogen (BUN) [2]. Another study evaluated the safety of chronic K. parviflora ethanol extract ingestion in Wistar rats (both male and female) at different doses for six months. The body weight measurements revealed that K. parviflora extract at highest dose of 500 mg/kg have considerably lowered the body weight of male rats when compared with control groups. While hematological parameters were within the normal range in both the sexes. Triglyceride levels had decreased significantly in male rats with highest (500 mg/kg) dose of K. parviflora extract but the glucose and cholesterol levels were increased in female rats receiving the same dose than their control vehicles. Histological evaluation of vital organs showed no notable toxicity related to the black ginger extract [3].
Moringa oleifera (M. oleifera) leaves have been reported to possess potential hypotensive and hypocholesterolemic and hypoglycemic activities and the toxicological studies showed that M. oleifera leaves extracts are safe. Awodele et al. conducted an acute toxicity study using M. oleifera leaves extracts in male Wistar albino mice by oral and intraperitoneal administrations up to 6400 mg/kg and 2000 mg/kg respectively. A 60 days oral sub-chronic toxicity test was performed using 250, 500 and 1500 mg/kg doses daily. Clinical biochemistry, hematological and histopathological examinations were showed no toxicity, suggesting that the water extract of M. oleifera leaf was safe by oral administration [4].
In an acute oral toxicity study by Adedapo et al., a safety evaluation of aqueous extract of M. oleifera leaves was carried out by the acute toxicity test with male Wistar rats. The extract did not show any animal death even at 2000 mg/kg dose level [5]. Moreover, the report of Asiedu-Gyekye et al. suggested that the water extract of M. oleifera leaf is non-toxic even at high dosage of 5000 mg/kg [6]. These results may evident that the aqueous M. oleifera leaf extract up to 2 g/kg dose was non-lethal, as orally administrated 2 g/kg dose was described to be the upper limit for acute toxicity studies for medicinal plants [4,7].
Pomegranate (Punica granatum or P. granatum) is an interesting field of research because of its constituent’s bioactivities especially due to the high content of polyphenols. Pomegranate peel and their extracts are utilized for the synthesis of various nanoparticles particularly silver nanoparticles in normal conditions [8]. Although, the in-depth toxicity studies of P. granatum has not been conducted. Common consumption and multiple safety investigations have demonstrated that pomegranate fruits are not toxic [5,9,10]. Pomegranate peel containing much more polyphenols, which are major source of antioxidants and can be used for anti-inflammatory, antitumor, anticancer molecules and regulating blood fat [11]. The methanol extract of pomegranate peel has been efficient to improve the activity of hepatic enzymes such as catalase, peroxidase and superoxide dismutase which have involved in resisting reactive oxygen species (ROS). Moreover, histopathological studies revealed that feeding of the pomegranate peel extract inhibited the CCl4 toxicity on the liver due to the polyphenols present in the extract [12]. The toxicology studies of the methanol extract of P. granatum peel in BALB/c mice showed no toxicity when administered orally up to 7.5 mg/kg [13]. Similarly, the methanol extract of P. granatum peel tested on brine shrimp assay and the results indicated that the extract had a LC50 value of 1.42 mg/mL [14]. In another study, 731 mg/kg was the LD50 value of the ethanol P. granatum fruit extract after intraperitoneal injection in mice [10].
Fitnox is a unique blend of three, M. oleifera leaf, P. granatum peel and K. parviflora extracts entrapped in a natural matrix developed by Aurea Biolabsas as described in our earlier studies [15,16]. This dietary ingredient has been shown to enhance the nitrate, nitrite, red blood cell (RBC) and dopamine levels in before and after exercise periods and may increase the physical endurance and stamina [15,16]. A study in humans showed that consumption of Fitnox for 22 days resulted in no adverse effects [15]. The aim of the study described here, was to evaluate the 90 days sub-chronic oral toxicity effects of Fitnox, in Wistar rats.
2. Materials and methods
2.1. Test products
The test ingredient, Fitnox, developed by Aurea Biolabs consists of the extracts of moringa leaf (45–50%), black ginger (15–20%) and pomegranate peel (25–30%) and was formulated by the Polar Non-polar Sandwich (PNS) technology [17] as previously described [15]. In brief, M. oleifera leaves were extracted using aqueous ethanol and K. parviflora rhizomes were extracted using ethanol. Methoxy flavones in the black ginger extract was enriched by re-purification and these two extracts were spray dried with water extract of pomegranate peel to obtain the product with layered matrix. The stability of Fitnox is up to 10 months. The tested sample contained total methoxy flavones (10.5%) form black ginger, total polyphenols (13.6%) from pomegranate peel and total saponins (22.2%) from moringa extracts.
2.2. Ethics and approvals
The study was conducted in complains with Organization for Economic Co-operation and Development (OECD) OECD C (97)186/Final guidelines, established by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). In addition, this study agreed the “OECD Principles of Good Laboratory Practice” (Organization for Economic Co-operation and Development, ENV/MC/CHEM (98) 17, revised in 1997) and the “OECD Guideline for The Testing of Chemicals 408, Repeated-Dose 90-day Oral Toxicity Study in Rodents” (Organization for Economic Co-operation and Development, adopted 21st September 1998). The experimental plan was evaluated and accepted by the Institutional Animal Ethics Committee (IAEC) (Approval No.: IAEC/ABMRCP/2016-2017/14). The experiments were conducted in agreement with the CPCSEA (Government of India) and the Guide for the Care and Use of Laboratory Animals.
2.3. Animals and study procedure
Five weeks aged male and female Wistar rats were obtained from Biogen Laboratory Animal Facility, Bengaluru (971/BC/06/CPCSEA). Body weight ranged from 100 to 160 g for males (n = 20) and from 110 to 140 g for females (n = 20). Animals were caged in stainless steel wire mesh cages (maximum of three animals in a cage) under standard laboratory conditions. The animals were provided food with laboratory animal feed ad libitum produced by Pranav Agro Industries Limited, Sangli, Maharastra till the end of the acclimatization, treatment and recovery periods. Water was provided in plastic containers fitted with stainless steel sipper tubes was provided ad libitum. The temperature and relative humidity were noted daily once.
An online randomization program (http://www.randomization.com) was used for the selection of animals by body weight was conducted by on the last day of acclimatization, such that mean body weight difference of rats did not exceed ± 20% of the mean body weight of each sex. The forty animals were divided equally into four groups - Group G1 (control male), G2 (control female), G3 (treatment male, Fitnox, 1000 mg/kg) and G4 (treatment female, Fitnox, 1000 mg/kg).
Fitnox and the control (starch) were administered orally. In the present study, a maximum dose of 1000 mg/kg body weight/day were chosen for assessing repeated dose toxicity. The required quantity of test substance was prepared freshly on daily basis by mixing with distilled water by stirring to maintain homogeneity. Rats were administered the test substance suspension or control (10 ml/kg body weight/day) once daily till the end of the study. Body weight was determined before administration and thereafter every week till 90th day. The volume of prepared formulations varied depend on the weekly measured body weights of the animals. All the animals were monitored at 2, 4 and 24 h after dosing and there after daily once for mortality throughout the study (i.e. 90 days). The physical and gross behavioral were examined every 7 days of treatment.
All the rats were monitored once daily to examine clinical observations and twice daily for mortality and morbidity. Detailed clinical observations were conducted prior to the first exposure (pre-dose) and once a week there after. All the animals were assessed for the following clinical signs: changes in eyes, skin, fur, mucous membranes, secretions and excretions, autonomic activity and changes in gait. Also, behavioral examinations such as posture and response to handling were carried out for all animals in control and treated group prior to beginning the test item administration and every 30 days during the study. These behavioral examinations included the muscle movements, stereotypies, repetitive circling and bizarre behaviors were also noticed. Daily feed in-take was analyzed from total food consumption over one week.
Functional observation and neurological examinations such as optical response, proprioception and auditory response was conducted in 30-day intervals on all existing rats throughout the 90 day study. Locomotor response using actophotometer and grip strength assessment using rota rod apparatus also examined at the 90th day. Grip strength was assessed using a rota rod apparatus (VJ Instruments, Mumbai, India).
After the completion of the experiment, rats were fasted overnight and anesthetized on day 91 and blood was taken from retro-orbital plexus puncture. Blood samples were collected in tests tubes with anticoagulant dipotassium ethylene di-amide tetra acetic acid (K2-EDTA) and stored at 4 °C till carried out the hematology tests. Similarly, the blood samples collected for clinical biochemistry tests were centrifuged at 3000 rpm for 10 min to obtain clear serum and kept at −20 °C till analyzed.
2.4. Clinical biochemistry and hematological tests
Clinical biochemistry tests included the following parameters-serum glutamic-pyruvic transaminase (SGPT), γ-glutamyl transpeptitase (GGT), autologous conditioned plasma (ACP), bilirubin, total protein, albumin, blood urea nitrogen (BUN), glucose, cholesterol and triglycerides were analyzed. Hematology testing parameters were analyzed using Nihon Kohden Hematology Analyzer MEK 6550K Celltac. These analysis included parameters such as white blood cell count (WBC), lymphocytes, monocytes, granulocytes, red blood cell count (RBC), hemoglobin, hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelets, plateletcrit (PCT), mean platelet volume (MPV) and platelet distribution width (PDW).
2.5. Histopathology analysis
Following humanly sacrifice, organs from all animals were collected and trimmed off any adherent tissue for the necropsy examinations and dehydrated. The paraffin-embedded tissues were sliced into 5 μm thick sections and stained with hematoxylin and eosin (H&E) for histopathological evaluation for both control and test groups. The microphotographs were taken and examined the various organs tissue architecture.
2.6. Statistical analysis
The obtained raw data were statistically analyzed using Graph Pad Prism software. The data on body weight and body weight gain, feed consumption, hematological and clinical biochemistry calculations were conducted statistically using Two-way ANOVA with Tukey post hoc test for difference between baseline and 90 days, between control and intervention group. All analyses and correlations were assessed at 95% level of confidence (p < 0.05). All the values were expressed as mean ± standard error of the mean (SEM). The statistically significant changes obtained for all tests are summarized in tables.
3. Results
During the entire study, oral dose of Fitnox produced neither product-related indications of toxicity or drug related death in any of the animals. There were few clinical signs noticed across all the treatment groups during the experiment period. The nasal discharge, which was evident in most animals, was not considered to be a treatment-related effect due to the pungent odor of the test item. There was no abnormal and stereotypic behavior in posture and clonic or tonic movements observed.
3.1. Functional observational series and neurological evaluation
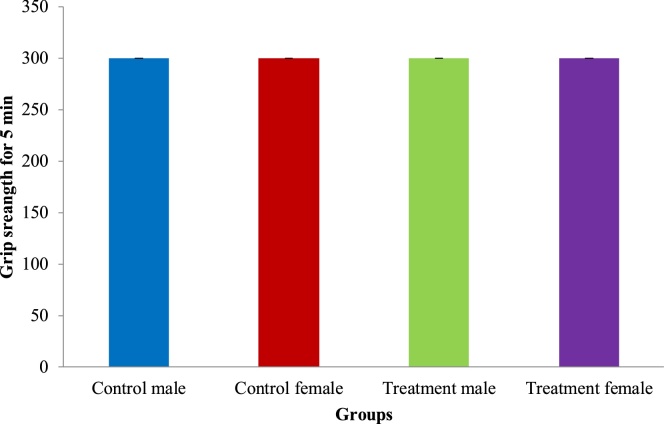
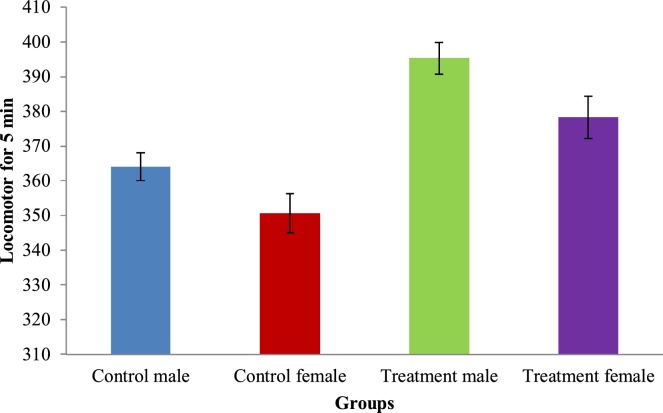
The following functional observations and neurological evaluations were analyzed during the last week of the experiment period for treated animals: auditory response, visual response, proprioception and grip strength assessment using wire mesh (Cage grill). There is no abnormal changes observed in both control and test groups during these observations. Results of muscle grip strength by rotarod apparatus and locomotor by actophotometer are given in Fig. 1, Fig. 2. No abnormal alterations were noticed in any of the values of neurological evaluation and functional observations of the animals across all the treatment groups during the study period. Statistically difference between control intergroup were shows non-significant.
Fig. 1.
Effect of sports nutrition powder on muscle grip strength by rotarod apparatus during 90 days repeated dose toxicity study. Values are Mean ± SEM.
Fig. 2.
Effect of sports nutrition powder on locomotor by actophotometer during 90 days repeated dose toxicity study. Values are Mean ± SEM.
3.2. Gross behavioral examinations
Behavioral examinations were carried out for all animals in control and treated group prior to the test item administration and every 30 days during the study. No abnormal changes were observed for various parameters of neurological evaluation and functional observations of the animals across all groups during the study period. Statistical difference between control intergroup were showed non-significant.
3.3. Body weight
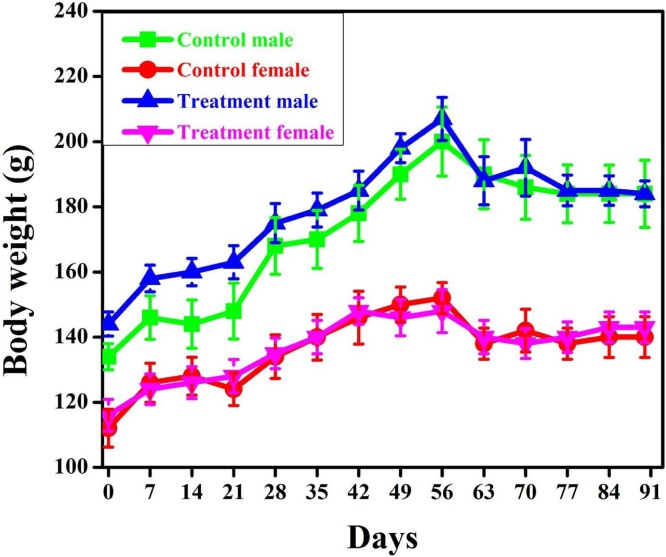
The summary of weekly body weight calculations of male and female rats are presented in Fig. 3. No Statistically significant differences in body weight gain were observed.
Fig. 3.
Effect of sports nutritional powder on body weight of rats at weekly interval during 90 days repeated dose toxicity study. Values are Mean ± SEM.
3.4. Clinical biochemistry
The summary of serum clinical biochemistry results are presented in Table 1. No statistically significant changes were detected in the clinical biochemistry parameters in males of treatment groups compared to male control animals and female treatment groups compared to female control animals.
Table 1.
Effect of Fitnox on clinical biochemistry parameters.
| Biochemical parameters | Groups |
|||||
|---|---|---|---|---|---|---|
| Control male | Fitnox- male | p value | Control female | Fitnox - female | p value | |
| SGPT (U/I) | 61.67 ± 5.3 | 55.67 ± 4.48 | ns | 61.00 ± 4.61 | 64.67 ± 2.38 | ns |
| GGT (U/I) | 1.00 ± 0.8 | 1.67 ± 0.33 | ns | 1.33 ± 0.7 | 2.67 ± 0.33 | ns |
| ACP (U/I) | 24.33 ± 1.76 | 26.33 ± 3.20 | ns | 15.33 ± 2.8 | 16.00 ± 1.08 | ns |
| Bilirubin (mg/dl) | 0.13 ± 0.03 | 0.17 ± 0.06 | ns | 0.10 ± 0.00 | 0.20 ± 0.05 | ns |
| Total protein (mg/dl) | 5.53 ± 0.34 | 7.20 ± 1.11 | ns | 6.50 ± 0.20 | 6.23 ± 0.51 | ns |
| Albumin (mg/dl) | 2.40 ± 0.25 | 2.43 ± 0.06 | ns | 2.60 ± 0.35 | 2.83 ± 0.13 | ns |
| BUN (U/I) | 27.67 ± 3.38 | 23.00 ± 0.37 | ns | 27.00 ± 6.92 | 25.33 ± 1.76 | ns |
| Glucose (mg/dl) | 91.33 ± 1.76 | 95.67 ± 5.60 | ns | 92.33 ± 4.37 | 96.00 ± 3.05 | ns |
| Cholesterol (mg/dl) | 47.67 ± 4.37 | 45.67 ± 3.18 | ns | 52.00 ± 5.07 | 52.33 ± 2.96 | ns |
| TG (mg/dl) | 65.00 ± 2.0 | 59.00 ± 1.52 | ns | 73.33 ± 3.18 | 72.00 ± 1.52 | ns |
Values are in Mean ± SEM, ns: not significant.
3.5. Clinical pathology and chemistry
The summary of hematological parameter estimations of male and female animals are presented in Table 2. There were no statistically significant changes detected in hematological parameters between male or female animals and their respective treatment groups. But statistically significant (p < 0.01) increase noticed in platelets in Fitnox for male rats (414.33 × 103 μl) than control male rats (257.67 × 103 μl). Similarly, statistically significant (p < 0.01) increase observed in platelets in Fitnox for female rats (378.67 × 103 μl) than control female rats (293.33 × 103 μl).
Table 2.
Effect of Fitnox on hematological parameters.
| Blood parameters | Groups |
|||||
|---|---|---|---|---|---|---|
| Control male | Fitnox - male | p value | Control female | Fitnox - female | p value | |
| WBC (X 103 μl) | 6.70 ± 0.1 | 6.33 ± 0.1 | ns | 5.30 ± 0.1 | 7.27 ± 0.1 | ns |
| Lymphocytes (%) | 61.13 ± 1.1 | 70.03 ± 2.2 | ns | 51.23 ± 1.3 | 67.57 ± 2.3 | ns |
| Monocytes (%) | 14.23 ± 0.4 | 9.40 ± 0.2 | ns | 12.53 ± 0.3 | 9.77 ± 0.3 | ns |
| Granulocytes (%) | 24.63 ± 0.3 | 20.57 ± 0.5 | ns | 36.23 ± 0.5 | 21.00 ± 0.4 | ns |
| RBC (X 106 μl) | 5.91 ± 0.1 | 7.08 ± 0.2 | ns | 6.63 ± 0.2 | 6.95 ± 0.2 | ns |
| Hemoglobin g/dl | 12.70 ± 0.3 | 15.30 ± 0.4 | ns | 14.50 ± 0.3 | 14.87 ± 0.4 | ns |
| HCT (%) | 34.30 ± 1.1 | 39.80 ± 1.4 | ns | 38.00 ± 2.1 | 39.07 ± 1.5 | ns |
| MCV (fl) | 57.97 ± 3.2 | 56.27 ± 3.1 | ns | 57.27 ± 2.3 | 56.20 ± 2.6 | ns |
| MCH (pg) | 21.33 ± 1.1 | 21.60 ± 1.0 | ns | 21.80 ± 1.2 | 21.33 ± 1.1 | ns |
| MCHC (g/dl) | 36.83 ± 1.4 | 38.40 ± 1.3 | ns | 38.13 ± 1.2 | 38.00 ± 1.4 | ns |
| RDW (%) | 13.40 ± 0.3 | 12.30 ± 0.2 | ns | 12.80 ± 0.4 | 11.90 ± 0.2 | ns |
| Platelets (X 103 μl) | 257.67 ± 4.5 | 414.33 ± 5.2** | p < 0.01 | 293.33 ± 4.2 | 378.67 ± 5.4** | p < 0.01 |
| PCT (%) | 0.17 ± 0.02 | 0.26 ± 0.02 | ns | 0.24 ± 0.01 | 0.20 ± 0.01 | ns |
| MPV (fl) | 7.13 ± 0.5 | 6.37 ± 0.3 | ns | 6.50 ± 0.4 | 6.80 ± 0.2 | ns |
| PDW (fl) | 6.87 ± 0.3 | 5.83 ± 0.4 | ns | 5.77 ± 0.3 | 6.10 ± 0.2 | ns |
Values are in Mean ± SEM, ns: not significant.
significant at p < 0.01.
3.6. Histopathology
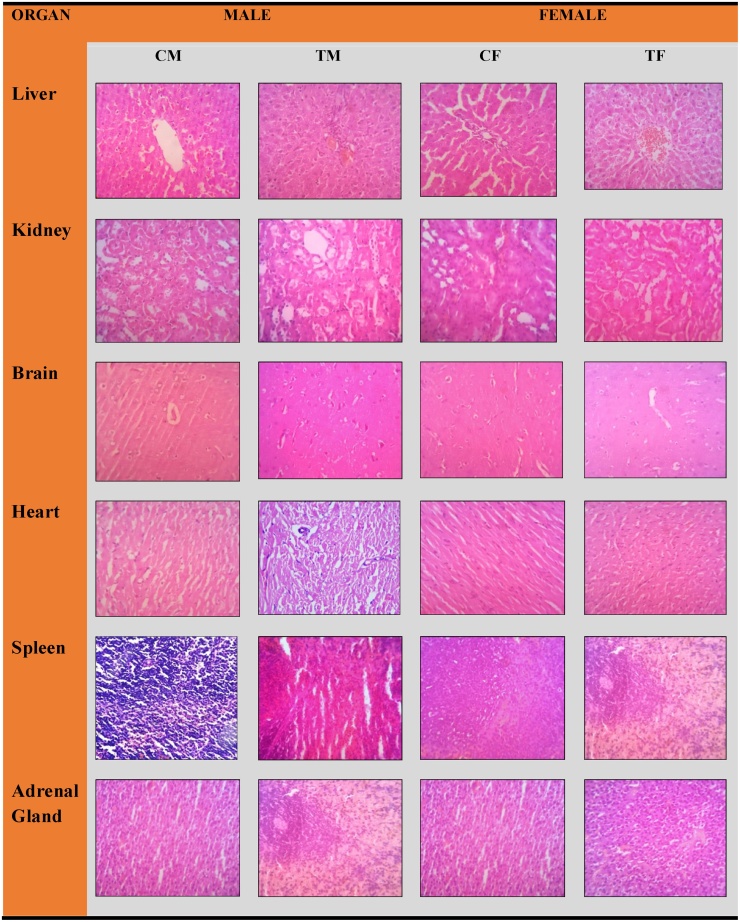
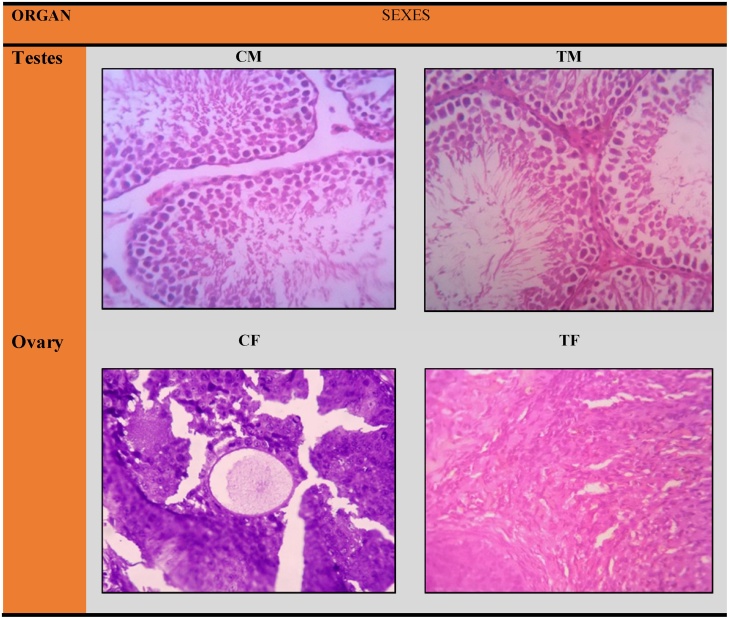
Histopathological examinations were conducted for liver, kidney, brain, heart, spleen, adrenal gland, testes and ovaries (Fig. 4, Fig. 5). The stained sections were subjected to detailed histopathological examination by the pathologist. There were no test product related adverse changes in any of the organs examined from both sexes at the treated group. Histopathological examination did not reveal any abnormalities. All the animals survived throughout the 90 day intervention period.
Fig. 4.
Histology of vital organs namely liver, kidney, brain, heart, spleen and adrenal gland in tissues in rat during 90 days repeated dose toxicity studies. CM-Control Male, CF-Control Female and TM-Treated Male, TF-Treated Female at Dose 1000 mg/kg B.wt. All Sections are stained with H&E x 400.
Fig. 5.
Histology of reproductive organs namely testes and ovary tissues in rat during 90 days repeated dose toxicity studies. CM-Control Male, CF-Control Female and TM-Treated Male, TF-Treated Female at Dose 1000 mg/kg B.wt. All Sections are stained with H&E x 400.
4. Discussion
Ayurveda has been long practiced in India as it is inexpensive, comfortably obtainable and Ayurvedic herbs are relatively safe. Additionally, there is an instant growth in the concern of Ayurveda due to the exploration for other possible options. In the Ayurvedic practice, herbal combinations are often utilized to increase the biological activity of biomolecules or prevent the adverse/toxic impact of compounds [[18], [19], [20], [21], [22]].
Oral administration of Fitnox at a dose of 1000 mg/kg/day for 90 days showed no treatment related signs of toxicity or mortality in either sex. The body weight of male and female rats gradually increased during the study and the final day body weight of male (184 g) and female (143 g) are similar with that of the controls (Fig. 3). There were no significant variations in food and water intake of the test animals during the 90-day treatment period when compared to the control groups, suggested that Fitnox did not create any variations in fat metabolism, carbohydrate or protein in animals. The significant increase in food and water intake is taken into account as being accountable for the increment in weight gain in animals [1]. The weight alterations are the foremost receptive indicators of the final health standing of animals [23]. It is clear that the Fitnox didn't seem to interfere with the usual metabolism of animals as nonsignificant changes observed from tested animals with the control groups. Weight loss is mainly happens due to the loss of appetite which leads to the disturbances of the metabolism of carbohydrate, protein or fat [24].
Hepatic and renal function analysis is a necessary thing within the toxicity analysis of any plant extracts as they are important for the functioning of an organism [25]. Impact of Fitnox in liver and kidney functions was assessed by the analysis of the serum hematological parameters and clinical biochemistry analyses. These results are depicted in Table 1, Table 2. In clinical biochemistry parameters (Table 1), the serum glutamic-pyruvic transaminase (SGPT) and gamma-glutamyl transpeptidase (GGT) changes are not significant in both control and tested animals in both sexes. In male group SGPT values were decreased to 55.67 U/I when compared with control (61.67 U/I) and in female group it was slightly increased from 61 U/I (control) to 64.67 U/I. (test group) Similarly, GGT values also slightly increased in both sexes. High levels of SGPT and GGT are the indicators of liver diseases or hepatotoxicity [26] and the nonsignificant changes in SGPT and GGT in both male and female rats at all groups propose that oral administration of Fitnox does not disturb the liver function in the rats. A slight increase observed in total protein in male rats (5.53 mg/dl for control and 7.2 mg/dl for test) and albumin in both male (2.4 mg/dl for control and 2.43 mg/dl for test) and female (2.6 mg/dl for control and 2.83 mg/dl for test) as compared with the control, may be a sign of the increased synthetic function of the liver. Low serum albumin is due to the infection or continuous loss of albumin [27], thus, these nonsignificant results of Fitnox treated groups additionally recommended that the extract has no adverse effects on the hepatocellular functions of the liver. Moreover, there were no significant changes in glucose and cholesterol indicates the safety of the Fitnox. BUN measurements give rise to a view of kidney dysfunction and the values were slightly decreased in both sexes and nonsignificant. The lower BUN levels in tested animals compared with the controls indicated the well-functioning of the renal systems [28].
Effect of Fitnox on hematological parameters is given in the Table 2. The assessment of hematological parameters can be used to decide various status of the body and to assess stress due to inflammations. These results also gives the scope of the harmful effect of Fitnox on the hematopoietic system. These hematological evaluations help to predict the toxicity in human and the changes in the hematological values in animal studies can be effectively transferred to the human toxicology [29]. Table 2 indicates the non-significant effect of the Fitnox on hematological parameters such as RBC, WBC and hemoglobin. The non-significant changes in these parameters in both sexes revealed that Fitnox does not have any adverse effect in body. Lymphocytes values slightly increased in male (70% as compared with 61%) and in female (67% as compared with 51%) after the administration of Fitnox. This increment in both the sexes may suggest that Fitnox helps the immune system by the responds to inflammatory process. Similarly, monocytes and granulocytes values also did not show any significant changes when compared with the control groups, validated the above findings. So it is clear from the hematological parameters that Fitnox is not only safe but helps the immune response system also.
Histological sections of the visceral organs (liver, kidney, brain, heart, spleen and adrenal gland) and reproductive organs (testes and ovary tissues) after the oral administration of Fitnox are shown in Fig. 4, Fig. 5 respectively. The morphology, cell structure and functions of the animal organs did not show any unfavorable effects, indicates Fitnox is not toxic. Same way, no abnormalities in histopathology were noticed in the microscopic analysis of the vital and reproductive organs in control groups. As discussed above, the non-significant increases observed in bilirubin, SGPT and GGT values (Table 1) conforms histopathology results and administration of Fitnox did not alter hepatocytes and metabolism in liver. Similarly, the non-significant BUN values in the subchronic administration of Fitnox revealed the safety of the formulation to functional nephrons. The histological sections of kidney tissue showed no abnormalities and justified the above findings. Hence, the results recorded in this study demonstrate that Fitnox can be considered as safe as it did not cause adverse changes in vital or reproductive organs.
Natural products, such as extracts of K. parviflora, P. granatum peel and M. oleifera leaf have been the used to treat many inflammatory conditions. The current subchronic study was attempted to assess the toxicity of Fitnox in rat model for 90 days. Hence, in this study, Fitnox at a dose of 1000 mg/kg/day had no adverse effect on the tested rats up to 90 days of observation.
5. Conclusion
Fitnox did not produce any mortality at 1000 mg/kg per oral dose, nor did it produce any observable toxic effect, or significant changes in mean body weight. Based on the observations recorded over the course of the 90 day study, it can be found that Fitnox has no toxic potential even at the oral dose of 1000 mg/kg in rats. There were no biologically significant, treatment related adverse effects on body weights, food consumption, hematology and clinical biochemistry parameters of animals at all groups when compared to control groups. Similarly, there were no test product related histopathological changes in treated animals of either sex. There were no treatment related adverse outcomes in any of the organs analyzed from either sex in. Hence, the NOAEL (No-Observed Adverse Effect Level) for the present study could be 1000 mg/kg body weight for both the sexes.
Conflict of interest
Three of the authors (J.J., A.A., and S.G.) are employees of Aurea Bio Labs Ltd., a research subsidiary of Plant Lipids Ltd. All other authors have no conflicts to report.
Transparency document
References
- 1.Ping K.Y., Darah I., Chen Y., Sreeramanan S., Sasidharan S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. Biomed. Res. Int. 2013;182064:1–14. doi: 10.1155/2013/182064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudwan P., Saenphet K., Saenphet S., Suwansirikul S. Effect of Kaempferia parviflora Wall. ex. Baker on sexual activity of male rats and its toxicity. Southeast Asian J. Trop. Med. Public Health. 2006;37:210–215. [PubMed] [Google Scholar]
- 3.Chivapat S., Chavalittumrong P., Attawish A., Rungsipipat A. Chronic toxicity study of Kaempferia parviflora Wall ex. Extract. Thai J. Vet. Med. 2010;40:377–383. [Google Scholar]
- 4.Awodele O., Oreagba I.A., Odoma S., da Silva J.A., Osunkalu V.O. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae. J. Ethnopharmacol. 2012;139:330–336. doi: 10.1016/j.jep.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Adedapo A.A., Mogbojuri O.M., Emikpe B.O. Safety evaluations of the aqueous extract of the leaves of Moringaoleifera in rats. J. Med. Plants Res. 2009;3:586–591. [Google Scholar]
- 6.Asiedu-Gyekye I.J., Frimpong-Manso S., Awortwe C., Antwi D.A., Nyarko A.K. Micro and macro elemental composition and safety evaluation of the nutraceutical Moringa oleifera leaves. J. Toxicol. 2014;786979 doi: 10.1155/2014/786979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu F.C., Jessup D.C., Lavallée A. Toxicity of pesticides in young versus adult rats. Food Cosmet. Toxicol. 1965;3:591–596. doi: 10.1016/s0015-6264(65)80206-1. [DOI] [PubMed] [Google Scholar]
- 8.Edison T.J., Sethuraman M.G. Biogenic robust synthesis of silver nanoparticles using Punica granatum peel and its application as a green catalyst for the reduction of an anthropogenic pollutant 4-nitrophenol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013;104:262–264. doi: 10.1016/j.saa.2012.11.084. [DOI] [PubMed] [Google Scholar]
- 9.Patel C., Dadhaniya P., Hingorani L., Soni M.G. Safety assessment of pomegranate fruit extract: acute and subchronic toxicity studies. Food Chem. Toxicol. 2008;46:2728–2735. doi: 10.1016/j.fct.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Vidal A., Fallarero A., Pena B.R., Medina M.E., Gra B., Rivera F., Gutierrez Y., Vuorela P.M. Studies on the toxicity of Punica granatum L. (Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2003;89:295–300. doi: 10.1016/j.jep.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Shaban N.Z., El-Kersh M.A., El-Rashidy F.H., Habashy N.H. Protective role of Punicagranatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem. 2013;141:1587–1596. doi: 10.1016/j.foodchem.2013.04.134. [DOI] [PubMed] [Google Scholar]
- 12.Murthy K.N.C., Jayaprakasha G.K., Singh R.P. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J. Agric. Food Chem. 2002;50:4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 13.Jahromi S.B., Pourshafie M., Mirabzadeh E., Abbasian S. Punica granatum peel extract toxicity in mice. Jundishapur J. Nat. Pharm. Prod. 2015;10:e23770. [Google Scholar]
- 14.Nisha M., Xavier R., Sreeramanan S., Rajasekaran A., Sasidharan S., Latha L.Y. Antimicrobial activity and toxicity of Punica granatum L. peel. J. Appl. Biol. Sci. 2008;2:57–59. [Google Scholar]
- 15.Gopi S., Jacob J., Varma K., Amalraj A., Sreeraj T.R., Kunnumakkara A.B., Divya C. Natural sports supplement formulation for physical endurance: a randomized, double-blind, placebo-controlled study. Sport Sci. Health. 2017;13:183–194. [Google Scholar]
- 16.Jacob J., Gopi S., Divya C. A randomized single dose parallel study on enhancement of nitric oxide in serum and saliva with the use of natural sports supplement in healthy adults. J. Diet. Suppl. 2017:1–12. doi: 10.1080/19390211.2017.1331944. [DOI] [PubMed] [Google Scholar]
- 17.Amalraj A., Jude S., Varma K., Jacob J., Gopi S., Oluwafemi O.S., Thomas S. Preparation of a novel bioavailable curcuminoid formulation (Cureit™) using Polar-Nonpolar-Sandwich (PNS) technology and its characterization and applications. Mater. Sci. Eng. C. 2017;75:359–367. doi: 10.1016/j.msec.2017.02.068. [DOI] [PubMed] [Google Scholar]
- 18.Patwardhan B., Warude D., Pushpangadan P., Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid. Based Complement. Altern. Med. 2005;2:465–473. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gertsch J. Botanical drugs, synergy, and network pharmacology: forth and back to intelligent mixtures. Planta Med. 2011;77:1086–1098. doi: 10.1055/s-0030-1270904. [DOI] [PubMed] [Google Scholar]
- 20.Pandey M.M., Rastogi S., Rawat A.K. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid. Based Complement. Altern. Med. 2013;376327:1–12. doi: 10.1155/2013/376327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonti M., Casu L. Traditional medicines and globalization: current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013;4(92):1–13. doi: 10.3389/fphar.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ragozin B.V. The history of the development of ayurvedic medicine in Russia. Anc. Sci. Life. 2016;35:143–149. doi: 10.4103/0257-7941.179868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vij V.A., Joshi A.S. Effect of excessive water intake on body weight, body mass index, body fat, and appetite of overweight female participants. J. Nat. Sci. Biol. Med. 2014;5:340–344. doi: 10.4103/0976-9668.136180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choe S.S., Huh J.Y., Hwang I.J., Kim J.I., Kim J.B. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016;7:1–16. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oyagbemi A.A., Omobowale T.O., Azeez I.O., Abiola J.O., Adedokun R.A., Nottidge H.O. Toxicological evaluations of methanolic extract of Moringa oleifera leaves in liver and kidney of male Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2013;24:307–312. doi: 10.1515/jbcpp-2012-0061. [DOI] [PubMed] [Google Scholar]
- 26.Habib-ur-Rehman M., Mahmood T., Salim T., Afzal N., Ali N., Iqbal J., Tahir M., Khan A. Effect of silymarin on serum levels of ALT and GGT in ethanol induced hepatotoxicity in albino rats. J. Ayub Med. Coll. Abbottabad. 2009;21:73–75. [PubMed] [Google Scholar]
- 27.Cisneros F.J., Gough B.J., Patton R.E., Ferguson S.A. Serum levels of albumin, triglycerides, total protein and glucose in rats are altered after oral treatment with low doses of 13-cis-retinoic acid or all-trans-retinoic acid. J. Appl. Toxicol. 2005;25:470–478. doi: 10.1002/jat.1082. [DOI] [PubMed] [Google Scholar]
- 28.Juraschek S.P., Appel L.J., Anderson C.A., Miller E.R., 3rd Effect of a high- protein diet on kidney function in healthy adults: results from the Omni Heart trial. Am. J. Kidney Dis. 2013;61:547–554. doi: 10.1053/j.ajkd.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacanaro C.P., Dias S.R., Serafim L.R., Costa M.P., Aguilar E., Paes P.R., Alvarez-Leite J.I., Rabelo E.M. Evaluation of biochemical, hematological and parasitological parameters of protein-deficient hamsters infected with Ancylostoma ceylanicum. PLoS Negl. Trop. Dis. 2014;8(9):1–10. doi: 10.1371/journal.pntd.0003184. e3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.