Abstract
The transmission of hospital-acquired Carbapenem-resistant Enterobacteriaceae (CRE) is a serious and growing concern in hospitals worldwide. Previous research of CRE found that traditional patient-to-patient transmission of the bacteria does not fully account for all cases of transmission. Recent efforts to further understand modes of transmission found identical genomes of CRE in patient sinks as was found in cultures collected from patients, indicating that environmental reservoirs could be playing a larger role in transmission than was first realized. This study evaluated imputation methods for linking multiscale clinical and environmental microbiological data. We then utilized the imputed data set to model the risk of CRE presence in sinks between culture dates. We demonstrated that imputation based on expert knowledge of the unique factors of the physical hospital layout and patterns of occurrence throughout hospital sinks provided the best representation of sink positivity and also identified several significant risk factors for explaining environmental contamination. This work helps to more clearly define the mechanism and risk of transmission from a wastewater source to hospitalized patients in a world with increasingly antibiotic-resistant bacteria which can thrive in wastewater environments and cause infections in vulnerable patients.
Introduction
The spread of hospital-acquired antibiotic-resistant bacteria is a serious and growing problem. The Center for Disease Control and Prevention (CDC) estimates that more than two million people are sickened each year with antibiotic- resistant infections resulting in at least 23,000 deaths [1]. Of the many bacteria that are growing in drug resistance, there is particular concern with gram-negative pathogens as they are becoming increasingly resistant to nearly all drugs, including Carbapenems. Carbapenems are widely considered to be the strongest class of antibiotic in use today and are typically administered as a last resort in treating gram-negative infections [1]. Because of their resistance to the strongest antibiotics available, infections caused by Carbapenem-resistant Enterobacteriaceae (CRE), most commonly found in a patient’s gastrointestinal tract, are difficult to treat and can result in death [2].
Traditionally, understanding and tracking of hospital acquired infections has focused on direct contact transmission where infected and colonized patients act as reservoirs of transmission between uncolonized patients and hospital workers [3]. A large healthcare facility in the United States has been tracking a low-level spread of a nosocomial CRE pathogen between August 2007 and September 2015 with approximately 450 patient colonizations. Since the discovery of CRE transmission, active surveillance of all patients has been instituted and strict contact precautions have been in place for patients who revealed a positive culture for the bacteria. Additionally, large-scale cleaning, disinfecting, sterilization, replacement, and limited-use interventions have been conducted, but these methods have produced only temporary successes in reducing the spread of the bacteria. Recent efforts to further understand transmission of the waterborne bacteria led hospital epidemiologists to begin sampling wet surfaces including patient sinks, toilets, and hoppers for the CRE pathogen. As a result, it has become increasingly recognized that non-patient reservoirs within the hospital setting may play a larger role in the transmission of drug resistant pathogens than was first realized [4] [5].
Beginning in September of 2013, nearly 3500 samples of sink drains, sink p-traps, toilets, hoppers, and other wet surfaces have been collected periodically throughout the hospital looking for the presence of CRE. Sampling found identical genomes in samples of patients as was found in the sinks, indicating that sinks, or other environmental factors, could be acting as reservoirs for transmission [6]. However, manpower and financial constraints limited the frequency and volume of samples taken over time leaving a large gap in knowledge of the lifecycle of the bacteria in sinks and resulting in a sparse data set with samples at irregularly spaced intervals. In order to understand the sink positivity between sample dates and to utilize the environmental microbiological sample results for further modeling, the data must be at equally spaced intervals, and thus missing data must be cleaned by removal or imputation.
Missing data are often unavoidable in epidemiological and clinical research. They can bias study results distorting statistical parameter estimates and decreasing the statistical power of a study. Numerous methodologies have been proposed to handle missing data [7]. We hypothesized that using standard methods of imputation would not provide the best representation of the environmental samples in this study because the study data include all three types of missing data, and the percentage of missing observations is significantly larger than the number of non-missing data when translated to daily measurements for modeling.
To determine which imputation method provides better representation of the presence of CRE in patient sinks over the study period, we examined two non-standard imputation methods as well as two regularly practiced methods. After determining the imputation method that resulted in the most accurate representation of the environmental sample results, we used the imputed data set for further modeling to understand the significant risk factors in predicting the presence of CRE in patient sinks.
The objectives of this study are twofold: 1) to evaluate imputation strategies for continuous representation of irregular sampled environmental microbiological data and 2) to use the selected imputation approach for modeling of the environmental reservoir positivity as a function of other significant risk factors including colonized patients, environmental interventions, and characteristics of patient room sinks.
1. Related Work
1.1. Patient Infection Risk Factors
The transmission of CRE infection occurs when a non-colonized person comes into direct contact with an infected or colonized patient, through intermediate carriers such as healthcare workers, or through contact with contaminated environmental reservoirs such as sinks and toilets, among others. Previous studies [8,9] from the US and Israel have shown that the primary risk factors for patient acquisition include ICU stays, long-term hospitalization, transplantation and antibiotics. Another outbreak of CRE at the Tisch Hospital at New York Medical Center included 24 infected patients in intensive care units (ICUs) over the course of a year. Similar to other outbreaks, risk factors for infection during this outbreak included prolonged hospital stay, a stay in the ICU, and ventilator usage [10].
1.2. Environmental Risk Factors
Risk factors from the studies conducted in the US and Israel did not completely explain infection transmission. Previous studies [4,11,12] provide evidence that environmental reservoirs are a source of infection and transmission. Additionally, a study from Spain [4] described an outbreak due to multidrug-resistant Klebsiella oxytoca in an ICU where damp environmental reservoirs were linked to bacterial transmission. In that study samples collected from sinks, drainpipes, and traps showed that only one storage sink, which had its drainpipes connected to two other sinks, was found to be positive. The connecting drainpipes were also found to be positive. Furthermore, this study showed that the outbreak was completely eradicated after replacing the horizontal drainage system that connected the two impacted sinks. In conclusion, this study stated that wet environmental reservoirs should be considered when strictly applied traditional control measures are not efficacious.
A study from France [12] found that sinks were frequently contaminated in ICUs as a result of their use in disposing of patient bodily fluids and were a potential source of extended-spectrum beta lactamase-producing Enterobacteriaceae (ESBLE), thus increasing risk in the environment of patients as a consequence of the splash-back effect. Recent research from Wolf et al. [11] demonstrated that sinks acted as a source of infection by verifying that the ESBLEs recovered from patients were identical to those that had been previously recovered from sinks. The outbreak described in a Colombian [13] study found that the likely cause of the infections was the improper design of sinks; in particular, the joints of the sinks to the walls were not sealed, leading to facilitation of colonization.
1.3. Modeling Infection Transmission
Methods including logistic regression have been used in previous studies to identify risk factors associated with nosocomial transmission. A study [14] conducted at Roosevelt hospital in New York City used logistic regression models to evaluate efficacy of infection control measures in preventing the transmission of multidrug-resistant tuberculosis. The study found that distance from infected patient room is a significant predictor of nosocomial transmission.
The logistic regression approach has also been used in some studies [15,16] to identify patient risk factors for CRE transmission in hospital settings. Research by Papadimitriou-Olivgeris et al. [15] focused on patient characteristics, diagnosis, and procedures to determine that prior ICU stay, duration of previous hospitalization, diagnosis of chronic obstructive pulmonary disease, carbapenem administration, and beta-lactamase administration were significant risk factors. Similarly, Tuon et al. [16] considered procedures such as mechanical ventilation and found that urinary catheter devices and central venous catheter devices were significant risk factors, along with advanced age and antibiotic exposure to ciproflaxin.
Work by [17] focused on modeling the nosocomial transmission of carbapenem-resistant bacteria using logistic regression and random forest models to determine important risk factors for CRE transmission. Both models showed that distance to the infected room was one of the significant predictors. One of the models showed that the proximity to sinks is important in predicting patient infection. This study also found that the cumulative presence of positive patients in the same room as a sink, distance from the bed to sink, and sink design are significant predictors of sink positivity. However, the model was constructed using limited data and a restricted time range, which leaves many questions about the role of sinks in the spread of infections.
Studies [4,11–13] have demonstrated that sinks play a role in infection transmission but did not highlight additional environmental risk factors responsible for sink contamination. This study improves on the understanding of sink contamination by highlighting important variables. This study is similar to [17] in terms of the modeling approach used, but it differs in the level of spatiotemporal variables from environmental data. In addition to examining the presence of positive patients in the same room over time, this study also considers the status of neighboring rooms and sinks as potential risk factors.
1.4. Imputation in Healthcare Studies
Missing data is a frequent issue in epidemiological and health sciences research. While most modeling techniques would simply call for the removal of incomplete cases, doing so can cause bias and loss of information [18]. Imputation methodologies can be used to replace missing data with substituted values in order to create a complete data set. Standard methods for imputation such as k-nearest neighbor, last observation carried forward, and mean value can lead to bias and are not effective in handling categorical variables [19]. These methods are also typically used to address only one of the three types of missing data - missing completely at random, missing at random, and missing not a random.
Missing data in health care settings is a result of many complex sources of information and their incompatibility between each other and also due to the personal nature of care given that varies among patients [20]. A recent study of outcomes of trauma cases found that using a combination of existing imputation methods, specific to each individual data set in the analysis significantly improved the accuracy of predicted mortality after trauma over the more common method of handing missing data by simply removing all unknown cases [20]. Additionally, a study on classification of respiratory patterns involving imputation of missing data found that self-organizing maps (SOM) machine learning techniques were more effective in predicting patterns than other more commonly used statistical methods such as mean/mode imputation and multiple linear regression [21].
2. Methods
2.1. Data
This Institutional Review Board (IRB) approved study used clinical and environmental data from a major U.S. hospital. Clinical data included culture dates of approximately 130 patients who tested positive for CRE and their room movement information from September 2013 to September 2015. Environmental data included 967 positive and negative swab and liquid samples from 166 sink drains and sink p-traps from floor 3-8 in the hospital over the same 740 day period. Twenty six attributes describing the physical characteristics of the sink were manually collected. The attributes include surrounding countertop area, faucet, exposed piping, and the presence of other wet areas such as showers, hoppers, and toilets. Lastly, dates and units of any clinical interventions that were performed during the period in question were collected.
For this analysis, our environmental microbiological sample dataset was transformed into to a sink-day format that includes a record for each sink and each day during the study period. We defined positive (“1”) population as the set of sink-days for which a known positive culture was found, negative (“0”) population as the set of sink-days for which a known negative culture was found, and unknown (“U”) population as the set of sink-days for which there was missing sample data or no environment sample taken. Of our data set with approciamtely 81,000 cases, only about 1,0 (1.2%) had known culture values.
Table 1 describes the variables used in this study. Variables Lag0, Lag!, Lag2, and Lag3 indicate whether on the given day, a positive patient stayed for any length of time in the given room. The lag number corresponds to the time with Lag0 indicating the current day, Lagl indicating one day prior, and Lag2 indicating two days prior. If a positive patient moved through the room, a binary response of “1” was indicated at the appropriate lag value. Similarly, CumSum7 and CumSum14 were created to demonstrate the cumulative number of days in the past 7 or 14 days respectively that a positive patient was in the given room with the given sink. Variables InterventionO, Intervention!, Intervention2, and Intervention3 were used to indicate whether an intervention was performed on the current day or one of the prior three days. The timing of different interventions was considered since infection control leadership hypothesized that these interventions influenced environmental positivity. SinkDesign represents 25 unique sink characteristics throughout the 166 sampled sinks. SinkBedDis represents the distance of the patient bed to the sink.
Table 1:
Description of Variables for Imputation of Sink Positivity
| Variable | Description | Input Values |
|---|---|---|
| U nit | unit of hospital | ICU1, ICU2, ICU3 TCV, NIMU |
| PTBR | patient (PT) or bathroom sink (BR) | PT, BR |
| LagO | presence of positive patient for sink/day at time t=0 | “0”, “1” |
| Lag! | presence of positive patient at time t-1 | “0”, “1” |
| Lag2 | presence of positive patient at time t-2 | “0”, “1” |
| Lag3 | presence of positive patient at time t-3 | “0”, “1” |
| InterventionO | intervention in place at time t=0 | “0”, “1” |
| Intervention! | intervention in place at time t-1 | “0”, “1” |
| Intervention2 | intervention in place at time t-2 | “0”, “1” |
| Intervention3 | intervention in place at time t-3 | “0”, “1” |
| CumSum7 | cumulative number of positive patients in 7 days prior | [0, 7] |
| CumSum!4 | cumulative number of positive patients in 14 days prior | [0,14] |
| SinkDesign | sink design based on unique values from sink variable description | 25 designs “A-Y” |
| SinkBedDis | measurement of distance from sink to bed | [4,14] feet |
2.2. Imputation of Environmental Sample Data
We evaluated four imputation methods, two non-standard methods based on expert guidance (Linear and Midpoint methods) and two traditional imputation methods that are frequently used in health care studies (Logistic Regression and Multivariate Imputation by Chained Equations). In the linear imputation method, a positive sample is carried forward to the next negative sample while linearly imputing the probability of the sink being positive between negative and positive observations. The midpoint method carries the first observation forward to the midpoint between two observations and carries the next observation backward to the midpoint. The logistic regression method trains a set of data on the given samples and predictor variables and predicts on the missing samples. The mutivariate imputation by chained equations (MICE) method uses the statistical package MICE in R to perform multiple imputation over 10 iterations of known variables to predict a binary response [22] [23].
For the purpose of imputation, we consider each sink as a univariate time series. Each imputation method was implemented on each sink over the period of 740 days resulting in a value for each sink-day that will be used in modeling. Predicted values are replaced by any known values. Figure 1 shows visual depiction of the sample data and each of the four methods on one sink over the first 350 days.
Figure 1.
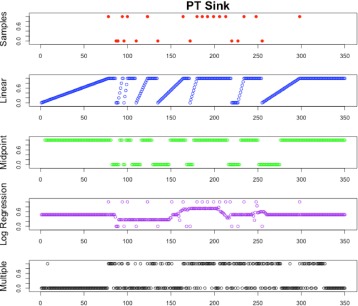
Example plot of Imputation Methods for Patient Sink
2.3. 2.3 Modeling and Feature Selection
We employed two modeling techniques to identify risk factors that affect sink positivity. First, Bayesian model averaging (BMA) [24] was used to explore all possible subsets of variables from Table 2 and identify the most significant predictors. Based on each subset model’s posterior probability, which can be estimated using Bayesian information criterion (BIC), the models that were at least 20 times less likely than the best model were removed. Among the rest of the models, any model which was less probable than its nested subset model was also excluded. The remaining models were averaged to estimate variable importance as well as mean and standard deviation of the coefficients. Secondly, a random forest model using an ensemble technique was developed to examine variable importance and to predict sink positivity. The random forest model was trained with 10-fold cross validation, using 500 trees with a maximum node size of 5.
Table 2:
Variable Selection for Sink Risk Modeling
| Variable | Values | Description |
|---|---|---|
| P | +/- | Patient was positive on current day |
| Plag7 | +/- | Patient positive in last 7 days in same room |
| Plag14 | +/- | Patient positive in last 14 days in same room |
| Plag21 | +/- | Patient positive in last 21 days in same room |
| Plag28 | +/- | Patient positive in last 28 days in same room |
| status(P1) | +/- | Patient in adjacent rooms that share common plumbing with next room was positive |
| status(P2) | +/- | Patient in adjacent rooms that do not share common plumb-ing with next room was positive |
| status(S1) | +/- | Patient in adjacent rooms that share common plumbing were positive |
| status(S2) | +/- | Patient in adjacent rooms that do notshare common plumb-ing were positive |
| I3 | 0 or 1 | Intervention occurred in the same room in last 3 days |
| I5 | 0 or 1 | Intervention occurred in the same room in last 5 days |
| I7 | 0 or 1 | Intervention occurred in the same room in last 7 days |
| Slag30 | 0 or 1 | Same room sink was positive in last 30 days |
3. Results
3.1. Imputation Methods
We evaluated the four imputation methods using the data that consists of 967 known positive samples. We began by evaluating a simple generalized linear model (logistic regression) of the given samples as a response to the extracted and selected features in Table 1 where gt annotates the probability of a positive sink given the selected set of predictor variables.
| (1) |
Next, we sequentially add in an imputed value from each method at t-3 (3 days prior to sink-day), t-7 (7 days prior to sink-day), and t-15 (15 days prior to sink-day) to determine which imputation method provided the most increase in the prediction accuracy of the sink based on 10-fold cross validation.
| (2) |
Our baseline data set consisted of the original 967 sink-days while the imputed data sets provides us the ability to use over 80,000 completed sink-days. Table 3 shows the performance of the four imputation methods. It indicates that regardless of time interval, the Midpoint imputation method provides the highest accuracy of prediction of positive sink compared to the baseline (without any imputed values).
Table 3:
Imputation Method Accuracy Results
| Method | t-3 | t-7 | t-15 |
|---|---|---|---|
| Linear | 82.4% | 79.9% | 78.1% |
| Midpoint | 98.2% | 86.1% | 80.5% |
| Logistic Regression | 69.7% | 72.0% | 70.4% |
| Mice | 71.2% | 71.0% | 71.3% |
| Baseline (no imputation) | 70.4% |
3.2. Logistic Regression Model
Based on the results of BMA, we selected the variables whose importance was higher than 10 percent for the final logistic regression model. Table 4 shows the odds ratios and confidence intervals of the variables included in the final model. The odds ratio denotes the increase in probability of a sink becoming positive given the presence of any variable. The confidence interval of odds ratio was calculated using a bootstrap method.
Table 4:
A demographic breakdown of the overall ALS cohort side-by-side with the ALS + PBA subcohort. None of the difference in prevalence were statistically significant.
| Variable | Odds Ratio | 95% C.I | |
|---|---|---|---|
| (P) | Same Room Patient Status | 1.92 | 1.46-2.53 |
| (Plag14) | Positive patient last 14 days | 1.78 | 1.52-2.08 |
| (P1) | Adjacent Room1 Patient status | 1.13 | 0.92- 1.40 |
| (P2) | Adjacent Room2 Patient Status | 1.70 | 1.37-2.09 |
| (S1) | Adjacent Room1 Sink Status | 1.80 | 1.68 - 1.93 |
| (S2) | Adjacent Room2 Sink Status | 1.13 | 1.03 - 1.24 |
| (Slag30) | Sink status in last 30 days | 13.25 | 12.39 - 14.18 |
| (I7) | Interventions carried out | 0.50 | 0.45 - 0.56 |
The results showed that the odds of a sink becoming positive increases by almost 13-fold (95% CI: 12.39-14.18) when the status of the sink was positive in the last 30 days. Intervention has a negative effect on sink positivity. This is consistent with the general understanding that interventions would reduce the odds of sink contamination for a short duration. The odds ratio reduces by 50% if an intervention is performed within the last 7 days. Instances of positive sinks in adjacent rooms1 which share common plumbing increase the chances of a sink becoming positive by 80% (95% CI:1.68-1.93). Instances of positive sinks in adjacent rooms2 that do not share common plumbing increase the chances of a sink becoming positive by only 13% (95% C.I: 1.03-1.24).
The presence of a positive patient in the same room increases the odds of a sink becoming positive by 1.92-fold (95% CI: 1.46-2.53), whereas the presence of any positive patient within the past 14 days increases the odds by 1.78-fold (95% CI: 1.52-2.08). Furthermore, the presence of a positive patient in an adjacent room1 which shares common plumbing increases the odds of sink positivity by 1.13-fold (95% CI: 0.92-1.40), while the presence of a patient in adjacent room that do not share common plumbing increases the odds by 1.70-fold (95% CI: 1.37-2.09). The overall significance of the model was evaluated using the likelihood ratio test, and the result indicates the model is significant at α=0.05 level.
3.2.1. Random Forest Models
The results for Random Forest are shown in Figure 2, which summarizes the importance of variables by mean decrease accuracy (MDA). MDA can be used as a feature selection method because it shows the impact of each variable on the model accuracy. The results show that 7 of the 9 variables included in the logistic regression were also included in the random forest model, while the accuracy slightly increased (81%) compared to Logistic regression (80%). Both models show identical true positive rate, which is important in our case since the cost associated with misclassification of a positive sink as negative is higher than falsely classifying negative sink as positive. Additionally, we see a higher specificity based on random forest model compared to logistic regression. Table 5 summarizes the results from both models.
Figure 2.
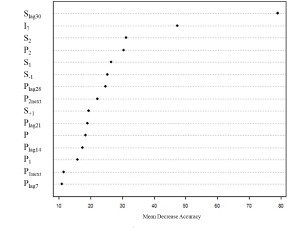
Variable Importance - Random Forest Model
Table 5:
Logistic Regression and Random Forest Model Metrics
| Model | Accuracy | AUC | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| 1 | Logistic Regression | 80% | 78.8% | 84.3% | 73.2% |
| 2 | Random Forest | 81% | 80.4% | 84.3% | 76.6% |
Conclusions
The modeling results indicate that variables such as presence of positive sink in adjacent rooms that share common plumbing, status of the patient in the same room, status of sink in the past 30 days, presence of a positive patient in last 14 days, presence of a positive patient in the adjacent room and interventions performed in the past 7 days are significant risk factors in explaining sink contamination. The findings on the presence of a positive patient in last 14 days, status of sink in the last 30 days and interventions implemented in the last 7 days are consistent with the understanding shared by infection control practitioners we consulted with.
Previous research [25] has shown that biofilms found in sinks were linked to outbreaks. Some research [26,27] has also shown that these biofilms are resistant to traditional disinfectant methods. Our results indicating the significance of adjacent rooms that share common plumbing can be explained by the probable presence of biofilms in sink drain walls, ptraps or drainpipes connecting the two room sinks. Despite the timely implementation of intervention strategies, it could be likely that the presence of biofilms contributed to the adjacent sinks becoming positive.
Another spatial factor that we found to be significant in the model is the presence of a positive patient in the adjacent room. The model results show that the odds of sink positivity increase by 1.13-1.70 fold when the adjacent rooms have a positive patient. We would expect this to be true given that positive patients in the adjacent room would use the sink (adjacent room sink). Thus, it is likely that they would contaminate the sink of the room that they are staying in.
Developing an imputation method for bacterial presence between known samples provided valuable insight into the growth and movement of the bacteria throughout sinks in the hospital. Additionally, the ability to complete a data set of environmental testing allows for the capability for it to be included in other models, such as patient risk modeling, that will provide a more comprehensive predictive capability. In modeling the presence of CRE in sinks, this research aimed to understand what factors contribute to sink positivity. Knowledge gain in both capacities can lead to better understanding the role of environmental reservoirs in the spread of the bacteria and could be used to support changes to hospital policy and procedures that ultimately aid in the containment or eradication of the bacteria from the hospital.
Acknowledgment
This work was supported by the Coulter Translational Research Partnership and the University of Virginia Health Center.
References
- [1].Frieden T. “Antibiotic resistance threats in the United States, 2013,”. Tech. Rep. 2013 [Google Scholar]
- [2].Kallen M, Ricks P, Edwards J, MStat A. S, Fridkin S, Rasheed J. K, Lonsway D, Herrera R, McDonald L. C, Patel J, et al. “Vital signs: carbapenem-resistant enterobacteriaceae,”. Morb Mortal Wkly Rep. 2013;62:165170. [PMC free article] [PubMed] [Google Scholar]
- [3].Wilcox J. B. Hospital-Acquired Infections. New York, New York: US:Nova; 2009. [Google Scholar]
- [4].Vergara-López S, Domínguez M, Conejo M, Pascual A, Rodríguez-Baño J. “Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-β-lactamase-producing klebsiella oxytoca,”. Clinical Microbiology and Infection. 2013;19(11):E490–E498. doi: 10.1111/1469-0691.12288. [DOI] [PubMed] [Google Scholar]
- [5].Kotay S, Cha i. W, Guilford W, Barry K, Mathers A. “Spread from the sink to the patient: In situ study using green fluorescent protein (gfp)-expressing escherichia coli to model bacterial dispersion from hand-washing sink-trap reservoirs,”. AEM. 2017;83.8 doi: 10.1128/AEM.03327-16. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5377511/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barnes L, Brown D, Lobo J, Mathers A, Papin J. “Superbug tracker,” 2015, design of Context-Aware Surveillance System for Nosocomial Outbreaks involving Non-Patient Reservoirs. Technical report [Google Scholar]
- [7].Sterne J. A. C, White I. R, Carlin J. B, Spratt M, Royston P, Kenward M. G, Wood A. M, Carpenter J. R. “Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls,”. BMJ. 2009;338 doi: 10.1136/bmj.b2393. [Online]. Available: http://www.bmj.com/content/338/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patel G, Huprikar S, Factor S. H, Jenkins S. G, Calfee D. P. “Outcomes of carbapenem-resistant klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies,”. Infection control and hospital epidemiology. 2008;29(12):1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- [9].Schwaber M. J, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. “Predictors of carbapenem-resistant klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality,”. Antimicrobial agents and chemotherapy. 2008;52(3):1028–1033. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Woodford N, Tierno P. M, Young K, Tysall L, Palepou M.-F. I, Ward E, Painter R. E, Suber D. F, Shungu D, Silver L. L, et al. “Outbreak of klebsiella pneumoniae producing a new carbapenem-hydrolyzing class a β- lactamase, kpc-3, in a new york medical center,”. Antimicrobial agents and chemotherapy. 2004;48(12):4793–4799. doi: 10.1128/AAC.48.12.4793-4799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wolf I, Bergervoet P, Sebens F, Van den Oever H, Savelkoul P, Van der Zwet W. “The sink as a correctable source of extended-spectrum β-lactamase contamination for patients in the intensive care unit,”. Journal of Hospital Infection. 2014;87(2):126–130. doi: 10.1016/j.jhin.2014.02.013. [DOI] [PubMed] [Google Scholar]
- [12].Roux D, Aubier B, Cochard H, Quentin R, van der Mee-Marquet N, et al. “Contaminated sinks in intensive care units: an underestimated source of extended-spectrum beta-lactamase-producing enterobacteriaceae in the patient environment,”. Journal of Hospital Infection. 2013;85(2):106–111. doi: 10.1016/j.jhin.2013.07.006. [DOI] [PubMed] [Google Scholar]
- [13].Crespo M, Woodford N, Sinclair A, Kaufmann M, Turton J, Glover J, Velez J, Castaneda C, Recalde M, Livermore D. “Outbreak of carbapenem-resistant pseudomonas aeruginosa producing vim-8, a novel metallo- β-lactamase, in a tertiary care center in cali, Colombia,”. Journal of clinical microbiology. 2004;42(11):5094–5101. doi: 10.1128/JCM.42.11.5094-5101.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stroud L. A, Tokars J. I, Grieco M. H, Crawford J. T, Culver D. H, Edlin B. R, Sordillo E. M, Woodley C. L, Gilligan M. E, Schneider N, et al. “Evaluation of infection control measures in preventing the nosocomial transmission of multidrug-resistant mycobacterium tuberculosis in a new york city hospital,”. Infection Control & Hospital Epidemiology. 1995;16(03):141–147. doi: 10.1086/647075. [DOI] [PubMed] [Google Scholar]
- [15].Papadimitriou-Olivgeris M, Marangos M, Fligou F, Christofidou M, Bartzavali C, Anastassiou E. D, Filos K. S. “Risk factors for kpc-producing klebsiella pneumoniae enteric colonization upon icu admission,”. Journal of antimicrobial chemotherapy. 2012;67(12):2976–2981. doi: 10.1093/jac/dks316. [DOI] [PubMed] [Google Scholar]
- [16].Tuon F. F, Rocha J. L, Toledo P, Arend L. N, Dias C. H, Leite T. M, Penteado-Filho S. R, Pilonetto M, Zavascki A. P. “Risk factors for kpc-producing klebsiella pneumoniae bacteremia,”. Brazilian Journal of Infectious Diseases. 2012;16(5):416–419. doi: 10.1016/j.bjid.2012.08.006. [DOI] [PubMed] [Google Scholar]
- [17].Stern J, Hewitt S, Guilfoyle M, Mishra C, Mathers A, Lobo J, Brown D, Barnes L. in Systems and Information Engineering Design Symposium (SIEDS), 2015. IEEE; 2015. “Modeling nosocomial transmission of carbapenem-resistant bacteria,”; pp. 176–181. [Google Scholar]
- [18].Horton N. J, Kleinman K. P. “Statistical computing and graphics-statistical computing software reviewsmuch ado about nothing: A comparison of missing data methods and software to fit incomplete data regression models,”. American Statistician. 2007;61(1):79. doi: 10.1198/000313007X172556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sterne J. A, White I. R, Carlin J. B, Spratt M, Royston P, Kenward M. G, Wood A. M, Carpenter J. R. “Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls,”. Bmj. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mirkes E. M, Coats T. J, Levesley J, Gorban A. N. “Handling missing data in large healthcare dataset: A case study of unknown trauma outcomes,”. Computers in Biology and Medicine. 2016;75:203–216. doi: 10.1016/j.compbiomed.2016.06.004. [Online]. Available: http://dx.doi.org/10.1016/j.compbiomed.2016.06.004. [DOI] [PubMed] [Google Scholar]
- [21].Hernández-Pereira E. M, Álvarez-Estévez D, Moret-Bonillo V. “Automatic classification of respiratory patterns involving missing data imputation techniques,”. Biosystems Engineering. 2015;138:65–76. [Online]. Available: http://www.sciencedirect.com/science/article/pii/S1537511015001117. [Google Scholar]
- [22].Buuren S, Groothuis-Oudshoorn K. “mice: Multivariate imputation by chained equations in r,”. Journal of statistical software. 2011;(45)(3) [Google Scholar]
- [23].Azur M, Stuart E, Frangakis C, Leaf P. “Multiple imputation by chained equations: What is it and how does it work?”. Int J Methods Psychiatr Res. 2011;20(1) doi: 10.1002/mpr.329. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3074241/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Raftery A. E, Painter I. S. “Bma: an r package for bayesian model averaging,”. R news. 2005;5(2):2–8. [Google Scholar]
- [25].Hota S, Hirji Z, Stockton K, Lemieux C, Dedier H, Wolfaardt G, Gardam M. A. “Outbreak of multidrug-resistant pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design,”. Infection Control & Hospital Epidemiology. 2009;30(01):25–33. doi: 10.1086/592700. [DOI] [PubMed] [Google Scholar]
- [26].Presterl E, Suchomel M, Eder M, Reichmann S, Lassnigg A, Graninger W, Rotter M. “Effects of alcohols, povidone-iodine and hydrogen peroxide on biofilms of staphylococcus epidermidis,”. Journal of Antimicrobial Chemotherapy. 2007;60(2):417–420. doi: 10.1093/jac/dkm221. [DOI] [PubMed] [Google Scholar]
- [27].Buckingham-Meyer K, Goeres D. M, Hamilton M. A. “Comparative evaluation of biofilm disinfectant efficacy tests,”. Journal of microbiological methods. 2007;70(2):236–244. doi: 10.1016/j.mimet.2007.04.010. [DOI] [PubMed] [Google Scholar]


