Abstract
Management of heart failure is complex, often involving interaction with multiple providers, monitoring of symptoms, and numerous medications. Employing principles of user-centered design, we developed a high- fidelity prototype of a mobile system for heart failure self-management and care coordination. Participants, including both heart failure patients and health care providers, tested the mobile system during a one-hour one-on-one session with a facilitator. The facilitator interviewed participants about the strengths and weaknesses of the prototype, necessary features, and willingness to use the technology. We performed a qualitative content analysis using the transcripts of these interviews. Fourteen distinct themes were identified in the analysis. Of these themes, integration, technology literacy, memory, and organization were the most common. Privacy was the least common theme. Our study suggests that this integration is essential for adoption of a mobile system for chronic disease management and care coordination.
Introduction
Heart failure is a major public health concern, affecting more than 5.7 million Americans1 and projected to increase 46% by 2030.2 Heart failure is the most common cause of hospitalization among adults over age 653 and accounts for more than 30 billion dollars in health care spending each year.1 Management of heart failure is complex, often involving interaction with multiple providers, continuous monitoring of symptoms and measurements, and numerous medications. Newly diagnosed heart failure patients are faced with the challenge of adapting to these major lifestyle changes and often struggle to successfully self-manage.4 As a result, heart failure readmissions are common. More than 50% of patients are readmitted within six months of initial discharge.3 Additionally, heart failure patients often suffer from one or more comorbidities; a large national study of Medicare patients found that 40% of heart failure patients also had diabetes, and around one-third had chronic obstructive pulmonary disease.5 A recent systematic review of depression in heart failure patients found an average prevalence of depression of 29%.6 Thus, patients with heart failure often have the task of managing multiple chronic conditions, adding further complexity to self- management.
Several heart failure management interventions have been associated with decreased readmissions and mortality.7–10 Characteristics of these interventions associated with improved outcomes include face-to-face contact,8 use of multidisciplinary teams,8,9 and longer intervention duration.10 However, because these interventions are extremely resource-intensive scalability is limited. A 2016 systematic review found 34 existing mobile systems for heart failure self-management. While data collected from these types of systems have the potential to inform healthcare decisions, existing applications are largely limited to the collection of physical measurements and symptom tracking and lack integration with the health system.11 Furthermore, most existing mobile systems for care management do not facilitate two-way communication, which has been found to be preferred by patients using heart failure monitoring systems.12 Finally, most self-management applications are focused on a single disease, rather than allowing patients to manage multiple conditions. These limitations reflect a lack of attention to patient-centeredness in the design of current mobile systems. Patient-centered applications are defined as “systems that enable a partnership among practitioners, patients, and their families (when appropriate) to ensure that procedures and decisions respect patients’ needs and preferences.”13 Such systems can facilitate care coordination, in which all members of the care team work collaboratively with the patient to make decisions about care and facilitate delivery of services.14 This type of shared decision-making requires that patients have a comprehensive understanding of their health condition, treatment options, and the roles and responsibilities of each care team member.15–17 User- centered design can help to create patient-centered applications for care coordination by understanding how patients would use the system in their daily lives and building features and functionalities around patient preferences and use cases.13,1 19 User-centered design may also increase the likelihood of adoption and satisfaction with the system.20,21 The OnPoint study was initiated to improve understanding of how patient- centered mobile systems may improve care coordination with for people with multiple chronic conditions, such as those with heart failure and other comorbidities.
Objectives
The objectives of this study were to 1) assess the major strengths and weaknesses of a high-fidelity prototype of the OnPoint application, a mobile system for the management of complex chronic disease, with patients with heart failure and key members of the care team and 2) identify additional functions and features that would support adoption of the application by patients and members of the care team.
Materials and methods
We used principles of user-centered design to design and evaluate a high-fidelity prototype of OnPoint, a patient-centered mobile application for collaborative chronic disease management. A high-fidelity prototype is an application that is similar to the intended final product, allowing the user to experience realistic interactions; this is in contrast to a low-fidelity prototype, which may be rendered on paper or screenshots. 22 In a previous paper we reported on interviews with key informants, including patients, clinicians, caregivers, and health coaches that resulted in a list of key user functions and user scenarios, around which we created the prototype.23 The prototype focused on the medication reconciliation and medication taking, priorities identified by both patients and providers: constructing a comprehensive medication list, creating a medication schedule, filling a pillbox, and tracking medications. Other features included care team contacts, tracking weight/blood pressure, managing appointments, and screening for symptoms. When possible, we created digital representations that complemented the tools that patients use in daily life for care coordination (the user-interface metaphor).24,25 For instance, because patients often use pillboxes to manage medications, we created an interface that evokes a pillbox metaphor to connect the digital representation on the tablet to the patient’s physical pillbox.26 The novel medication management features allow a user to take a picture of the medication bottle label which scans the data into the application, inserts a picture of the pill itself, sorts medications into appropriate day/time compartment in the rendered physical pillbox, which all serve to help the patient organize medications accurately and efficiently. The high-fidelity prototype was developed using Ionic, an open-source software development kit that allows creation of hybrid mobile applications.27 Adobe PhoneGap was deployed to allow development of a cross-platform application that would run on both iOS and Android.26 Figure 1 shows several screens from the high-fidelity prototype.
Figure 1.
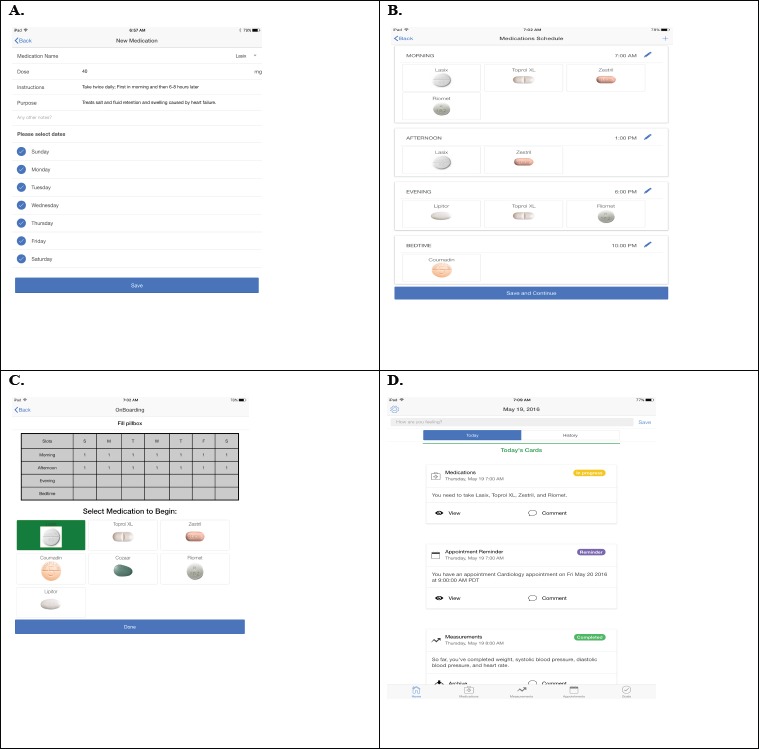
Selected medication features of OnPoint high-fidelity prototype, including A. Medication setup, B. Medication scheduling, C. Pillbox filling feature, and D. Timeline view showing list of today’s tasks
We recruited participants from the UC Davis Health System to test the high-fidelity prototype. We selected patient participants who had experience living with or managing heart failure along with another health condition as well as clinicians or health coaches who provide care for heart failure patients. Participants tested the mobile system on a 7.9-inch internet-connected iPad mini tablet during a one-hour one-on-one session with a facilitator. Following a structured patient scenario, the facilitator instructed the participant to attempt each feature of the prototype. For 1) the medication management feature, participants were asked to a) add several medications, b) create a schedule of medications, c) fill a pillbox using the application, d) record the taking and skipping of medications, and e) view medication history. For 2) the measurements feature, participants were asked to a) set up new measurements (weight, blood pressure, and heart rate), b) add measurements to the schedule, c) record measurements, and d) view measurement history. For 3) the appointments feature, participants were asked to enter new appointments and view appointments in the timeline. Finally, participants were asked to use 4) the comments feature of the timeline to record notes and questions to use share with other members of the care team. The symptom management feature was not ready to be tested at the time of the interviews, and was therefore not included in this evaluation. We applied a think-aloud methodology to elicit participants’ thoughts and perceptions of use of the application.28,29 The facilitator took notes capturing both verbalized feedback and visually observed challenges with using the application. The facilitator then interviewed participants about the strengths and weaknesses of the prototype, additional necessary features and functionalities, and willingness to use the technology, following a semistructured interview guide.
Field notes and interview notes were analyzed using a three-phase qualitative content analysis process: 1) immersion, consisting of numerous close readings of the transcripts, 2) reduction, in which themes were identified, defined, and coded, and 3) interpretation, in which themes were organized and conclusions were drawn and verified.30–32 Initial codes were inductively developed by one investigator (SH) on analysis of thefirst three interview transcripts. The codes and their associated themes were discussed in detail with a second investigator (KK) and revised. The revised coding scheme was applied to the remaining transcripts. A final review of transcripts and codes was completed by the two investigators with discussion of any discrepancies with the goal of consensus on issues identified. A basic quantitative content analysis consisting of counting the number of times a theme was mentioned by any participant was calculated to provide a high level indication of the themes that are “top of mind” among the participant group. The UC Davis Institutional Review Board concluded that this study was not human subjects research (IRB# 782917-1).
Results
Eight individuals participated in the testing of the prototype. These included five patients with heart failure who also manage multiple health conditions, including cancer, diabetes, hypertension, and depression. Three care team members participated including a cardiologist, a cardiac care nurse, and a primary care health coach. Themes fell into three main categories: strengths and potential benefits, potential barriers to use, and features and functions for future development. Fourteen distinct themes were identified during the content analysis. These themes are shown in Table 1 along with a count of the number of times the theme was mentioned, and the proportion (%) of patient and care team members who mentioned the theme at least once.
Table 1.
Definitions, overall number of mentions, and percent of participants who mentioned the theme, for each theme identified.
| Theme | Definition used for coding | Number of mentions | % of patients | % of care team |
|---|---|---|---|---|
| Strengths and Potential Benefits to Use of OnPoint Prototype | ||||
| Memory | Remembering tasks and activities | 12 | 80.0 | 66.7 |
| Organization | Arranging tasks and activities; structuring and storing information in a standard way | 12 | 60.0 | 66.7 |
| Convenience | Ease of using the system | 7 | 60.0 | 33.3 |
| Sharing | Sharing of information with others, including clinicians, caregivers, family | 7 | 40.0 | 100.0 |
| members, and other members of the care | ||||
| team | ||||
| Efficiency | Speed of tasks and activities | 2 | 20.0 | 33.3 |
| Potential Barriers to Use of OnPoint Prototype | ||||
| Technology literacy | Affordances for users with different levels of familiarity with technology to use technology effectively | 16 | 80.0 | 100.0 |
| Burden | Challenges facing users that may inhibit effective use of the system | 10 | 40.0 | 66.7 |
| User types | Differences between user personalities and | 4 | 40.0 | 33.3 |
| profiles that may affect how the system is | ||||
| used | ||||
| Connectivity | Reliance of a technology system on wireless Internet | 3 | 0.0 | 66.7 |
| Privacy | Protection of personal information | 1 | 0.0 | 33.3 |
| Features and Functions for Future Development | ||||
| Integration | Linkage with other data sources, applications, or devices | 20 | 80.0 | 100.0 |
| Personalization | Tailoring of the system to meet an individual’s specific needs | 9 | 60.0 | 66.7 |
| Communication | Exchange of information between patients and providers | 6 | 40.0 | 66.7 |
| Tracking | Recording information (patient-generated) | 5 | 60.0 | 0.0 |
Integration was the most common theme overall, mentioned by 80% of patient participants and all of the clinician participants. Participants stressed that the usefulness of a mobile system depends upon its ability to integrate with other important sources of information. These sources include other applications such as calendars and applications that allow users to track salt, fluids, and other nutrition information; they also include devices such as physical activity trackers and Wi-Fi-enabled scales and heart rate monitors. The ability to link the application to clinical data, including lab results and prescription information, was especially important to patient participants, particularly for those taking medications like warfarin that require continuous monitoring. Integration may also help to decrease the burden on patients that may be associated with setup and management of a mobile application:
“The application would need to be smart about how to pull data from multiple places; I wouldn’t want to enter the same information in two different places.” - Patient participant
Technology literacy, the second most common theme, was mentioned by both patient and clinician participants as a potential challenge to implementation. They expressed concern that those with low technology literacy, particularly many elderly patients, would need substantial support from others to set up and use the system. For this reason, participants thought that this group of people might be reluctant to adopt the technology:
“Setting up the application by yourself would be really difficult, particularly if you’re not familiar with technology.” –Health coach
Memory was the next most common theme. Several participants described memory as a common problem for heart failure patients. Participants believed that the system would improve the ability to remember both routine and infrequent tasks:
“A lot of people have brain fog from heart failure- mental reminders are helpful so you know which pills have been taken and which haven’t.” -Cardiac nurse
Participants felt that the system would improve the ability to organize data in one place so that information was available when it was needed. Patient participants reported that they currently used a variety of methods and systems to manage information; patients felt that this was a burden for daily self-management as well as for sharing information.
“Right now, I have tracking on lots of different devices, papers, calendars… it would be much easier if it was all in one place.” – Patient participant
“If you go to the ER, you could just show all of the information. It would be so much easier to provide your information when someone needed it because it’s all in one place.” – Patient participant
Importantly, participants also saw the benefit of the mobile system as something that could help them better understand their condition and their care plan, an important component of shared decision-making.
“This would be useful for familiarizing yourself with your medications… I realize I need a closer look at what’s going on with my meds.” –Patient participant
“I like that you can see the purpose of all the [medications] – you can really know what you’re taking and why.” –Patient participant
Several themes were more important to either patient participants or care team participants. Tracking, or the recording of patient-generated information, was mentioned by 60% of patient participants but mentioned by none of the care team participants. Conversely, several care team participants spoke about connectivity issues, while none of the patient participants mentioned connectivity as a potential barrier to use.
Privacy was the least common theme, mentioned by only one of the care team participants:
“Personal information protection is a concern - not everyone wants to have their wife or husband or adult children know what they’re taking.” – Cardiologist
Discussion
In their review of 34 mobile applications for heart failure, Masterson Creber et al. found that most applications are focused on building healthy habits, rather than on shared disease management.11 One reason for this may be the difficulty of integration with existing health care systems. Currently, incompatibility between systems and data sharing policies inhibit integration with mobile applications. 33–35 In our study, the voice of patients themselves brings attention to a key challenge for chronic disease management: the challenge of unifying multiple data streams to create a single personalized system tailored to each individual patient’s needs. This type of system requires not only the ability to gather and organize patient data but also the capacity to learn and respond to information. Integration with the electronic health record (EHR) is one example of this. Clinicians commonly rely on EHR systems and patient portals for managing patient data and communicating with patients. Health system regulations, in addition to feasibility and workflow considerations, prevent adoption of a system that is separate from the EHR. Because shared information and communication are crucial components of successful disease management and effective care coordination, integration with the EHR should be a priority for any chronic disease management system. Our study suggests that integration is also crucial to patients’ adoption of a mobile system for chronic disease management. Because chronic disease patients often manage multiple comorbidities and have varying nutrition and activity requirements, fluid restrictions, or other specific measurements, integration with other devices and applications is necessary to ensure completeness of a shared care plan. A useful application will also have the ability to integrate with the patient’s existing management strategies, such as calendar or pharmacy applications. This is in line with previous findings on user preferences for selfmanagement applications. 36 Our study suggests that integration of an application with existing external data sources and systems is more important than developing additional features; this is an important implication for prioritizing future work in this area. In addition, our findings indicate that there is a need for organized summary data for all types of users: patients, caregivers, and care team. Each type of user requires different types of information to make informed decisions. Development of data visualizations for these various users will require in-depth exploration into how users make decisions about care, and how data collected by a mobile system can assist in the decision-making process.
To create a usable application for chronic disease management, concerns about technology literacy must be addressed. Many previous studies have used mobile systems to deliver chronic disease management interventions.37–42 However, these studies often suffer from low usage of the technology, which may be in part a function of low technology literacy. To date, there have been few studies examining technology literacy in the context of mobile applications for disease management. Principles of user-centered design can help to create a system that can be used effectively even by those with little previous experience with mobile applications.
Although privacy was not mentioned by patient participants in this study, concerns about privacy are often mentioned with respect to use of technology. Some studies that have found low levels of concern about privacy threats with regard to mobile technologies. In a national public opinion survey of radio frequency identification (RFID) technology for healthcare, Katz and Rice found only a minority of participants who were concerned about threats to privacy.43 In a qualitative study by Pinnock et al., neither patients nor healthcare professionals considered privacy to be a major issue with regard to adoption of a healthcare management technology.44 Nevertheless, a recent statewide survey found high levels of concern regarding privacy in sharing of electronic health data for provision of healthcare and willingness to agree to share these data. Hence, assurance of privacy should continue to be considered in development and implementation of mobile care coordination solutions.
Findings of our study suggest several implications for building systems for care coordination. First, while reminders and tracking can be useful management tools, they are not sufficient for facilitating shared care. The patient participants in our study desired a thorough understanding of the health condition and care plan. Improved understanding of medications was seen as especially useful for patients. This suggests that patient- centered mobile systems should have the capacity to enrich a patient’s understanding of the disease and the treatment plans, instead of simply recording it. These findings align with existing literature on shared care and shared decision-making, which emphasize the informed participation of the patient in the creation of a care plan.15–17 Second, our findings indicate that the ability to share information quickly and easily is an important part of a mobile system for chronic disease management. Access to the same information by everyone involved in the patient’s care will likely allow for improved coordination and collaboration among the care team, characteristics of care found to be associated with improved health outcomes.45
There are several limitations to our study. First, we interviewed a small convenience sample of potential users, recruited from a single academic medical center in California. Although appropriate for a usercentered design study,46 the sample was not large enough to quantify differences between types of users or to discuss how preferences differed by age, disease severity, familiarity with technology, or other characteristics. Second, four of the participants (three patients participants and the cardiologist) were also involved in previous design phases, where they provided details on how they manage care and what aspects of care management and coordination are most important to them. For this reason, they may have been more inclined to provide positive feedback on the high-fidelity prototype, biasing the study results. As is common for qualitative studies such as the one present in this paper, the findings are not intended to be generalizable.
Next steps
We are currently using the feedback provided by patient and care team participants to develop the OnPoint prototype into a fully functional application for chronic disease management. The working version of the system will include additional features and integration with other systems, including specifications for EHR integration. We plan to use a participatory design process to test the application with patient and clinician participants in simulated home and clinical environments; we expect that this will provide insight into how a system can be optimized for different types of users. Our next steps will also include the creation and testing of data visualizations, to explore how patient-generated data collected by the application can be used for care coordination and shared decision-making. Understanding how these types of data will be shared and used is essential for building a system that can support coordination and management of multiple complex chronic diseases.
Conclusion
Although many mobile applications have been developed for the management of chronic disease, none has achieved widespread adoption. Our study provides insight into potential reasons for this by highlighting key barriers and facilitators of adoption of a mobile system for heart failure care coordination. The findings of this project emphasize the need for an integrated solution. Such a system will have the potential to improve selfmanagement for patients managing multiple chronic conditions. It will also facilitate collaboration between patients and their care team, by allowing for the development and management of a shared care plan. Integration of the resulting care plan with the electronic health record is crucial for meaningful sharing of patient-generated information between patients and healthcare providers. The unique requirements, restrictions, and circumstances facing each patient require a flexible system that has the ability to capture and summarize information from multiple sources.
Acknowledgements
We thank Bjoern Hartmann, Dan Gillette, Angela Hsueh, Bill Kim, and Amy Wang for collaborating on the development of the prototype. This project was funded by University of California, Center for Information Technology in the Interest of Society (CITRIS).
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):447. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the united states a policy statement from the american heart association. Circulation: Heart Failure. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai AS, Stevenson LW. Rehospitalization for heart failure predict or prevent. Circulation. 2012;126(4):501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 4.Carlson B, Riegel B, Moser DK. Self-care abilities of patients with heart failure. Heart & Lung: The Journal of Acute and Critical Care. 2001;30(5):351–359. doi: 10.1067/mhl.2001.118611. [DOI] [PubMed] [Google Scholar]
- 5.Havranek EP, Masoudi FA, Westfall KA, Wolfe P, Ordin DL, Krumholz HM. Spectrum of heart failure in older patients: results from the National Heart Failure project. American heart journal. 2002;143(3):412–417. doi: 10.1067/mhj.2002.120773. [DOI] [PubMed] [Google Scholar]
- 6.Sokoreli I, de Vries J, Pauws S, Steyerberg E. Depression and anxiety as predictors of mortality among heart failure patients: systematic review and meta-analysis. Heart failure reviews. 2016;21(1):49–63. doi: 10.1007/s10741-015-9517-4. [DOI] [PubMed] [Google Scholar]
- 7.Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC cardiovascular disorders. 2006;6(1):1. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sochalski J, Jaarsma T, Krumholz HM, et al. What works in chronic care management: the case of heart failure. Health Affairs. 2009;28(1):179–189. doi: 10.1377/hlthaff.28.1.179. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission. Journal of the American College of Cardiology. 2004;44(4) doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 10.Jonkman NH, Westland H, Groenwold RH, et al. What are effective program characteristics of self-management interventions in patients with heart failure? An individual patient data meta-analysis. Journal of Cardiac Failure. 2016;22(11):861–871. doi: 10.1016/j.cardfail.2016.06.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masterson Creber RM, Maurer MS, Reading M, Hiraldo G, Hickey KT, Iribarren S. Review and Analysis of Existing Mobile Phone Apps to Support Heart Failure Symptom Monitoring and Self-Care Management Using the Mobile Application Rating Scale (MARS) JMIR mHealth and uHealth. 2016;4(2) doi: 10.2196/mhealth.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert NM, Dinesen B, Spindler H, et al. Factors associated with telemonitoring use among patients with chronic heart failure. Journal of telemedicine and telecare. 2016;1357633X16630444 doi: 10.1177/1357633X16630444. [DOI] [PubMed] [Google Scholar]
- 13.Demiris G, Afrin LB, Speedie S, et al. Patient-centered applications: use of information technology to promote disease management and wellness. [A white paper by the AMIA knowledge in motion working group]. Journal of the American Medical Informatics Association. 2008;15(1):8–13. doi: 10.1197/jamia.M2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald KMS, Vandana Bravata, Dena M. Lewis, Robin Lin, Nancy Kraft, Sally A McKinnon, Moira Paguntalan, Helen Owens, Douglas K. Closing the quality gap: a critical analysis of quality improvement strategies (Vol 7: care coordination) AHRQ Technical Reviews. 2007 [PubMed] [Google Scholar]
- 15.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean?(or it takes at least two to tango) Social science & medicine. 1997;44(5):681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 16.Katz SJ, Belkora J, Elwyn G. Shared decision making for treatment of cancer: challenges and opportunities. Journal of Oncology Practice. 2014;10(3):206–208. doi: 10.1200/JOP.2014.001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards A, Elwyn G. Shared decisionmaking in health care: Achieving evidence-based patient choice. Oxford University Press; 2009. [Google Scholar]
- 18.Årsand E, Demiris G. User-centered methods for designing patient-centric selfhelp tools. Informatics for health and social care. 2008;33(3):158–169. doi: 10.1080/17538150802457562. [DOI] [PubMed] [Google Scholar]
- 19.Dabbs ADV, Myers BA, Mc Curry KR, et al. User-centered design and interactive health technologies for patients. Computers, informatics, nursing: CIN. 2009;27(3):175. doi: 10.1097/NCN.0b013e31819f7c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCurdie T, Taneva S, Casselman M, et al. mHealth consumer apps: the case for user-centered design. Biomedical instrumentation & technology. 2012;46(s2):49–56. doi: 10.2345/0899-8205-46.s2.49. [DOI] [PubMed] [Google Scholar]
- 21.Endsley MR. Designing for situation awareness: An approach to user-centered design. CRC press; 2016. [Google Scholar]
- 22.Walker M, Takayama L, Landay JA. Highfidelity or low-fidelity, paper or computer? Choosing attributes when testing web prototypes. Paper presented at: Proceedings of the human factors and ergonomics society annual meeting. 2002 [Google Scholar]
- 23.Haynes S, Kim K. A mobile care coordination system for the management of complex chronic disease. Studies in health technology and informatics. 2016;225 [PubMed] [Google Scholar]
- 24.Neale DC, Carroll JM. The role of metaphors in user interface design. Handbook of human-computer interaction. 1997;2:441–462. [Google Scholar]
- 25.Carroll JM, Thomas JC. Metaphor and cognitive representation of computing systems. IEEE Transactions on Systems, Man, & Cybernetics. 1982 [Google Scholar]
- 26.Hsueh A. OnPoint: A Social and Mobile Platform to Optimize Health Services for Complex, Chronic Care Management. University of California Berkeley; 2016. [Google Scholar]
- 27.Ionic Framework [computer program] Version 1.3.22016
- 28.Jaspers MW, Steen T, Van Den Bos C, Geenen M. The think aloud method: a guide to user interface design. International journal of medical informatics. 2004;73(11):781–795. doi: 10.1016/j.ijmedinf.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Jaspers MW. A comparison of usability methods for testing interactive health technologies: methodological aspects and empirical evidence. International journal of medical informatics. 2009;78(5):340–353. doi: 10.1016/j.ijmedinf.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Forman J, Damschroder L. Empirical methods for bioethics: A primer. Emerald Group Publishing Limited; 2007. Qualitative content analysis; pp. 39–62. [Google Scholar]
- 31.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qualitative health research. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 32.Coffey A, Atkinson P, Omarzu J. Making sense of qualitative data. Psyccritiques. 1997;42(7):650. [Google Scholar]
- 33.Pagliari C, Detmer D, Singleton P. Potential of electronic personal health records. BMJ: British Medical Journal. 2007;335(7615):330. doi: 10.1136/bmj.39279.482963.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deering MJ, Siminerio E, Weinstein S. Issue brief: patient-generated health data and health IT. Office of the National Coordinator for Health Information Technology. 2013:1–11. [Google Scholar]
- 35.Huba N, Zhang Y. Designing patient-centered personal health records (PHRs): health care professionals’ perspective on patient-generated data. Journal of medical systems. 2012:1–13. doi: 10.1007/s10916-012-9861-z. [DOI] [PubMed] [Google Scholar]
- 36.Hilliard ME, Hahn A, Ridge AK, Eakin MN, Riekert KA. User preferences and design recommendations for an mHealth app to promote cystic fibrosis self-management. JMIR mHealth and uHealth. 2014;2(4) doi: 10.2196/mhealth.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Årsand E, Tatara N, Østengen G, Hartvigsen G. Mobile phone-based self-management tools for type 2 diabetes: the few touch application. Journal of diabetes science and technology. 2010;4(2):328–336. doi: 10.1177/193229681000400213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. Journal of the American Heart Association. 2013;2(6):e000568. doi: 10.1161/JAHA.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mertens A, Brandl C, Miron-Shatz T, et al. A mobile application improves therapy-adherence rates in elderly patients undergoing rehabilitation: a crossover design study comparing documentation via iPad with paper-based control. Medicine. 2016;95(36) doi: 10.1097/MD.0000000000004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc™ mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes technology & therapeutics. 2008;10(3):160–168. doi: 10.1089/dia.2008.0283. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Pérez B, de la Torre-Díez I, Lopez-Coronado M, Sainz-De-Abajo B. Comparison of mobile apps for the leading causes of death among different income zones: a review of the literature and app stores. JMIR mHealth and uHealth. 2014;2(1):e1. doi: 10.2196/mhealth.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz JE, Rice RE. Public views of mobile medical devices and services: A US national survey of consumer sentiments towards RFID healthcare technology. International journal of medical informatics. 2009;78(2):104–114. doi: 10.1016/j.ijmedinf.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Professional and patient attitudes to using mobile phone technology to monitor asthma: questionnaire survey. Primary care respiratory journal. 2006;15(4):237–245. doi: 10.1016/j.pcrj.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies G, Harris M, Perkins D, et al. Coordination of care within primary health care and with other sectors: a systematic review. Australian Primary Health Care Research Institute Report. 2006 [Google Scholar]
- 46.Nielsen J. Why You Only Need to Test with 5 Users 2000. https://www.nngroup.com/articles/why-you-only-need-to-test-with-5-users/


