Abstract
We consider the risk of adverse drug events caused by antibiotic prescriptions. Antibiotics are the second most common cause of drug related adverse events and one of the most common classes of drugs associated with medical malpractice claims. To cope with this serious issue, physicians rely on guidelines, especially in the context of hospital prescriptions. Unfortunately such guidelines do not offer sufficient support to solve the problem of adverse events. To cope with these issues our work proposes a clinical decision support system based on expert medical knowledge, which combines semantic technologies with multiple criteria decision models. Our model links and assesses the adequacy of each treatment through the toxicity risk of side effects, in order to provide and explain to physicians a sorted list of possible antibiotics. We illustrate our approach through carefully selected case studies in collaboration with the EpiCURA Hospital Center in Belgium.
Introduction
Adverse drug events are one of the most important causes of mortality in the healthcare context. They claim each year between 700,000 and 1.5 million casualties in the United States1. From these, antibiotics are the second most common cause of drug related adverse events2,3,4 and one of the most common classes of drugs associated with medical malpractice claims5. Every antibiotic treatment is associated with a risk rate, i.e., an assessment of the degree of risk, which takes into account both the severity and the frequency of undesirable side effects. Determining the best treatments is highly dependent on the considered acceptance risk. The acceptance risk of a particular treatment varies according to the vulnerability of the patient. Therefore, physicians are in need of support that can give them both an overview of the situation, but also a detailed description of the risk and the vulnerability of each patient.
For many hospitals, guidelines constitute the solution to guide antibiotic prescriptions, by linking infection diagnosis to the relevant antibiotic therapies. As an example, our collaborators in the EpiCURA Hospital Center have been using a hundred-page guideline since 20116. Unfortunately, there is evidence showing that little or nothing has really changed regarding the malpractice issue in spite of the existence of guidelines. The main causes of this situation are that a) the physician cannot have a global view of the guidelines or complete explanations for the suggested treatment; b) in their current textual form these guidelines are completely static, making them hard to use and adapt to either the specific needs of the patient or the changing environment context (e.g. usage in emergency rooms).
Using decision support systems (DSS) in a clinical context is mainly about providing recommendations to physicians. Some of these Clinical DSS are using semantic technologies. From this perspective, Bright and coauthors7 have developed a formal ontology to structure the empiric antibiotic therapy guidelines of the New-York Presbyterian Hospital (NYP). The guidelines in this study have been explicitly entered in Protégé. This work was able to generate three kinds of prescribing alerts when the guidelines were not respected. Despite its advantages, this approach has serious limitations. Its main drawback is that both the basic data and the relationships between them should be explicitly entered in the system (i.e. the system cannot generalize to new data), making maintainability difficult, if not impossible. Other funded projects around semantic technologies for medical procedures such as REMINE8 and PSIP9 use data mining techniques to reduce drug adverse effects by taking into account the patient’s medical record.
Despite these efforts there is currently no widely accepted standardized framework that will help physicians in their day to day prescriptions needs, although some researchers have tried to move towards this direction10,11. The broadest approach has been taken by Doulaverakis and coauthors12,13 to cover drug to drug interactions and drug to diseases interaction but, apparently, with no consideration of patient vulnerability and the risk of the drug side effects. Other Clinical DSS use Multiple Criteria Decision Aiding14 (MCDA). For example, the AHP15 (Analytic Hierarchy Process) method has been used16 to analyze and compare the treatment options for eradication of H. pylori infection in children. The main limit of this work is the lack of structured objective knowledge. The recommendation is highly sensitive to the stakeholders evaluations and weights.
Clinical DSS have another important task which is to provide physicians with explanations for recommendations. In a previous work17, we combined semantic technologies with MCDA to sort alternatives (antibiotics) by their suitability to the subject (patient) but we did not consider the toxicity risk as is done by the physicians (assessing the risk level by combining severity and frequency).
Our objective in this work is to build a model for a clinical DSS that links knowledge structures for assessing the antibiotic prescription risk. The method we propose integrates MCDA with ontologies in order to provide to physicians sorted list of antibiotics assessed according to their toxicity risk for a given patient with an infection disease.
We use ontologically structured knowledge about the pharmacological characteristics of antibiotics and an ontology describing the critical criteria of patients. These ontologies are then linked through a set of rules structured in a Majority Rule Sorting model (MR Sort) with Veto18. This model, which is a simplified version of ELECTRE TRI22, assesses the alternatives by sorting them into ordered categories. This process results in antibiotic prescription recommendations categorized according to the risk of their side effect toxicity. We model the relations between concepts by simple interpretable rules, involving a small number parameters, to guarantee generality and maintainability of the knowledge model.
The main contributions of this paper are:
A new model for integrating MCDA with ontologies for clinical Decision Support Systems
An adapted version of the ELECTRE TRI – Majority Rule Sorting model (MR Sort) with Veto that is tailored to prescription recommendation with explanation.
An experimental validation of the above model for categorization of antibiotics through risk assessment of side-effect toxicity
The rest of the paper is organized as follows: The next section (“The system”) describes our adaptation of the ELECTRE TRI - MR Sort rule, as well as our model for integrating the method with the systematic representation of patient profiles and antibiotic knowledge in ontological structures. Section “Illustration” details our validation of antibiotics categorization through the risk of side-effect toxicity. Finally in the “Conclusion” section, we present further research perspectives.
The system
The Semantic Model Our solution models a patient P that hosts pathogens which cause a bacterial infection. This patient goes to the hospital to seek medical care and requires a treatment by antibiotics. The first step of the decision support model for antibiotics prescription is to gather knowledge.
For this purpose we use an ontology OP that models the patient and his/her relevant characteristics. These involve gender, age, comorbidities, allergies, and all the necessary patient information in order to assess the efficiency and the hazard of an antibiotic. All these characteristics influence the choice of an antibiotic in a way which is specified in reasoning rules. Indeed, a given antibiotic could suit a pregnant woman but not an old diabetic man, or conversely.
The variable SPj → {0,1, 2} represents the sensitivity indication of patient to the side effect Sj. A value 0 means “no sensitivity”, a value 1 means “minor sensitivity”, while 2 represents “major sensitivity”.
Our second ontology OA provides us with the set of side effects Sj of a given antibiotic Ai.
The variable RSAij is the risk of side effect Sj caused by antibiotic Ai. It represents the relation between an antibiotic Ai and a side effect Sj with indication of harmfulness. RSAij combines the intrinsic (i.e., independent of the antibiotic) evaluation of the side effect severity GSj → {0,1, 3} with the side effect frequency indicator of the antibiotic FSAij → {0,1, 2}. The following combination rules have been established with the help of an infectious diseases specialist.
The values of the severity GSj are presented in Table 1. Value 0 indicates that side effect Sj is not severe. Value 1 indicates that the side effect Sj is severe. Similarly, value 3 corresponds to a harmful side effect. The frequency values FSAij are presented in Table 2. Value 0 represents a less than 1/10,000 chance that antibiotic Ai has side effect Sj. Similarly, values 1 and 2 represent the chance being between 1/10,000 and 1/100 and more than 1/100, respectively.
Table 1:
Severity
| Description of the severity GSj of the side effect j | Level |
|---|---|
| Not severe | 0 |
| Severe | 1 |
| Harmful | 3 |
Table 2:
Frequency
| Description of the frequency FSAij of side effect Sj due to antibiotic Ai | Level |
|---|---|
| Does not exist and Very rare (< 1/10,000) | 0 |
| Rare and Infrequent (≥ 1/10,000 to < 1/100) | 1 |
| Frequent and Very frequent (≥ 1/100) | 2 |
Currently we deal with a total of 45 side effects organized in 8 categories for 60 antibiotics and 22 patient criteria.
The MR Sort model with Veto for assigning objects to ordered categories We use the MR Sort (Majority Rule Sorting) model with Veto (18,19,21) to classify the antibiotics for a given patient in ordered categories. MR Sort with Veto is a variant of ELECTRE TRI, which is a decision model belonging to the family of outranking methods22,23. The goal of MR Sort (and ELECTRE TRI)24 is to sort alternatives in ordered categories based on their performance on several criteria. In our context, the alternatives are antibiotics Ai and the criteria are the side effects Sj. The performance of an antibiotic w.r.t. a side effect for a given patient is determined by three elements: the severity GSj, the frequency FSAij and the sensitivity of the patient to the side effect SPj.
Each category Ck, k = 1,…, K, is associated a lower profile t(Ck), which is a vector of levels on each criterion representing the minimal requirements to belong to category Ck. Actually, not all these requirements are to be fulfilled, but only a sufficiently large majority of them, in order for an alternative to be assigned to this category. The majority condition can be implemented by assigning weights to the criteria and selecting a majority threshold; the condition is fulfilled whenever the sum of the weights of the criteria on which the alternative is at least as good as the profile passes the majority threshold. A simpler version of this rule consists of counting the criteria on which the alternative is at least as good as the profile; the condition is fulfilled whenever this number passes a minimal fixed number of criteria (This is tantamount to assigning equal weights to all criteria). An additional condition for being assigned into category Ck is that none of the alternative performances is “unacceptably bad”. Unacceptably bad performances are determined by veto thresholds on each criterion. Whenever the performance of an alternative on some criterion is worse than the corresponding profile performance by some specified quantity, a veto is activated which precludes assignment into the category.
To sum up, the principle implemented in MR-Sort with Veto in order to assign alternatives into categories is the following: An alternative is assigned to category Ck or better (i.e. Ck+1 up to CK) if the performances of the alternative are at least as good as these of the profile t(Ck) on a majority of criteria and none of these performances falls below the veto threshold on each criterion.
In this work, we chose to use MR Sort with Veto for two reasons. On the one hand, it gives us structured rules to link the antibiotic knowledge structure to that of the patient. On the other hand, it provides us with an assessment of the suitability of the antibiotics to the patient. In addition, our method assigns the antibiotics (alternatives) in three categories (K = 3): R (“recommended”), P (“possible”) and TBA (“to be avoided”), according to their toxicity risk.
The MR Sort with Veto assignment principles are applied to the context of antibiotic prescription, yielding the following rules:
An antibiotic is assigned to category R for a given patient if it has only a small number of side effects that the patient is sensitive to and if there is no unacceptable side effect (risk) for the same patient (no veto against R).
A similar rule applies for an antibiotic being assigned to category P. The number of side effects tolerated is higher than those for category R and the list of unacceptable side effects (risk) can possibly be smaller.
If none of these conditions are fulfilled, the antibiotic is assigned to category TBA.
Coupling ordered classification with the Semantic Model MR Sort with Veto is combined with the Semantic models to assess one by one each antibiotic Ai (as illustrated in Fig. 1).
Figure 1:
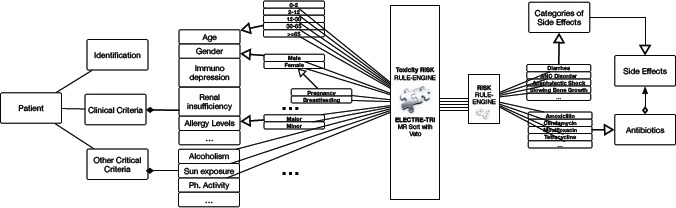
Using MR Sort method with Veto in the Semantic Model to link and to assess antibiotics for a patient by toxicity risk
These assignment principles are implemented using the following mathematical representation:
Ai, i ∈ 1,…,n denotes the antibiotics that are potentially considered, i.e., antibiotics that cover the germs infecting the patient.
Sj, j ∈ 1,…, m denotes the possible side effects of an antibiotic.
- CSAij, i ∈ 1,…, n, j ∈ 1,…, m is a variable taking its values from {0,1,2}. It represents the level of risk of side effect Sj of antibiotic Ai for the patient (taking into account the sensitivity of the patient to side effect Sj.
CSAi is a number associated to antibiotic Ai. It counts the number of risky side effects Sj such that CSAi,j = 0.
Two tolerance levels λR, λP with λR < λP determine the maximal number of side effects (risk) that are compatible with an assignment in categories R and P, respectively.
An unacceptable risk (CSAj > 1) can prevent assignment of Ai to category R or P. The list of unacceptable risks for an assignment to category R (resp. P) is a subset Veto[R] (resp. Veto[P]) of the set of all side effects risks. The vetoes are presented in Table 3.
Table 3:
Vetoes
| Antibiotic (GSj, FSAij) | (0,0) | (0,1) | (1,0) | (0,2) | (1,1) | (1,2) | (3,0) | (3,1) | (3,2) |
|---|---|---|---|---|---|---|---|---|---|
| Patient SPj | |||||||||
| 0 | R | R | R | R | R | R | R | R | R |
| 1 | R | P | P | P | P | P | P | TBA | TBA |
| 2 | P | P | P | P | TBA | TBA | P | TBA | TBA |
For a given Patient, the assignment of a suitable antibiotic Ai to the class R, P or TBA is summarized in Table 4. CSAj counts the number of side effects the patient is sensitive to. The first tolerance level, λR, is the maximum number of side effects the antibiotic could have to be in the R (recommended) category. The second tolerance level, λP, is the maximum number of side effects the antibiotic could have to be in the P (possible) category. In our application, λR was empirically set to 6 and λP to 12. Some side effects can induce a high risk on a patient, which explains the usefulness of vetoes (Table 3). The first veto, Veto[P], is put when the antibiotic has an unbearable side effect for the patient. This antibiotic could not be prescribed, even though it only has this side effect. For example, this veto would be raised if the antibiotic contains penicillin (RSAij = 3) and if the patient has a major allergy to penicillin (SPj = 2). With a Veto[P], the considered antibiotic is put in the TBA category. Similarly, a second veto, Veto[R], is put when the antibiotic has a very rare severe impact on the patient health (RSAij = 1), which, however, is not unbearable. For instance, an antibiotic which contains penicillin would get this Veto[R] if the patient has a minor allergy to penicillin (SPj = 1). With the Veto[R], the considered antibiotic Ai is put either in the P (possible) or in the TBA (to be avoided) category, depending on the value of CSAi.
Table 4:
Rules for assigning antibiotics to classes R, P and TPA
| CSAi < λR and no veto [R] | Recommended |
| CSAi < λP, no veto [P] and not Recommended | Possible |
| CSAi> λP or veto [P] | To be avoided |
Illustration
In order to illustrate the model, we built a scenario through several meetings with practitioners of the EpiCURA Hospital Center6 (infectious diseases specialist, microbiologist) through which we were able to tune the sensitivities and the thresholds of our model. For the purpose of illustration, we present the following case:
Bill is a 68 years old man, he is suffering of alcoholism problems and he is diabetic. He comes to the emergency room with an increased temperature of 40.1C and he has an inflammation in his leg (see Fig. 2). It is later revealed, that he got injured 2 days before when he was gardening. The diagnostic yielded is “Erysipelas”.
Figure 2:
Erysipelas around the ankle25
The guidelines (Fig. 3) inform us about the pathogens which cause the infection. In our example, the pathogens in question are Staphylococcus Aureus (MSSA) and Group A streptococci (GAS), as indicated in the second line of Fig. 3. To suggest an appropriate antibiotic, the guidelines distinguish three situations with respect to penicillin: (a) a patient who is not allergic to penicillin, (b) a patient with a minor allergy and (c) a patient with a major allergy. In the latter two cases, it suggests two different antibiotics.
Figure 3:

Guidelines recommendation for Erysipelas
Table 5 gives us the set of side effects (we did not present all the side effects that Bill is sensitive to, because for this use case the set of all antibiotics (Table 6) that can help Bill don’t have them; in terms of frequency, FSAij = 0), that Bill is sensitive to. SPj denotes the sensitivity of Bill to the side effect Sj, j = a,…, h.
Table 5:
Sensitivities of Bill
| SPa | SPb | SPc | SPd | SPe | SPf | SPg | SPh |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
Table 6:
Frequency, severity and risk of side effects per antibiotic
| Antibiotics | Sa | Sb | Sc | Sd | Se | Sf | Sg | Sh | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSa = 3 | GSb = 3 | GSc = 0 | GSd = 1 | GSe = 1 | GSf = 3 | GSg = 3 | GSh = 3 | |||||||||
| FSAia | RSAia | FSAib | RSAib | FSAic | RSAic | FSAid | RSAid | FSAie | RSAie | FSAif | RSAif | FSAig | RSAig | FSAih | RSAih | |
| Oxacillin | 0 | 2 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| Cefazolin | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 3 | 1 | 3 | 0 | 2 |
| Ceftriaxone | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 3 | 1 | 3 | 0 | 2 |
| Amoxicillin Clavulanic | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| Piperacillin Tazoboctam | 0 | 2 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| Vancamycin | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 3 | 0 | 2 |
| Moxifloxacin | 0 | 2 | 1 | 3 | 0 | 0 | 2 | 2 | 0 | 1 | 2 | 3 | 0 | 2 | 1 | 3 |
| Clindamicin | 2 | 3 | 0 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 2 |
The list shown in Table 6 gives us: The set of antibiotics which are effective against or cover the germs causing Bill’s infection; the set of side effects Sj; the severity evaluation of the side effects GSj; the frequency FASij and the risk RSAij, where i = 1,.., 8 is the set of antibiotics and j = a,.., h is the set of side effects.
We sort this list by suitability to Bill. The output of our system for this case is the following:
P: Oxacillin, CSA = 2, Veto[R]
P: Amoxicillin-Clavulanic, CSA = 1, Veto[R]
P: Piperacillin-Tazoboctam, CSA = 2, Veto[R]
TBA: Moxifloxacin, CSA = 4, Veto[P]
TBA: Cefazolin, CSA = 2, Veto[P]
TBA: Ceftriaxone, CSA = 2, Veto[P]
TBA: Vancomycin, CSA = 2, Veto[P]
TBA: Clindamycin, CSA = 2, Veto[P]
Note that, currently, in order to get these results (without using our system), the physician has to manually cross-check and combine several different sections of the guidelines.
More precisely, for a patient that is as much vulnerable as Bill, at least one of the vetoes [R] and [P] are activated and therefore, no antibiotic is assigned to category “R”. For this kind of situation, indeed, we rarely find antibiotics sorted in the “Recommended” category. The best alternatives are usually sorted in the “Possible” category. The advantage of this kind of output is that it provides the prescribing physician with a global view of the sorted treatments. In addition it gives him/her a detailed explanation of the toxicity risk assessment for each particular patient.
To describe in further details our model, we present here a second scenario. For this scenario we consider the case of “Abscess Perirectal” from the guidelines (as seen in Fig. 4).
Figure 4:
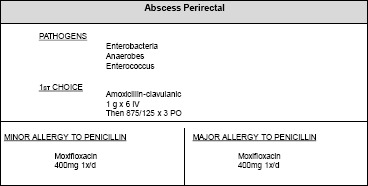
Guidelines recommendation for Abscess Perirectal
The guidelines inform us about the pathogens which cause the infection in the second line of Fig. 4. For this example, the pathogens are Enterobacteria, Anaerobes, Enterococcus. To suggest an appropriate antibiotic, the guidelines distinguish three possible contexts with respect to penicillin: (a) a patient who is not allergic to penicillin, (b) a patient with a minor allergy and (c) a patient with a major allergy. In the last two cases, the guidelines suggest the same antibiotic.
The list presented in Table 7 gives the set of antibiotics which are effective against the germs causing the infection (Amoxicillin_Clavulanic, Piperacillin_Tazoboctam, Moxifloxacin). The set of side effects Sj, where j = 1,.., 22 is presented underneath. These side effects can be given by the antibiotics: Amoxicillin_Clavulanic, Piperacillin_Tazoboctam and Moxifloxacin.
Table 7:
Frequency, severity and risk of 22 side effects for 3 antibiotics
| Antibiotics | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GS1 = 1 | GS2 = 3 | GS3 = 0 | GS4 = 1 | GS5 = 1 | GS6 = 3 | GS7 = 1 | GS8 = 8 | |||||||||
| FSAi1 | RSAi1 | FSAi2 | RSAi2 | FSAi3 | RSAi3 | FSAi4 | RSAi4 | FSAi5 | RSAi5 | FSAi6 | RSAi6 | FSAi7 | RSAi7 | FSAi8 | RSAi8 | |
| Amoxicillin_Clavulanic | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 |
| Piperacillin_Tazoboctam | 2 | 2 | 0 | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 2 |
| Moxifloxacin | 2 | 2 | 1 | 3 | 0 | 0 | 2 | 2 | 1 | 2 | 2 | 3 | 1 | 2 | 1 | 3 |
| S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | |||||||||
| GS9 = 3 | GS10 = 3 | GS11 = 1 | GS12 = 1 | GS13 = 3 | GS14 = 1 | GS15 = 1 | GS16 = 1 | |||||||||
| FSAi9 | RSAi9 | FSAi10 | RSAi10 | FSAi11 | RSAi11 | FSAi12 | RSAi12 | FSAi13 | RSAi13 | FSAi14 | RSAi14 | FSAi15 | RSAi15 | FSAi16 | RSAi16 | |
| Amoxicillin_Clavulanic | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 |
| Piperacillin_Tazoboctam | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 |
| Moxifloxacin | 1 | 3 | 1 | 3 | 1 | 2 | 1 | 2 | 1 | 3 | 1 | 2 | 1 | 2 | 1 | 2 |
| S17 | S18 | S19 | S20 | S21 | S22 | |||||||
| GS17 = 3 | GS18 = 3 | GS19 = 3 | GS20 = 1 | GS21 = 3 | GS22 = 3 | |||||||
| FSAi17 | RSAi17 | FSAi18 | RSAi18 | FSAi19 | RSAi19 | FSAi20 | RSAi20 | FSAi21 | RSAi21 | FSAi22 | RSAi22 | |
| Amoxicillin_Clavulanic | 0 | 2 | 0 | 2 | 1 | 3 | 0 | 1 | 1 | 3 | 0 | 2 |
| Piperacillin_Tazoboctam | 0 | 2 | 0 | 2 | 1 | 3 | 1 | 2 | 1 | 3 | 0 | 2 |
| Moxifloxacin | 1 | 3 | 1 | 3 | 0 | 2 | 0 | 1 | 0 | 2 | 2 | 3 |
The severity evaluation of the side effects GSj; the frequency FASij and the risk RSAij (where i = 1,.., 3 is the set of antibiotics and j = 1,.., 22 is the set of side effects) are all presented in Table 7. The case description follows:
| 1 | Diarrhea | 2 | Fulminant Hepatitis | 3 | Gastro Intestinal Troubles | 4 | Hepatic Troubles |
| 5 | Rash | 6 | Hematologic Troubles | 7 | Nausea and vomiting | 8 | Heart Failure |
| 9 | SNC Troubles | 10 | Neuropathy | 11 | Dizziness | 12 | Photosensitization |
| 13 | Ototoxicity | 14 | Benign Intracranial Hypertension | 15 | Cholestatic jaundice | 16 | Insomnia |
| 17 | Lyell syndrome | 18 | Steven Johnson Syndrome | 19 | Allergic Reaction | 20 | Cutaneous eruptions |
| 21 | Anaphylactic shock | 22 | Prolongation of QT interval |
Alex is a 29 years old patient, who is suffering from “Abscess Perirectal”. He is otherwise in good health without any history of medical incidents. His laboratory tests reveal that he does not have allergies and his creatinine level is 80ml/min.
For this case of infection, a surgical drainage is essential with antibiotic prescribing. Table 8 indicates the sensitivities SPj of Alex to the side effects Sj, j = 1 22.
Table 8:
Sensitivities of Alex
| SP1 | SP2 | SP3 | SP4 | SP5 | SP6 | SP7 | SP8 | SP9 | SP10 | SP11 |
| 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| SP12 | SP13 | SP14 | SP15 | SP16 | SP17 | SP18 | SP19 | SP20 | SP21 | SP22 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
We sort this list by suitability for Alex. The output of our system for this case is the following:
P: Amoxicillin-Clavulanic, CSA = 1, Veto[R]
P: Piperacillin-Tazoboctam, CSA = 2, Veto[R]
P: Moxifloxacin, CSA = 1, Veto[R]
If Alex has a “Major allergy to penicillin”, the sensitivities SPi9 and SP21 change: SPi9 = 2 and SP21 = 2. The output of the system becomes:
P: Moxifloxacin, CSA = 1, Veto[R]
TBA: Amoxicillin-Clavulanic, CSA = 3, Veto[P]
TBA: Piperacillin-Tazoboctam, CSA = 4, Veto[P]
The recommendations of our system match the ones in the guidelines (as seen in Fig. 4). Even in the case of “Major allergy to penicillin”, Moxifloxacin remains the only option.
Conclusion
We have developed a novel approach to help physicians with antibiotic prescriptions. Our solution combines semantic technologies with our own adaptation of the MR Sort method with Veto.
Our method sorts antibiotics in three categories: R (“recommended”), P (“possible”) and TBA (“to be avoided”) based on a small number of general rules. It is able to take into account a patient’s specific clinical criteria as well as generalize to new cases when – for example – a new antibiotic is added to the knowledge base. Using input from practitioners in the EpiCURA Hospital Center6 we have tuned the sensitivities and thresholds of our model and were able to validate our approach through examples that score prescription recommendations according to the risk of side effect toxicity.
In terms of future work we would like to apply our model to other types of drugs to determine if our method is applicable beyond antibiotics We plan to expand our model to other dimensions of the prescription problem including: costs, drug-drug interaction and drug-disease interaction among others.
Aknowledgement
We are grateful to Dr Jean Pierre Sabot – medical director of the EpiCURA Hospital Center – for his warm welcome and availability considering our research. We also want to thank Dr. Sammy Place (infectiologist), Dr. Lorenzo Filippin (microbiologist) as well as all the EpiCURA Hospital Center staff for numerous helpful meetings that allowed us to improve and validate our approach.
References
- 1.Laura L. Incentives Push More Doctors to E-Prescribe. The Wall Street Journal. 2009 Jan;(B7-8) [Google Scholar]
- 2.T. Gandhi, S. Weingart, J. Borus, A. Seger, J. Peterson, E. Burdick, et al. Adverse Drug Events in Ambulatory Care. New England Journal of Medicine. 2003;348(16) doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 3.Gurwitz J, Field T, Harrold L, Rothschild J, Debellis K, Seger A, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9) doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 4.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse Drug Events Occurring Following Hospital Discharge. Journal of General Internal Medicine. 2005 Mar; doi: 10.1111/j.1525-1497.2005.30390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothschild J, Federico F, Gandhi T, Kaushal R, Williams D, Bates D. Analysis of medication-related malpractice claims: causes, preventability, and costs. Arch Intern Med. 2002 doi: 10.1001/archinte.162.21.2414. [DOI] [PubMed] [Google Scholar]
- 6.Place S, Mathieu D. Recommandations pour l’usage des antibiotiques: traitement empirique des problèmes infectieux courants. Guidelines. Baudour, Belgium: Centre Hospitalier EpiCURA, Reseau hospitalier de medecine. 2011 [Google Scholar]
- 7.Bright T, Furuya E, Kuperman G, Cimino J, Bakken S. Development and evaluation of an ontology for guiding appropriate antibiotic prescribing. Journal of Biomedical Informatics. 2012 doi: 10.1016/j.jbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceusters W, Capolupo M, Moor De G, Devlies J. In Proceedings of the 2008 conference on Formal Ontology in Information Systems. The Netherlands: Amsterdam; 2008. Introducing realist ontology for the representation of adverse events; pp. 237–250. [Google Scholar]
- 9.Beuscart R, McNair P, Brender J, PSIP consortium. Patient safety through intelligent procedures in medication: the PSIP project. Studies in Health Technology and Informatics. 2009;148:6–13. [PubMed] [Google Scholar]
- 10.Stephens S, Morales A, Quinlan M. Applying semantic web technologies to drug safety determination. Intelligent Systems, IEEE. 2006;21(1):82–88. [Google Scholar]
- 11.Adnan M, Warren J, Orr M. Ontology based semantic recommendations for discharge summary medication information for patients. IEEE 23rd International Symposium on Computer-Based Medical Systems (CBMS) 2010:456–461. [Google Scholar]
- 12.Doulaverakis C, Nikolaidis G, Kleontas A, Kompatsiaris I. GalenOWL:Ontology based drug recommendations discovery. J Biomed Semantics. 2012;3(14) doi: 10.1186/2041-1480-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doulaverakis C, et al. Panacea, a semantic-enabled drug recommendations discovery framework. J. Biomed. Semant. 2014;5:13. doi: 10.1186/2041-1480-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belton V, Stewart T. Dordrecht: Kluwer Academic; 2002. Muliple Criteria Decision Analysis: An Integrated Approach. [Google Scholar]
- 15.Saaty TL. New York: McGraw-Hill; 1980. The Analytic Hierarchy Process, Planning, Piority Setting, Resource Allocation. [Google Scholar]
- 16.Erjaee A, Bagherpour M, Razeghi S, Dehghani SM, Imanieh MH, Haghighat M. A multi-criteria decision making model for treatment of Helicobacter pylori infection in children. Hong Kong J Paediatr. 2012;17(4):23742. [Google Scholar]
- 17.Ben Souissi S, Abed M, Elhiki L, Fortemps P, Pirlot M. Categorizing the suitability of an alternative for a subject: An application to antibiotics prescription recommendation. IEEE Symposium Series on Computational Intelligence (SSCI). Athens. 2016:1–8. [Google Scholar]
- 18.Leroy A, Mousseau V, Pirlot M. Learning the Parameters of a Multiple Criteria Sorting Method Based on a Majority Rule. Lecture notes in artificial intelligence. 2011;6992:219–233. [Google Scholar]
- 19.Bouyssou D. An axiomatic approach to noncompensatory sorting methods in MCDM I: The case of two categories. European Journal of Operational Research. 2007;178(1):217–245. [Google Scholar]
- 20.D. Bouyssou, T. Marchant. An axiomatic approach to noncompensatory sorting methods in MCDM II: More than two categories. European Journal of Operational Research. 2007;178(1):246–276. [Google Scholar]
- 21.Sobrie O, Lazouni M, Mahmoudi S, Mousseau V, Pirlot M. A new decision support model for preanesthetic evaluation. Computer Methods and Programs in BioMedicine. 2016;133:183–193. doi: 10.1016/j.cmpb.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Roy B, Bouyssou D. Aide Multicritère à la décision: Méthodes et Cas. Economica. 1993 [Google Scholar]
- 23.Figueira J, Mousseau V, Roy B. Boston, Dordrecht, London: Springer Verlag; 2005. ELECTRE methods: Multiple Criteria Decision Analysis: State of the Art Surveys; pp. 133–162. [Google Scholar]
- 24.Mousseau V, Slowi#x0144;ski R, Zielniewicz P. A user-oriented implementation of the ELECTRE TRI method integrating preference elicitation support. Computers & Operations Research. 2000;27(7-8):757–777. [Google Scholar]
- 25.Lachmann HJ, Hawkins PN. Developments in the scientific and clinical understanding of autoinflammatory disorders. Arthritis Res Ther. 2009;11(1):212. doi: 10.1186/ar2579. [DOI] [PMC free article] [PubMed] [Google Scholar]


