Abstract
Introduction:
The aim of this study was to understand the mechanism by which iatrogenic root dentin removal influences radicular stress distribution and subsequently affects the resistance to vertical root fractures (VRF) in endodontically treated teeth.
Materials and Methods:
The experiments were conducted in two phases. Phase 1: freshly extracted premolar teeth maintained in phosphate-buffered saline were instrumented to simulate three different degrees of dentin removal, designated as low, medium, and extreme groups. Micro-Ct analyzes were performed to quantitatively determine: (a) the amount of dentin removed, (b) the remaining dentin volume, and (c) the moment of inertia of root dentin. The specimens were then subjected to thermomechanical cycling and continuous loading to determine (a) the mechanical load to fracture and (b) dentin microcracking (fractography) using scanning electron microscopy. Phase 2: Finite element analysis was used to evaluate the influence of dentin removal on the stress distribution pattern in root dentin. The data obtained were analyzed using one-way ANOVA and Tukey's post hoc test (P < 0.05).
Results:
Phase 1: A significantly greater volume of dentin was removed from teeth in extreme group when compared to low group (P < 0.01). The mechanical analysis showed that the load to fracture was significantly lower in teeth from extreme group (P < 0.05). A linear relationship was observed between the moment of inertia and load to fracture in all experimental groups (R2 = 0.52). Fractography showed that most microcracks were initiated from the root canal walls in extreme group. Phase 2: The numerical analysis showed that the radicular stress distribution increased apically and buccolingually with greater degree of root canal dentin removal.
Conclusions:
The combined experimental/numerical analyses highlighted the influence of remaining root dentin volume on the radicular bending resistance, stress distribution pattern, and subsequent propensity to VRF.
Keywords: Biomechanics, dentin, endodontics, VRF
INTRODUCTION
Endodontically treated teeth have been suggested to have greater susceptibility to vertical root fractures (VRFs).[1,2,3,4] Iatrogenic and noniatrogenic factors that result in the removal of substantial amounts of root dentin or produce microdefects in root dentin may increase the propensity of fractures in endodontically treated teeth.[3,4] Any gross changes in the root canal morphology, loss of circumferential dentin, altered canal curvature, and altered canal cross-section configuration[5,6,7,8,9,10,11] may change the nature of functional stress distribution within the root dentin, subsequently increasing the susceptibility for VRF in such teeth.[5,6,7,8,9,10,11] However, the precise mechanism by which iatrogenic root dentin removal would influence the resistance to fracture in endodontically treated teeth is not well understood.
Finite element (FE) analysis has been used widely to study the stress/strain responses of dental structures to functional forces.[6,9,11] Using the FE models of mandibular first molar, it was demonstrated that canal enlargement results in increased concentration of stresses on the canal wall at the orifice level in the coronal third of root.[11] It was highlighted that the magnitude of radicular stresses formed during loading was higher when the root canals were prepared to larger diameters.[6] The larger diameters of prepared canals were noted to increase the magnitude of radicular stresses by up to 37%.[8] Nevertheless, another study has suggested that the reduced dentin thickness did not necessarily increase the fracture susceptibility in endodontically treated teeth while changing the canal configuration from oval to round relieves internal stress despite substantial loss of proximal dentin.[10]
Different degrees of dentin loss may occur during root canal instrumentations. This loss of root canal dentin would alter the biomechanical response of the tooth. Any significant alteration in the biomechanical response of a tooth may influence its resistance to fracture. Although previous static and cyclic load-based mechanical testing has emphasized the importance of preserving root dentin to retain the mechanical integrity of endodontically treated teeth,[5,7,8] the specific impact of iatrogenic dentin loss on the biomechanical behavior of root dentin is not well understood.[12,13,14,15] The goal of this study was to understand the effect of different degrees of root dentin removal on the load to fracture and stress distribution of endodontically treated teeth using both experimental and numerical methods. The current hybrid approach, which combines both experimental and numerical analyzes, would provide a better understanding on how root dentin removal during instrumentation would influence the susceptibly to VRF in endodontically treated teeth.
MATERIALS AND METHODS
Experimental analysis
Sample preparation
Ethics approval for the use of extracted teeth for this study was attained from the University of Toronto Ethics Review Board. Forty noncarious human mandibular premolar teeth, extracted for orthodontic reasons, were collected from patients from age group of 20–40 years, with mature root, straight root, and single (verified radiographically) and were selected for this study. The tooth specimens were transilluminated and examined under a stereomicroscope to exclude the presence of any cracks or craze lines. These specimens were stored in phosphate-buffered saline water at 4°C until use.
The teeth were mounted in a custom-made device, and micro-computed tomography (CT) images (1172 High-resolution μCT, SkyScan, Belgium) (pretreatment scan) were acquired. The scan conditions used were as follows: slice thickness 18 μm, tube voltage 100 kV, and exposure dose 100 μA. The specimens were randomly divided into four groups as described in the following. Access cavities were prepared using diamond burs under water cooling as per conventional guidelines.[16] All root canals were negotiated with size 10 K-type files (Lexicon: Dentsply Tulsa Dental Specialist, Germany). The working length was measured from the pretreatment scan at a reference point 0.5 mm short of the portal of exit and confirmed radiographically. A glide path was established with a size 15 K-type file. Each root canal was instrumented to the working length with instruments as indicated below.
In Group 1 (Low), the canals were enlarged up to ISO K-type file #20 (Lexicon: Dentsply Tulsa Dental Specialist, Germany) to simulate the low amount of dentin removal. In Group 2 (Medium), the canals were enlarged to ISO size 20 and then Gates Glidden Drills #1-2 (Lexicon: Dentsply Tulsa Dental Specialist, Switzerland) were used at 600 rpm to enlarge the coronal third of root canal. This step was followed by apical enlargement up to K-type file #35 to simulate a medium amount of dentin removal. In Group 3 (extreme), the coronal third of the canals was further enlarged up to Gates Glidden Drill #4 and apical enlargement up to K-type file #50 to simulate an extreme amount of dentin removal. Group 4 (Control) was the control group, in which the root canals were uninstrumented. Throughout the instrumentation procedures in the groups,[1,2,3] the root canals were irrigated with distilled water using a ProRinse side-vented 30 G needle (Dentsply Tulsa Dental Specialties, Tulsa, OK) at standardized intervals and the canals were dried with paper points.
Determination of dentin volume removed and moment of inertia
Micro-CT scanning was repeated (as above) on the root canal instrumented teeth (posttreatment scan). Manual volumetric segmentation of the pre- and post-treatment images (Amira 5.2.2, Visage Imaging, San Diego, CA, USA) was used to determine the amount of dentin removed. Amira software was used to calculate the cross-sectional area and radius of gyration to calculate the moment of inertia. Moment of inertia is a geometric property of a structure that measures the distribution of material about a given axis, representing the ability to resist bending or torsion. One hundred slices obtained from the cervical aspect of the root dentin postinstrumentation to determine the moment of inertia using the following equation.
k = √I/A I = k2A
Where k is the radius of gyration, I is the moment of Inertia, and A is the cross-sectional area. The moment of inertia was determined to examine the relationship between the ability to resist bending and load to fracture in the specimens.
Thermal and mechanical cyclic testing
Thermal and mechanical cycling was performed to simulate aging and mastication of root dentin during function. Before the thermal and mechanical cycling, the teeth crowns were sectioned off at the cementoenamel junction (CEJ) under water cooling with a diamond disk. The teeth were embedded in cylindrical molds of polymethylmethacrylate (Palapress Vario, Heraeus-Kulzer, Germany) with a 200 μm thick layer of polyether material (Impregum, 3M Espe, Seefeld, Germany) surrounding the root surfaces to mimic periodontal ligament (PDL). The CEJ was positioned approximately 1.5 mm above the level of mold to simulate bone crest. All the teeth were then aged under thermal and mechanical load cycles in a chewing simulator (TCML, Chewing Simulator, EGO, Regensburg, Germany). The thermal cycling consisted of 6000 cycles × 5°/55°; each cycle was 2 min. The specimens were simultaneously subjected to mechanical load cycles of 1.2 × 106 cycles of 50 N at frequency of 1.6 Hz. This mechanical/thermal load cycles simulated 5 years of clinical function, based on the masticatory loads, speed of mandibular movements, and rate of chewing.[17,18,19] All specimens were kept hydrated in deionized water throughout the experiments. These thermomechanically cycled specimens were used to determine the load to fracture and subsequent fractographic analysis.
Determination of the load to fracture
The load to fracture was determined on tooth specimens previously subjected to thermal and mechanical cycling. Twenty-eight samples (n = 7 from each group 1–4), were subjected to compressive loading to failure (Zwick 1446, Ulm, Germany). Vertical load was applied with a cylindrical tip (radius of 4 mm), centered over the occlusal aspect of the tooth, using a crosshead speed of 1 mm/min, within a custom stainless steel loading fixture. The specimens were loaded till the load dropped suddenly observed in load-displacement curve.
Fractographic analysis
Scanning electron microscopy (SEM) (Quanta FEG 400, FEI Company, Oregon, USA) was used to determine the presence of microcracks in root dentin. Three specimens in each group previously subjected to mechanical/thermal cyclic loading were examined. Cross-sectional specimens (3 mm thick) were prepared under water cooling with a diamond disk from the root specimens at apical, middle, and coronal levels. Only those microcracks that initiated from the root canal walls were analyzed as the representative initiation of VRF.
Numerical analysis
Segmentation of tooth and generation of finite element analysis models
An extracted human premolar tooth with a single and straight root canal was used. The tooth was positioned in a custom-made mount and μCT imaged intact. Root canal instrumentation was performed sequentially and μCT images were acquired at each stage to simulate three different degrees of dentin removal on a single tooth (control, low, medium, and extreme groups, as described earlier).
From each scan, the intensity-based manual image segmentation of the tooth and supporting structures (PDL and bone) was generated on a slice-by-slice basis using Visage Imaging Amira 5.2.2 software. The images were saved as a STL file and were subsequently imported into an ICEM CFD 14.5 platform and assembled into 4-noded tetrahedral meshes (Ansys Inc., Southpointe, Canonsburg, PA, USA). These meshes were generated as finite element analysis models representing different levels of dentin removal as in control, low, medium, and extreme groups (described previously).
Finite element analysis
Meshes were imported into ABAQUS 6.12 (Providence, RI, USA) to assign different material properties, apply boundary condition, and conduct stress analysis. All model materials were considered to be homogenous, isotropic, and linearly elastic. The mechanical properties of the dentin, PDL, and bone specified in the model are shown in Table 1.[20,21,22,23] The interfaces between the components were treated as perfectly bonded interfaces. The base of the model was constrained to a zero displacement boundary (restraining all forms of translational movements), and a load of 100 N was applied on the coronal aspect of the root. The resulting stress distribution patterns were evaluated at the cervical, middle, and apical portions of the root.
Table 1.
Material properties of structures considered for the finite element method model

Statistical analysis
The mean dentin volume removed was compared between the low, medium, and extreme groups using a one-way ANOVA and post hoc Tukey testing. A similar analysis was also used to compare the load-to-fracture data between all groups. All statistical analyses were performed to a 95% level of confidence (α = 0.05).
RESULTS
Experimental analysis
Determination of dentin volume removed
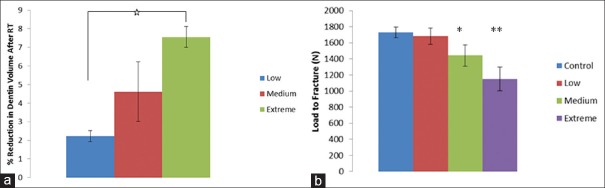
The μCT segmentations showed a significant difference between the volume of dentin removed in the low group and the extreme group (P < 0.05). The volume of dentin removed was lowest in the low group (2.63 ± 0.24%) and highest in the extreme group (7.34 ± 0.69%) [Figure 1a]. It was also found that the dentin volume removed in medium group showed a wide variation in the volumetric change (1.05%–12.36%), resulting in the lack of significant differences between this group and the extreme or low groups [Figure 1a].
Figure 1.
(a) The percentage reduction in dentin volume simulated with different levels of dentin removal reported as a mean ± standard error, (b) load to fracture values reported as a mean ± standard error (*statistically significant with control, **statistically significant with low)
Load-to-fracture analysis
Figure 1b shows the load to fracture obtained for different experimental and control groups used in this study. The load to fracture was significantly lower in the extreme group when compared to the low and control groups (P < 0.05). There were no statistically significant differences in the load to fracture for the medium group as compared to the other experimental groups (P > 0.05).
Load to fracture versus moment of inertia
The correlation between the moment of inertia and the load to fracture was examined for all the experimental samples. It was observed that there was a linear relationship (R2 = 0.52) between the load to fracture and the moment of inertia [Figure 2]. This indicated that the decrease in moment of inertia of root dentin during loading would lead to increased flexure of the root dentin and subsequent reduction in load to fracture.
Figure 2.
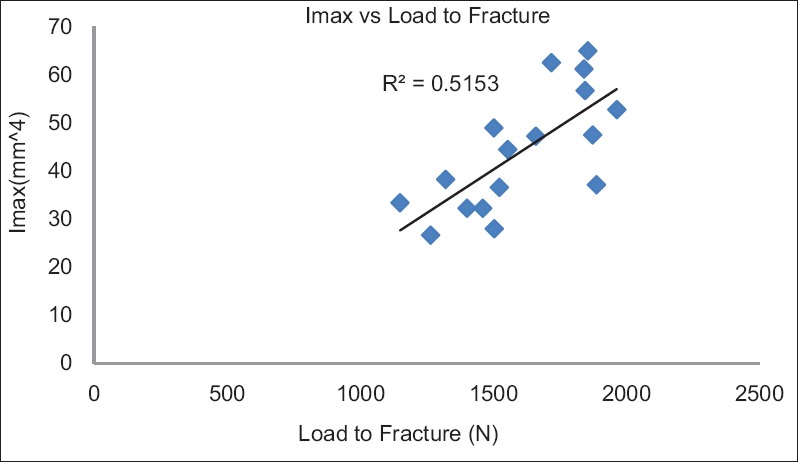
Shows the correlation between the load to fracture and moment of inertia
Fractographic analysis
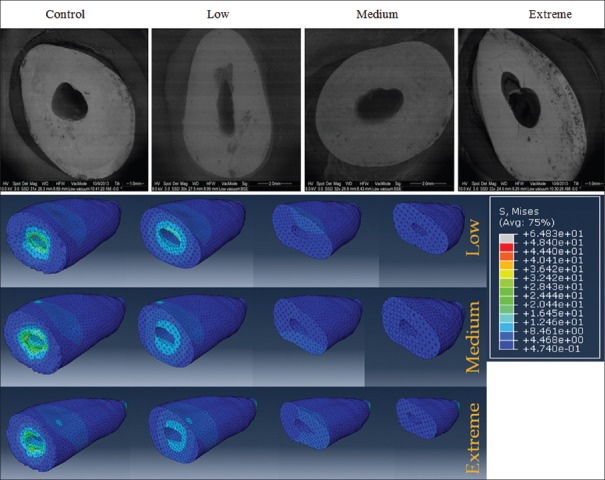
The fractography showed that a considerable number of microcracks were initiated from the root canal wall in the extreme group. These microcracks in the root dentin of the extreme groups were largely observed in the apical and cervical aspects of the root dentin [Figure 3a]. However, no cracks, which initiated from the root canal surface, were observed in the medium and low groups.
Figure 3.
(a) Microcrack analysis of samples after mechanical cycling load based on the different degree of dentin removal and (b) stress distribution analysis
Numerical analysis
Stress distribution pattern
In root dentin with minimal removal of dentin (low group), the distinct stress distribution patterns were observed at the cervical region of the root [Figure: 3b (top row)]. With increase in root canal dentin removal, the stress distribution pattern was shifted into the middle third of the root [Figure 3b (middle and bottom rows)]. In all experimental groups, stresses were distributed circumferentially around the canal wall in the mesiodistal direction. However, with an increase in root canal dentin removal, the stress distribution pattern became more conspicuously in the buccolingual direction.
DISCUSSION
Micro-CT is a nondestructive technique that allowed evaluation of tooth in series of cross-sectional slices, which are later reconstructed to determine various parameters such as root canal morphology, volume of dentin removed, and remaining dentin thickness.[24,25,26,27] Previous investigations using micro-CT showed that the largest degree of dentin loss occurred during caries removal (~8%), whereas the root canal preparation did not result in significant loss of dentin (1%).[12] Elnaghy and Elsaka showed that there was no significant difference in the volume of dentin removed and centering ratio among the teeth instrumented with ProTaper Next instruments with/without Glide Path.[28] In the current study, the micro-CT-based analysis showed that the amount of root dentin removed during instrumentation differed only between the low and extreme instrumentation groups. There was no significant difference in the amounts of dentin removed between the medium and the low/extreme groups. This finding suggested that the amount of dentin removed during root canal instrumentation was not only influenced by the instrumentation protocol but also depended on the initial root canal geometry and remaining dentin volume. Similar findings were reported in several previous studies.[24,25,26,29,30]
The resistance to fracture of endodontically treated teeth is a particular concern, since the mechanical integrity of the remaining teeth structure may be compromised by different pathological and iatrogenic reasons.[31] In this study, the teeth were loaded without considering the endodontic access cavities to avoid (a) the confounding effects such as cuspal flexure and (b) different bonded/unbounded filling materials. In this manner, the effect of root canal dentin loss on the mechanical integrity of remaining root dentin could be directly assessed. Further, the teeth were subjected to cyclic loading under fully hydrated conditions to simulate functional chewing forces in an oral environment. The cyclic loading of 1,200,000 cycles utilized in this study simulated about 5 years of clinical functioning.[17,18,19] The findings from the cyclic loading experiments followed by static loading demonstrated that there is a significant difference in the loads to fracture of extreme group as compared to low and control groups. However, there was no significant difference between loads to fracture for medium group compared to other groups.
Comparison between the root canal dentin removal and load to fracture showed that there was a consistent decrease in the load to fracture with a decrease in remaining dentin volume. Nevertheless, there existed statistically significant decrease in the load to fracture only in the medium group (when compared to the control group) and in the extreme group (when compared to the low group). This observation may perhaps suggest that the remaining dentin volume influenced the resistance to fracture in endodontically treated root only after a critical degree of dentin removal. It is also key to realize that other associated factors such as canal geometry and pretreatment canal volume would also influence the resistance of fracture. When the moment of inertia, which is the resistance to bending, is correlated with the load to fracture, it was noted that the load to fracture depended on the distribution of dentin material around the root canal. This effect was confirmed by considering the radius of gyration in the calculation of moment of inertia, which determines the distribution of dentin material around the centroid of the root canal. Thus, removal of dentin away from the canal center reduced the moment of inertia resulting increased root flexure. A previous study has highlighted a conspicuously increased deformation in single-rooted teeth following instrumentation and postpreparation.[31]
Teeth in the oral cavity serve as a mechanical device for mastication of food. During chewing, the intact natural teeth experienced flexing or bending stress when biting forces act on them.[32] Thus examining the nature of stress distribution within root would aid in understanding how root canal dentin removal would alter the stress distribution pattern in root during function. The pattern of stress distribution on the root dentin is critical in propagating cracks that lead to VRF.[32] VRF is defined as longitudinally oriented fracture of the tooth that originates from the apical region of the root and propagates toward the coronal aspect of the root. They are generally found in the buccolingual direction of the root.[33] Although they may originate in proximity to the root canal wall, they may be complete or incomplete in nature.[34,35] Therefore, only those cracks that initiated from the canal wall were recorded using SEM. The microcrack analyses of the root specimens subjected to different degrees of dentin removal demonstrated that higher dentin removal from the root canal initiated a greater number of microcracks and root fractures.
The current numerical analysis and mechanical experiments demonstrated that functional stresses were predominantly distributed circumferentially at the cervical dentin. However, with increasing root dentin removal, the stress patterns shifted more apically and along the buccolingual plane. The increased stress distribution in the apical direction and in the buccolingual direction can be attributed to the increasing root flexure, resulting from dentin removal. This altered stress distribution pattern may contribute to fracture that propagates from the apical portions of the root to the coronal portions in the buccolingual direction.[35,36]
CONCLUSIONS
This biomechanical study demonstrated that extreme degree of iatrogenic root canal dentin removal, particularly away from the root canal center, would result in a decreased moment of inertia and root flexure. The increased root flexure would shift the radicular stress distribution from the cervical dentin to the apical dentin in the buccolingual plane. Thus, it could be suggested that the resistance to VRF after root canal preparation was influenced by the remaining dentin volume and moment of inertia of the root dentin. A small amount of root canal dentin removal by root canal instrumentation did not compromise the mechanical integrity of root.
Financial support and sponsorship
This study was financially supported by Natural Sciences and Engineering Research Council of Canada-Discovery grant.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tamse A, Fuss Z, Lustig J, Kaplavi J. An evaluation of endodontically treated vertically fractured teeth. J Endod. 1999;25:506–8. doi: 10.1016/S0099-2399(99)80292-1. [DOI] [PubMed] [Google Scholar]
- 2.Touré B, Faye B, Kane AW, Lo CM, Niang B, Boucher Y, et al. Analysis of reasons for extraction of endodontically treated teeth: A prospective study. J Endod. 2011;37:1512–5. doi: 10.1016/j.joen.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Meister F, Jr, Lommel TJ, Gerstein H. Diagnosis and possible causes of vertical root fractures. Oral Surg Oral Med Oral Pathol. 1980;49:243–53. doi: 10.1016/0030-4220(80)90056-0. [DOI] [PubMed] [Google Scholar]
- 4.Joffe E. Management of vertical root fracture in endodontically treated teeth. N Y State Dent J. 1992;58:25–7. [PubMed] [Google Scholar]
- 5.Abdo SB, Darrat AA, Masaudi SM, Luddin N, Husien A, Khamis MF. Comparison of over flared root canals of mandibular premolars filled with MTA and resin based material: An in vitro study. Smile Dent J. 2012;7:38–42. [Google Scholar]
- 6.Ricks-Williamson LJ, Fotos PG, Goel VK, Spivey JD, Rivera EM, Khera SC, et al. A three-dimensional finite-element stress analysis of an endodontically prepared maxillary central incisor. J Endod. 1995;21:362–7. doi: 10.1016/S0099-2399(06)80971-4. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox LR, Roskelley C, Sutton T. The relationship of root canal enlargement to finger-spreader induced vertical root fracture. J Endod. 1997;23:533–4. doi: 10.1016/S0099-2399(97)80316-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Yue L, Wang JD, Gao XJ. The correlation between the enlargement of root canal diameter and the fracture strength and the stress distribution of root. Zhonghua Kou Qiang Yi Xue Za Zhi. 2006;41:661–3. [PubMed] [Google Scholar]
- 9.Sathorn C, Palamara JE, Palamara D, Messer HH. Effect of root canal size and external root surface morphology on fracture susceptibility and pattern: A finite element analysis. J Endod. 2005;31:288–92. doi: 10.1097/01.don.0000140579.17573.f7. [DOI] [PubMed] [Google Scholar]
- 10.Lertchirakarn V, Palamara JE, Messer HH. Patterns of vertical root fracture: Factors affecting stress distribution in the root canal. J Endod. 2003;29:523–8. doi: 10.1097/00004770-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Lertchirakarn V, Palamara JE, Messer HH. Finite element analysis and strain-gauge studies of vertical root fracture. J Endod. 2003;29:529–34. doi: 10.1097/00004770-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Ikram OH, Patel S, Sauro S, Mannocci F. Micro-computed tomography of tooth tissue volume changes following endodontic procedures and post space preparation. Int Endod J. 2009;42:1071–6. doi: 10.1111/j.1365-2591.2009.01632.x. [DOI] [PubMed] [Google Scholar]
- 13.Pilo R, Shapenco E, Lewinstein I. Residual dentin thickness in bifurcated maxillary first premolars after root canal and post space preparation with parallel-sided drills. J Prosthet Dent. 2008;99:267–73. doi: 10.1016/S0022-3913(08)60059-1. [DOI] [PubMed] [Google Scholar]
- 14.Kalburge V, Yakub SS, Kalburge J, Hiremath H, Chandurkar A. A comparative evaluation of fracture resistance of endodontically treated teeth, with variable marginal ridge thicknesses, restored with composite resin and composite resin reinforced with ribbond: An in vitro study. Indian J Dent Res. 2013;24:193–8. doi: 10.4103/0970-9290.116676. [DOI] [PubMed] [Google Scholar]
- 15.Mireku AS, Romberg E, Fouad AF, Arola D. Vertical fracture of root filled teeth restored with posts: The effects of patient age and dentine thickness. Int Endod J. 2010;43:218–25. doi: 10.1111/j.1365-2591.2009.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S, Rhodes J. A practical guide to endodontic access cavity preparation in molar teeth. Br Dent J. 2007;203:133–40. doi: 10.1038/bdj.2007.682. [DOI] [PubMed] [Google Scholar]
- 17.Rosentritt M, Behr M, Gebhard R, Handel G. Influence of stress simulation parameters on the fracture strength of all-ceramic fixed-partial dentures. Dent Mater. 2006;22:176–82. doi: 10.1016/j.dental.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Rosentritt M, Siavikis G, Behr M, Kolbeck C, Handel G. Approach for evaluating the significance of laboratory simulation. J Dent. 2008;36:1048–53. doi: 10.1016/j.jdent.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Aboushelib MN. Simulation of cumulative damage associated with long term cyclic loading using a multi-level strain accommodating loading protocol. Dent Mater. 2013;29:252–8. doi: 10.1016/j.dental.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Geramy A, Eghbal MJ, Ehsani S. Stress distribution changes after root canal therapy in canine model: A finite element study. Iran Endod J. 2008;3:113–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Meijer HJ, Starmans FJ, Steen WH, Bosman F. A three-dimensional, finite-element analysis of bone around dental implants in an edentulous human mandible. Arch Oral Biol. 1993;38:491–6. doi: 10.1016/0003-9969(93)90185-o. [DOI] [PubMed] [Google Scholar]
- 22.Fill TS, Carey JP, Toogood RW, Major PW. Experimentally determined mechanical properties of, and models for, the periodontal ligament: Critical review of current literature. J Dent Biomech 2011. 2011:312980. doi: 10.4061/2011/312980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gröning F, Fagan MJ, O'Higgins P. The effects of the periodontal ligament on mandibular stiffness: A study combining finite element analysis and geometric morphometrics. J Biomech. 2011;44:1304–12. doi: 10.1016/j.jbiomech.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Peters OA, Schönenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J. 2001;34:221–30. doi: 10.1046/j.1365-2591.2001.00373.x. [DOI] [PubMed] [Google Scholar]
- 25.Peters OA, Laib A, Göhring TN, Barbakow F. Changes in root canal geometry after preparation assessed by high-resolution computed tomography. J Endod. 2001;27:1–6. doi: 10.1097/00004770-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Peters OA, Peters CI, Schönenberger K, Barbakow F. ProTaper rotary root canal preparation: Effects of canal anatomy on final shape analysed by micro CT. Int Endod J. 2003;36:86–92. doi: 10.1046/j.1365-2591.2003.00626.x. [DOI] [PubMed] [Google Scholar]
- 27.Paqué F, Ganahl D, Peters OA. Effects of root canal preparation on apical geometry assessed by micro-computed tomography. J Endod. 2009;35:1056–9. doi: 10.1016/j.joen.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Elnaghy AM, Elsaka SE. Evaluation of root canal transportation, centering ratio, and remaining dentin thickness associated with ProTaper next instruments with and without glide path. J Endod. 2014;40:2053–6. doi: 10.1016/j.joen.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Siqueira JF, Jr, Alves FR, Versiani MA, Rôças IN, Almeida BM, Neves MA, et al. Correlative bacteriologic and micro-computed tomographic analysis of mandibular molar mesial canals prepared by self-adjusting file, reciproc, and twisted file systems. J Endod. 2013;39:1044–50. doi: 10.1016/j.joen.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 30.Peters OA, Boessler C, Paqué F. Root canal preparation with a novel nickel-titanium instrument evaluated with micro-computed tomography: Canal surface preparation over time. J Endod. 2010;36:1068–72. doi: 10.1016/j.joen.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Lang H, Korkmaz Y, Schneider K, Raab WH. Impact of endodontic treatments on the rigidity of the root. J Dent Res. 2006;85:364–8. doi: 10.1177/154405910608500416. [DOI] [PubMed] [Google Scholar]
- 32.Kishen A. Mechanisms and risk factors for fracture predilection in endodontically treated teeth. Endod Top. 2006;13:57–83. [Google Scholar]
- 33.Rivera EM, Walton RE. Cracking the Cracked Tooth Code: Detection and Treatment of Various Longitudinal Tooth Fractures. Chicago, USA: American Association of Endodontists Colleagues for Excellence; 2008. [Google Scholar]
- 34.Lertchirakarn V, Palamara JE, Messer HH. Load and strain during lateral condensation and vertical root fracture. J Endod. 1999;25:99–104. doi: 10.1016/S0099-2399(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 35.Kishen A, Kumar GV, Chen NN. Stress-strain response in human dentine: Rethinking fracture predilection in postcore restored teeth. Dent Traumatol. 2004;20:90–100. doi: 10.1111/j.1600-4469.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- 36.Asundi A, Kishen A. Advanced digital photoelastic investigations on the tooth-bone interface. J Biomed Opt. 2001;6:224–30. doi: 10.1117/1.1344587. [DOI] [PubMed] [Google Scholar]