Abstract
Background
Energy balance–related risk factors for colon cancer recurrence and mortality—type II diabetes, hyperinsulinemia, inflammation, and visceral obesity—are positively correlated with consumption of refined grains and negatively correlated with consumption of whole grains. We examined the relationship between the consumption of refined and whole grains with cancer recurrence and mortality in a cohort of patients with colon cancer.
Methods
We conducted a prospective observational study of 1024 patients with stage III colon cancer who participated in a randomized trial of postoperative chemotherapy. Patients reported consumption of refined and whole grains using a food frequency questionnaire during and six months after chemotherapy. The primary outcome was disease-free survival (DFS). Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox regression models. All P values are two-sided.
Results
During a median follow-up of 7.3 years, 394 patients experienced a DFS event. The hazard ratio for DFS was 1.56 (95% CI = 1.09 to 2.24) for patients consuming three or more servings per day of refined grains compared with patients consuming less than one serving per day (Ptrend = .005). The hazard ratio for DFS was 0.89 (95% CI = 0.66 to 1.20) for patients consuming three or more servings per day of whole grains compared with patients consuming less than one serving per day (Ptrend = .54). The hazard ratio for DFS of substituting one serving per day of refined grain with one serving per day of whole grain was 0.87 (95% CI = 0.79 to 0.96, P = .007).
Conclusions
The choice of grain consumed may be associated with cancer recurrence and mortality. Future studies are necessary to confirm our findings and to inform the design of randomized trials.
Each year, 83 000 people are diagnosed with stage I–III colon cancer in the United States (1). Despite surgical resection, either alone or in combination with adjuvant chemotherapy, 25%−30% of patents will experience recurrent and metastatic disease within three years of diagnosis, and 91% of those who recur within three years die by five years (2). Consequently, patients with colon cancer are motivated to understand how lifestyle behaviors, such as their dietary choices, may influence cancer recurrence and mortality (3). Consumption of a Western-style diet (4), high-glycemic carbohydrates (5), red and processed meats (6), and sugar-sweetened beverages are associated with a higher risk of recurrence and mortality (7,8). Conversely, coffee (9), milk (10), and modest alcohol consumption are associated with a lower risk of recurrence and mortality (8). Evidence describing other dietary constituents will inform clinical recommendations for patients and guide the design of randomized trials of dietary modification (11).
Whole grains contain endosperm, germ, and bran, whereas refined grains have the germ and bran removed during the milling process. The consumption of refined grains is associated with an increased risk of developing type II diabetes (12–14). The consumption of refined grains is positively correlated with hyperinsulinemia (15), inflammation (16), and visceral obesity (17). This is relevant to patients with colon cancer because type II diabetes (18,19), hyperinsulinemia (20), inflammation (21), and visceral obesity are risk factors for cancer recurrence and mortality (22). Conversely, the consumption of whole grains is associated with a lower risk of developing type II diabetes (23). The consumption of whole grains is negatively correlated with hyperinsulinemia (24), inflammation (25), and visceral obesity (17). Randomized trials in healthy men and women demonstrate that substituting refined grains with whole grains reduces insulin resistance (26,27), inflammation (28,29), and body fat (29,30).
We prospectively examined the relationship between the consumption of refined grains and whole grains with cancer recurrence and mortality in a cohort of patients with stage III colon cancer enrolled in a National Cancer Institute–sponsored randomized clinical trial of postoperative chemotherapy. We hypothesized that 1) higher consumption of refined grains would be associated with an increased risk of cancer recurrence and mortality; 2) higher consumption of whole grains would be associated with a decreased risk of cancer recurrence and mortality, and 3) substitution of refined grains with whole grains would be associated with a decreased risk of cancer recurrence and mortality.
Methods
Study Population
Patients in this prospective cohort study participated in the National Cancer Institute (NCI)–sponsored Cancer and Leukemia Group B (CALGB; now Alliance for Clinical Trials in Oncology) 89803 postoperative chemotherapy trial for stage III colon cancer (31). Patients were recruited from the United States and Canada. The primary aim of CALGB 89803 was to compare weekly 5-fluorouracil and leucovorin with weekly irinotecan, 5-fluorouracil, and leucovorin (ClinicalTrials.gov NCT000038350). Between May 1999 and May 2001, the trial enrolled 1264 patients. After the enrollment of 87 patients, an amendment required patients to complete a self-administered questionnaire that quantified diet and lifestyle behaviors midway through chemotherapy (approximately four months after surgical resection, questionnaire 1 [Q1], and again six months after completion of chemotherapy [14 months after surgical resection; Q2]).
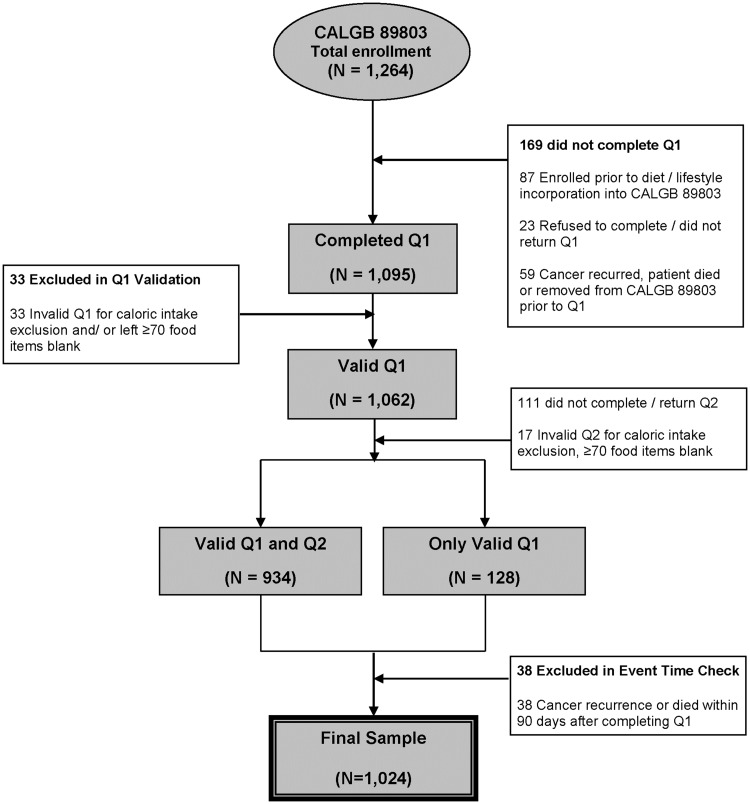
Eligible patients underwent a complete surgical resection of the primary tumor within 56 days of trial enrollment, had regional lymph node metastases but no evidence of distant metastases, had a baseline Eastern Cooperative Oncology Group performance status of 0 to 2, and had adequate bone marrow, renal, and hepatic function. Patients were excluded from this analysis if they reported unreasonable energy intake (<600 or >4200 calories per day for men; <500 or >3500 calories per day for women) or left 70 or more food items blank (see Dietary Assessment below). To avoid bias in dietary assessment because of declining health, patients were excluded if they experienced cancer recurrence or death within 90 days of completing the questionnaire (Q1). Figure 1 describes the derivation of the final sample size of 1024 patients included in this study. There were no substantive differences in the baseline characteristics of patients included in the dietary analysis, and the remaining patients enrolled in CALGB 89803 (5).
Figure 1.
Derivation of cohort. Caloric intake exclusion: <600 calories or >4200 calories per day for men and <500 calories and >3500 calories per day for women. Q1 = questionnaire 1 (midway through adjuvant therapy); Q2 = questionnaire 2 (six months after completion of adjuvant therapy).
All patients signed informed consent, which was approved by the National Cancer Institute Cancer Treatment Evaluation Program and each participating site’s institutional review board.
Dietary Assessment
The consumption of refined and whole grain foods was quantified using semiquantitative food frequency questionnaires (FFQ) that included 131 food items, vitamin and mineral supplements, and open-ended sections for other supplements and foods not specifically listed (32). For each food, a common unit or portion size (eg, slice of bread) was specified, and the patient was asked to report how often, on average over the previous three months, they consumed that portion size. Up to nine frequency responses were possible, which ranged from never to six or more times per day. Type and brand of cereal were also assessed. We computed nutrient intakes by multiplying the frequency of consumption of each food by the nutrient content of the specified portions using composition values from the Department of Agriculture. All nutrient values were energy-adjusted using the residuals method (33).
Foods were classified as refined or whole grains using the technique developed by Jacobs and colleagues (34). Refined grains included sweet rolls, cake deserts, white bread, pasta, English muffins, muffins, biscuits, refined grain cereals, white rice, pancakes, waffles, and pizza. Whole grains included dark bread, whole grain ready-to-eat cereals (≥25% whole grain content by weight), popcorn, cooked oatmeal, wheat germ, brown rice, bran, and other grains. A full description of the FFQ, reproducibility, and validity statistics has been reported previously (32,35). The performance of the FFQ to quantify individual grain products is high; the correlation between the FFQ and detailed diet records is 0.75 for cereal, 0.71 for white bread, and 0.77 for dark bread (36). Variables including prudent and Western dietary patterns, the healthy eating index, and glycemic load were calculated using the dietary assessment following techniques described previously (8).
Patients who completed Q1 and whose cancer had not recurred prior to Q1 completion were included in these analyses. The median time from study entry to Q1 (range) was 3.5 (0.2–9.9) months. We updated dietary exposures based on the results of Q2 using cumulative averaging, with weighting that is proportional to times between Q1 and Q2 and then between Q2 and the event (or censoring) time (4). This technique accounts for changes in diet that may occur over time, thereby maximizing the information obtained from the available data.
Study End Points
The primary end point of this study was disease-free survival (DFS), and secondary end points included recurrence-free survival (RFS) and overall survival (OS). DFS was defined as the time from completion of Q1 to cancer recurrence, occurrence of a new primary colon cancer, or death from any cause, whichever occurred first. RFS was defined as the time from completion of Q1 to cancer recurrence or occurrence of a new primary colon cancer; patients who died without known disease recurrence were censored at the last documented physician evaluation. OS was defined as the time from completion of Q1 to death from any cause.
Statistical Analysis
In the clinical trial, there were no statistical differences in DFS, RFS, or OS between treatment arms (31). Therefore, data for patients in both arms were combined and analyzed according to frequency categories of dietary intake. For the primary analysis, consumption of refined and whole grain foods was categorized into three groups (<1, 1–2, and ≥3 servings per day). Cox proportional hazards regression was used to determine the simultaneous influence of other potential confounding variables. Three models were built to incrementally examine the association between grain intake and the study end points. Model 1 was adjusted for age and energy intake; model 2 was adjusted for demographic, clinical, and behavioral variables; model 3 was adjusted for the covariates in model 2, plus the complementary grain measure: whole grain intake (for refined grain models) and refined grain intake (for whole grain models). We used time-varying covariates to adjust for body mass index (BMI), physical activity, total energy intake, whole grain intake, and refined grain intake. Other covariates, including age at Q1, sex, race, performance status (physician estimate of patient ability for self-care, daily activity, and physical ability), depth of invasion through bowel wall (T stage), number of positive lymph nodes (N stage), location of the primary tumor, and chemotherapy treatment arm, were entered into the model as fixed covariates.
We tested for linear trends across frequency categories of consumption by assigning each patient the median value for each category and modeling the value as a continuous variable. We graphically examined the frequency of grain consumption as a continuous variable using nonlinear restricted cubic splines. The proportionality of hazards assumption was examined by including time-dependent covariates in the regression models and visually inspecting log-log plots. To evaluate the effect of substituting refined grains with whole grains, we built substitution models by simultaneously entering whole and refined grain intakes into a model as continuous variables, then exponentiating the difference in coefficients to obtain an estimate that is on the hazard ratio scale (37). The hazard ratios derived from these models are interpreted as the estimated effect of substituting one daily serving of refined grain with one daily serving of whole grain.
All analyses were performed with SAS, version 9.4 (SAS Institute, Cary, NC). A P value of less than .05 was considered statistically significant. All P values are two-sided. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center at Duke University Medical Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on November 9, 2009.
Results
Baseline Characteristics
Baseline characteristics of the 1024 patients by frequency of consumption of refined and whole grains are displayed in Table 1. Those in the highest category of refined grain intake were more likely to be male and report a higher consumption of coffee, sugar-sweetened beverages, and poorer overall dietary patterns, compared with patients in the lowest category of refined grain intake. Those in the highest category of whole grain intake were more likely to be male and of white race, to have fewer positive lymph nodes and better performance status, and to report higher physical activity, higher consumption of cereal fiber, and more favorable overall dietary patterns, compared with patients in the lowest category of whole grain intake.
Table 1.
Baseline characteristics of 1024 patients by servings of refined and whole grains
| Refined grain intake, servings/d |
Whole grain intake, servings/d |
|||||||
|---|---|---|---|---|---|---|---|---|
| <1 | 1–2 | ≥3 | <1 | 1–2 | ≥3 | |||
| (n = 160) | (n = 565) | (n = 299) | P | (n = 294) | (n = 481) | (n = 249) | P | |
| Age, median (IQR), y | 61 (54–69) | 59 (51–68) | 62 (51–70) | .11 | 59 (50–68) | 60 (52–68) | 61 (52–70) | .20 |
| Sex, No. (%) | <.001 | <.001 | ||||||
| Male | 79 (49.4) | 284 (50.3) | 213 (71.2) | 177 (60.2) | 241 (50.1) | 158 (63.5) | ||
| Female | 81 (50.6) | 281 (49.7) | 86 (28.8) | 117 (39.8) | 240 (49.9) | 91 (36.5) | ||
| Race, No. (%) | .18 | .04 | ||||||
| White | 136 (85.0) | 503 (89.0) | 270 (90.3) | 247 (84.0) | 436 (90.7) | 226 (90.8) | ||
| Black | 16 (10.0) | 39 (6.9) | 13 (4.4) | 27 (9.2) | 29 (6.0) | 12 (4.8) | ||
| Other | 8 (5.0) | 23 (4.1) | 16 (5.3) | 20 (6.8) | 16 (3.3) | 11 (4.4) | ||
| Baseline performance status, No. (%)* | .72 | .002 | ||||||
| 0 | 121 (75.6) | 419 (74.2) | 210 (70.2) | 199 (67.7) | 367 (76.3) | 184 (73.9) | ||
| 1 − 2 | 36 (22.5) | 135 (23.9) | 82 (27.4) | 90 (30.6) | 109 (22.7) | 54 (21.7) | ||
| Missing/unknown | 3 (1.9) | 11 (1.9) | 7 (2.4) | 5 (1.7) | 5 (1.0) | 11 (4.4) | ||
| Invasion through bowel wall by T stage, No. (%) | .18 | .21 | ||||||
| T1 − 2 | 19 (11.9) | 88 (15.6) | 30 (10.0) | 33 (11.2) | 69 (14.3) | 35 (14.1) | ||
| T3 − 4 | 138 (86.2) | 465 (82.3) | 260 (87.0) | 256 (87.1) | 403 (83.8) | 204 (81.9) | ||
| Missing/unknown | 3 (1.9) | 12 (2.1) | 9 (3.0) | 5 (1.7) | 9 (1.9) | 10 (4.0) | ||
| Positive lymph nodes, No. (%) | .22 | .04 | ||||||
| 1 − 3 | 110 (68.7) | 361 (63.9) | 174 (58.2) | 180 (61.2) | 314 (65.3) | 151 (60.7) | ||
| ≥4 | 47 (29.4) | 195 (34.5) | 118 (39.5) | 110 (37.4) | 162 (33.7) | 88 (35.3) | ||
| Missing/unknown | 3 (1.9) | 9 (1.6) | 7 (2.3) | 4 (1.4) | 5 (1.0) | 10 (4.0) | ||
| Location of primary tumor, No. (%) | .78 | .12 | ||||||
| Right | 4 (2.5) | 23 (4.1) | 9 (3.0) | 12 (4.1) | 13 (2.7) | 11 (4.4) | ||
| Left | 59 (36.9) | 222 (39.3) | 105 (35.1) | 123 (41.8) | 182 (37.8) | 81 (32.5) | ||
| Transverse | 61 (38.1) | 207 (36.6) | 115 (38.5) | 99 (33.7) | 186 (38.7) | 98 (39.4) | ||
| Multiple | 33 (20.6) | 104 (18.4) | 61 (20.4) | 56 (19.0) | 93 (19.3) | 49 (19.7) | ||
| Missing | 3 (1.9) | 9 (1.6) | 9 (3.0) | 4 (1.4) | 7 (1.5) | 10 (4.0) | ||
| Treatment arm, No. (%) | .24 | .24 | ||||||
| FU/LV | 88 (55.0) | 272 (48.1) | 156 (52.2) | 157 (53.4) | 229 (47.6) | 130 (52.2) | ||
| IFL | 72 (45.0) | 293 (51.9) | 143 (47.8) | 137 (46.6) | 252 (52.4) | 119 (47.8) | ||
| BMI, median (IQR), kg/m2 | 27.5 (23.8–32.2) | 27.2 (23.8–30.9) | 27.5 (24.2–31.3) | .54 | 27.6 (23.5–32.3) | 27.4 (24.4–31.1) | 26.6 (23.6–30.7) | .29 |
| Physical activity, median (IQR), MET-h/w | 5.0 (1.7–14.8) | 4.6 (0.9–15.4) | 5.1 (1.1–17.8) | .47 | 3.7 (0.6–9.6) | 5.1 (1.3–15.1) | 7.1 (1.6–22.5) | <.001 |
| Current use of aspirin, No. (%) | 38 (23.8) | 156 (27.6) | 87 (29.1) | .47 | 82 (27.9) | 131 (27.2) | 68 (27.3) | .98 |
| Dietary intake, median (IQR) | ||||||||
| Alcohol consumption, g/d | 0.1 (0.0–1.8) | 0.4 (0.0–2.0) | 0.2 (0.0–3.5) | .70 | 0.2 (0.0–3.5) | 0.2 (0.0–2.0) | 0.4 (0.00–2.0) | .77 |
| Coffee intake, cups/wk | 3.0 (0.0–17.5) | 7.0 (0.5–17.5) | 7.0 (0.5–17.5) | .001 | 7.0 (0.4–17.5) | 6.5 (0.5–17.5) | 7.0 (0.2–17.0) | .85 |
| Sugar sweetened beverages, s/wk | 1.0 (0.4–3.8) | 2.4 (0.5–6.0) | 3.0 (0.7–7.0) | <.001 | 3.1 (0.6–7.0) | 1.7 (0.5–5.7) | 1.6 (0.5–5.5) | .02 |
| Cereal fiber, g/d | 5.6 (3.6–8.1) | 5.7 (4.3–7.3) | 5.4 (4.3–7.0) | .48 | 4.1 (3.1–4.9) | 5.8 (4.7–7.2) | 7.8 (6.2–9.9) | <.001 |
| Western diet pattern, No. < median (%) | 124 (77.5) | 329 (58.2) | 59 (19.7) | <.001 | 147 (50.0) | 252 (52.4) | 113 (45.4) | .20 |
| Prudent diet pattern, No. < median (%) | 97 (60.6) | 266 (47.1) | 149 (49.8) | .01 | 197 (67.0) | 228 (47.4) | 87 (34.9) | <.001 |
| AHEI dietary pattern, No. < median (%) | 63 (39.4) | 254 (45.0) | 195 (65.2) | <.001 | 189 (64.3) | 221 (45.9) | 102 (41.0) | <.001 |
| Glycemic load, No. < median (%) | 96 (60.0) | 278 (49.2) | 138 (46.2) | .02 | 168 (57.1) | 246 (51.1) | 98 (39.4) | <.001 |
Baseline performance status: 0 indicates fully active; 1 indicates restricted in physically strenuous activity but ambulatory and able to perform light work; 2 indicates ambulatory and capable of all self-care but unable to perform any work activities, up to approximately 50% of waking hours. AHEI = Alternate Healthy Eating Index 2010; BMI = body mass index; FU/LV = fluorouracil and leucovorin; IFL = irinotecan, fluorouracil, and leucovorin; IQR = interquartile range; MET-h/w = metabolic equivalent task hours per week; s = servings.
Associations Between Cancer Recurrence and Mortality With Grain Intake
The median follow-up time from completion of Q1 was 7.3 years. During follow-up, we observed 394 DFS events, 350 RFS events, and 311 OS events.
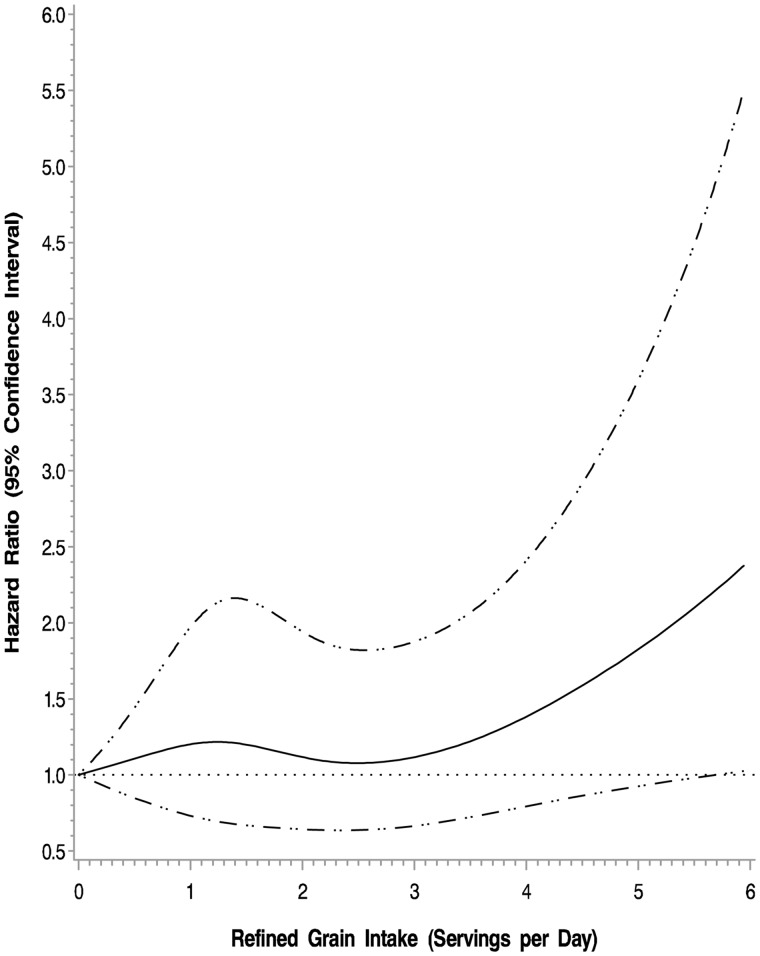
In the adjusted cubic spline model, higher intake of refined grains was associated with an increased risk of cancer recurrence and mortality (P = .03) (Figure 2). The associations between cancer recurrence and mortality with refined and whole grain intake are displayed in Table 2. The multivariable-adjusted hazard ratio for DFS was 1.56 (95% CI = 1.09 to 2.24) for those consuming three or more servings per day of refined grains compared with those consuming less than one serving per day (Ptrend = .005). Results were similar for RFS and OS. Exclusion of patients who experienced a DFS event in the first 90 to 365 days after Q1 did not substantively alter the above-described results.
Figure 2.
Adjusted cubic spline model for intake of refined grains and association with colon cancer recurrence and mortality (disease-free survival). Models are adjusted for age, sex, race, performance status, T stage, positive lymph nodes, location of primary tumor, treatment arm, body mass index, physical activity, total energy, and whole grain intake.
Table 2.
Associations between colon cancer recurrence and mortality with refined and whole grain intake*
| Grain intake, servings/d |
||||
|---|---|---|---|---|
| Outcome and exposure | <1 | 1–2 | ≥3 | Ptrend |
| Disease-free survival | ||||
| Refined grain intake | ||||
| No. of events/No. at risk | 53/160 | 204/565 | 137/299 | |
| Model 1† | 1.00 (referent) | 1.18 (0.87 to 1.61) | 1.75 (1.24 to 2.46) | <.001 |
| Model 2‡ | 1.00 (referent) | 1.16 (0.85 to 1.58) | 1.58 (1.12 to 2.24) | .003 |
| Model 3§ | 1.00 (referent) | 1.15 (0.84 to 1.58) | 1.56 (1.09 to 2.24) | .005 |
| Whole grain intake | ||||
| No. of events/No. at risk | 129/294 | 173/481 | 92/249 | |
| Model 1† | 1.00 (referent) | 0.77 (0.61 to 0.97) | 0.81 (0.62 to 1.07) | .19 |
| Model 2‡ | 1.00 (referent) | 0.81 (0.64 to 1.03) | 0.85 (0.64 to 1.13) | .32 |
| Model 3§ | 1.00 (referent) | 0.84 (0.66 to 1.07) | 0.89 (0.66 to 1.20) | .54 |
| Recurrence-free survival | ||||
| Refined grain intake | ||||
| No. of events/No. at risk | 48/160 | 177/565 | 125/299 | |
| Model 1† | 1.00 (referent) | 1.11 (0.80 to 1.54) | 1.73 (1.21 to 2.49) | <.001 |
| Model 2‡ | 1.00 (referent) | 1.08 (0.78 to 1.50) | 1.59 (1.10 to 2.29) | .001 |
| Model 3§ | 1.00 (referent) | 1.08 (0.77 to 1.50) | 1.57 (1.08 to 2.30) | .003 |
| Whole grain intake | ||||
| No. of events/No. at risk | 111/294 | 155/481 | 84/249 | |
| Model 1† | 1.00 (referent) | 0.81 (0.64 to 1.04) | 0.89 (0.67 to 1.20) | .53 |
| Model 2‡ | 1.00 (referent) | 0.82 (0.64 to 1.06) | 0.90 (0.67 to 1.21) | .58 |
| Model 3§ | 1.00 (referent) | 0.86 (0.67 to 1.12) | 0.97 (0.71 to 1.33) | .98 |
| Overall survival | ||||
| Refined grain intake | ||||
| No. of events/No. at risk | 38/160 | 158/565 | 115/299 | |
| Model 1† | 1.00 (referent) | 1.33 (0.92 to 1.90) | 2.09 (1.40 to 3.10) | <.001 |
| Model 2‡ | 1.00 (referent) | 1.32 (0.92 to 1.90) | 1.89 (1.27 to 2.81) | <.001 |
| Model 3§ | 1.00 (referent) | 1.32 (0.91 to 1.90) | 1.88 (1.25 to 2.85) | .001 |
| Whole grain intake | ||||
| No. of events/No. at risk | 108/294 | 130/481 | 73/249 | |
| Model 1† | 1.00 (referent) | 0.69 (0.54 to 0.89) | 0.74 (0.55 to 1.01) | .09 |
| Model 2‡ | 1.00 (referent) | 0.78 (0.60 to 1.02) | 0.81 (0.59 to 1.11) | .24 |
| Model 3§ | 1.00 (referent) | 0.81 (0.62 to 1.06) | 0.86 (0.62 to 1.20) | .46 |
Two-sided P values. Trend across quintiles. CI = confidence interval; HR = hazard ratio.
Model 1: adjusted for age and time-varying total energy.
Model 2: adjusted for age, sex, race, performance status, T stage, positive lymph nodes, location of primary tumor, treatment arm, time-varying body mass index, physical activity, and total energy.
Model 3: adjusted for age, sex, race, performance status, T stage, positive lymph nodes, location of primary tumor, treatment arm, time-varying body mass index, physical activity, total energy, and whole grain intake (for refined grain models) or refined grain intake (for whole grain models).
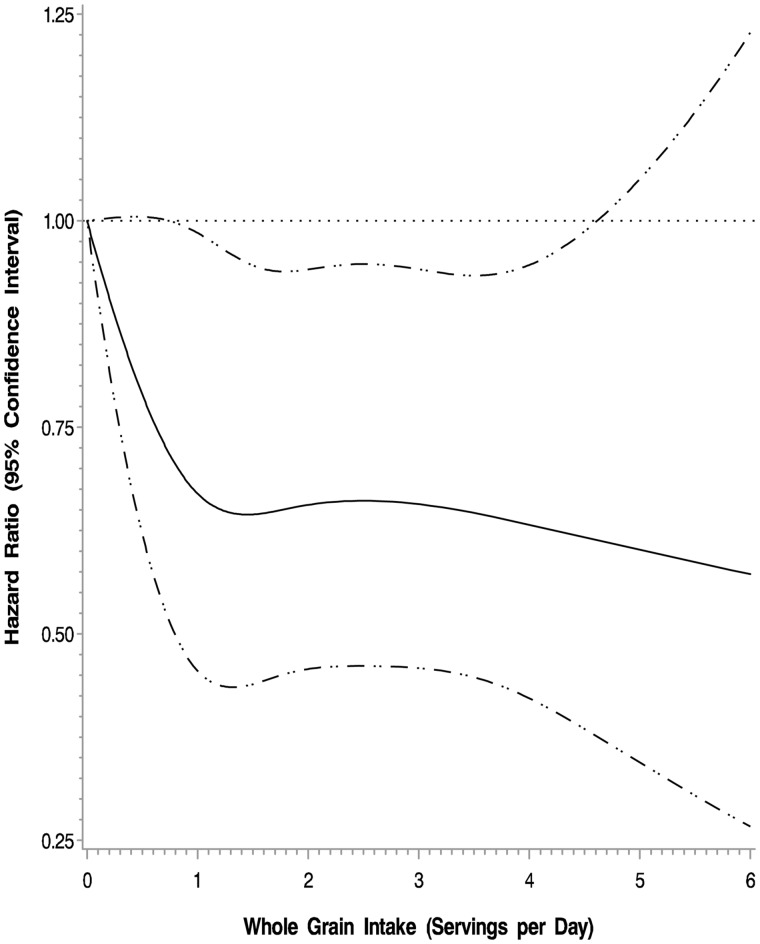
In the adjusted cubic spline model, higher intake of whole grains was associated with a reduced risk of cancer recurrence and mortality (P = .05) (Figure 3). The multivariable-adjusted hazard ratio for DFS was 0.89 (95% CI = 0.66 to 1.20) for those consuming three or more servings per day of whole grains compared with those consuming less than one serving per day (Ptrend = .54). Results were similar for RFS and OS. Exclusion of patients who experienced a DFS event in the first 90 to 365 days after Q1 did not substantively alter the above-described results.
Figure 3.
Adjusted cubic spline model for intake of whole grains and association with colon cancer recurrence and mortality (disease-free survival). Models are adjusted for age, sex, race, performance status, T stage, positive lymph nodes, location of primary tumor, treatment arm, body mass index, physical activity, total energy, and refined grain intake.
Substitution Analysis
The consumption of refined grains was negatively correlated with the consumption of whole grains (r = –.19, P < .001). We therefore sought to estimate the influence of substituting one daily serving of refined grain with one daily serving of whole grain. In substitution analysis, the multivariable-adjusted hazard ratio for DFS by substituting one daily serving of refined grain with one daily serving of whole grain was 0.87 (95% CI = 0. 79 to 0.96, P = .007). Results were similar for RFS (HR = 0.86, 95% CI = 0.77 to 0.96, P = .006) and OS (HR = 0.87, 95% CI = 0.78 to 0.97, P = .01).
Discussion
In this prospective cohort of patients with stage III colon cancer who participated in an NCI-sponsored randomized trial of postoperative chemotherapy, higher consumption of refined grains was associated with an increased risk of cancer recurrence and mortality, and the substitution of refined grains with whole grains was associated with a decreased risk of cancer recurrence and mortality. These data add to an evidence base that has documented the importance that dietary choices may have on clinical outcomes in this population.
Type II diabetes (18,19), hyperinsulinemia (20), inflammation (21), and visceral obesity are risk factors for cancer recurrence and mortality (22). Many of these risk factors have been proposed as biological mediators of the relationship between energy balance–related lifestyle factors (diet, physical activity, obesity) and clinical outcome in patients with cancer (38,39). The consumption of refined grains is associated with an increased risk of developing type II diabetes (12–14) and positively correlated with hyperinsulinemia (15), inflammation (16), and visceral obesity (17). Conversely, the consumption of whole grains is associated with a lower risk of developing type II diabetes (23) and negatively correlated with hyperinsulinemia (24), inflammation (25), and visceral obesity (17). The observed relationship between the consumption of refined grains with cancer recurrence and mortality is therefore biologically possible. This mechanistic hypothesis in oncology parallels that of cardiology, where the consumption of refined grains is associated with an increased risk of incident cardiovascular disease and cardiovascular-specific mortality (40).
A novel aspect of our study was the observation that substituting one daily serving of refined grains with one daily serving of whole grains was associated with a 13% lower risk of cancer recurrence or mortality during the follow-up period. Clinical trials in healthy men and women demonstrate that substituting refined grains with whole grains reduces insulin resistance (27), inflammation (28), and body fat (30). For example, in a randomized crossover trial, six weeks of a whole grain diet reduced concentrations of fasting insulin compared with a refined grain diet (−15.0±5.5 pmol/L, P = .03) (27). Similarly, in a 12-week randomized trial, a whole grain diet reduced body fat compared with a refined grain diet (−1.9%, P = .04) (30). These data suggest that randomized trials of dietary modification are feasible and induce physiologic changes that may be associated with clinical outcome. A decade ago, the initial observation was made that a Western-style diet was associated with cancer recurrence and mortality in patients with colon cancer (4). Since that time, the specificity of our understanding regarding what dietary constituents may influence clinical outcome has evolved, and now includes high-glycemic carbohydrates (5), red and processed meats (6), and sugar-sweetened beverages (7,8), as well as coffee (9), milk (10), and modest alcohol consumption (8). These observational data will be useful to inform and tailor the design of a dietary modification intervention trial in patients with colon cancer, a trial that will eventually need to be tested with a disease end point to provide persuasive evidence to inform clinical practice.
The principal strength of this study was embedding the FFQ in a randomized clinical trial. Consequently, this study had a large sample size with a well-defined study population. Follow-up in this study was standardized, such that all patients underwent quarterly medical examinations to identify recurrent disease. Although the FFQ was self-reported, it was collected prospectively, such that any ascertainment bias in the FFQ would be nondifferential and shift our effect size estimates toward the null. We excluded patients who experienced recurrent disease or mortality within 90 days of completing the FFQ to minimize the potential of occult disease inducing changes in dietary intake. We adjusted for variables that may confound the relationship between the consumption of refined and whole grains with cancer recurrence and mortality; several of these variables were time-varying, allowing us to account for changes in BMI, physical activity, and other aspects of dietary intake that may have occurred after completing chemotherapy.
The principal limitation of this study is the observational design, such that we cannot rule out the possibility of residual confounding. Although the FFQ used in the study was self-reported, it is correlated with plasma concentrations of carotenoids, tocopherols, and fatty acids in patients with colon cancer (32). Future studies should consider the potential utility of predicting prognosis with objective measures of dietary intake, such as metabolites in urine or plasma samples (41,42). The patients in the current study elected to enroll in a clinical trial and may not be representative of colon cancer patients in the US population with respect to demographic and lifestyle behaviors. We did not have information about type of surgery, postoperative complications, and presurgery diet and lifestyle measures, which precludes our ability to adjust for these potentially important variables.
In summary, this prospective cohort of patients with stage III colon cancer who participated in an NCI-sponsored randomized trial of postoperative chemotherapy demonstrated that a higher consumption of refined grains was associated with an increased risk of cancer recurrence and mortality, and the substitution of refined grains with whole grains was associated with a decreased risk of cancer recurrence and mortality. Although this observational study does not provide definitive evidence for causality, these data may inform clinical recommendations for patients and inform hypotheses to guide the design of future observational studies and randomized trials of dietary modification in patients with colon cancer.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology and U10CA180820 to the ECOG-ACRIN (CA60138, U10CA180857, U10CA180836, U10CA180867). Additional support was provided by Pharmacia & Upjohn Company, now Pfizer Oncology. Dr. Brown is supported by a grant from the National Cancer Institute (K99CA218603). Dr. Ogino is supported by a grant from the National Cancer Institute (R35CA197735). Drs. Fuchs and Meyerhardt are supported in part by grants from the National Cancer Institute (R01CA118553, R01CA149222, R01CA169141, and P50CA127003). The sponsors did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Notes
Affiliations of authors: Dana-Farber/Partners CancerCare, Boston, MA (JCB, SZ, RJM, SO, KN, JAM); Duke Cancer Institute, Duke University Medical Center, Durham, NC (DN); Memorial Sloan Kettering Cancer Center, New York, NY (LBS); Toledo Community Hospital Oncology Program, Toledo, OH (RBM); Hôpital du Sacré-Coeur de Montréal Montreal, Canada (RW); Edward-Elmhurst Heatlhcare, Naperville, IL (AH); Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL (AB); Virginia Oncology Associates, Norfolk, VA (DA); Southeast Clinical Oncology Research (SCOR) Consortium, Mission Hospitals, Inc., Asheville, NC (MM); University of Chicago Comprehensive Cancer, Chicago, IL (HK); University of California at San Francisco Comprehensive Cancer Center, San Francisco, CA (AV); Harvard T.H. Chan School of Public Health, Boston, MA (SO, YL, WCW, ELG); Brigham and Women’s Hospital, Boston, MA (SO, XZ, ELG); Yale Cancer Center, Yale School of Medicine, New Haven, CT (CSF).
ClincalTrials.gov registration: NCT000038350.
The views expressed in the article are those of the authors and not an official position of their affiliated institutions or funders.
References
- 1. Siegel R, Desantis C, Jemal A.. Colorectal cancer statistics, 2014. CA Cancer J Clin .2014;642:104–117. [DOI] [PubMed] [Google Scholar]
- 2. Sargent DJ, Wieand HS, Haller DG et al. , . Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;2334:8664–8670. [DOI] [PubMed] [Google Scholar]
- 3. James-Martin G, Koczwara B, Smith E, Miller M.. Information needs of cancer patients and survivors regarding diet, exercise and weight management: A qualitative study. Eur J Cancer Care. 2014;233:340–348. [DOI] [PubMed] [Google Scholar]
- 4. Meyerhardt JA, Niedzwiecki D, Hollis D et al. , . Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;2987:754–764. [DOI] [PubMed] [Google Scholar]
- 5. Meyerhardt JA, Sato K, Niedzwiecki D et al. , . Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Natl Cancer Inst. 2012;10422:1702–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCullough ML, Gapstur SM, Shah R, Jacobs EJ, Campbell PT.. Association between red and processed meat intake and mortality among colorectal cancer survivors. J Clin Oncol. 2013;3122:2773–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuchs MA, Sato K, Niedzwiecki D et al. , . Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance). PLoS One. 2014;96:e99816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fung TT, Kashambwa R, Sato K et al. , . Post diagnosis diet quality and colorectal cancer survival in women. PLoS One. 2014;912:e115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guercio BJ, Sato K, Niedzwiecki D et al. , . Coffee intake, recurrence, and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance). J Clin Oncol. 2015;3331:3598–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang B, McCullough ML, Gapstur SM et al. , . Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: The Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2014;3222:2335–2343. [DOI] [PubMed] [Google Scholar]
- 11. Rock CL, Doyle C, Demark-Wahnefried W et al. , . Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;624:243–274. [DOI] [PubMed] [Google Scholar]
- 12. Nanri A, Mizoue T, Noda M et al. , . Rice intake and type 2 diabetes in Japanese men and women: The Japan Public Health Center-based Prospective Study. Am J Clin Nutr. 2010;926:1468–1477. [DOI] [PubMed] [Google Scholar]
- 13. Sun Q, Spiegelman D, van Dam RM et al. , . White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;17011:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villegas R, Liu S, Gao YT et al. , . Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med. 2007;16721:2310–2316. [DOI] [PubMed] [Google Scholar]
- 15. Newby PK, Maras J, Bakun P, Muller D, Ferrucci L, Tucker KL.. Intake of whole grains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J Clin Nutr. 2007;866:1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masters RC, Liese AD, Haffner SM, Wagenknecht LE, Hanley AJ.. Whole and refined grain intakes are related to inflammatory protein concentrations in human plasma. J Nutr. 2010;1403:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKeown NM, Troy LM, Jacques PF, Hoffmann U, O'Donnell CJ, Fox CS.. Whole- and refined-grain intakes are differentially associated with abdominal visceral and subcutaneous adiposity in healthy adults: The Framingham Heart Study. Am J Clin Nutr. 2010;925:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeon JY, Jeong DH, Park MG et al. , . Impact of diabetes on oncologic outcome of colorectal cancer patients: Colon vs. rectal cancer. PLoS One. 2013;82:e55196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyerhardt JA, Catalano PJ, Haller DG et al. , . Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;213:433–440. [DOI] [PubMed] [Google Scholar]
- 20. Wolpin BM, Meyerhardt JA, Chan AT et al. , . Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;272:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C.. C-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: A systematic review. PloS one. 2015;1012:e0143080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao J, Mazurak VC, Olobatuyi TA, Caan BJ, Prado CM.. Visceral adiposity and cancer survival: A review of imaging studies. Eur J Cancer Care (Engl) .2018;272:e12611. [DOI] [PubMed] [Google Scholar]
- 23. Aune D, Norat T, Romundstad P, Vatten LJ.. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;2811:845–858. [DOI] [PubMed] [Google Scholar]
- 24. McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF.. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham offspring Study. Am J Clin Nutrit. 2002;762:390–398. [DOI] [PubMed] [Google Scholar]
- 25. Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB.. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;292:207–211. [DOI] [PubMed] [Google Scholar]
- 26. Malin SK, Kullman EL, Scelsi AR et al. , . A whole-grain diet reduces peripheral insulin resistance and improves glucose kinetics in obese adults: A randomized-controlled trial. Metabolism .2018;82:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pereira MA, Jacobs DR Jr., Pins JJ et al. , . Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;755:848–855. [DOI] [PubMed] [Google Scholar]
- 28. Vanegas SM, Meydani M, Barnett JB et al. , . Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutrit. 2017;1053:635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roager HM, Vogt JK, Kristensen M et al. , . Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut .2017; pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kristensen M, Toubro S, Jensen MG et al. , . Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr. 2012;1424:710–716. [DOI] [PubMed] [Google Scholar]
- 31. Saltz LB, Niedzwiecki D, Hollis D et al. , . Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol. 2007;2523:3456–3461. [DOI] [PubMed] [Google Scholar]
- 32. Meyerhardt JA, Heseltine D, Campos H et al. , . Assessment of a dietary questionnaire in cancer patients receiving cytotoxic chemotherapy. J Clin Oncol. 2005;2333:8453–8460. [DOI] [PubMed] [Google Scholar]
- 33. Willett W, Stampfer MJ.. Total energy intake: Implications for epidemiologic analyses. Am J Epidemiol. 1986;1241:17–27. [DOI] [PubMed] [Google Scholar]
- 34. Jacobs DR, Meyer KA, Kushi LH, Folsom AR.. Whole-grain intake may reduce the risk of ischemic heart disease death in postmenopausal women: The Iowa Women's Health Study. Am J Clin Nutrit. 1998;682:248–257. [DOI] [PubMed] [Google Scholar]
- 35. Willett WC, Sampson L, Stampfer MJ et al. , . Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;1221:51–65. [DOI] [PubMed] [Google Scholar]
- 36. Salvini S, Hunter DJ, Sampson L et al. , . Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;184:858–867. [DOI] [PubMed] [Google Scholar]
- 37. Mekary RA, Willett WC, Hu FB, Ding EL.. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;1704:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown JC, Meyerhardt JA.. Obesity and energy balance in GI cancer. J Clin Oncol. 2016;3435:4217–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;83:205–211. [DOI] [PubMed] [Google Scholar]
- 40. Aune D, Keum N, Giovannucci E et al. , . Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Playdon MC, Moore SC, Derkach A et al. , . Identifying biomarkers of dietary patterns by using metabolomics. Am J Clin Nutrit. 2017;1052:450–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Playdon MC, Sampson JN, Cross AJ et al. , . Comparing metabolite profiles of habitual diet in serum and urine. Am J Clin Nutr. 2016;1043:776–789. [DOI] [PMC free article] [PubMed] [Google Scholar]