Supplemental Digital Content is available in the text.
Abstract
Background:
Cell-enriched fat grafting has shown promising results for improving graft survival, although many questions remain unanswered. A large animal model is crucial for bridging the gap between rodent studies and human trials. We present a step-by-step approach in using the Göttingen minipig as a model for future studies of cell-enriched large volume fat grafting.
Methods:
Fat grafting was performed as bolus injections and structural fat grafting. Graft retention was assessed by magnetic resonance imaging after 120 days. The stromal vascular fraction (SVF) was isolated from excised fat and liposuctioned fat from different anatomical sites and analyzed. Porcine adipose-derived stem/stromal cells (ASCs) were cultured in different growth supplements, and population doubling time, maximum cell yield, expression of surface markers, and differentiation potential were investigated.
Results:
Structural fat grafting in the breast and subcutaneous bolus grafting in the abdomen revealed average graft retention of 53.55% and 15.28%, respectively, which are similar to human reports. Liposuction yielded fewer SVF cells than fat excision, and abdominal fat had the most SVF cells/g fat with SVF yields similar to humans. Additionally, we demonstrated that porcine ASCs can be readily isolated and expanded in culture in allogeneic porcine platelet lysate and fetal bovine serum and that the use of 10% porcine platelet lysate or 20% fetal bovine serum resulted in population doubling time, maximum cell yield, surface marker profile, and trilineage differentiation that were comparable with humans.
Conclusions:
The Göttingen minipig is a feasible and cost-effective, large animal model for future translational studies of cell-enriched fat grafting.
INTRODUCTION
Autologous fat grafting has become a widely used tool in plastic surgery for tissue augmentation and for restoring volume defects.1–5 Major differences in graft retention (10–90%) has been reported over the years,6–10 but extensive research and increasing experience with the technique has resulted in improved and more consistent graft retentions in recent reports.11 Despite this, the quest for improving fat graft retention even further continues and enriching fat grafts with either stromal vascular fraction (SVF) cells12–17 or ex vivo expanded adipose-derived stromal/stem cells (ASCs)18,19 have shown promising results. However, many questions regarding the mechanism of action and fate of ASCs remain unclear, and the optimal concentration and most effective cellular enrichment composition are unknown. To answer these questions, an animal model with fat volume and composition similar to humans is needed.
Most previous animal studies have used xenogeneic models with human cells and fat tissue grafted in very small volumes into immune-deficient rodents,20,21 which is far from the clinical setting. Therefore, an intermediate large animal model with superior comparability to humans and the option of studying larger volumes of fat grafts is imperative.
No such model currently exists, and therefore, we decided to investigate and validate the Göttingen minipig for future studies of cell-enriched fat grafting due to the similarities between these pigs and humans in terms of anatomy and pathophysiology.22,23 Regarding the scope of fat grafting, an important factor is that Göttingen minipigs build up a thick subcutaneous fat layer when fed to obesity, which is essential for performing standard large-volume liposuction. Additionally, obese Göttingen minipigs weigh no more than 60–70 kg, which allows for handling during surgery and magnetic resonance imaging (MRI). Finally, these minipigs are widely used experimental animals in other fields of research.24,25
The primary aim of this study was to investigate the efficacy and translatability of the Göttingen minipig as an animal model for future studies of autologous cell-enriched fat grafting of larger volumes. We therefore performed feasibility studies of both the in vitro and in vivo aspects of the technique and compared the obtained data with existing human data on ASC expansion and autologous fat grafting. We investigated:
1) SVF isolation from excised fat versus liposuction from different anatomical sites;
2) large volume liposuction and conventional fat grafting (nonenriched) via both bolus injection and structural fat grafting;
3) fat graft retention assessed by MRI at day 120;
4) ASC cultures with different growth supplements [fetal bovine serum (FBS), pooled porcine platelet lysate (pPPL), pooled human platelet lysate (pHPL), and porcine serum (PS)] with respect to population doubling time (PDT), maximum cell yield, expression of surface markers, and differentiation potential; and
5) the feasibility of ASC expansion for large-volume cell-enriched fat grafting.
MATERIALS AND METHODS
Animals
Adult female Göttingen minipigs weighing approximately 70 kg were used in accordance with The Danish Animal Experiments Inspectorate, permission 2015-15-0201-00681.
Harvesting of Fat for SVF Isolation: Techniques and Anatomical Sites
To identify the effect of different harvesting techniques and different donor sites on SVF yield, we performed syringe-aspiration and surgical excision of adipose tissue from the neck, back, and abdomen. Fifty milliliters of lipoaspirate and 20 grams of excised fat were harvested from all 3 donor sites, and the SVF yield was determined by cell counting.
Large Volume Liposuction and Fat Grafting
A GID-700 canister was used for collecting and washing the lipoaspirate. Tumescent solution was installed before suction-assisted liposuction, which was performed with a pressure no lower than -0.6 bar. The temperature in the canister was kept above 30°C to prevent solidification of the lipoaspirate. Fat grafting was performed with either a subcutaneous bolus injection of 30 mL lipoaspirate injected on the abdomen of the animal (n = 4) or via a structural fat grafting technique using a fat graft of 150–185 mL injected into the pig’s breast (n = 4).
Magnetic Resonance Imaging
Volume retention of the bolus fat grafts was calculated as described previously26 directly after grafting and again after 120 days. A region of interest (ROI) was drawn around the bolus graft on all slices (Fig. 1). ROIs were then multiplied by slice thickness and summed to determine the overall graft volume.
Fig. 1.

MR images and delineation of the ROI (subcutaneously implanted bolus fat graft). A, Immediate postgrafting MR images. D, The same areas on day 120. The inset images (B, C, E and F) show the graft areas magnified with and without delineation for reference. MR, magnetic resonance.
Volume retention of the porcine breast was measured as described previously.27 First, the cranial/caudal and lateral/medial border of the breast were outlined on the image acquired directly after grafting. The cranial/caudal borders were translated to a fixed distance from the nipple of the grafted breast, and the lateral border was translated into a fixed distance from the midline. The distances from these fixed pointers were noted and used on the subsequent scan after 120 days (Fig. 2).
Fig. 2.

MR images with delineation of the ROI (large-volume structural fat graft in the breast area of the pig). This example pig received bilateral structural fat grafting. A, Immediate postgrafting MR images. B, The same areas on day 120. Note the nipple, which serves as a reference point for setting the cranial/caudal borders. MR, magnetic resonance.
Isolation and Culture of ASCs
The tissues were incubated with collagenase type 4 at 37°C with constant rotation for 90 minutes before neutralizing with culture medium (CM) consisting of Dulbecco’s modified Eagle’s medium, 1% penicillin-streptomycin, and 10% FBS. The suspension was filtered (100-µm filter) and centrifuged at 1,200 g for 10 minutes. The pellet was resuspended in CM and seeded in 75 cm2 culture flasks with 10,000 cells/cm2. Cells were cultured at 37°C in an atmosphere of 5% carbon dioxide and humidified air. The CM was changed every 3–4 days. At confluence, cells were passaged using TrypLE Select 1X for 20 minutes at 37°C, neutralized with CM, and centrifuged at 300 g for 6 minutes. The pellet was resuspended and reseeded.
Culture Expansion of ASCs for Large-volume Fat Grafting
Up to 350 mL of lipoaspirate was incubated with collagenase at 37°C for 90 minutes while shaken at 140 rpm. The suspension was neutralized with CM, filtered (200-µm filter) and centrifuged at 1,200 g for 10 minutes. The pellet was resuspended in CM, and cells were seeded in one 6,320 cm2 10-layer Cell Factory at densities of 20,000–40,000 cells/cm2. The CM, which contained 20% FBS, was changed only once on day 6, and at 90–100% confluence (day 14), the cells were passaged and reseeded in three 8,216 cm2 13-layer Cell Factory’s at densities of 20,000–30,000 cells/cm2 without changing the CM (according to unpublished data) for 1 additional week.
Production of Pooled Human Platelet Lysate
pHPL was produced as described previously18,28 by using otherwise discarded outdated platelet concentrates initially intended for human use.
Production of Pooled Porcine Platelet Lysate
Blood from exsanguinated slaughter pigs was collected and separated by centrifugation and leukocyte depletion filtration before a freeze/thaw cycle for the lysis and release of growth factors. pPPL was pooled and sterile filtered before storing at -80°C.
Proliferation Assay
ASCs were isolated and cultured 2 passages in CM supplemented with 1 of 5 growth supplements: 10% FBS, 20% FBS, 10% pPPL, 10% pHPL, or 10% PS. Cells were seeded in 25 cm2 flasks with 5,000 cells/cm2, and the CM was changed every fourth day. One flask from all cell lines was collected and counted in technical triplicates every other day.
The yields were plotted with time on a linear x axis and cell densities on a base 10 logarithmic y axis. Semi-logarithmic fit lines were fitted to the linear growth phase (ie, the log phase) by means of the least-squares methods.
The PDT was calculated using the formula: PDT = (t2 - t1) · log(2)/(log(final cell density at t2) - log(initial cell density at t1)), where t1 is the beginning of the log phase, and t2 is the end of the log phase as defined by the fit lines.
Trilineage Differentiation
ASCs were cultured to passage 5 in CM containing 10% FBS, 20% FBS, 10% pHPL, 10% pPPL, or 10% PS and then induced to trilineage differentiation using a STEMPRO Differentiation Kit. Cells were cultured for 14 or 21 days before staining for adipogenic, osteogenic, and chondrogenic differentiation.
Flow Cytometry
ASCs cultured with each of the 5 different growth supplements were phenotypically characterized using flow cytometry with anti-CD44, anti-CD90, anti-CD105, and anti-CD45 antibodies. Fluorescence minus one staining served as controls.
Statistical Analysis
Statistical analysis was performed using SAS Enterprise Guide 7.1, and graphs were generated in Prism 7. A 2-way analysis of variance (ANOVA) was used for comparing differences in SVF cell yields between different harvest techniques and donor sites with Tukey’s multiple comparisons test used for post hoc analysis. To compare the total cell yield in the proliferation assay and differences in the PDTs, 1-way ANOVA was performed with Bonferroni-adjusted P values. The normal distribution of all groups was assessed by the Shapiro-Wilk test, and a 2-sided P value of < 0.05 was considered statistically significant. All data are presented as the mean ± SD.
RESULTS
Harvest Techniques and Donor Sites
The SVF cell yield obtained by surgical excision was significantly higher than that obtained by liposuction in all 3 donor sites (P ≤ 0.026). In addition, the number of SVF cells obtained by liposuction from the abdomen was significantly higher than that obtained from the back (P = 0.043), as shown in Figure 3. For histological features of the 3 donor sites, see Supplemental Digital Content 1 (see figure, Supplemental Digital Content 1, which displays histological features of the different donor sites, http://links.lww.com/PRSGO/A741).
Fig. 3.

The mean SVF cells/g fat obtained by syringe liposuction from the back, neck, and abdomen regions were 4.92 × 105 ± 1.96 × 105, 6.08 × 105 ± 0.75 × 105, and 7.72 × 105 ± 2.10 × 105 SVF cells/g fat, respectively, and those obtained by surgical excision were 9.93 × 105 ± 2.32 × 105, 10.8 × 105 ± 2.82 × 105, and 11.4 × 105 ± 3.40 × 105 SVF cells/g fat, respectively (n = 4). In contrast, as shown in Table 1, suction-assisted liposuction from the abdomen in larger volumes yielded a mean of 9.95 × 105 SVF cells/g fat. This difference could be explained by the different harvesting techniques, but most likely because the pigs undergoing syringe liposuction were exsanguinated before the procedure, and thus, a smaller amount of blood cells were mixed with the lipoaspirate, that is, in the SVF.
Fat Grafting and Volume Retention Calculated from MRI
MRI revealed that the initial volume of the 4 bolus grafts was 30.22 ± 0.78 mL, corresponding well to the injected volumes. On day 120, the average residual volume was 4.61 ± 0.96 mL, demonstrating graft retention of 15.28 ± 3.31% of the initial volume.
The average retention of the 4 structural fat grafts was 53.55 ± 1.95% (Figs. 1, 2).
For histology of the different fat grafts before and 120 days after grafting see Supplemental Digital Content 2 (see figure, Supplemental Digital Content 2, which displays histology of the fat grafts before and 120 days after grafting, http://links.lww.com/PRSGO/A742).
Isolation and Culture of ASCs
ASCs cultured in pPPL, 10% FBS, and 20% FBS appeared elongated and spindle shaped and reached complete confluence without losing their plastic adherent properties as seen in Figure 4. ASCs cultured with PS initially displayed the same appearance but turned into large spheres with low adherence, whereas ASCs cultured in pHPL were smaller, less elongated, more pellucid, and less adherent to the plastic surface. See full figure of the cell morphology in Supplemental Digital Content 3 (see figure, Supplemental Digital Content 3, which displays full figure of the cell morphology, http://links.lww.com/PRSGO/A743).
Fig. 4.

ASC morphology in pPPL cultured cells. Populations of plastic adherent, ASCs occurred from both lipoaspirate and excised adipose tissue with an elongated, spindle shaped appearance. After passage and reseeding with 5,000 cells/cm2, confluency was reached after 5–7 days with the use of 10% pPPL. These ASCs also had the ability to become “overconfluent” before reaching replicative senescence. Magnification: 10× original magnification. For full figure of the ASC morphology, see Supplemental Digital Content 3.
Large-volume Culture Expansion
The average amount of fat used in large-volume SVF isolation was 260 g, but 1,000–1,500 g could be easily obtained by liposuction. The average yield of isolated SVF was 9.95 × 105 ± 3.14 × 105 SVF-cells/g fat. Subsequent culture expansion for 3 weeks resulted in an average of 1.63 × 109 ± 0.32 × 109 total ASCs per sample, equivalent to an increase by more than 1,600 times. Details are given in Table 1.
Table 1.
Data for ASC Culture Expansion in 6 Minipigs

Trilineage Differentiation Assay
All ASCs could differentiate into adipocytes, chondrocytes, and osteocytes regardless of the growth supplement used for culture as seen in Figure 5 and the full figure of trilineage differentiation(see figure, Supplemental Digital Content 4, which displays full figure of trilineage differentiation, http://links.lww.com/PRSGO/A744).
Fig. 5.
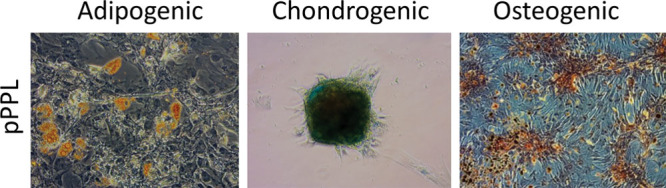
Differentiation capacity of porcine ASCs. Cells were expanded in culture with 5 different growth supplements before trilineage differentiation was induced. Oil red O, Alcian Blue, and Alizarin red S staining were used to visualize lipid vacuoles, proteoglycans and calcium complexes, respectively. Here only ASCs cultured in pPPL are shown. For the full figure, see Supplemental Digital Content 4. Adipogenic differentiation: 20× original magnification. Chondrogenic and osteogenic differentiation: 10× original magnification.
Flow Cytometry
ASCs cultured in 10% FBS, 20% FBS, pPPL, and PS were positive for CD44, CD90, and CD105, although cells cultured in 10% PS appeared to have a slightly lower expression of CD44. In contrast, ASCs cultured in pHPL were negative for CD105 and exhibited reduced expression of CD90 and CD44. All cells were negative for CD45 regardless of the CM used (see figure, Supplemental Digital Content 5, which displays Figure of the phenotypic analysis by flow cytometry, http://links.lww.com/PRSGO/A745).
Proliferation Assay
All cells showed an initial lag phase of growth for approximately 2 days. For the ASCs cultured in 20% FBS, pPPL, and pHPL, the lag phase was followed by a short log phase of growth for 4–5 days before reaching a plateau around days 6–8. The ASCs cultured in 10% FBS and PS showed a longer log phase of growth for approximately 8–10 days before reaching a plateau around days 10–14. There was no significant difference in PDT between ASCs cultured in 20% FBS, pPPL, or pHPL, but the PDTs were significantly shorter compared with ASCs cultured in 10% FBS and PS (P < 0.05; Fig. 6).
Fig. 6.

Growth curves of ASCs cultured in different growth supplements (n = 3). The fit lines were calculated with the following formula: y = 10ax + b (where a is the slope, and b is the intercept), with x = (log10[y] – b) / a. Lower right: The mean cell density of all growth supplements over time.
During the plateau phase (approximately days 16–28), ASCs cultured in 20% FBS exhibited the highest maximum yield of 17.7 × 104 ± 0.94 × 104 ASCs/cm2, corresponding to approximately 340% more ASCs than those grown in PS. There was also a 250% increase in ASCs grown in 20% FBS compared with those grown in 10% FBS (Fig. 7).
Fig. 7.

Mean cell densities are shown for the ASCs during the plateau phase (n = 3). Significant differences were found among all groups when multiple comparisons were performed (1-way ANOVA). The highest Bonferroni-adjusted P value is shown. 10% FBS = 7.19 × 104 ± 0.97 × 104 ASC/cm2, 20% FBS = 17.70 × 104 ± 0.94 × 104 ASC/cm2, pPPL = 12.10 × 104 ± 1.29 × 104 ASC/cm2, pHPL = 8.72 × 104 ± 1.63 × 104 ASC/cm2, and PS = 5.20 × 104 ± 0.80 × 104 ASC/cm2.
DISCUSSION
Porcine ASCs have previously been isolated and characterized from excised fat tissue.29–31 However, contrary to previous animal studies where graft volumes have seldom exceeded 1 mL,20,21 the development of a translational model for clinically relevant fat grafting of larger volumes requires the use of lipoaspirate instead of excised fat.
No previous studies have reported isolation of porcine ASCs from large-volume lipoaspirates. We were able to extract 1,000–1,500 mL of lipoaspirate with little effort and no surgical complications.
In large-volume SVF isolations acquired by suction-assisted liposuction, we demonstrated an average yield of 9.95 × 105 ± 3.14 × 105 live SVF cells/g adipose tissue. This amount of SVF cells correlates to results from human studies in which the average SVF yield has been reported to range from 7.19 × 105 to 9.55 × 105 live cells/g adipose tissue.32–34 In addition, similar to previous human studies, we demonstrated a significantly higher SVF yield from adipose tissue harvested by surgical excision compared with liposuction,35–37 and a higher density of SVF cells in the lipoaspirate from the abdomen compared with the back.35,38,39
To our knowledge, this is also the first report of structural fat grafting into the breast area of Göttingen minipigs. This specific recipient site and technique of structural fat graft injection were chosen to mimic the gold standard of clinical fat grafting in humans. After 120 days, we found a mean residual volume of 53.55%, which is comparable with humans where residual volumes of 38.1–54% have been reported in prospective, controlled clinical studies with no preconditioning of the recipient site.12,13,17,27,40,41 We also investigated subcutaneous bolus injections of 30-mL fat grafts and found an average residual volume of 15.28% similar to the 16.3% reported in the only previous human study that employed this technique.18
For large-volume ASC-enriched fat grafting to be feasible in a clinical setting, it is imperative to obtain a sufficient number of cells within an acceptable timeframe. Additionally, regarding translational tissue, PDTs similar to human studies may hold promising biological significance. Because studies of human ASCs have repeatedly identified human platelet lysates as superior to FBS for ensuring fast and reproducible culture expansion and because animal-based growth supplements are associated with the risk of xeno-immunization and transmission of animal pathogens,42–48 we tested both FBS, pHPL, and allogeneic alternatives, that is, pPPL and PS.
Porcine ASCs have previously been cultured in autologous porcine platelet lysate,49 which cannot be produced in the quantities needed for large-volume culture expansion. We present for the first time a feasible and cost-effective method to produce allogeneic pPPL. Minipigs and domestic pigs are considered the same species, and therefore, the blood from both animals can be used to produce pPPL. This is advantageous, as large quantities of blood can be collected from slaughter pigs and used to culture ASCs from minipigs. Pigs and humans have similar platelet counts in the range of 150–500 × 109/l50,51; however, porcine platelets are smaller, why the centrifugation force and time for production of Platelet Rich Plasma are different, in addition to leucocyte filtration. We found that pPPL provided the shortest PDT (26.48 hours) and was correlated with culture expansion of human ASCs, for which the use of pHPL also conferred shorter PDTs compared with FBS.42,43,52,53 Additionally, the total pPPL cell density of 1.21 × 105 at the plateau was comparable with studies of human ASCs in which cell densities at the plateau phase were reported in the range of 1.50 × 105 ASC/cm2 with the use of 10% pHPL.26,42 However, FBS remains the standard growth supplement for culturing ASCs in animal studies, and growth rates and cell densities comparable with those from 10% pPPL could be achieved with the use of 20% FBS, as shown in Figures 6, 7. The differentiation potential and surface marker profiles for culture in pPPL and 20% FBS were not significantly different, indicating that they are equally translatable to human data. Additionally, 20% FBS produced the highest total cell density at the plateau phase with 1.77 × 105 ASC/cm2, which can be advantageous if large-volume ASC expansion is needed. Moreover, FBS is readily obtainable from commercial suppliers.
Porcine ASCs have previously been characterized,29,54,55 and in particular, Casado et al.30 reported their characterization including mesenchymal stem cell markers (CD29, CD44, CD45, CD90, and CD105), histocompatibility molecules (SLA-I and SLA-II), and cell adhesion molecules (CD11a, CD11b, CD18, and CD61), revealing that porcine ASCs are similar to human ASCs. In the present study, we show that cells isolated and expanded in culture had CD44+, CD90+, CD105+, and CD45− phenotypes when cultured in allogeneic serum/platelet lysate or FBS. In contrast, cells cultured in the xenogeneic pHPL showed a marked reduction in the surface expression of CD44 and CD90 and were negative for CD105, indicating possible alteration of the cells. Nonetheless, we found that the porcine ASCs were able to differentiate into adipogenic, chondrogenic, and osteogenic lineages regardless of the growth supplement used for expansion.
To summarize the in vitro studies, we found that porcine ASCs cultured in either 10% pPPL or 20% FBS demonstrated morphologies, growth patterns, PDTs, maximum cell densities, surface markers, and differentiation capacities that were comparable with human ASCs cultured in pHPL.
CONCLUSIONS
In this study, we have presented a novel large-animal model tailored to investigate cell-enriched fat grafting in clinically relevant volumes, using the Göttingen minipig. We highlight the many similarities between the minipig model and humans in terms of the fat harvest technique, donor sites, SVF cell yield, fat graft retention rates measured with MRI, ASC proliferation capacities, surface marker profiles, and differentiation capabilities. We have proven that the Göttingen minipig is a feasible large-animal model suitable as a solid alternative for future studies of cell-enriched fat grafting of larger volumes. The Göttingen minipig may thus be used to bridge the gap between rodent models and humans to accelerate the translation of stem cell technology to clinical practice.
ACKNOWLEDGMENT
The authors thank the staff at the Department of Experimental Medicine, Panum Institute, University of Copenhagen for professional and dedicated assistance with the minipigs during the study period.
Supplementary Material
Footnotes
Published online 4 April 2018.
Preliminary results presented at IFATS San Diego 2016.
Supported by The Danish Cancer Society (Kræftens Bekæmpelse) and The Research Foundation of Rigshospitalet (Rigshospitalets Forskningspuljer).
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by The Danish Cancer Society.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Zielins ER, Brett EA, Longaker MT, et al. Autologous fat grafting: the science behind the surgery. Aesthet Surg J. 2016;36:488–496.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001;28:111–119.. [PubMed] [Google Scholar]
- 3.Kanchwala SK, Holloway L, Bucky LP. Reliable soft tissue augmentation: a clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Ann Plast Surg. 2005;55:30–35.; discussion 35. [DOI] [PubMed] [Google Scholar]
- 4.Khouri R, Del Vecchio D. Breast reconstruction and augmentation using pre-expansion and autologous fat transplantation. Clin Plast Surg. 2009;36:269–80, viii.. [DOI] [PubMed] [Google Scholar]
- 5.Khouri RK, Smit JM, Cardoso E, et al. Percutaneous aponeurotomy and lipofilling: a regenerative alternative to flap reconstruction? Plast Reconstr Surg. 2013;132:1280–1290.. [DOI] [PubMed] [Google Scholar]
- 6.Delay E, Garson S, Tousson G, et al. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29:360–376.. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Luo X, Lu Y, et al. Is the resorption of grafted fat reduced in cell-assisted lipotransfer for breast augmentation? Ann Plast Surg. 2015;75:128–134.. [DOI] [PubMed] [Google Scholar]
- 8.Herold C, Ueberreiter K, Busche MN, et al. Autologous fat transplantation: volumetric tools for estimation of volume survival. A systematic review. Aesthetic Plast Surg. 2013;37:380–387.. [DOI] [PubMed] [Google Scholar]
- 9.Ross RJ, Shayan R, Mutimer KL, et al. Autologous fat grafting: current state of the art and critical review. Ann Plast Surg. 2014;73:352–357.. [DOI] [PubMed] [Google Scholar]
- 10.Wetterau M, Szpalski C, Hazen A, et al. Autologous fat grafting and facial reconstruction. J Craniofac Surg. 2012;23:315–318.. [DOI] [PubMed] [Google Scholar]
- 11.Khouri RK, Jr, Khouri RK. Current clinical applications of fat grafting. Plast Reconstr Surg. 2017;140:466–86.. [DOI] [PubMed] [Google Scholar]
- 12.Gentile P, Orlandi A, Scioli MG, et al. A comparative translational study: the combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med. 2012;1:341–351.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentile P, Scioli MG, Orlandi A, et al. Breast reconstruction with enhanced stromal vascular fraction fat grafting: what is the best method? Plast Reconstr Surg Glob Open. 2015;3:e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55.; discussion 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009;4:265–273.. [DOI] [PubMed] [Google Scholar]
- 16.Dos Anjos S, Matas-Palau A, Mercader J, et al. Reproducible volume restoration and efficient long-term volume retention after point-of-care standardized cell-enhanced fat grafting in breast surgery. Plast Reconstr Surg Glob Open. 2015;3:e547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tissiani LA, Alonso N. A prospective and controlled clinical trial on stromal vascular fraction enriched fat grafts in secondary breast reconstruction. Stem Cells Int. 2016;2016:2636454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kølle SF, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382:1113–1120.. [DOI] [PubMed] [Google Scholar]
- 19.Koh KS, Oh TS, Kim H, et al. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg. 2012;69:331–337.. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen BS, Lykke Sørensen C, Vester-Glowinski PV, et al. Effect, feasibility, and clinical relevance of cell enrichment in large volume fat grafting: a systematic review. Aesthet Surg J. 2017;37:S46–S58.. [DOI] [PubMed] [Google Scholar]
- 21.Toyserkani NM, Quaade ML, Sørensen JA. Cell-assisted lipotransfer: a systematic review of its efficacy. Aesthetic Plast Surg. 2016;40:309–318.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casal M, Haskins M. Large animal models and gene therapy. Eur J Hum Genet. 2006;14:266–272.. [DOI] [PubMed] [Google Scholar]
- 23.Larsen MO, Rolin B. Use of the Göttingen minipig as a model of diabetes, with special focus on type 1 diabetes research. ILAR J. 2004;45:303–313.. [DOI] [PubMed] [Google Scholar]
- 24.Harding J, Roberts RM, Mirochnitchenko O. Large animal models for stem cell therapy. Stem Cell Res Ther. 2013;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubessa M, Polkoff K, Bionaz M, et al. Use of pig as a model for mesenchymal stem cell therapies for bone regeneration. Anim Biotechnol. 2017:1–13.. [DOI] [PubMed] [Google Scholar]
- 26.Herly M, Ørholt M, Glovinski PV, et al. Quantifying long-term retention of excised fat grafts: a longitudinal, retrospective cohort study of 108 patients followed for up to 8.4 years. Plast Reconstr Surg. 2017;139:1223–1232.. [DOI] [PubMed] [Google Scholar]
- 27.Glovinski PV, Herly M, Müller FC, et al. Avoiding a systematic error in assessing fat graft survival in the breast with repeated magnetic resonance imaging. Plast Reconstr Surg Glob Open. 2016;4:e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glovinski PV, Herly M, Mathiasen AB, et al. Overcoming the bottleneck of platelet lysate supply in large-scale clinical expansion of adipose-derived stem cells: a comparison of fresh versus three types of platelet lysates from outdated buffy coat-derived platelet concentrates. Cytotherapy. 2017;19:222–234.. [DOI] [PubMed] [Google Scholar]
- 29.Williams KJ, Godke RA, Bondioli KR. Isolation and culture of porcine adipose tissue-derived somatic stem cells. Methods Mol Biol. 2011;702:77–86.. [DOI] [PubMed] [Google Scholar]
- 30.Casado JG, Gomez-Mauricio G, Alvarez V, et al. Comparative phenotypic and molecular characterization of porcine mesenchymal stem cells from different sources for translational studies in a large animal model. Vet Immunol Immunopathol. 2012;147:104–112.. [DOI] [PubMed] [Google Scholar]
- 31.Niada S, Ferreira LM, Arrigoni E, et al. Porcine adipose-derived stem cells from buccal fat pad and subcutaneous adipose tissue for future preclinical studies in oral surgery. Stem Cell Res Ther. 2013;4:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dos-Anjos Vilaboa S, Navarro-Palou M, Llull R. Age influence on stromal vascular fraction cell yield obtained from human lipoaspirates. Cytotherapy. 2014;16:1092–1097.. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez J, Pratta AS, Abbassi N, et al. Evaluation of three devices for the isolation of the stromal vascular fraction from adipose tissue and for ASC culture: a comparative study. Stem Cells Int. 2017;2017:9289213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown JC, Shang H, Li Y, et al. Isolation of adipose-derived stromal vascular fraction cells using a novel point-of-care device: cell characterization and review of the literature. Tissue Eng Part C Methods. 2017;23:125–135.. [DOI] [PubMed] [Google Scholar]
- 35.Iyyanki T, Hubenak J, Liu J, et al. Harvesting technique affects adipose-derived stem cell yield. Aesthet Surg J. 2015;35:467–476.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreml S, Babilas P, Fruth S, et al. Harvesting human adipose tissue-derived adult stem cells: resection versus liposuction. Cytotherapy. 2009;11:947–957.. [DOI] [PubMed] [Google Scholar]
- 37.Duscher D, Luan A, Rennert RC, et al. Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells. J Transl Med. 2016;14:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332:415–426.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faustini M, Bucco M, Chlapanidas T, et al. Nonexpanded mesenchymal stem cells for regenerative medicine: yield in stromal vascular fraction from adipose tissues. Tissue Eng Part C Methods. 2010;16:1515–1521.. [DOI] [PubMed] [Google Scholar]
- 40.Peltoniemi HH, Salmi A, Miettinen S, et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: a prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;66:1494–1503.. [DOI] [PubMed] [Google Scholar]
- 41.Choi M, Small K, Levovitz C, et al. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;131:185–191.. [DOI] [PubMed] [Google Scholar]
- 42.Trojahn Kølle SF, Oliveri RS, Glovinski PV, et al. Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy. 2013;15:1086–1097.. [DOI] [PubMed] [Google Scholar]
- 43.Fekete N, Gadelorge M, Fürst D, et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy. 2012;14:540–554.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih DT, Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. N Biotechnol. 2015;32:199–211.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doucet C, Ernou I, Zhang Y, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–236.. [DOI] [PubMed] [Google Scholar]
- 46.Lange C, Cakiroglu F, Spiess AN, et al. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213:18–26.. [DOI] [PubMed] [Google Scholar]
- 47.Bernardo ME, Cometa AM, Pagliara D, et al. Ex vivo expansion of mesenchymal stromal cells. Best Pract Res Clin Haematol. 2011;24:73–81.. [DOI] [PubMed] [Google Scholar]
- 48.Bieback K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother. 2013;40:326–335.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aldén A, Gonzalez L, Persson A, et al. Porcine platelet lysate as a supplement for animal cell culture. Cytotechnology. 2007;55:3–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaar M, Secher NH, Gam CM, et al. Interindividual variation in platelets and the cardiovascular response to haemorrhage in the pig. Blood Coagul Fibrinolysis. 2011;22:92–97.. [DOI] [PubMed] [Google Scholar]
- 51.Ross DW, Ayscue LH, Watson J, et al. Stability of hematologic parameters in healthy subjects. Intraindividual versus interindividual variation. Am J Clin Pathol. 1988;90:262–267.. [DOI] [PubMed] [Google Scholar]
- 52.Juhl M, Tratwal J, Follin B, et al. Comparison of clinical grade human platelet lysates for cultivation of mesenchymal stromal cells from bone marrow and adipose tissue. Scand J Clin Lab Invest. 2016;76:93–104.. [DOI] [PubMed] [Google Scholar]
- 53.Rauch C, Feifel E, Amann EM, et al. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011;28:305–316.. [DOI] [PubMed] [Google Scholar]
- 54.Wang KH, Kao AP, Wangchen H, et al. Optimizing proliferation and characterization of multipotent stem cells from porcine adipose tissue. Biotechnol Appl Biochem. 2008;51:159–166.. [DOI] [PubMed] [Google Scholar]
- 55.Williams KJ, Picou AA, Kish SL, et al. Isolation and characterization of porcine adipose tissue-derived adult stem cells. Cells Tissues Organs. 2008;188:251–258.. [DOI] [PubMed] [Google Scholar]


