Abstract
Background:
Traditionally, tissue expanders (TEs) for breast reconstruction have been placed beneath the pectoralis major muscle with or without acellular dermal matrix. More recently, full acellular dermal matrix coverage has been described for prepectoral TE placement. Our study aims to explore differences in clinical and quality-of-life (QOL) outcomes for prepectoral versus subpectoral TE breast reconstruction.
Methods:
We identified patients who underwent postmastectomy breast reconstruction with prepectoral or subpectoral TE placement between 2011 and 2015 and completed QOL surveys. Primary outcomes were postoperative pain and QOL scores. Secondary outcomes were clinical outcomes. We used Wilcoxon rank-sum test, chi-square test, and linear regression to compare outcomes. Postoperative follow-up for each patient was at least 60 days, except that of pain scores, which were at least 30 days. Mean age was 49 ± 10 years.
Results:
Twenty-six prepectoral TE patients and 109 subpectoral TE patients met inclusion criteria. Pain scores were significantly lower at 12 hours, 1 day, 7 days, and 30 days postoperatively for the prepectoral group, compared with the subpectoral group, even after adjusting for confounding variables [PO12H: Sub-Pectoral (SP) median (interquartile range), 7 (5–8), Pre-Pectoral (PP), 5 (2.5–7.5), P value = 0.004; PO1D: SP, 5 (4–6), PP 3 (2–4), P value = < 0.001; PO7D: SP, 2 (0–4), PP, 0 (0–2), P value = 0.004; PO30D: SP, 0 (0–2), PP, 0 (0–0), P value = 0.039)]. Breast-Q scores were not significantly different between study groups. RAND-36 Physical Health scores were lower among prepectoral TE patients.
Conclusions:
Prepectoral TE breast reconstruction presents an opportunity to improve upon current reconstructive methods and does result in significantly lower pain scores. The associated risks have yet to be fully described and are important considerations, as these prepectoral patients had lower physical health outcome scores.
BACKGROUND
Breast cancer is the second leading cause of cancer deaths among women in the United States. The American Cancer Society estimated that 231,840 new cases of invasive breast cancer and 40,200 deaths due to the disease would occur in 2015. This represents a 19.8% increase in new cases of breast cancer over 2009 estimates.1 In addition to breast-conserving treatments, mastectomy can be an important and definitive locoregional treatment option for certain patients. Mastectomy rates have significantly increased in the United States over the past decade, from 40% in 2005 to 51% in 2011.2,3 Postmastectomy breast reconstruction plays an important role in breast cancer survivorship for many women and has been shown to improve quality of life scores,4,5 with benefits extending to a woman’s sense of sexuality, body image, and self-esteem.6
Breast reconstruction after mastectomy can be performed using implants or autologous tissue transfer. Though autologous transfer results in the closest resemblance to the original breast, particularly in radiated patients, implant-based breast reconstruction is now the most widely used approach in the United States,3,7 with this technique on the rise. Implant-based reconstructions are commonly performed in a single- or 2-staged fashion. The immediate placement of prosthetic implants in single-stage reconstructions can be comforting to women after mastectomy; however, this type of reconstruction relies more heavily on the quality of the mastectomy flaps and thereby increases the risk of postoperative skin necrosis.8 In 2-stage reconstructions, a tissue expander (TE) is placed at the time of mastectomy, to preserve the natural footprint of the breast, and then exchanged for a prosthetic implant once the skin envelope has been expanded to the desired volume.6,9
Traditionally, 2-stage breast reconstruction was performed using total submuscular coverage of the TE/implant beneath the pectoralis major and serratus anterior.10 Although cosmetic results have been described to be excellent, total submuscular reconstruction is associated with significant postoperative pain, injury-induced muscular deficit, potential for breast animation deformity (in greater than 70% of patients), lateral deviation of the breast mound with poor inframammary fold definition, and insufficient lower pole fullness.6,11 To reduce manipulation of the pectoralis muscle, acellular dermal matrix (ADM), either human or animal derived, has emerged to reinforce the lower pole of the breast pocket. These dual plane reconstructions, also known as partial subpectoral/partial sub-ADM, minimize elevation of the pectoralis muscle and thereby avoid some of the complications described above.11 Moreover, they offer comparable cosmetic outcomes, a similar safety profile, better early fill volumes, and less postoperative pain.10 Prepectoral, also known as subcutaneous, implant placement with complete ADM coverage has recently been described in breast reconstruction in the literature.12 Lower postoperative pain scores and favorable cosmetic results speak for the potential utility of prepectoral reconstruction.13–16
Prepectoral TE breast reconstruction with human ADM presents an opportunity to improve upon current reconstructive methods and minimize complications such as postoperative pain and breast animation deformity that are associated with manipulation of the pectoralis muscle. However, the risks associated with prepectoral breast reconstruction are important considerations for patients when deciding on a type of reconstruction and these risks have yet to be fully explored.17
To date, little is known regarding the effectiveness of total ADM-covered devices using a 2-stage reconstructive approach in the subcutaneous plane.6,12,17 To our knowledge, this study is the first retrospective review of clinical and quality of life outcomes associated with ADM reinforcement of breast pocket tissues in 2-stage tissue expansion and implant placement in the subcutaneous (prepectoral) plane. The aim of this study to describe clinical and quality of life outcomes in women who have undergone unilateral or bilateral mastectomy followed by immediate, unilateral, or bilateral prepectoral TE breast reconstruction reinforced with ADM coverage and compare the results with those of women who have undergone mastectomy followed by immediate, subpectoral TE breast reconstruction reinforced with partial ADM coverage.
METHODS
This study was a retrospective review of adult (aged 18 years or older) female patients who underwent unilateral or bilateral mastectomy followed by immediate breast reconstruction over a 5-year period (2011–2016). Retrospective chart reviews of our breast reconstruction patients were reviewed and approved by the Institutional Review Board at Johns Hopkins School of Medicine. All patients were operated on by 2 board-certified plastic and reconstructive surgeons at our academic institution.
A total of 135 patients underwent unilateral or bilateral mastectomy with immediate breast reconstruction over the study period and completed our postoperative follow-up questionnaires. These were divided into 2 cohorts for comparison based on the plane of placement of TEs as the primary predictor variable: prepectoral versus subpectoral. Prepectoral reconstructions were those in which the TE was placed above the pectoralis muscle (subcutaneously), without manipulating the pectoralis muscle. Subpectoral reconstructions were those in which a TE was placed below the pectoralis muscle after manipulation with the use of ADM with inferior pole reinforcement.
Secondary predictor variables were demographic factors, health status, and oncologic and surgical factors potentially related to the outcomes of interest. A careful review of all medical records was performed to record demographic data (ie, age, body mass index (BMI), and smoking status), medical comorbidities (ie, diabetes mellitus, hypertension, coronary artery disease), oncological data (ie, cancer stage, history of radiation therapy, history of chemotherapy), and surgical variables (ie, use of ADM, use of tumescent technique for mastectomy, history of breast reduction, and placement of TE).
Primary outcome variables were postoperative pain scores and subjective assessment of patient satisfaction. Postoperative pain scores were collected from the charts of included patients, as the recording of pain scores is included in the postoperative standard of care. Secondary outcome variables were complication rates and number of readmission/reoperations. Complications recorded hematoma, seroma, minor infection (ie, clinical signs of infection successfully treated with outpatient antibiotic therapy), major infection (ie, infections requiring hospitalization and intravenous antibiotic therapy and/or any surgical treatment), minor skin necrosis (ie, partial-thickness necrosis treated with local wound care), or major skin necrosis (ie, full-thickness necrosis requiring local wound care or surgical debridement and closure). Reoperations were calculated for each patient as any operative intervention beyond the planned number of procedures for the method of reconstruction. Planned procedures such as nipple reconstruction or tattoo were excluded. Subjective outcomes were assessed by means of BREAST-Q and RAND-36 surveys given to the study pool.18,19
Data were recorded over the study period using Microsoft Excel and entered into a statistical database, Stata v13.1 (StataCorp LP, College Station, Tex.) for analysis. Wilcoxon rank-sum test, chi-square test, multiple linear regression, and multivariable linear regression were computed to compare the prepectoral and subpectoral groups with regard to the predictor variables to identify any confounders. Outcome measures, including pain scores, were compared by placement of TE for breast reconstruction, and multiple regression models were constructed to calculate adjusted associations between the placement of TEs and objective and subjective outcomes. For all analysis, a value of P < 0.05 was considered significant. A power analysis confirmed that the sample size that was available for analysis would provide more than 80% power to detect a clinically important difference in the primary outcome assuming an alpha error of 0.05.20
RESULTS
Over the 5-year study period, a total of 135 patients underwent unilateral or bilateral mastectomy with staged breast reconstruction and completed our postoperative follow-up questionnaire. Twenty-six patients underwent staged breast reconstruction with prepectoral TEs and ADM. One hundred nine patients underwent breast reconstruction with subpectoral TEs and ADM. Postoperative follow-up for each patient was at least 60 days, except for pain scores (which was at least 30 days). Summary statistics for the study population are listed in Table 1.
Table 1.
Comparison of Subpectoral and Prepectoral TE Breast Reconstruction Groups
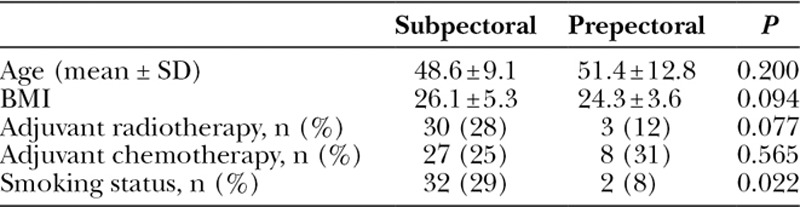
Pain scores were significantly lower at 12 hours, 1 day, 7 days, and 30 days postoperatively for the prepectoral TE patient group, compared with the subpectoral TE group. This statistical significance was seen even after adjusting for confounding variables, including age group, BMI class, smoking status, and history of radiation therapy and chemotherapy (Table 2).
Table 2.
Postoperative Pain Scores of Subpectoral Versus Prepectoral Procedures
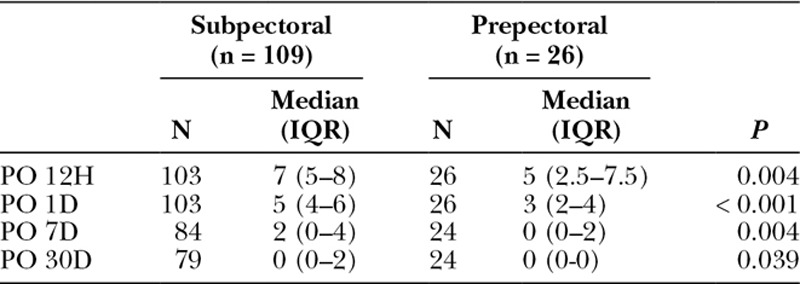
BREAST-Q scores were not significantly different between study groups, even after adjusting for the same confounding variables. RAND-36 Summary Physical Health scores were lower among prepectoral TE patients [median, 56; interquartile range (IQR), 44–85 points] than subpectoral TE patients (median, 83; IQR, 61–94 points; P = 0.046), which persisted after adjusting for confounding (12.4 points lower for prepectoral TE; 95% CI, 0.6–24.2 points lower; P = 0.039). RAND-36 Summary Mental Health scores were not significantly different (P = 0.519). Fewer patients developed complications in the prepectoral group than the subpectoral group (17.4% versus 30.7%); however, this difference was not statistically significant (P = 0.127), even in adjusted analysis (P = 0.148). Breast-Q and RAND-36 survey response rate was approximately 50%. Quality of life data are shown in Table 3 and Figure 1. Most common complications were surgical-site infection and seroma for the subpectoral group and nipple ischemia and seroma for the prepectoral group. Complication data are summarized in Table 4. Preoperative and postoperative images from 1 patient included in our study can be seen in Figure 2.
Table 3.
Patient-reported Quality of Life Scores
Fig. 1.
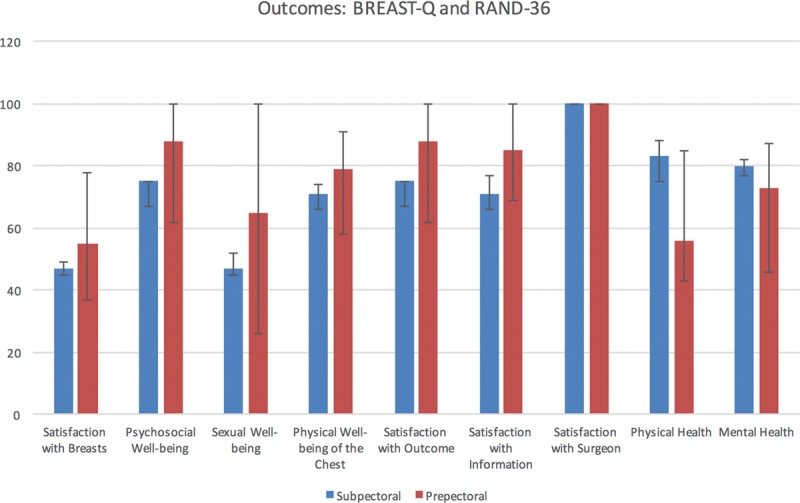
Outcomes: BREAST-Q and RAND-36.
Table 4.
Complication Rates of Subpectoral Versus Prepectoral Procedures
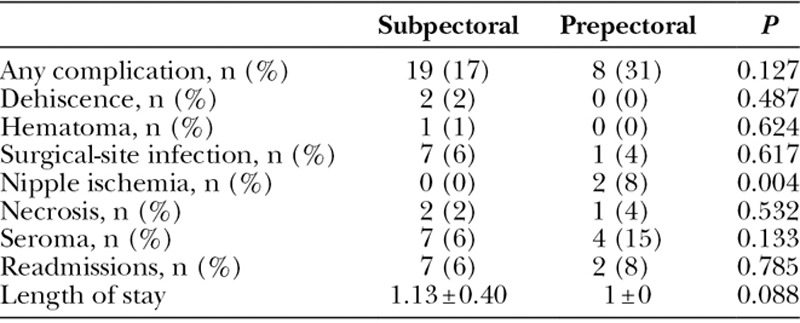
Fig. 2.
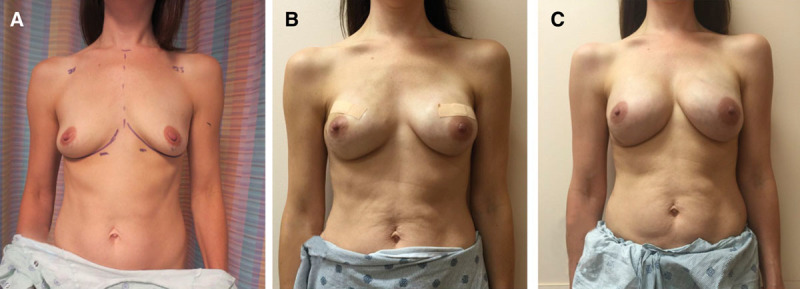
Prepectoral preoperative and postoperative images. A, Displays breasts preoperatively. B, Displays breast reconstruction results at postoperative day 21. C, Displays breast reconstruction results at 8 months from initial mastectomy.
DISCUSSION
Breast cancer remains a significant public health concern, with over 200,000 new cases diagnosed in per year.21 Breast reconstruction plays a pivotal role in the management of breast cancer, with over 105,000 breast reconstructions performed annually,22 and as evidenced by the well-documented restoration of positive self-image and psychological well-being following reconstruction of the postmastectomy defects.23,24 Furthermore, new advances and techniques continue to evolve, allowing for more natural, efficient methods of breast reconstruction and optimizing patient satisfaction. In an era of cost containment and emphasis on efficient practices, a critical analysis of the various methodologies for breast reconstruction and their outcomes are essential.
Subpectoral TE placement breast reconstruction continues to be the most common method, but this type of reconstruction is associated with significant postoperative pain, injury-induced muscular deficit, and potential for breast animation deformity (in greater than 70% of patients).6,11,25,26 The breast reconstruction techniques in the prepectoral (subcutaneous) plane was first described by Snyderman and Guthrie27 in the 1970s. However, these prepectoral reconstruction techniques were found to have early associations with a high incidence of complications, including mastectomy skin flap necrosis, implant extrusion, and capsular contracture.28–30 The recent emergence of ADM products as an adjunct to immediate postmastectomy breast reconstruction has been shown to decrease the risks of overstretching the lower pole of the breast, implant rippling, and capsular contracture.16
After the first stage of the breast reconstruction (the TE placement), it has been shown that a low complication rate of 8.6% is attainable in nonsmokers with a low BMI (less than 29), small and nonptotic breasts, and no history of radiation.16 Recent literature has further shown that prepectoral TE placement for immediate postmastectomy breast reconstruction has several potential benefits, ranging from a faster tissue expansion, the ability to fill the TE with greater volumes, decreased time to reach final expansion, a reduction in postoperative pain, and fewer postoperative expansion visits.16
As the prepectoral TE techniques allow for increased postoperative filling volumes, there will be fewer clinic visits, less postoperative pain, lower costs, less time constraints for patients, and a better acceptance of treatment plan.16 The ability to have greater and faster early expansion leads to better preservation of the mastectomy skin flap, which will result in a better overall cosmetic result. However, fast and early expansion also adds stress to the skin flap, putting the tissue at risk of necrosis, which is completely dependent on the quality of the mastectomy (if the blood supply is sufficient). Further, the ability to position the TE more toward the midline as to better define the medial footprint improves the patient’s resulting medial cleavage.16
It has been shown that the shape of the reconstructed breast is mainly affected by the skin envelope.31 With the use of TEs that help produce thin and distensible capsules,32 reconstruction of the skin envelope has become easier. In recent years, several reconstructive surgeons have reported a better control of the breast shape with prepectoral TE placement when compared with subpectoral TE placement.32,33 Further, studies have shown that prepectoral TE placement results in thinner capsule formation, which is beneficial to predict and control the shape of the reconstructed breast.32
Our comparison analysis of 135 immediate breast reconstructions using either prepectoral or subpectoral TE breast reconstruction reveals comparative BREAST-Q subjective satisfaction scores and RAND-36 Summary Mental Health scores. Comparable postoperative subjective satisfaction scores illustrate that both methods achieve successful cosmetic and reconstructive results. On the other hand, RAND-35 Summary Physical Health scores were lower among patients who underwent prepectoral TE placement when compared with those who underwent subpectoral TE placement, even after confounding. We may not be able to attribute these lower quality of life scores to the surgery because we do not have baseline scores due to lack of preoperative subjective survey data. We need to see additional studies with a higher study population to be able to determine if these findings are related to the prepectoral surgery technique.
However, our study also revealed that patients undergoing prepectoral TE placement had significantly lower postoperative pain scores compared with those undergoing subpectoral TE placement. We have seen that with the lack of manipulation of the pectoralis muscle, there are much lower postoperative pain scores. By avoiding the manipulation of the pectoralis muscle, we can forego the several complications that commonly occur in patients who underwent subpectoral TE placement.
Prepectoral TE breast reconstruction with complete ADM coverage presents an opportunity to improve upon current reconstructive methods for select mastectomy patients. Decreased pain and improved quality of life is something that we as physicians should all ascribe to providing for our patients. Surgical dogma of placing a breast TE below a serratus and pectoralis muscle has been replaced with subpectoral and subpectoral with ADM for inferior pole projection. Prepectoral staged and immediate breast reconstruction is perhaps an evolution in mastectomy reconstruction whose time has now arrived. Current use of synthetic meshes and biological matrices and newer ones in the future will allow us as reconstructive surgeons to recapitulate the lost breast and restore femininity without obligate pain and suffering. Current and newer saline and silicone implants coordinated with synthetic meshes and biological matrices in the prepectoral plan will allow plastic surgeons to potentially innovate the aesthetics of breast reconstruction. The most natural breast reconstructions are often those using autologous adipose tissue, particularly in radiated patients. These reconstructions are in the prepectoral plane. As plastic surgeons, this is the anatomical space we should favor if human anatomy allows us to obtain aesthetically and functional outcomes. However, before the decision is finalized, the risks associated with this procedure have yet to be fully described and are important considerations for patients and providers when choosing among reconstruction options. As plastic surgeons, we strive every day to make our procedures better for our patients. We are a unique society of surgeons in that we are always iterating and always striving for perfection for our patients.
This study has limitations, given the methodology and assessed outcomes. As a retrospective study, there is an element of selection bias in the patients that were selected for surgery type that cannot be avoided. Although we were able to obtain full data regarding complications and revisions for consecutive patients over a 5-year period, our Breast-Q and RAND-36 survey response rate was approximately 50%. This may result in skewed results, as patients who were, on average, more satisfied with the results of the operation may be more likely to respond, upwardly biasing the satisfaction scores. Further, our study does have a limited follow-up for secondary complications, including pain, and we were unable to account for all confounding variables. Cost analysis and comparison was not within the scope of the study but would be an important undertaking for future clinical studies. Future studies evaluating the cost for both methods of TE breast reconstruction and revision rates within a defined long-term postoperative period would be valuable.
CONCLUSIONS
Patients undergoing prepectoral TE breast reconstruction have significantly lower postoperative pain scores and relatively lower complication rates, with regard to dehiscence, hematoma, surgical-site infections, and length of stay, when compared with patients undergoing subpectoral TE breast reconstruction. We believe that prepectoral reconstruction improves patient satisfaction and quality of life and lowers postoperative pain scores for select patients. Carefully designed, prospective studies are needed to establish high-quality data to facilitate appropriate selection of patients who may benefit from this procedure.
Footnotes
Published online 20 April 2018.
Disclosure: The Johns Hopkins Department of Plastic Surgery receives research support from LifeCell Corp. (Branchburg, N.J.), and educational support from LifeCell Corp. (Branchburg, N.J.), Integra Lifesciences (Plainsboro, N.J.), and Sientra (Santa Barbara, Calif.). Dr. Justin Sacks is a consultant/speaker for LifeCell. Dr. Rosson has licensed IP to Aegeria Soft Tissue, Inc., which is not related to the subject material of this study. All other authors do not have any financial conflict of interests. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Breast Cancer Facts and Figures 2015–2016. American Cancer Society. Available at http://www.cancer.org/.
- 2.Lucas DJ, Sabino J, Shriver CD, et al. Doing more: trends in breast cancer surgery, 2005 to 2011. Am Surg. 2015;81:74–80.. [PubMed] [Google Scholar]
- 3.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23.. [DOI] [PubMed] [Google Scholar]
- 4.Edsander-Nord A, Brandberg Y, Wickman M. Quality of life, patients’ satisfaction, and aesthetic outcome after pedicled or free TRAM flap breast surgery. Plast Reconstr Surg. 2001;107:1142–1153.; discussion 1154. [DOI] [PubMed] [Google Scholar]
- 5.Girotto JA, Schreiber J, Nahabedian MY. Breast reconstruction in the elderly: preserving excellent quality of life. Ann Plast Surg. 2003;50:572–578.. [DOI] [PubMed] [Google Scholar]
- 6.Harless C, Jacobson SR. Current strategies with 2-staged prosthetic breast reconstruction. Gland Surg. 2015;4:204–211.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cemal Y, Albornoz CR, Disa JJ, et al. A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg. 2013;131:320e–326e.. [DOI] [PubMed] [Google Scholar]
- 8.Basta MN, Gerety PA, Serletti JM, et al. A systematic review and head-to-head meta-analysis of outcomes following direct-to-implant versus conventional two-stage implant reconstruction. Plast Reconstr Surg. 2015;136:1135–1144.. [DOI] [PubMed] [Google Scholar]
- 9.Susarla SM, Ganske I, Helliwell L, et al. Comparison of clinical outcomes and patient satisfaction in immediate single-stage versus two-stage implant-based breast reconstruction. Plast Reconstr Surg. 2015;135:1e–8e.. [DOI] [PubMed] [Google Scholar]
- 10.Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740.. [DOI] [PubMed] [Google Scholar]
- 11.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e–410e.. [DOI] [PubMed] [Google Scholar]
- 12.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167.. [DOI] [PubMed] [Google Scholar]
- 13.Woo A, Harless C, Jacobson SR. Revisiting an old place: single-surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast J. 2017;23:545–553.. [DOI] [PubMed] [Google Scholar]
- 14.Hammond DC, Schmitt WP, O’Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg. 2015;135:1540–1544.. [DOI] [PubMed] [Google Scholar]
- 15.Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous direct-to-implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open. 2015;3:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:e77–e86.. [DOI] [PubMed] [Google Scholar]
- 17.Lee KT, Mun GH. Updated evidence of acellular dermal matrix use for implant-based breast reconstruction: a meta-analysis. Ann Surg Oncol. 2016;23:600–610.. [DOI] [PubMed] [Google Scholar]
- 18.Pusic AL, Klassen AF, Cano SJ. Use of the BREAST-Q in clinical outcomes research. Plast Reconstr Surg. 2012;129:166e–167e.; author reply 167e. [DOI] [PubMed] [Google Scholar]
- 19.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–353.. [DOI] [PubMed] [Google Scholar]
- 20.Cano SJ, Klassen AF, Scott A, et al. Interpreting clinical differences in BREAST-Q scores: minimal important difference. Plast Reconstr Surg. 2014;134:173e–175e.. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Breast Cancer Statistics. Available at http://www.breastcancer.org/symptoms/understand_bc/statistics. Accessed December 15, 2016.
- 22.2013 Plastic Surgery Statistics Report. 2013American Society of Plastic Surgeons. [Google Scholar]
- 23.Pusic AL, Matros E, Fine N, et al. Patient-reported outcomes 1 year after immediate breast reconstruction: results of the Mastectomy Reconstruction Outcomes Consortium Study. J Clin Oncol. 2017:Jco2016699561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2000;106:1014–1025.; discussion 1026. [DOI] [PubMed] [Google Scholar]
- 25.Wallace MS, Wallace AM, Lee J, et al. Pain after breast surgery: a survey of 282 women. Pain. 1996;66:195–205.. [DOI] [PubMed] [Google Scholar]
- 26.Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg. 2009;33:44–48.. [DOI] [PubMed] [Google Scholar]
- 27.Snyderman RK, Guthrie RH. Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg. 1971;47:565–567.. [DOI] [PubMed] [Google Scholar]
- 28.Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69:195–208.. [DOI] [PubMed] [Google Scholar]
- 29.Lapin R, Elliott M, Juri H. The use of an integral tissue expander for primary breast reconstruction. Aesthetic Plast Surg. 1985;9:221–226.. [DOI] [PubMed] [Google Scholar]
- 30.Artz JS, Dinner MI, Sampliner J. Breast reconstruction with a subcutaneous tissue expander followed with a polyurethane-covered silicone breast implant. Ann Plast Surg. 1988;20:517–521.. [DOI] [PubMed] [Google Scholar]
- 31.Hudson DA. Factors determining shape and symmetry in immediate breast reconstruction. Ann Plast Surg. 2004;52:15–21.. [DOI] [PubMed] [Google Scholar]
- 32.Tomita K, Yano K, Nishibayashi A, et al. Effects of subcutaneous versus submuscular tissue expander placement on breast capsule formation. Plast Reconstr Surg Glob Open. 2015;3:e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tadiparthi S, Alrawi M, Collis N. Two-stage delayed breast reconstruction with an expander and free abdominal tissue transfer: outcomes of 65 consecutive cases by a single surgeon. J Plast Reconstr Aesthet Surg. 2011;64:1608–1612.. [DOI] [PubMed] [Google Scholar]


