Summary:
Hyaluronic acid (HA) is present in the connective tissues of the skin and decreases with age. HA fillers are popular as facial rejuvenation treatments. They are generally considered safe; however, complications, such as cutaneous necrosis and blindness due to vascular embolism, sometimes occur. Because vascular embolisms are likely associated with the deep placement of HA fillers, a strategy that involves injection into superficial regions (the conventional method) is commonly used to reduce risks. However, deep injections to achieve revolumization are becoming common, even in high-risk areas for intravascular infusion. We aimed to study the usefulness of the ultrasonography-guided cannula method for preventing intravascular infusion of HA fillers. An HA filler was injected into the region just under the dermis on the left side of the face of a 38-year-old man using the conventional method, and another HA filler was injected into the periosteum on the right side using the ultrasonography-guided cannula method. The skin blood flow on both sides was compared using laser speckle flowgraphy (LSFG). The ultrasonography-guided method was successful in detecting the cannula and the blood vessel, and the HA filler was safely injected into a deep region. Using LSFG, a difference in skin blood flow between the 2 methods was detected. The ultrasonography-guided cannula method was effective in aiding the safe injection of an HA filler in a deep high-risk area and maintained skin blood flow. LSFG may be adopted to evaluate skin blood flow after HA filler injections.
INTRODUCTION
Hyaluronic acid (HA) is natively present in the connective tissues of the skin, and its amount decreases with age.1 HA is a glycosaminoglycan biopolymer with alternating D-glucuronic acid and N-acetyl-D-glucosamine units.1 HA fillers are glycosaminoglycan complex sugars, comprising alternating D-glucuronic acid and N-acetyl-D-glucosamine units.2 Because they can achieve long-sustained effects, they are increasingly used in nonsurgical facial rejuvenation treatments.1–5 Although HA fillers are generally considered safe, complications sometimes occur, and the incidence of complications is increasing worldwide.2–7 The most concerning complications are cutaneous necrosis and blindness due to vascular embolisms, and preventing them is important for clinicians.3–7 Although the nasolabial folds and alar base are considered high-risk areas for vascular embolisms, HA fillers are increasingly injected into these areas on patients’ requests.6 Several strategies, such as injecting into superficial regions (the conventional method), have been proposed to prevent intravascular infusion, and most complications are avoidable; nevertheless, the possibility of intravascular infusion still exists.3–5,7 In this study, we conducted an exploratory use and provided case evidence of the usefulness of the ultrasonography-guided cannula method for preventing the vascular embolism that can be caused by injection of HA fillers. Moreover, we used 2-dimensional laser speckle flowgraphy (LSFG), a newly developed technology, to evaluate skin blood flow after the injections.8–10 Recently, LSFG has been used for assessing blood flow not only in experimental systems, but also in clinical analyses.8–10 Because LSFG uses a laser to estimate blood flow, LSFG allows for the noninvasive and quantitative evaluation of blood flow in an area of interest without the use of contrast agents.8–10 To our knowledge, this study is the first to use LSFG to evaluate skin blood flow before and after the injection of an HA filler.
CASE REPORT
The patient was a 38-year-old man. After obtaining written informed consent, an HA filler (Juvederm Vista Ultra XC: 0.70 ml, Allergan plc) was injected into the region just under the dermis in the left nasolabial fold and alar base using the conventional method. The same HA filler (Juvederm Vista Ultra XC: 0.70 ml, Allergan plc) was injected into the periosteum in the right nasolabial fold and alar base using the ultrasonography-guided cannula method (Figs. 1, 2). Branches of the facial artery supply blood to the alar base; if vascular embolisms arise here, considerable serious cutaneous necrosis of the nasal wings can occur.4,5 However, the cannula and the blood vessel were successfully detected using ultrasonography, and the filler could be injected into the periosteum, easily avoiding the blood vessels (Fig. 3). Using LSFG as described in previous reports,8–10 we evaluated the skin blood flow before and after the injections. We focused on the alar base in evaluating the skin blood flow, as it is considered the most sensitive area for ischemic states (Table 1). LSFG revealed a reduction in skin blood flow in both sides of the face; however, the blood flow reduction was less with the ultrasonography-guided cannula method (Table 1).
Fig. 1.
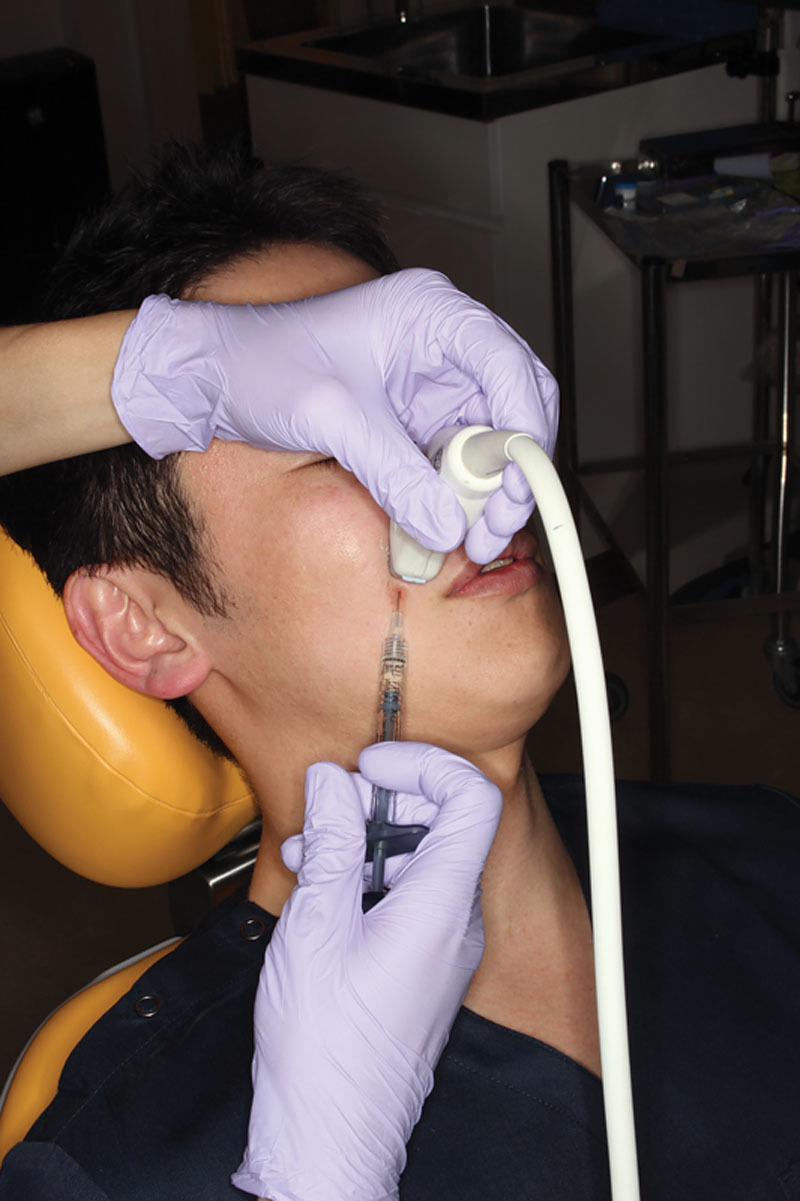
Injection into the right nasolabial folds and alar base using the ultrasonography-guided cannula method.
Fig. 2.
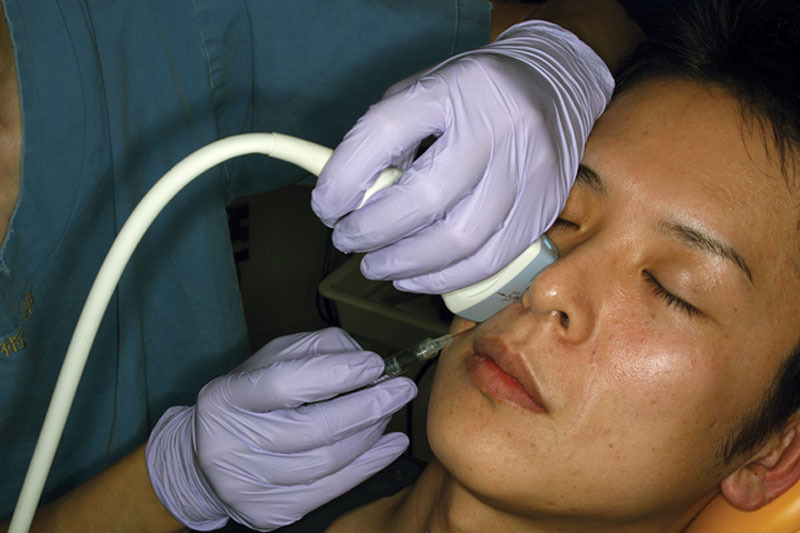
Injection into the right nasolabial folds and alar base using the ultrasonography-guided cannula method.
Fig. 3.
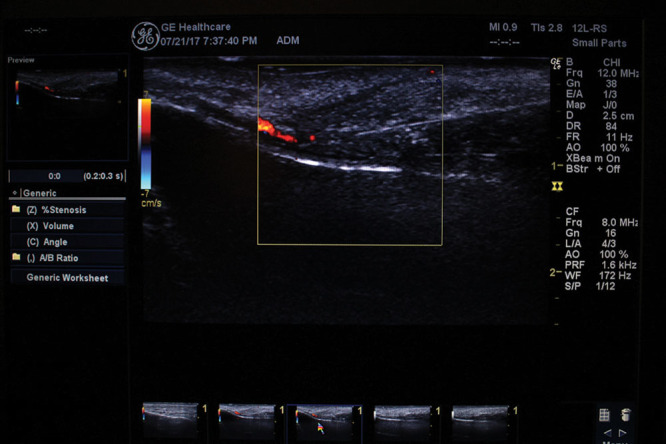
The cannula and the blood vessel clearly detected using ultrasonography.
Table 1.
Longitudinal Change in the LSFG Parameters before and after the HA Filler Injections

DISCUSSION
We explored 2 important clinical issues. Our exploratory use of this method provided case evidence of the usefulness of an ultrasonography-guided cannula for preventing vascular embolism that can be caused by injection of HA fillers. Moreover, we used LSFG to evaluate skin blood flow after the injections.
First, HA fillers are generally safe and are 1 of the most popular cosmetic injectables for facial rejuvenation worldwide.1–7 However, they are associated with some complications, such as skin necrosis and blindness due to vascular embolisms, and the increasing incidence of these complications is a severe problem.3–7 These complications can be mostly prevented by using appropriate techniques; however, the nasolabial folds, alar base, and the glabellar region are still high-risk areas because of the small-caliber vessels and inadequate collateral circulation in these regions.3–5,7 Although knowledge of the anatomy of the injection regions can minimize risks, the depth and distribution of the facial artery branches can sometimes vary.4,5 Recently, some reports have suggested strategies to reduce the risk of these complications, such as avoiding the use of smaller needles, aspirating before injection, injecting smaller volumes slowly, and injecting into a more superficial region.3–5,7 However, the use of the combination of larger volumes and deeper injection placement is increasingly becoming common to achieve revolumization.5,7 Some studies also recommend the use of hyaluronidase, a mucolytic enzyme that can hydrolyze HA fillers, if complications arise, but it is not completely effective in some patients.2,5,6 Therefore, we believe that prevention of these complications is critical. Our study showed the potential benefits of the ultrasonography-guided cannula method. Under ultrasonography guidance, the blood vessels can be easily visualized and the tip of the cannula can be identified, thereby reducing the risk of intravascular infusion of the filler.
Second, because LSFG can identify ischemic states,9 we used it to evaluate skin blood flow after the injections, which can help predict the development of skin necrosis.9 In this study, LSFG allowed for quick and easy blood flow evaluations and revealed a reduction in skin blood flow after injection with both the conventional method and ultrasonography-guided cannula method. However, the ultrasonography-guided cannula method was more effective in maintaining skin blood flow than the conventional method. We speculate that temporary subcutaneous stretching might have exerted pressure on the subdermal vascular network, causing a reduction in blood flow after injection into superficial regions with the conventional method.
One limitation of this exploratory study is that we studied only a single patient and could not confirm if reduction in the skin blood flow would be significant or cause necrosis. Another limitation is that we did not take cost and time issues into consideration. Although further studies are necessary to corroborate our findings, we conclude that the ultrasonography-guided cannula method could reduce the risks associated with intravascular HA filler injections and suggest that LSFG might be a potential technology for the quick evaluation of skin blood flow to predict the cutaneous necrosis associated with HA filler injections.
Footnotes
Published online 20 April 2018.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (Restylane) in facial cosmetic surgery: review and technical considerations. Plast Reconstr Surg. 2007;120:41S–54S.. [DOI] [PubMed] [Google Scholar]
- 2.DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561–575.. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197–1201.. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31:110–121.. [DOI] [PubMed] [Google Scholar]
- 5.Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097–1117.. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthet Surg J. 2015;35:844–849.. [DOI] [PubMed] [Google Scholar]
- 7.DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014;34:584–600.. [DOI] [PubMed] [Google Scholar]
- 8.Kunikata H, Aizawa N, Kudo M, et al. Relationship of ocular microcirculation, measured by laser speckle flowgraphy, and silent brain infarction in primary aldosteronism. PLoS One. 2015;10:e0117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagami G, Sari Y, Nagase T, et al. Evaluation of the usefulness of skin blood flow measurements by laser speckle flowgraphy in pressure-induced ischemic wounds in rats. Ann Plast Surg. 2010;64:351–354.. [DOI] [PubMed] [Google Scholar]
- 10.Furuta T, Sone M, Fujimoto Y, et al. Free flap blood flow evaluated using two-dimensional laser speckle flowgraphy. Int J Otolaryngol. 2011;2011:297251. [DOI] [PMC free article] [PubMed] [Google Scholar]


